Abstract
Patients with COVID-19 have shown melatonin deficiency. We evaluated the efficacy and safety of administration oral melatonin in patients with COVID-19-induced pneumonia. Patients were randomly assigned in a 1:1 ratio to receive melatonin plus standard treatment or standard treatment alone. The primary outcomes were mortality rate and requirement of IMV. The clinical status of patients was recorded at baseline and every day over hospitalization based on seven-category ordinal scale from 1 (discharged) to 7 (death). A total of 226 patients (109 in the melatonin group and 117 in the control group) were enrolled (median age; in melatonin group: 54.60 ± 11.51, in control group: 54.69 ± 13.40). The mortality rate was 67% in the melatonin group and 94% in the control group (OR; 7.75, 95% CI, 3.27–18.35, P < 0.001). The rate of IMV requirement was 51.4% in the melatonin group and 70.9% in the control group, for an OR of 2.31 (95% CI, 1.34–4.00, P < 0.001). The median number of days to hospital discharge was 15 days (13–17) in the melatonin group and 21 days (14–24) in the control group (OR; 5.00, 95% CI, 0.15–9.84, P = 0.026). Time to clinical status improvement by ≥ 2 on the ordinal scale in was 12 days (9–13) in the melatonin group and 16 days (10–19) in the control group (OR; 3.92, 95% CI, 1.69–6.14, P = 0.038). Melatonin significantly improved clinical status with a safe profile in patients with severe COVID-19 pneumonia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10787-022-01096-7.
Keywords: Melatonin, COVID-19, Cytokine storm, Inflammation, Antioxidant
Introduction
Coronavirus disease 2019 (COVID-19) was a newly emerged infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Baloch et al. 2020). This disease has several consequences, including inflammation, sepsis, cytokine storm, hypoxia, fibrosis, neurological disorders, and liver dysfunction (Bouck et al. 2021). Acute respiratory distress syndrome (ARSD) is the most important complication in COVID-19 patients (Mazeraud et al. 2022). Also, the SARS-CoV-2 impair coagulation process that causes disorders as disseminated intravascular coagulation (DIC) and venous thromboembolism (VTE) (Al-Samkari et al. 2020). Although, there are currently some vaccines and drugs to inhibit COVID-19, but due to the appearance of various variants of the virus, efforts to find a best protocol treatment for the disease are ongoing (Cook et al. 2022).
Studies have shown that the serum level of melatonin in patients with COVID-19 has decreased due to disruption in melatonin synthesis pathway in the body by SARS-CoV-2 (Camp et al. 2021; Sen 2021). Melatonin is a nocturnal hormone secreted by the pineal gland with antioxidant, anti-inflammatory potential (Sánchez-López et al. 2018), immunoregulatory (Marzougui et al. 2021), and ion chelator activity (Sen 2021) Besides, it also has a main role in sleep regulation (Zisapel 2018). Clinical studies demonstrated, melatonin could significantly decrease sepsis reactions in newborns (Gitto et al. 2001; Henderson et al. 2018). Ex vivo model has supported that melatonin could prevent sepsis by regulating oxidative stress, and cytokine responses (Galley et al. 2014). Melatonin could improve the risk of thrombosis by reducing clotting factor activity and plasma level of FVIII:C, VII (FVII:C), and fibrinogen (Wirtz et al. 2008). Melatonin could decrease proinflammatory cytokines in patients with advanced nonalcoholic steatohepatitis (NASH) (Cichoz-Lach et al. 2010). In patients with neoplasms, melatonin could improve clinical status by reducing immunomodulating cytokines IL-2 and IL-12. IL-2 could induce lymphocytosis and IL-12 alone could cause lymphocytopenia (Lissoni 2000). Clinical study on patients with ARSD indicated that melatonin reduces inflammation by inhibiting histone-induced NLRP3 inflammasome activation and suppressing the release of extracellular histones (Zhang et al. 2016). It has also shown that the administration of melatonin could reduce thrombosis, sepsis, and mortality rate in hospitalized patients with COVID-19 (Hasan et al. 2022).
Considering the above-mentioned, it is possible that many consequences caused by COVID-19 were due to melatonin deficiency in these patients. Also, the need for high efficient pharmacological agent for the management of COVID-19. Therefore, in this study, we evaluated the efficacy and safety of addition oral melatonin to standard treatment of hospitalized patients with COVID-19-induced pneumonia.
Materials and methods
Study Design
This is a single-center, open-label, randomized clinical trial with a parallel-group design to assess the efficacy and safety of melatonin in the treatment of patients with confirmed COVID-19 admitted to the intensive care unit (ICU). Patients were recruited between March 1, 2021, and Nonmember 30, 2021. The trial was conducted at Shahid Mohammadi Hospital affiliated with Hormozgan University of Medical Sciences in Bandar Abbas, Iran. Ethics approval was obtained from the ethics committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1399.526). This study was registered on the Iranian Registry of Clinical Trials (IRCT20200506047323N7), registered on February 16, 2021. The protocol of this trial was also published previously. The study was also undertaken in accordance with the guidelines of the Declaration of Helsinki and the principles of the International Conference on Harmonization Good Clinical Practice.
Participants
All patients with age of ≥ 20 years (weight ≥ 35 kg) with a confirmed diagnosis of COVID-19 based on the positive real-time polymerase chain reaction (RT-PCR) test and requirement of admission in ICU due to an oxygen saturation (SpO2) of < 88%, respiratory rate (RR) of > 30, PaO2/FiO2 ≤ 300 mmHg, or/and lung involvement > 50% in chest CT scan imaging were eligible to enroll in this trial. Patients with underlying diseases, including convulsive disorders, chronic hepatic and renal diseases, use of invasive mechanical ventilation (IMV), history of known allergy to melatonin, and during pregnancy or breastfeeding period were excluded. A written informed consent was obtained from all patients.
Randomization
Eligible patients based on the inclusion and exclusion criteria were randomly assigned to either the melatonin group or control group in a 1:1 ratio using an interactive web-based system. A stratified block randomization was used with a block size of six to create the randomization sequence. Sealed envelopes were used to protect the randomization sequence. A unique code was allocated to every patient to conceal their identity, and patients were assigned to the groups based on their unique code.
Intervention
Patients in the melatonin group received the standard treatment regimen for severe COVID-19 pneumonia, according to the protocol of the Iranian Ministry of Health and Medical Education plus melatonin soft gelatin capsule (Danna Pharmaceutical Company, Iran) at a dose of 5 mg twice a day for 7 days. Patients in the control group received the standard treatment regimen. Other supportive and routine care will be the same in both groups.
Outcomes
In this trial, the time of ICU admission was defined as the baseline, and patients were followed up for 28 days. A seven-category ordinal scale was recorded at baseline and every day over hospitalization to assess the clinical status of patients, based on the following categories; 1, discharged or ready to discharge; 2, non-ICU hospitalization without supplemental oxygen; 3, non-ICU hospitalization with supplemental oxygen; 4, non-ICU/ICU hospitalization with noninvasive ventilation; 5, ICU hospitalization with IMV; 6, ICU hospitalization with mechanical ventilation and additional organ support or extracorporeal membrane oxygenation; and 7, death.
The primary endpoints of this trial were the number of deaths until day 28, and need for invasive mechanical ventilation. Secondary outcomes were the clinical status of patients on the ordinal scale at days 7 and 14, length of stay (LOS) in hospital until discharge, the number of days until improvement by at least two categories on the seven-category ordinal scale, LOS in ICU and the number of ventilator-free days.
Statistical analysis
According to the previous studies, the study sample size was calculated based on the assumption that the clinical improvement by day 7 would be 80% in the treatment group and 55% in the control group (Davoudi-Monfared et al. 2020; Dequin et al. 2020; Rahmani et al. 2020). Considering a power of 80% and a significance level of 0.05, this trial needed 95 participants in each arm. Accounting for a probable 20% dropout rate, 114 patients were required in each group.
Continuous variables were presented as median (interquartile range), and categorical variables were expressed as frequency (percentages) of patients in each category. Mann–Whitney U test was applied to compare clinical status, LOS, and ventilator-free days between the studied groups. Moreover, mortality and IMV rates were compared using Fisher’s exact test. Between-group difference and Odds ratio (OR) were also calculated. The daily changes of vital signs, arterial blood gasses (ABGs), and laboratory findings were compared between the groups using the Generalized Estimation Equation (GEE) model considering the time, treatment, and their interaction in the analysis. The SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and p < 0.05 was considered as statistically significant.
Results
Study population
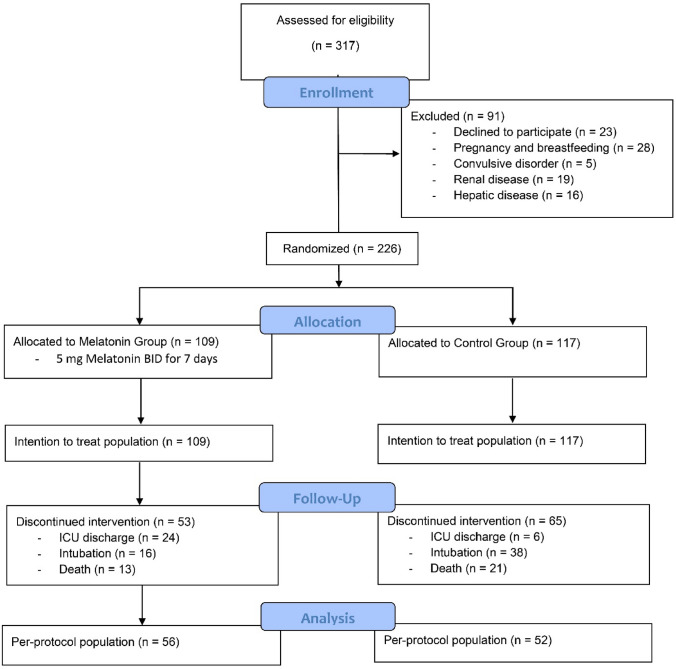
A total of 317 patients with laboratory-confirmed SARS-CoV-2 infection were screened. 91 patients were excluded based on exclusion criteria. After assessment of eligibility criteria, 226 patients were randomly allocated to the melatonin (n = 109) and control (n = 117) groups, their data analyzed as intent-to-treat population. Fifty-three patients in the melatonin group and 65 patients in the control group could not complete their intervention for at least 7 days because of intubation, or death, or ICU discharge before 7 days, and the remaining patients completed the treatment at least for 7 days and were used for analysis as per-protocol population (Fig. 1). None of the patients discontinued their intervention because of adverse drug events.
Fig. 1.
Flowchart of patient enrollment and selection
Demographic characteristics of patients were generally similar between the two trial groups. The mean age of the patient was 54.60 ± 11.51 years in the melatonin group and 54.69 ± 13.40 years in the control group, and about 50% of patients in each group were male. Symptoms onset until intervention and ward hospitalization before intervention were similar between the two trial group. Baseline results of lung imaging were also well balanced between the studied groups. The percentage of patients who received antiviral and glucocorticoid treatments was not statically different between the groups (Table 1).
Table 1.
Demographic, baseline lung imaging, and clinical characteristics of the patients
| Characteristic | Melatonin (n = 109) | Control (n = 117) | P-value |
|---|---|---|---|
| Age, year | 54.60 ± 11.51 | 54.69 ± 13.40 | 0.954 |
| 20–39 | 6 (5.5) | 14 (12.0) | |
| 40–69 | 96 (88.1) | 91 (77.8) | 0.112 |
| 70–99 | 7 (6.4) | 12 (10.3) | |
| Male gender | 47 (43.1) | 49 (41.9) | 0.893 |
| Underlying disease | |||
| Hypertension | 34 (31.2) | 26 (22.2) | 0.135 |
| Diabetes mellitus | 36 (33.0) | 30 (25.6) | 0.244 |
| Hyperthyroidism | 22 (20.2) | 11 (9.4) | 0.024 |
| Hypothyroidism | 7 (6.4) | 2 (1.7) | 0.092 |
| Asthma | 7 (6.4) | 4 (3.4) | 0.362 |
| Thalassemia | 9 (8.3) | 5 (4.3) | 0.273 |
| Coronary artery disease | 9 (8.3) | 5 (4.3) | 0.273 |
| Rheumatoid arthritis | 5 (4.6) | 3 (2.6) | 0.487 |
| Malignancy | 8 (7.3) | 4 (3.4) | 0.241 |
| Imaging findings | |||
| Right upper zone infiltrate | 58 (53.2) | 61 (52.1) | 0.895 |
| Right middle zone infiltrate | 101 (92.7) | 106 (90.6) | 0.637 |
| Right lower zone infiltrate | 109 (100) | 117 (100) | – |
| Left upper zone infiltrate | 47 (43.1) | 45 (38.5) | 0.500 |
| Left middle zone infiltrate | 99 (90.8) | 102 (87.2) | 0.405 |
| Left lower zone infiltrate | 109 (100) | 117 (100) | – |
| Symptoms onset until hospitalization | 9.84 ± 1.66 | 9.89 ± 1.66 | 0.839 |
| Symptoms onset until Intervention (admitted ICU) | 17.11 ± 2.16 | 17.08 ± 2.04 | 0.905 |
| Ward LOS before Intervention | 7.27 ± 1.60 | 7.19 ± 1.41 | 0.697 |
| Drugs treatments | |||
| Antiviral drugs | 57 (52.3) | 56 (47.9) | 0.594 |
| Glucocorticoids | 44 (40.4) | 56 (47.9) | 0.285 |
Values were presented as mean ± SD for continuous variables and n (%) for categorical variables. Baseline was defined as the time of admission in ICU before the administration of melatonin. Comparison between groups was performed using the t test or Fisher’s exact test. p < 0.05 was considered as statistically significant
Outcomes
Primary outcomes
In-hospital mortality until day 28 was reported in 73 patients (67%) in the melatonin group and 110 (94%) in the control group, for an OR of 7.75 (95% CI; 3.27–18.35, P < 0.001). The rate of mortality over the study is illustrated in (Supplementary Fig. 2). The rate of IMV was 51.4% in the melatonin group and 70.9% in the control group, for an OR of 2.31 (95% CI, 1.34–4) (Supplementary Fig. 2).
Secondary outcomes
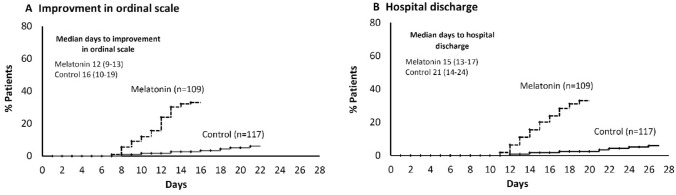
The clinical status on the 7-category ordinal scale at day 7 was 4.0 (ICU hospitalization with noninvasive ventilation, 4.0–4.0) in the melatonin group and 4.0 (4.0–5.0) in the control group (P = 0.014). The clinical status at day 14 was also lower in the melatonin group (3, non-ICU hospitalization with supplemental oxygen; 2.0–4.0) compared to the control group (4; 3–5) (P < 0.001). The information regarding time to the improvement of clinical status is illustrated in (Fig. 2, Supplementary Fig. 1, and Supplementary Table 2). The LOS in the hospital was 15 days (13–17) in the melatonin group and 21 days (CI, 14–24) in the control group (P = 0.026). The time to clinical improvement (by ≥ 2 on an ordinal scale) was 12 days (9–13) in the melatonin group and 16 days (10–19) in the control group (P = 0.038). The LOS in ICU was 9 days (6–10) in the melatonin group and 13 days (7–15) in the control group, for a difference of 3.59 days (95% CI, 1.95–5.24). Patients in the melatonin group had a more number of ventilator-free days (15.5 days; 7–18) than those in the control group (6 days; 4–9), for a difference of 6 days (95% CI, 3.98–8.03). All outcomes are summarized in (Table 2 and Supplementary Table 1).
Fig. 2.
Change in clinical status A hospital discharge, B over the study
Table 2.
Clinical outcomes of the patients
| Outcomes | Melatonin (n = 109) | Control (n = 117) | Difference or Odds Ratio | P-value |
|---|---|---|---|---|
| Primary outcome | ||||
| In-hospital mortality until day 28 | 73/109 (67.0) | 110/117 (94.0) | 7.75 (3.27–18.35) | < 0.001 |
| Invasive mechanical ventilation | 56/109 (51.4) | 83/117 (70.9) | 2.31 (1.34–4.00) | < 0.001 |
| Secondary outcome | ||||
| Clinical status at day 7a | 4.0 (4.0–4.0) | 4.0 (4.0–5.0) | – 0.34 ( – 0.93–0.06) | 0.014 |
| Clinical status at day 14a | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | – 0.12 ( – 1.77–0.71) | < 0.001 |
| Time to clinical improvementb | 12.0 (9.0–13.0) | 16.0 (10.0–19.0) | 3.92 (1.69–6.14) | 0.038 |
| LOS in hospital until dischargec | 15.0 (13.0–17.0) | 21.0 (14.0–24.0) | 5.00 (0.15–9.84) | 0.026 |
| LOS in ICUc | 9.0 (6.0–10.0) | 13.0 (7.0–15.0) | 3.59 (1.95–5.24) | < 0.001 |
| Ventilator-free days | 15.5 (7.0–18.0) | 6.0 (4.0–9.0) | 6.00 (3.98–8.03) | < 0.001 |
Values were presented as median (IQR) for continuous variables and n (%) for categorical variables. Primary and secondary outcomes were analyzed in the intent-to-treat population (patients received at least one dose of melatonin). Comparison between groups was performed using the Mann–Whitney U test or the Fisher’s exact test. p < 0.05 was considered as statistically significant
ICU intensive care unit, LOS length of stay
aMedian value for clinical status on the ordinal scale at day 7 and 14
bMedian number of days until improvement by ≥ 2 on the ordinal scale in clinical status
cMedian number of days until hospital/ICU discharge for alive patients
The results of the GEE model for vital signs (Supplementary Fig. 3), ABGs (Supplementary Fig. 4), hematological findings (Supplementary Fig. 5 and 6), coagulation functions (Supplementary Fig. 7), biochemical characteristics (Supplementary Fig. 8), liver function tests (Supplementary Fig. 9), and infection-related indices (Supplementary Fig. 10), and clinical symptoms (Supplementary Fig. 11) are demonstrated in the Supplementary Appendix.
Safety outcomes
Overall, more adverse events were reported in the control group compared to the melatonin group. Dizziness were the most prevalent side effects in melatonin group. Stroke, gastrointestinal hemorrhage, and hematuria were the most prevalent in the control group. Other adverse events were no significant difference between the melatonin and control groups. (Table 3).
Table 3.
Adverse drug events of the patients
| Adverse drug events | Melatonin Group (n = 109) | Control Group (n = 117) | P value |
|---|---|---|---|
| QTc interval prolongation | 16(14.7) | 11(9.4) | 0.305 |
| Cerebral hemorrhage | 14(12.8) | 6(5.1) | 0.059 |
| Dizziness | 12(11) | 3(2.6) | 0.014 |
| Stroke | 9(8.3) | 21(17.9) | 0.048 |
| Myopathy | 12(11) | 15(12.8) | 0.688 |
| Palpitations | 16(14.7) | 21(17.9) | 0.591 |
| Tremor | 17(15.6) | 8(6.8) | 0.054 |
| Gastrointestinal hemorrhage | 7(6.4) | 19(16.2) | 0.023 |
| Intestinal cramps | 5(4.6) | 8(6.8) | 0.573 |
| MI | 12(11) | 11(9.4) | 0.826 |
| Rash | 28(25.7) | 18(15.4) | 0.069 |
| Hematuria | 8(7.3) | 25(21.4) | 0.004 |
Values were expressed as n (%). Comparison between groups was performed using Fisher’s exact test. Data were collected within period of randomization. The events were studied in the intent-to-treat population
Discussion
In this randomized, open-label single-center clinical trial with a parallel group, the efficacy and safety of oral melatonin was assessed in hospitalized patients with severe COVID-19 within 28 days. The mortality rate, need for IMV, clinical status, ventilator-free days, and hospital length of stay (LOS) were significantly improved in patients who received melatonin compared to the control group.
The length of stay (LOS) in ICU and hospital were significantly lower in patients of the melatonin group than in the control group. Dianatkhah et al. reported melatonin administration reduced the LOS in the ICU in patients with hemorrhagic stroke admitted to ICU (Dianatkhah et al. 2017). In another clinical trial on a patient admitted to ICU after coronary artery bypass grafting operation, melatonin had effectively reduced their LOS in ICU and hospital (Can et al. 2018).
Our results indicated a reduction in inflammation in COVID-19 patients. The inflammatory biomarkers during the first 7 days of intervention in patients with the same lung involvement were reduced. Inflammation and cytokine storms in the respiratory system were some of the serious problems in COVID-19 patients (Rodríguez-Rubio et al. 2020). Cichoz-Lach et al. indicate melatonin could reduce inflammation by pro-inflammatory cytokine (Cichoz-Lach et al. 2010). Another clinical study has shown melatonin decrease and increases in IL-2, and IL-12 levels play an immunomodulating role in lymphocytosis and lymphocytopenia, controlling inflammation in patients with neoplasm (Lissoni 2000). In another clinical trial performed on infants with chronic lung disease, melatonin has reduced mortality in the group who received melatonin compared to those who did not receive melatonin by reducing inflammatory factors and cytokines. In patients with non-small cell lung carcinoma, melatonin by controlling immune function/inflammation could delay mortality (Seely et al. 2021).
Our results demonstrated increment in hospital discharge which may be due to the reduction of sepsis reactions in COVID-19 patients. Sepsis was the chain extreme immunological response to an infection. Sepsis was a dangerous complication in COVID-19 patients, which often occurred in severe and critical COVID-19 patients. Gitto et al. had demonstrated in a clinical study on septic newborns, melatonin could control sepsis effectively. Melatonin could be able to prevent sepsis reactions in 24–48 h after intervention effectively (Gitto et al. 2001). A clinical study on the experimental human sepsis model had shown the administration of melatonin could reduce sepsis reaction by reducing pro-inflammatory cytokine. The results of this study also showed melatonin could prevent oxidative damages by playing its antioxidant role in reducing oxidative stress (Alamili et al. 2014). Other randomized controlled trials had indicated administration of melatonin along with standard therapy in patients with septic shock could significantly reduce multiple organ failure acute respiratory distress syndrome (Aisa-Alvarez et al. 2020). Thanon Hasan et al. had shown in patients with COVID-19 administration, melatonin along with standard therapy could reduce the mortality rate due to reduction sepsis reaction and thrombosis events (Hasan et al. 2022). Camp et al. had shown melatonin could reduce sepsis and cytokine storm in patient with COVID-19 by regulating immune response. In addition, melatonin could reduce thromboembolism events in patients with COVID-19 (Camp et al. 2021).
Our result had shown that clotting parameters such as D-dimer, PT, PTT had reduced, and INR had improved. Our study indicates melatonin could improve thrombosis status in COVID-19 patients. Reduction of mortality rate may be related to improvement in thrombosis status in these patients. Thrombosis events such as venous thromboembolism (VTE) and deep vein thrombosis (DVT) were the critical concern in COVID-19 patients, which caused the death of many of these patients. Wirtz et al. had shown melatonin could reduce thrombotic risk by reducing activation of fibrinogen, FVII: C in serum (Wirtz et al. 2008). A clinical study on tetraplegia patients who had a high risk of venous thrombosis and demonstrated that patients who received melatonin had improved endogenous thrombin potential (ETP) and thrombin generation peak compared to placebo group (Iversen et al. 2015). Other clinical study on tetraplegia patients were conducted in Norway had indicated melatonin could reduce the risk of venous thrombosis by reducing activation FVII (Aisa-Alvarez et al. 2020). A clinical study on COVID-19 patients showed that in patients who received venous melatonin, the thromboembolism occurrence was less compared to control group, D-dimer level had also reduced in serum. There was an improved thrombosis condition in melatonin group patients and reduced mortality rate in melatonin group compared to control group (Hasan et al. 2022).
We have assessed 16 clinical symptoms like chills, headache, sore throat, diarrhea, cough, dyspnea, phlegm, lethargy, muscular pain, tiredness, chest pain, nausea, anorexia, abdominal pain, smell loss, taste loss during the first 7 days after the intervention. Our results had shown melatonin had improved these clinical symptoms except phlegm. Anderson et al. demonstrated melatonin could improve some COVID-19 symptoms consistent with our obtained results (Anderson and Reiter 2020). Randomized controlled trial conducted in Lancaster County, Pennsylvania, United states had shown oral melatonin could improve clinical symptoms in mild–moderate COVID-19 patient during 14 days of intervention and improve life quality (Fogleman et al. 2022).
In adverse drug events assessing we observed gastrointestinal hemorrhage occurred significantly less in the melatonin group than in the control group. Anderson et al. indicated that melatonin had beneficial effects on the gut disorder and improved systemic homeostasis. There results indicated melatonin could prevent the occurrence of gastrointestinal disorders (Anderson and Maes 2020). A recent systematic review has reported treatment with melatonin has slight side effects, including sleepiness. But in some cases, adverse drug events such as dizziness were also observed (Foley and Steel 2019).
Our findings had shown mortality rate and need for IMV was significantly reduced. One of the leading causes of death and need of IMV in COVID-19 patients was the occurrence of acute respiratory distress syndrome (ARSD). Zhang et al. demonstrated that melatonin could reduce the occurrence of acute respiratory distress syndrome (ARDS) and acute lung injury in COVID-19 patients and reduce the mortality rate in these patients by its antioxidant and anti-inflammatory effects. (Zhang et al. 2020). Kleszczyński et al. indicated melatonin with immunoregulating effect could inhibit the inflammatory reactions in the lung and prevent the occurrence of ARDS and ALI in patient with COVID-19 (Kleszczyński et al. 2020). Other study had also shown melatonin had been able to reduce ARDS and ALI occurrence in patients with COVID-19 by its anti-inflammatory and immunomodulatory effects (Nair 2022). A clinical study was conducted in Mexico on patients admitted to ICU who had indicated that the use of melatonin could reduce ARDS occurrence and oxidative stress (Aisa-Alvarez et al. 2020). Schneider et al. had shown melatonin with its antioxidant effect reduced oxidative stress in patient with COVID-19. Furthermore, melatonin could reduce cytokine storms and severe respiratory inflammatory reactions by regulating immune responses in patients with COVID-19. As result, melatonin could reduce IMV requirement in patient with COVID-19 (Shneider et al. 2020).
The results of our study show that melatonin, due to its iron-chelating role, has been able to prevent the excessive increase of iron ions in COVID-19 patients, and the first 7-day ferritin test confirms it in these patients. The liver was primarily responsible for the scavenger of iron ions, and excess iron ion could damage liver cells. But, our result showed the liver biomarkers levels such as ALT, AST, and ALP during the first 7 days of the intervention demonstrated that liver status was improved in patients who received melatonin compared to the control group (Sen 2021). There was an indication that melatonin could reduce liver damage by its iron-chelating effect. These excess iron ions come from the SARS-CoV-2 attack to the 1-beta chain of hemoglobin and causes iron ions to separate from hemoglobin. Hemoglobin is primarily responsible for the exchange of oxygen and carbon dioxide gases. Hemoglobin loses its ability in the absence of bounded iron ions (Kronstein-Wiedemann et al. 2022). Patients with COVID-19 have respiratory disorder which reduces oxygen saturation, increases CO2 and HCO3- blood levels, disturbance in normal range of respiratory blood gases made loss of consciousness and mortality. Camp et al. study had shown melatonin could improve oxygen saturation (SpO2) level and reduced hypoxia in patient with COVID-19 by increasing the hemoglobin capacity to absorb oxygen (Camp et al. 2021). The results of our study had indicated the improvement of respiratory gases ventilation status in melatonin group compared to control group. This may have been effective in reducing needs IMV and hospital LOS and increasing ventilator-free days and discharge rate alive.
Melatonin has antiviral activity. Studies had shown supplemental melatonin helps eradicate the pathogenicity of Venezuelan equine encephalitis (VEE) virus, Semliki Forest virus (SFV), West Nile virus (WNV) (Bahrampour Juybari et al. 2020; Bonilla et al. 2004), especially the Ebola virus (Reiter et al. 2020) and severe acute respiratory syndrome (SARS) (Shiu et al. 2003). The SARS-CoV-2 had emerged from the mutation of SARS and very similar to SARS virus. Considering that melatonin had been effective against SARS, there was a possibility that it is also effective against SARS-CoV-2 and has reduced mortality and increased hospital discharges alive in melatonin group compared control group.
Considering the pharmacological properties of melatonin, including the abilities to mitigate inflammation, diminish redox stress, and moderate immune response, could improve the success of clinical management in patients with severe COVID-19 pneumonia.
The main limitation of the trial was the small number of enrolled patients. This study has been initially protocoled as a preliminary stage before multiple-center trials with larger sample size; more trials with larger sample sizes, different populations, and high-quality designs are needed to confirm the results of this preliminary study. However, being a natural molecule, well-studied, with high tolerability and low price, are the main advantages of melatonin. Therefore, oral melatonin administration could be effective in patients with severe COVID-19, in poor communities, particularly those do not have access to vaccines.
Conclusion
The use of oral melatonin in patients with severe COVID-19 along with standard treatment could reduce mortality, IMV, hospital LOS and improve clinical status. Moreover, ventilator-free days and hospital discharge alive increased. Inflammatory markers, arterial blood gases parameters and coagulating parameters had improved in melatonin group compared control group. Although melatonin had good effect in patients with severe COVID-19 but significantly increased the risk of dizziness in these patients. However, confirmation of the results of this preliminary trial requires more detailed, randomized trials in larger populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciably thank the trial patients and their families, whose help and participation made this work possible.
Author contributions
AA, AZ, and MF: conceptualization, methodology, software. MF, MM, and SH: data curation, writing-original draft preparation. MF, MV, OS, and MK: visualization, investigation. AAi: supervision. SH: software, validation. MF: writing- reviewing and editing.
Funding
This research was funded by the Hormozgan University of Medical Sciences (no; 990592).
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Other data requests will be considered by the management group upon written request to the corresponding authors.
Declarations
Conflict of interest
None of the authors have a conflict of interest to disclose.
Ethical approval and consent to participate
Ethics approval was obtained from the ethics committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1399.526). The study was also undertaken in accordance with the guidelines of the Declaration of Helsinki and the principles of the International Conference on Harmonization Good Clinical Practice.
Consent for publication
A written informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manoochehr Kamali, Email: M.kamali@hums.ac.ir.
Mohammad Fathalipour, Email: m.fathalipour@yahoo.com.
References
- Aisa-Alvarez A, Soto ME, Guarner-Lans V, Camarena-Alejo G, Franco-Granillo J, Martínez-Rodríguez EA, Gamboa Ávila R, Manzano Pech L, Pérez-Torres I. Usefulness of antioxidants as adjuvant therapy for septic shock a randomized clinical trial. Medicina (kaunas) 2020;56:619. doi: 10.3390/medicina56110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gögenur I. Melatonin suppresses markers of inflammation and oxidative damage in a human daytime endotoxemia model. J Crit Care. 2014;29:184.e9–184.e13. doi: 10.1016/j.jcrc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M. Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr Top Med Chem. 2020;20:524–539. doi: 10.2174/1568026620666200131094445. [DOI] [PubMed] [Google Scholar]
- Anderson G, Reiter RJ. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol. 2020;30:e2109. doi: 10.1002/rmv.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BahrampourJuybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S. Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res. 2020;287:198108. doi: 10.1016/j.virusres.2020.198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloch S, Baloch MA, Zheng T, Pei X. The Coronavirus disease 2019 (COVID-19) Pandemic. Tohoku J Exp Med. 2020;250:271–278. doi: 10.1620/tjem.250.271. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Valero N, Chacín-Bonilla L, Medina-Leendertz S. Melatonin and viral infections. J Pineal Res. 2004;36:73–79. doi: 10.1046/j.1600-079X.2003.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck EG, Denorme F, Holle LA, Middelton EA, Blair AM, de Laat B, Schiffman JD, Yost CC, Rondina MT, Wolberg AS, Campbell RA. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol. 2021;41:401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp OG, Bai D, Gonullu DC, Nayak N, Abu-Soud HM. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J Inorg Biochem. 2021;223:111546. doi: 10.1016/j.jinorgbio.2021.111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can MG, Ulugöl H, Güneş I, Aksu U, Tosun M, Karduz G, Vardar K, Toraman F. Effects of alprazolam and melatonin used for premedication on oxidative stress, glicocalyx integrity and neurocognitive functions. Turk J Anaesthesiol Reanim. 2018;46:233–237. doi: 10.5152/TJAR.2018.65475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M. The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J Physiol Pharmacol. 2010;61:577–580. [PubMed] [Google Scholar]
- Cook B, Mascolo M, Bass G, Duffy ME, Zehring B, Beasley T. Has COVID-19 Complicated eating disorder treatment? An examination of comorbidities and treatment response before and during the COVID-19 pandemic. Prim Care Companion CNS Disord. 2022;24:21m03087. doi: 10.4088/PCC.21m03087. [DOI] [PubMed] [Google Scholar]
- Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, Kazemzadeh H, Yekaninejad MS. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64:e01061–e1120. doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, François B, Aubron C, Ricard JD, Ehrmann S, Jouan Y, Guillon A, Leclerc M, Coffre C, Bourgoin H, Lengellé C, Caille-Fénérol C, Tavernier E, Zohar S, Giraudeau B, Annane D, le Gouge A. Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients With COVID-19: a randomized clinical trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianatkhah M, Najafi A, Sharifzadeh M, Ahmadi A, Sharifnia H, Mojtahedzadeh M, Najmeddin F, Moghaddas A. Melatonin supplementation may improve the outcome of patients with hemorrhagic stroke in the intensive care unit. J Res Pharm Pract. 2017;6:173–177. doi: 10.4103/jrpp.JRPP_17_49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogleman C, Cohen D, Mercier A, Farrell D, Rutz J, Bresz K, Vernon T. A pilot of a randomized control trial of melatonin and vitamin C for mild-to-moderate COVID-19. J Am Board Fam Med. 2022;35:695–707. doi: 10.3122/jabfm.2022.04.210529. [DOI] [PubMed] [Google Scholar]
- Foley HM, Steel AE. Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther Med. 2019;42:65–81. doi: 10.1016/j.ctim.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. 2014;56:427–438. doi: 10.1111/jpi.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, Cordaro S, Corona G, Trimarchi G, Barberi I. Effects of melatonin treatment in septic newborns. Pediatr Res. 2001;50:756–760. doi: 10.1203/00006450-200112000-00021. [DOI] [PubMed] [Google Scholar]
- Hasan ZT, Atrakji D, Mehuaiden DAK. The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int J Infect Dis. 2022;114:79–84. doi: 10.1016/j.ijid.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R, Kim S, Lee E. Use of melatonin as adjunctive therapy in neonatal sepsis: a systematic review and meta-analysis. Complement Ther Med. 2018;39:131–136. doi: 10.1016/j.ctim.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Iversen PO, Dahm A, Skretting G, Mowinckel MC, Stranda A, Østerud B, Sandset PM, Kostovski E. Reduced peak, but no diurnal variation, in thrombin generation upon melatonin supplementation in tetraplegia. A randomised, placebo-controlled study. Thromb Haemost. 2015;114:964–968. doi: 10.1160/TH15-05-0396. [DOI] [PubMed] [Google Scholar]
- Kleszczyński K, Slominski AT, Steinbrink K, Reiter RJ. Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients. 2020;12:2561. doi: 10.3390/nu12092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstein-Wiedemann R, Stadtmüller M, Traikov S, Georgi M, Teichert M, Yosef H, Wallenborn J, Karl A, Schütze K, Wagner M, El-Armouche A, Tonn T. SARS-CoV-2 infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism. Stem Cell Rev Rep. 2022;18:1809–1821. doi: 10.1007/s12015-021-10322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissoni P. Modulation of anticancer cytokines IL-2 and IL-12 by melatonin and the other pineal indoles 5-methoxytryptamine and 5-methoxytryptophol in the treatment of human neoplasms. Ann N Y Acad Sci. 2000;917:560–567. doi: 10.1111/j.1749-6632.2000.tb05421.x. [DOI] [PubMed] [Google Scholar]
- Marzougui H, Hammouda O, Bendhia I, Maaloul R, Agrebi I, Chaker H, Kammoun K, Benhmida M, Ayadi F, Kallel C, Driss T, Turki M, MasmoudI H, Hachicha H. Melatonin ingestion before intradialytic exercise improves immune responses in hemodialysis patients. Int Urol Nephrol. 2021;53:553–562. doi: 10.1007/s11255-020-02643-3. [DOI] [PubMed] [Google Scholar]
- Mazeraud A, Jamme M, Mancusi RL, Latroche C, Megarbane B, Siami S, Zarka J, Moneger G, Santoli F, Argaud L, Chillet P, Muller G, Bruel C, Asfar P, Beloncle F, Reignier J, Vinsonneau C, Schimpf C, Amour J, Goulenok C, Lemaitre C, Rohaut B, Mateu P, de Rudnicki S, Mourvillier B, Declercq PL, Schwebel C, Stoclin A, Garnier M, Madeux B, Gaudry S, Bailly K, Lamer C, Aegerter P, Rieu C, Sylla K, Lucas B, Sharshar T. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10:158–166. doi: 10.1016/S2213-2600(21)00440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AS. Perioperative melatonin in COVID-19 patients: benefits beyond sedation and analgesia. Med Gas Res. 2022;12:41–43. doi: 10.4103/2045-9912.325990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ, Fazeli MR, Ghazaeian M, Yekaninejad MS. Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol. 2020;88:106903. doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Ma Q, Sharma R. Treatment of ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 2020;3:43–57. doi: 10.32794/mr11250047. [DOI] [Google Scholar]
- Rodríguez-Rubio M, Figueira JC, Acuña-Castroviejo D, Borobia AM, Escames G, de la Oliva P. A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:699. doi: 10.1186/s13063-020-04632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-López AL, Ortiz GG, Pacheco-Moises FP, Mireles-Ramírez MA, Bitzer-Quintero OK, Delgado-Lara DLC, Ramírez-Jirano LJ, Velázquez-Brizuela IE. Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch Med Res. 2018;49:391–398. doi: 10.1016/j.arcmed.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Seely D, Legacy M, Auer RC, Fazekas A, Delic E, Anstee C, Angka L, Kennedy MA, Tai LH, Zhang T, Maziak DE, Shamji FM, Sundaresan RS, Gilbert S, Villeneuve PJ, Ashrafi AS, Inculet R, Yasufuku K, Waddell TK, Finley C, Shargall Y, Plourde M, Fergusson DA, Ramsay T, Seely AJE. Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): a randomized placebo controlled clinical trial. EClinicalMedicine. 2021;33:100763. doi: 10.1016/j.eclinm.2021.100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A. Deficient synthesis of melatonin in COVID-19 can impair the resistance of coronavirus patients to mucormycosis. Med Hypotheses. 2021;158:110722. doi: 10.1016/j.mehy.2021.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SY, Reiter RJ, Tan DX, Pang SF. Urgent search for safe and effective treatments of severe acute respiratory syndrome: is melatonin a promising candidate drug? J Pineal Res. 2003;35:69–70. doi: 10.1034/j.1600-079X.2003.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, Bärtschi C, Spillmann M, Ehlert U, von Känel R. Effect of oral melatonin on the procoagulant response to acute psychosocial stress in healthy men: a randomized placebo-controlled study. J Pineal Res. 2008;44:358–365. doi: 10.1111/j.1600-079X.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li X, Grailer JJ, Wang N, Wang M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX, Li X. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;60:405–414. doi: 10.1111/jpi.12322. [DOI] [PubMed] [Google Scholar]
- Zisapel Y. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol. 2018;175:3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Other data requests will be considered by the management group upon written request to the corresponding authors.