Abstract
Nanoparticles have aided in the development of nano-based sensors for diagnostic applications. However, use of nanoparticles in the development of sensing devices for multiple analyte detection is constrained due to their inability to detect several analytes with a single type of nanoparticle. The term “Janus particle” refers to micro or nanoscale particles that have been divided into sections or compartments, each of which has a distinct set of chemical or physical properties, producing multifunctional particles endowed with distinctive qualities. Furthermore, Janus particles have the ability to perform multiple functions within a single particle at the same time, with no interference from adjacent sections. This review focuses on the use of Janus particles in the fabrication of biosensors as well as in the investigation of various properties endowed by these Janus particles for their use as biosensors. It also discusses the various types of Janus particle-based biosensors that are currently available. Finally, the limitations of Janus particles in sensor technologies and their future scope have been discussed.
Graphical abstract
Keywords: Janus nanoparticle, Biosensing, Point of care
Introduction
Nanotechnology is a booming field with immense scope for fabricating materials of higher quality and enhanced properties. These properties include high surface to volume ratio, small size, ease of functionalization etc. Owing to these properties, nanoparticles have been employed for various applications like in drug delivery [1–3], sensing [4, 5] etc. Various sensing platforms have been developed using nanoparticles for detection of different analytes in diagnostics [6, 7], environment [8, 9], food industry [10, 11], defence [12–14] etc.
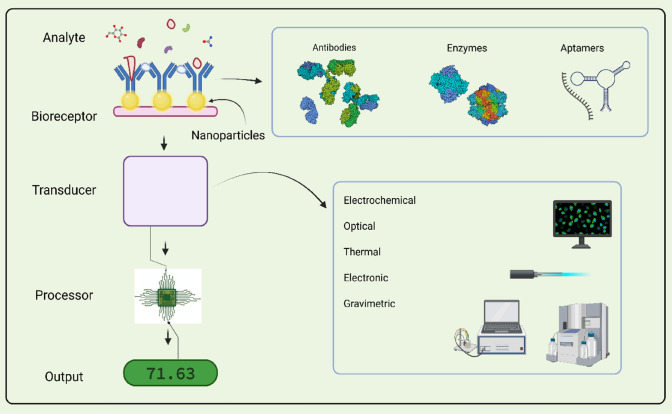
Nanoparticle-based sensors provide a promising future due to enhanced sensitivity, mechanical, optical, electrical properties, rapid detection and thus increasing the facets of the current biosensors (Fig. 1). Curtailment of the biosensor dimensions into micro or nano range can induce a better signal-to-noise ratio [15]. Apart from several advantages reported, the nanoparticle-based sensors also embody a couple of drawbacks. These include possibility of agglomeration of nanoparticles when they are not immobilized on a surface, multifunctionality for multi-analyte sensing, environment friendliness. Some nanoparticles such as the cerium oxide nanoparticles are readily reactive and sensitive to ROS (Reactive Oxygen Species). This sets a barrier for their application in detection of biomolecules in vivo [15].
Fig. 1.
Diagrammatic representation of nanoparticle-based biosensors. Created with BioRender
Despite of the fact that numerous macro, micro and nano isotropic particles have played crucial role in the development of the biomedical field, the Janus particles have attained an irreplaceable position in this field owing to their multifunctional property, cumulating multiple opposing properties in a single particle [16]. These properties of Janus particles in combination with nanotechnology can be instrumental in detection of multiple analytes without interference via different parts of a single particle [17, 18].
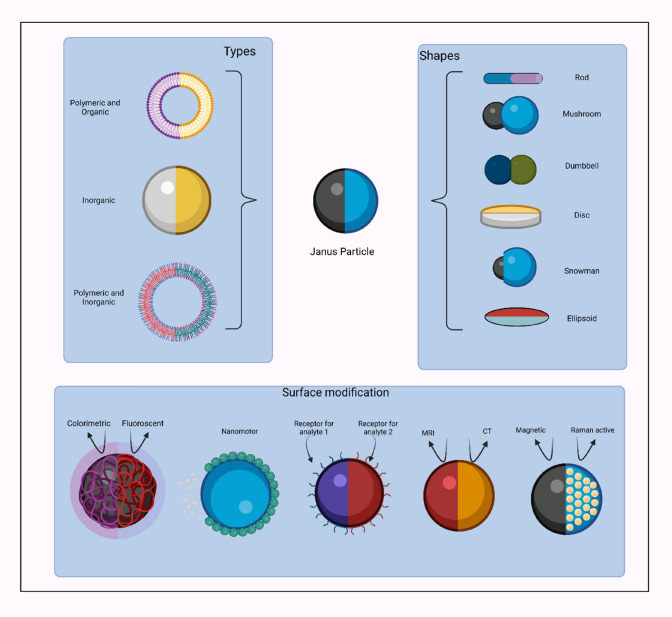
Janus particles are a unique set of colloidal particles in the nanoscale or microscale range, that can be explored for various applications with abundant fruitful ramifications. These particles have been inspired by the Roman God, Janus possessing two faces, each in the opposite direction. The Janus particles have a unique characteristic of exhibiting two different properties via different regions within a single particle as shown in Fig. 2 [19]. Casagrande and Veyssie in 1988 demonstrated the formulation of glass beads with a hydrophilic hemisphere attached to a hydrophobic one, later named as “Janus beads”. The term “Janus particles” was introduced by Pierre-Gilles de Gennes during his Nobel Laureate speech. These particles exhibited asymmetry, i.e., the Janus balance which means the specific area allotted to each patch of the particle can vary from two hemispheres to extremely small parts strewn. Calculation of the Janus balance value is paramount to delineate the best geometry for synthesis of efficient and stabilized Janus particles [20].
Fig. 2.
Schematic illustration depicting different types and shapes of Janus particle. Janus particles possess multifunctionality, endowing unique chemical or physical properties to the particle. Created with BioRender.com
Janus particles also have an analog in nature. Many fungi possess hydrophobin proteins which have a structure with a characteristic sequence of eight cysteine residues with conserved spacings in their primary sequence. They, thus exhibit hydrophobic and hydrophilic portions. Due to their the novel characteristics, multifunctional properties and extraordinary aggregation behaviour into superstructures of different length scales, the Janus particles find their use in numerous domains such as biochemistry, medicine, physics, material science, drug delivery etc [21].
With the correct use of the synergistic effect of Janus particles, their characteristics can be capitalized for valuable research and contriving devices for industrial use. However, their non-centrosymmetric feature makes their synthesis an arduous task. This resulted in a low production of Janus nanoparticles and thus dwindled the research conducted on these particles, further resulting in their stunted exploitation (2). Even so, recent techniques and neoteric methods of synthesis have provided an advantage for their augmented production (2). Thus, making the Janus nanoparticle suitable for an array of applications. Ranging from optical switchable devices, optical probes, self-propelling particles, catalysts, emulsifiers, disease detection, drug discovery and delivery, environmental monitoring, analysis of disease-causing microorganisms and markers that are indicators of disease in bodily fluids (blood, urine, saliva, sweat) and so forth (3). Moreover, various dyes and fluorescent particles (e.g., quantum dots), fluorophores and other such luminescent molecules can aid in the production of Janus particles for bio-imaging and bio-sensing purposes [22].
The review focuses on elucidating the structure of Janus particles and the diverse attributes offered by these non-centrosymmetric particles. It describes the contribution of the varied characteristics of Janus particles to enhance the properties of biosensors. Further, this review also gives a brief description of factors affecting the properties of Janus nanoparticles such as the composition, method of synthesis, temperature, etc. The review also provides a comprehensive account of different types of biosensors fabricated using Janus particles such as electrochemical, optical, optical-motile, etc.as well as how the utilization of Janus particles improves the LOD (Limit of detection) of sensors, resulting in highly sensitive and efficient biosensors. Lastly, the toxicity and exploration of the Janus particles for the fabrication of multi-analyte biosensors are also highlighted.
Properties of Janus nanoparticles
Janus nanoparticles own unique and unparalleled properties as aforementioned. As the development of these particles is very recent so far a few properties have been investigated and tested experimentally [23].
Optical properties
Optical properties of a particle or a substance describe the interaction of that particle or substance with light (reflection, refraction, etc.). With the advent of new research and experiments with metal nanoparticles, the exploitation of the optical properties of metal nanoparticles, metallic Janus particles and nanoparticle aggregates for biosensing has gained momentum [24].
Janus particles-based optical biosensors provide an efficacious solution for solving conundrums such as autofluorescence from samples and instrument optics. For example, the aspherical, magnetically modulated optical nanoprobes called MagMOONs, which are Janus particles undergo rotational diffusion, emitting flickering signals (Fig. 3). These MagMOONs provide a distinct and distinguishable signal, allowing facile biosensing as a blinking probe signal can be easily distinguished from the unmodulated background. This enables simple and sensitive sensing of low analyte concentrations despite autofluorescence and other background noise. Therefore, MagMOONs can further provide flexibility to the biosensing technique by broadening the range of dyes used and the experimental conditions under which these processes work. These particles are also capable of absorption and emission of different fluxes of light in different orientations due to internal reflection and self-absorption. MagMOONs are thus capable of upgrading various biomedical processes such as immunoassays, intracellular chemical sensors, cellular labels or tags, and protein folding studies by improving the signal-to-noise ratio [25].
Fig. 3.
Schematic representation of working principle of MagMOONS. A The aspherical MagMOONS align according the external magnetic field, generating a blinking on and off signal based on its rotation. B Experimental setup for the modulation of MagMOONS and separation of the blinking signals generated by the MagMOONS from the unmodulated background. Reprinted figure with permission from Anker JN, Behrend C, Kopelman R. Aspherical magnetically modulated optical nanoprobes (MagMOONs). J Appl Phys. 2003. Copyright (2003) by Journal of Applied Physics
As the Janus nanoparticles are having two different faces, they interact with light differently. These particles show dual emission characteristics with single excitation wavelength. These characteristics are also dependent on the environmental conditions like pH. Thus Janus nanoparticles can be utilized for developing pH sensors [26].
These observations imply that the optical properties of the Janus nanoparticles are dependent on the interactions of light with each face of the nanoparticle thus providing unique optical properties like absorption and emission of light in specific conditions like pH, orientation of the particles etc. resulting in flickering effect. This effect results in overcoming the limitations of autofluorescence experienced while using conventional nanoparticles.
Multifunctionality (non-centrosymmetry)
The presence of multiple domains in a single Janus particle may improve or create changes in the intrinsic properties (magnetic, electrical, optical, surface chemistry, etc.) of the particle. However, the unique properties of both segments of the particle collectively contribute to improvement and enhancement of the properties of the whole/complete particle [16]. Furthermore, novel functions can be established due to the spatial configuration of the different components in the Janus particle. The interface between the two separate components or domains of the particle also plays a crucial role in determining the surface area available for various reactions to take place [27]. These boundaries between the two components can be manipulated to increase or decrease the surface area for biorecognition or catalytic activity.
An important factor in a biosensor is the presence of an immobilization matrix which can ensure the efficient binding of the analyte and its stability, without its structural distortion [28]. This must be implemented without hindering the interaction of the analyte (direct or indirect) with the electrode. Janus particles resolve this concern due to their multifunctional nature. These particles allow the attachment of biomolecules such as antibodies, enzymes for their selective binding with target analytes. Moreover, different types of antibodies or enzymes can be attached to a single Janus particle thus highlighting its use for simultaneous, real-time multi-analyte sensing.
Inorganic Janus particles composed of metallic nanoparticles and oxide particles [29] or transition metal NPs and oxides [30] display high catalyst activity as the boundary of metals and oxide components serves as a site of binding for catalyst activation along with oxygen sorption [22]. Hence, this property of these particles can be utilized for biosensing as they prove to be superior to the existing core or shell particles as the multiple components present in the single particle interact independently with the external environment stimulus upon exposure to it [31].
This demonstrates that each of the two faces of Janus nanoparticles can act as two different catalytic sites or attachment sites for different reactions or bio recognition thus paving way for a possibility of utilizing these nanoparticles for multi-analyte detection.
Tunable nature
The composition, architecture and the surface chemistry play salient role for determining the properties and the applications of the Janus particles [16]. Janus particles possess surface chemistry variation due to their non-centrosymmetric physical or chemical nature. The heterogenicity offered by this idiosyncratic property of the Janus particles induces functionally distinct regions for selective attachment of different chemical species on the same molecule. Thus, these particles can serve as biosensing elements for multiple analytes simultaneously [16].
The reagents and chemicals used in the synthesis of Janus particles, also have a great influence on the chemical properties and the physical structure of these particles. The morphologies of the Janus particles can be tuned by the addition or substitution of certain chemicals with others. For example, in research study manifesting the fabrication of Polymeric Janus Nanoparticles for drug delivery, Resomer RG502 (PLA/PGA 50:50) and thiol-terminated PLGA solution (PLGA RG502SH, 25 mg/mL acetone solution) were used. The particles produced in this manner, when observed through the AFM (atomic force microscopy) showed the presence of ‘dents’ on Janus particles, making them appear like pitted olives. When the substitution of the thiol-terminated PLGA was done with RG504H having a carboxylate end group, spherical Janus particles lacking dents were obtained. It was assumed that the bulky thiol-terminated chain was responsible for the dents, however, the true underlying mechanism was not deciphered [18].
These studies infer that each of the faces of the Janus nanoparticles can be tuned to impart specific characteristics thus giving flexibility to tune the properties of these particles. This convenience of tuning the properties of Janus particles can play a vital role in designing and fabricating biosensors with multiple recognition sites.
Biocompatibility
Considering the fact that Janus particles used in the biomedical field are administered in the human body, their biocompatibility in vivo is of the utmost importance. They must not exert toxic effects on the cells, tissues and hinder the biochemical processes occurring in the body. Also, these particles need furnish/accomplish their function consistently, constraining the clearance of these particles from the immune system.
Janus particles generally synthesized for the in-vivo gastrointestinal (GI) delivery tasks are micromotors with a core–shell structure composed of a reactive metal (e.g., Zinc) and an inner inert shell (titanium dioxide (TiO2), various polymers) along with a small perforation on one side for reaction with the biological fuel. This composition of the particle makes it biocompatible and biodegradable [32].
The toxicity of Janus particles is a function of their composition and it primarily depends on their constituents. The toxicity of each component in the Janus particle contributes to the toxicity as well as determines the overall toxicity of the Janus particle as a whole.
Most of the Janus particles synthesized aim at targeting the diseased part of the body (either cells or tissues). Target-specific biosensors assist/help in detecting infections by sensing of disease-specific biomarkers. Moreover, the drugs aimed at attacking specific cells are expected to exert a precise cytotoxic effect only on their target. Janus particles can offer this target-specific toxicity with minimal toxic effects on the healthy cells. A study report is available on the fabrication of a multifunctional drug delivery system of poly (lactic-co-glycolic acid) (PLGA) drug-loaded magnetic Janus particles (DMJPs). According to this study afore mentioned Janus particles displayed a non-toxic effect on normal cells but generated extremely lethal effects in cancer cells. Such particles offer a more secure and accurate treatment for cancer [33].
Le et al. (2019) demonstrated the encapsulation of the two cancer drugs, curcumin (CUR) and DOX in a single biodegradable biphasic Polymer/Lipid Janus nanoparticle for lung cancer treatment by inhalation in A549 human lung cancer cells that were injected into the lung of nude mice. These drugs had different properties such as different solubility, pharmacology, etc. The incorporation of these two drugs with different physicochemical properties in a single Janus nanoparticle provided synergistic cytotoxic and genotoxic effects against A549 lung cancer cells in vitro. It was also observed that empty Janus NPs were found to be non-toxic in the mice. The in vitro and in-vivo studies of subcutaneous and orthotopic liver tumour models in mice clearly indicated that magnetic Janus nanoparticles had suppressed the tumour growth in the liver. Additionally, these Janus NPs exhibited a significant reduction in systemic toxicity [34]. According to the reports of Chen et al. (2020), cardanol mesoporous silica/silver Janus nanoparticles (C-MSN-Ag) showed no obvious cytotoxicity on the osteoblast cell line (MC3T3-E1) of rats as concluded by observing 95% cell viability post 24 and 48 h of incubation without significant changes over time. Therefore, these Janus particles exhibited a non-cytotoxic behaviour and thus were used for Janus haemostatic dressings [35].
Dehghani et al. (2020) reported use of different polymers as carriers of DOX. Their study involved synthesis of the Janus particles with combinations of different polymers. The toxicity of a polymer is dependent on its charge, density and molecular weight. It was observed that PHEMA-PMAA (poly 2-hydroxyethyl methacrylate-poly methacrylic acid) Janus particles showed no effect on the growth of MG-63 cells due to carboxyl groups of PMAA and mild properties relative to cell function. Nonetheless, the positive charge on PDMAEMA led to interactions between the particles and outer membrane barrier of cells that culminated in the inhibition of MG-63 cell proliferation by PHEMA-PDMAEMA (poly(2-hydroxyethyl methacrylate-poly 2-(dimethylamino) ethyl methacrylate) Janus particles [36].
These studies suggest that the toxicity of Janus nanoparticles is dependent on the combined toxicity of the materials that make up the two faces of these particles. However, insufficient data regarding the toxicity of Janus particles is recorded. Considering the use of these particles in vivo, for various biomedical purposes and their effect on the environment, implementing studies and collecting sufficient data of their toxicity is an urgent necessity.
Factors affecting properties of Janus nanoparticles
Composition
The variations in Janus particles are not just confined to their morphology and surface structure. The composition of the particle has an essential role to play in determining the intrinsic properties of the particle. Consolidation of different materials can be used to obtain a variety of Janus particles retaining desired properties. They can generally be classified as polymeric, inorganic and polymeric-inorganic Janus particles, depending on the material of synthesis [16].
Li et al. (2017) have reported the fabrication of multifunctional asymmetric diblock and triblock (SiO2 and EPMO i.e., silicon dioxide and ethane bridged periodic mesoporous organosilane, SiO2 and BPMO i.e., silicon dioxide and benzene bridged periodic mesoporous organosilica, SiO2 and EPMO and BPMO, etc.) mesoporous silica-based Janus nanocomposites, possessing 1D mesoporous organosilicate nanorod with a dense SiO2 nanosphere positioned on one side of the nanorod. The silica-based core–shell nanoparticles, used as seeds result in the synthesis of 1D asymmetric diblock and triblock mesoporous nanocomposites with enhanced physical and chemical properties such as high surface area, unique highly ordered 1D mesopore channels and functional asymmetry [37]. All these properties make it an excellent candidate for biosensing with increased sensitivity. This increased sensitivity is attained through larger surface area that provides greater chances for biorecognition process and enhances the single-molecule detection or sensing. These nanorods can be further functionalized with different inorganic nanocrystals, such as magnetic Fe3O4, upconversion nanoparticles (UCNPs) and Au nanorods (Au-NRs) etc. With the coating of these metallic materials on one side and recognizing elements on the other side, sensitive biosensors can be fabricated.
Intrestingly, Ye et al. (2019) reported the fabrication of Titanium oxide-platinum Janus micromotors. These particles got clustered together in the presence of UV light but drifted apart in the absence of the same. This property of the Janus particle is attributed to the incorporation of Titanium oxide that displays an enhanced photoactivity on exposure to UV illumination [38].
Tan et al. (2019) documented the use of 1H,1H,2H,2H-perfluorodecyltriethoxysilane (FAS) for the synthesis of Janus particles. FAS is amphiphobic with very low surface energy. It remained stable when reacted with acids, bases and other solvents. However, it displayed degradation when was exposed to UV light. Thus, the FAS particles when exposed to UV light produced Janus particles with a combination of amphiphobic and amphiphilic properties. A similar procedure was employed for the synthesis of hexadecyltrimethoxysilane (HDTMS, a hydrophobic and oleophilic alkylsilane with a 16-carbon chain). Janus particles were produced with a combination of hydrophobicity and oleophilicity on HDTMS-functionalized areas, and amphiphilicity on UV degraded areas. Such contrasting properties in a single particle can only be achieved by means of Janus particles [39].
Wang et al. (2020) demonstrated the fluorescent-based nanomotor biosensors for the detection of 2,4,6-trinitrophenol (TNP), an explosive in aqueous media. The Py-Azine COF is highly luminescent and contains sites for the assembly of various other molecules for interaction. This is implemented by the azine groups through the formation of hydrogen bonds, which lead to changes in fluorescence properties, allowing easy detection of analytes by fluorescence-based biosensing. Furthermore, The COF-based micromotors are composed of biodegradable polycaprolactone (PCL) polymeric microspheres that make the particle biodegradable, MnO2 microparticles allowing catalytic propulsion of the Janus particles and magnetic Fe3O4 nanoparticles for controlled movement of the Janus micromotors based sensors [40].
These observations imply that composition of the Janus nanoparticles play a crucial role in imparting functional characteristics to these particles. Stimulus responsive materials can be used during fabrication of the Janus nanoparticles, so to provide targeting capabilities to these materials for sensing applications.
Method of synthesis
Moreover, the selection of appropriate synthesis method for Janus particles is cardinal as it greatly influences the properties of the particles, offers ease of synthesis and determines toxicity to the environment. The conventional PVD (Physical Vapour Deposition) method is a better choice over electroplating for it offers a more uniform deposition, allows the deposition of a wider variety of materials and is environmentally friendly. Additionally, this method offers easy installation of catalytic and magnetic properties in the Janus particles, essential for multifarious applications [38].
Electrospraying is another technique to synthesize Janus particles of controlled sizes. For example, Janus particles of 135–3 μm were synthesized using electrospraying technique, by altering the electric field [41]. Furthermore, an oppositely charged twin-head electrospraying setup can be used for large-scale production of Janus particles with desired morphologies, composition and architecture [42].
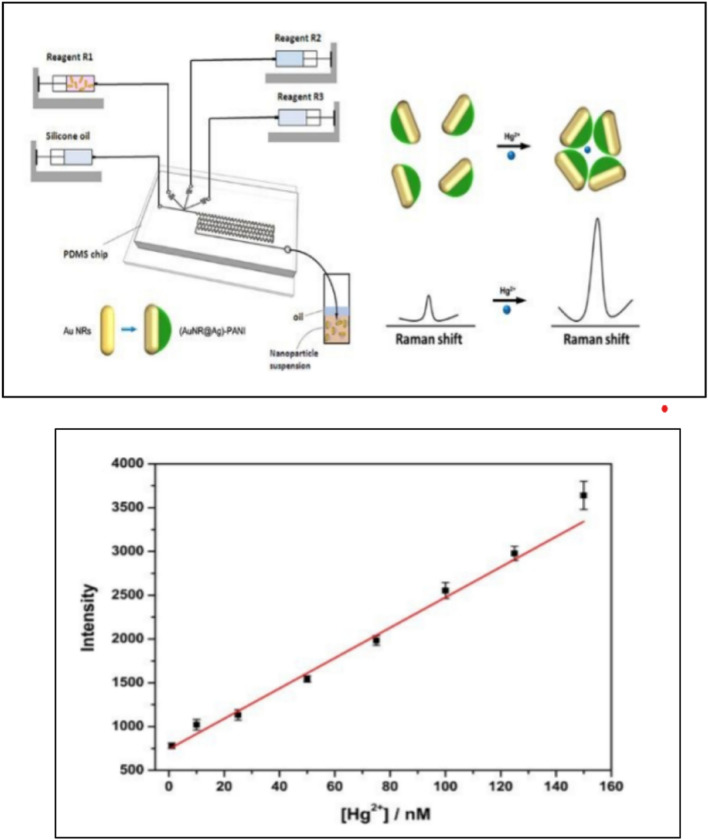
The droplet-based microfluidic method was used for the synthesis of Au nanorod (NR)@Ag)-polyaniline (PANI) Janus nanoparticles (SNPs) for the fabrication of surface-enhanced Raman scattering (SERS) sensors for detection of Hg2+ ions (Fig. 4). The synthesis method so chosen presented several advantages such as curtailed time of synthesis paired with precise control on the size and volume of the droplet, along with facile manipulation of every single droplet formed to produce uniform Janus particles [43].
Fig. 4.
A Schematic illustration of fabrication of Au nanorod (NR)@Ag)-polyaniline (PANI) Janus nanoparticles (JNPs) by droplet-based microfluidic method. The modification of Au nanorod to PANI JNPs is presented along with their effect on Raman intensity. B The linear calibration curves for the SERS intensity of PANI at 1379 cm-1 and different concentrations of Hg2 + ions. (From bottom to top: 1, 10, 25, 50, 75, 100, 125, and 150 nM, respectively). Reprinted figure with permission from Wang Y, Shang M, Wang Y, Xu Z (2019) Droplet-based microfluidic synthesis of (Au nanorod@Ag)-polyaniline Janus nanoparticles and their application as a surface-enhanced Raman scattering nanosensor for mercury detection. Anal Methods. Copyright (2019)
© Royal Society of Chemistry 2022
These studies suggest that precision in fabrication of single Janus nanoparticle is essential for their efficient synthesis. Microfluidic based method of synthesis is superior to other methods like physical vapour deposition, electrospraying etc. for fabrication of Janus nanoparticles as it provides uniformity in dual face Janus nanoparticles.
pH
Desired shapes of the Janus particles can be obtained as per the requirements of different applications by altering the pH values of the medium of synthesis. Seeded emulsion polymerization of MAA (methacrylic acid) in the presence of poly (2-hydroxyethyl methacrylate) (PHEMA) seeds at pH 1 and 4.6 produced snowman-like Janus particles. Whereas, polymerization of MAA at pH 10 contrived monodisperse core–shell particles. The same results were obtained with poly 2-(dimethylamino)ethyl methacrylate (PDMAEMA) by alternating the pH values of polymerization medium, obtaining dumbbell-like particles, monodisperse core–shell structures and snowman-shaped Janus particles. These results were attributed to the thermodynamic parameters and electrostatic interactions occurring in these chemical components [36].
The advantage of these pH-controlled Janus particles is that they can be employed in the fabrication of in-vivo biosensors which can travel to specific systems of the body and carry out sensing of the molecules which function at a specific pH in the body. Moreover, multi-analyte sensing can be carried out by incorporation of different segments in the Janus particle, each functioning at a different pH value.
These observations clearly indicate the role of pH in determining the morphology, by providing targeting capabilities and imparting multifunctionality to the particle. These parameters are crucial as these properties determine the efficiency of the biosensors.
Temperature
The temperature maintained during the synthesis of the Janus particles has a considerable influence over the morphology and various other factors of the Janus particles. For example, molecular dynamics simulations of Fe − Cu bimetallic nanoparticles carried out in a study demonstrated that cooling rate affected the morphology of Fe − Cu bimetallic nanoparticles, resulting into patchy, random mixture of Janus particles. A mixture of random Janus particles was obtained at different cooling rates. Experimental analysis demonstrated that the immiscibility and crystallization of Fe − Cu bimetallic Janus nanoparticles was obtained at extremely slow cooling rate [44].
Temperature plays an important role in the synthesis of Janus particles by the Pickering emulsion method. For example, in the wax–water system, untreated hydrophilic silica particlesat high temperatures adsorb to the oil-in-water emulsion interface which contains molten wax. The temperature is then reduced to solidify the wax and capture the particles. Subsequently, chemical modification of the particles is carried out at temperatures containing the solid oil phase, thus selectively modifying the faces of the particles that are free from wax (not buried in wax) [45].
Thus, temperature plays a vital role in fabrication of the Janus nanoparticles. The rate of heating and cooling determine the efficiency of structural as well as chemical modification of the Janus nanoparticle.
Janus nanoparticle-based sensing platforms
Electrochemical sensors
Electrochemical sensors are the sensors that convert the signals generated as a result of electrochemical reactions (the reaction occurring between the electrode and the analyte) into legible signals that are easy to interpret. These can be classified as potentiometric, conductometric, and amperometric/voltametric sensors. The multifunctional property of Janus particles results in a superior electrochemical biosensor with enhanced characteristics such as better transduction, multiple analyte enrichment and signal enhancement.
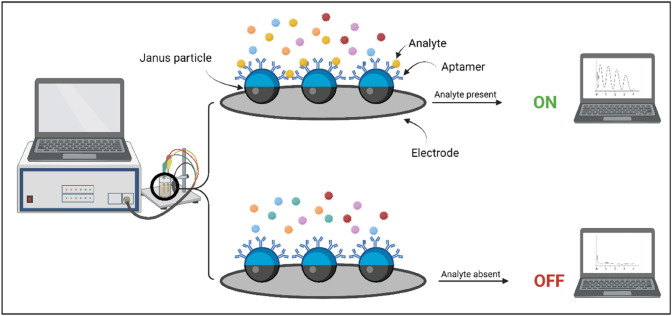
Among the various biosensors used, aptamer-based biosensors are eminent ones due to their properties such as high accuracy, economical and high stability and the prospect of assembling analyte-related 3D hairpin-shaped structures with these biomolecules. These aptamers can be attached to Janus particles for the specific binding of the analytes to the biosensor for their facile detection (23) (Fig. 5).
Fig. 5.
Illustration depicting Janus particle-based aptameric electrochemical biosensors. One compartment of the Janus particle possesses aptamers specific for the target analyte while the other compartment of the particle is attached to the electrode. A Attachment of analyte to the specific aptamer produces a signal. B The analyte does not bind to the specific aptamers; hence, no signal is generated. Created with BioRender.com
Paniagua et al. (2019) reported a Janus particle-based electrochemical biosensor for the detection of carcinoembryonic antigen (CEA). This disposable amperometric aptasensor was comprised of silica-gold Janus nanoparticles with horseradish peroxidase enzyme (signalling element) attached to the silica segment of the Janus particle, whereas, a biotin thiol-modified anti-CEA DNA hairpin aptamer (biorecognition element) was immobilized on the gold surface. This sensor permitted the occupancy of both, the biorecognition element and the signalling system in the different segments of the same molecule. This Janus particle-based aptasensor was able to detect CEA in the range from 1 to 5000 ng mL−1 (5.5 pM–28 nM) with a detection limit of 210 pg mL−1 (1.2 pM) [46]. The formation of non-conducting layer that results in higher concentration of capturing element causes decrease in electrochemical signal and thus can be a limiting factor in this sensor. Avoiding the agglomeration of the capturing element at higher concentration may help in improving the limit of detection.
Yang et al. (2019) reported a simpler approach for detection of Ochratoxin A(OTA) using Aptamer/NH2 Janus particles. Under optimum conditions, the sensor detected OTA concentration from 1 × 10–5 nM to 10 nM, and the detection limit is 3.3 × 10–3 pM under the condition of acceptable stability and reproducibility. The Janus/COOH-GN/GCE sensor thus produced was highly specific for the OTA toxins. These types of Janus-based sensors can provide blueprints for the production of multitudinous amounts of highly specific sensors for the detection of fatal pathogens and disease-causing agents [47]. Superior performance of this biosensor than the previous biosensor can be attributed to the use of graphene layer which play a vital role in better electron transfer and thus resulting in higher sensitivity.
Zhou et al. (2018) documented the detection of ractopamine (RAC) in human urine using aptamer/octadecanethiol Janus particles. The Gold component in the Janus nanoparticles imparts enhanced electrical conductivity along with increased sensitivity and selectivity which are the crucial parameters for a biosensor. The detection limit obtained for the sensor was 3.3 × 10−14 mol/L, which was lower than many of the existing methods [48]. Both these sensors utilized differential pulse voltammetry which is a far sensitive technique for quantification of analyte when compared to amperometric detection.
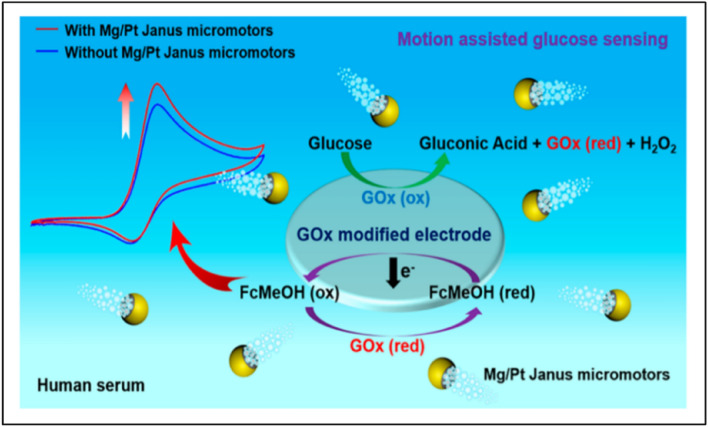
An electrochemical sensor was fabricated by Kong et al. (2019) using motile Mg/Pt (Magnesium–Platinum) Janus particles for enhanced detection of glucose in the human serum owing to the enhanced mass transfer in the solution by the rapid motion and bubbling effect of Janus micromotors as shown in Fig. 6. The Janus micromotors were capable of propelling through different viscosities and concentrations of glucose without the addition of toxic chemicals or fuels, using glucose oxidase instead, for the propulsion of micromotors. It was also observed that there was a linear increase in the signals when the concentrations of the Janus micromotors and glucose were increased. In the presence of 1 mg/mL of micromotors, the LOD and LOQ values obtained were 33.2 and 110.7 μM, respectively and were further increased on increasing the concentration of the micro motors [49].
Fig. 6.
Schematic illustration of motile Mg/Pt (Magnesium–Platinum) Janus particles-based electrochemical sensors for enhanced detection of glucose in the human serum using screen-printed electrode (SPE). The mass transfer generated by the micromotor motion and bubbling leads to the generation of an enhanced and increased signal output. Reprinted figure with permission from Kong L, Rohaizad N, Nasir MZM, Guan J, Pumera M (2019) Micromotor-Assisted Human Serum Glucose Biosensing. Anal Chem. Copyright (2019) by the American Chemical Society
These studies suggest that electrochemical sensors based on Janus nanoparticles show superior performance in terms of their limit of detection. However, limited nanomaterials have been utilized for the fabrication of these sensors. Hence, other cost-effective materials can be explored for devising Janus nanoparticles for their utilization in the development of electrochemical sensors.
Optical sensors
The structural and compositional characteristics of the Janus nanoparticles contribute to their unique interaction with electromagnetic radiations. These interactions have been exploited for the fabrication of optical biosensors. Based on the interaction and the subsequent changes brought by these interactions, biosensors developed accordingly are discussed in the following sections.
Fluorescence based sensors
Fluorescence is a widely used phenomenon for the detection and tracking of biomolecules and thus is helpful in inspecting various processes carried out in the human body, plants, microorganisms, etc. to elucidate cells functioning, for rapid drug discovery, and for detecting minute quantities of pathogens or DNA. Along with being a sensitive method it also permits real-time imaging of the molecules at ambient conditions with high spatial and spectral resolution. However, background fluorescence from samples and instrument optics restraints the accession of low levels of fluorescent signals, making the detection of low concentrations of analytes difficult.
Consequently, a technique was proposed to modulate an optical probe signal magnetically and separate it from the background fluorescence. Anker et al. (2003) documented the fabrication of aspherical, magnetically modulated optical nanoprobes called MagMOONs that undergo orientation in external fields due to magnetic shape anisotropy (Fig. 3). These particles can blink on and off with respect to their surrounding rotating magnetic fields. These MagMOONs are Janus particles having one side coated with a metal and the other side attached to fluorophores. The particle is then magnetized so that the uncoated light-emitting side becomes the magnetic north of the particle. The alignment of the MagMOONs in 90° marks the signal “ON” and shows fluorescence, whereas their alignment at 180° marks it “OFF” (with no fluorescence). Thus, 360° rotation of these particles emits flickering signals [25].
The flickering signals obtained from these MagMOONs can be used to ascertain the viscoelastic and other properties of the solution or environment containing the MagMOONs as the flickering signals depend on the viscous drag experienced by the mg during rotation. Nevertheless, the MagMOON rotation rate is independent of particle size if constant viscosity and magnetic susceptibility are maintained [25]. It is possible to devise accurate nano-viscometers for Nano thermometers using this technique. These MagMOONs can be contrived into superior biosensors by attachment of recognizing elements on the metal-deposited hemisphere of the Janus MagMOONs. Fabrication of highly sensitive and accurate optical biochemical sensors is possible since the flickering of the particles is not only sensitive to viscous drag, but also to electric and magnetic fields as well as to chemical attraction and biochemical forces [21]. Moreover, collaboration of MagMOONs with PEBBLES permits highly sensitive detection of intracellular analytes using a broad range of dyes [25].
The Janus hydrogel microparticles were designed and further used by Sun XT et al. (2017) for multi-target sensing of glucose (by fluorescence method) and cholesterol (by colorimetric method) (Fig. 7). The sensor so produced did not offer sensitivity as high as the pre-established electrochemical sensors or photometric assays for similar types of detection but were efficient enough in the direct detection of body fluid samples. It was also observed that the fluorescence of the particles increased with increase in the concentration of glucose for the fluorescence of the particles enhanced self-adsorption of the fluorochrome at greater glucose concentrations. This Janus particle biosensor also offered low interference from other biomolecules and also had high stability than the conventional Con A-based glucose sensing. The values of glucose and cholesterol observed after testing of the collected human serum sample were 91.7 mg/dL and 4.11 mM respectively with fasting-blood sample, which showed a slight deviation from the values of glucose and cholesterol obtained by the standard oxidase method which were 82.6 mg/dL and 4.42 mM correspondingly. Nonetheless, the use of biosensor is an apt choice for preliminary blood testing as it is a fast, cost-effective and easy method besides its sensitivity and accuracy could be improved in the future [17].
Fig. 7.
Schematic representation of multi-target sensing of glucose (by fluorescence method) and cholesterol (by colorimetric method) using Janus hydrogel microparticles. The hydrogel particles restrict the contents in the particles without any leakage while allowing the permeability of small molecules. A (Left hemisphere of the Janus particle) Glucose sensing is carried out by competitive binding assay using concanavalin A and fluorescein labelled dextran. The binding of FITC with Con A quenches the fluorescence. (Right hemisphere of the Janus particle) The γ-Fe2O3 nanoparticles embedded in the hydrogel were used as a catalyst for the oxidation of 3,3′,5,5′-Tetramethylbenzidine (TMB) by H2O2, an enzymatic hydrolysis product of cholesterol. B (Left hemisphere of the Janus particle) Glucose molecules bind to the Con A, releasing FITC and resurging the quenched fluorescence. The fluorescence of the particles was positively correlated with glucose concentration. (Right hemisphere of the Janus particle) Hydrogen peroxide, the enzymatic hydrolysis product of cholesterol oxidises TMB, generating a blue coloured oxTMB, in the presence of γ-Fe2O3 nanoparticles which serve as a catalyst. The colour transition is proportional to the concentration of cholesterol molecules in the sample. Created with BioRender.com
A basic ON–OFF (fluorescence) approach is used to detect the presence of lipopolysaccharides (LPS) toxins which are present in the outer membranes of gram-negative bacteria. The selective interaction of a component of LPS present in the sample with the respective fluorescent-labelled affinity peptide present on the Janus-based micromotor sensor, quenches the fluorescence and turns it off indicating the qualitative detection of LPS. A number of sensors have been proposed based on this strategy. This is a highly selective, facile and rapid method providing results within a few minutes [50–52].
For example, Pacheco et al. (2018) fabricated a micromotor-based fluorescent Janus sensors that demonstrated selective binding of 3-deoxy-d-manno-oct-2-ulosonic acid (KDO) from the endotoxin to the quantum dot comprising region of the Janus particle. This binding led to quenching of the fluorescence (turning off) indicating the presence of endotoxins while the other half was involved in the transportation of sensing probes as shown in Fig. 8. KDO present in the LPS residues contains an acyclic 1,2-diol on the exocyclic side chain which selectively binds to the PABA groups on the graphene quantum dots by reversible boronate formation creating a cross-linked QD-LPS structure. This covalent cross-linking produces surface states for fluorescence quenching. This offers a rapid detection technique, giving results within a few minutes, compared to other sensors designed and developed for toxin detection in food [50].
Fig. 8.
Schematic illustration depicting micromotor-based fluorescent Janus sensor. A The Janus particle is composed of quantum dots while the other half of the particle encapsulates platinum nanoparticles. PABA is attached to the quantum dots for selective binding with the KDO present in the LPS residues. The graphene quantum dot part endows the particle with fluorescence sensing while the platinum part, which serves as a catalyst for the reduction of hydrogen peroxide, enables motility. B The KDO segment of the bacterial LPS selectively binds to the PABA, generating a fluorescent signal. Created with BioRender.com
In few other studies, the same group reported WS2 Janus nanoparticles based “off–on” fluorescent sensors for detection of lipopolysaccharide (LPS) residues. Although the limit of detection of these sensors were not as low as previously reported but the time for detection was improved to 60 s. These sensors are expensive as these involve incorporation of expensive nanomaterials like platinum during their fabrication thus restricting their applicability as point of care devices [52, 52].
Janus emulsions or Janus particles composed of hydrocarbon and fluorocarbon oils were used as biosensors for the detection of anti-SARS-CoV‑2 Spike antibody. Equal amounts of fluorocarbon and hydrocarbon oils were used to form interfaces between the Janus droplets. Subsequently, the surrounding aqueous phase was functionalized with SARS-CoV-2 spike RBD and Goat Anti-Human IgG antibody, forming two complementary emulsions which were further used in 1:1 ratio for the selective detection of the antibody by agglutination reaction. These Janus particles were labelled with a non-emissive dye that blocks the emission of the absorbed light when present in its normal configuration and an emissive dye on the other half of the particle that allows the emission of the absorbed light, upon agglutination of the Janus particles around an anti-SARS-CoV-2 spike antibody (Fig. 9). Thus, a fluorescent signal was obtained based on the flipping of the Janus droplet in the presence or absence of the target antibody, and the consequent configuration of the emissive and non-emissive dyes on the particle. The detection limit of the sensor was as low as low as 0.2 μg/mL in 2 h [53].
Fig. 9.
Schematic illustration depicting fluorescence biosensor fabricated with Janus nanoparticles based on the orientation of aggregation of Janus nanoparticles. The Janus particle is coated with an emissive dye on one side and a blocking dye on the other side. The normal orientation of the particles when no analyte is present is with the blocking dye side on the top. In the presence of the analyte, the analyte selectively binds to the respective antibody and causes agglutination, turning the emissive dye side up, thus emitting fluorescence. Created with BioRender.com
Li et al. (2020) used a similar approach in the detection of Listeria monocytogenes wherein the binding between the Listeria and Listeria antibody resulted in the tilting of the Janus structure exposing the emissive dye segment on the Janus particle thus inducing fluorescence, and blocking the non-emissive dye segment (Fig. 9). The limit of detection for the biosensor is less than 100 CFUs per mL [54]. This method can be employed for the detection of other analytes using corresponding antibodies.
These results clearly emphasize on duality of the nanoparticle which play a vital role in enhancing the detection of the target analyte. The two faces of the Janus nanoparticle determine the action of the particle in the presence or absence of the target analyte hence playing a crucial role in their sensitive detection.
Colorimetric based sensors
Colorimetric sensors are the sensors which exhibit a colour change due to the specific reaction between the analyte and the biorecognition element. The intensity of the colour produced depends on the concentration of the analyte reacting with the biorecognition element. Janus particle-based colorimetric sensors prove to be superior to conventional colorimetric sensors as they can be attached with specific antibodies, aptamers, etc., for specific binding of the analyte molecules result in signal enhancement. Furthermore, they can be incorporated with a fluorescent and motile segment, giving rise to a multifunctional sensor in a single particle.
Russell et al. (2019) fabricated a colorimetric sensor using Janus particles. These Janus particles combined the particles responsible for both, motility and colour change. The change in colour occurred due to the binding of the PCT (Procalcitonin) biomarker present in the blood to the bioreceptors on Janus particles, in higher concentrations, as an indication of sepsis (Fig. 10). The intensity of the pixels produced by the particles could be measured with the assistance of a mobile application and thus results could be obtained within seconds and read in realtime [27].
Fig. 10.
A Schematic representation of the multifunctional Janus particles. The iron oxide core provides magnetic and colorimetric properties, whereas the Janus coating contains anti-PCT antibodies that endow the particles with biorecognition functions. An immunoassay renders the particles covered with catalase, which triggers colloid motion in the presence of H2O2. B PCT is specifically captured in blood by means of a competitive immunoassay consisting in a 10 min capture step and a 2 min wash procedure; C Signal generation mechanism: after spotting the particles on a piece of filter paper H2O2 is added; the catalase enzymes generate bubbles that propel the particles and disperse the colour within seconds. The subsequent change in pixel intensity is read in realtime with a mobile phone app. Reprinted figure with permission from Russell SM, Alba-Patiño A, Borges M, de la Rica R. Multifunctional motion-to-colour janus transducers for the rapid detection of sepsis biomarkers in whole blood. Biosens Bioelectron [Internet]. 2019.
Copyright © 2019 Elsevier B.V. All rights reserved
The predominant property of a Janus particle is its ability to conglomerate multiple regions with multiple characteristics in a single particle proficiently. This peculiarity offered by the Janus particle permits real-time sensing of multiple analytes. The same principle was utilized by Sun et al. (2017) for multi-target sensing of glucose and cholesterol using Janus hydrogel microparticles. This Janus particle-based optical sensor permits the fluorescent sensing of glucose (green colour) and colorimetric sensing of cholesterol (blue colour) in a single microparticle simultaneously, avoiding any interference in the sensing of either particle (Fig. 7). The hydrogel Janus microparticles confined the detecting probes in their respective domains by prohibiting the surrounding solution from turning blue and also avoided the interference between glucose and cholesterol sensing. These Janus particles can be later separated from the solution by magnets and reused for sensing other samples [17].
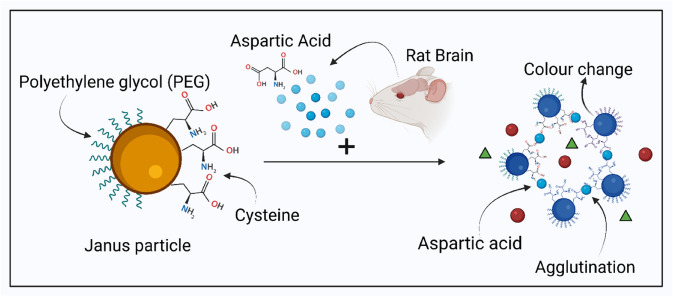
In another study, Xin-Yue et al. (2018) proposed a colorimetric sensor based on the orientation of aggregated Janus gold nanoparticles for the detection of aspartic acid (Asp) in rat brains. These particles were functionalized with polyethylene glycol (PEG) and cysteine for stability and selective binding of the analyte, respectively (Fig. 11). The binding of aspartic acid to the cysteine present on the Janus particles caused oriented and controlled aggregation of Janus particles leading to enhanced stability for longer durations, also offering a wider dynamic range (1.8 nM–180 μM) in comparison to the other ordinary colorimetric sensors. Additionally, it was observed that as the size of AuNPs increased from 13 to 45 nm, the detection limits decreased from 18 μM to 1.8 nM. Thus, the detection limit of this sensor can be enhanced by increasing the size of gold nanoparticles. Besides, the functionalization of the particle with PEG leads to steric repulsion among PEG chains and decreases Coulombic interaction causing highly specific interaction between the analyte and AuNPs in the real sample. Overall, a facile and economical sensor was fabricated, providing selective and sensitive detection, without the need for expensive instruments, complex protocols, or a large quantity of expensive raw materials [55].
Fig. 11.
Colorimetric sensor based on the orientation of aggregated Janus gold nanoparticles for the detection of aspartic acid (Asp) in rat brains. Janus particle is functionalized with PEG and Cysteine for selective detection of Aspartic acid present from rats’ brain. The aspartic acid in the sample selectively binds to the cysteine groups of the Janus particle leading to their agglutination. This agglutination induced colour change in the particles helps in the detection of aspartic acid. Created with BioRender.com
These reports demonstrate that multifunctionality of Janus nanoparticles can be utilized for developing biosensors with dual sensing mechanism. Hence it can be used for detecting two different analytes via same/different mechanisms with detection limit of up to 1 nM.
Raman based sensor
Raman spectroscopy is a highly specific technique to procure molecular information, saving the additional steps and efforts of molecular labelling. The non-destructive nature of the technique, the selectivity of Raman bands and the insensitivity to water make Raman spectroscopy a useful analytical tool for both qualitative and quantitative studies of organic as well as inorganic systems. Yet, the conventional Raman spectroscopy method cannot be used for low analyte or biomolecule concentrations.
Surface-enhanced Raman scattering (SERS) is a method that not only encompasses the conventional Raman spectroscopy benefits but also allows us to make low concentration detection of analyte possible. Owing to these benefits offered by the technique, various methods, both top down and bottom down approaches have been proposed to enhance and fabricate the SERS surface having greater sensitivity [56]. The SERS enhances the weak Raman signals by utilizing rough, nanosized metallic structures (usually noble metals) as substrate to which the target molecule is attached along with the localized surface plasmons [57]. Laser excitation of the sample and nano-substrate complex excites the “LSPR” or Localized surface plasmons into resonance. At this condition, both the laser excitation radiation and the scattered radiation from the sample are amplified.
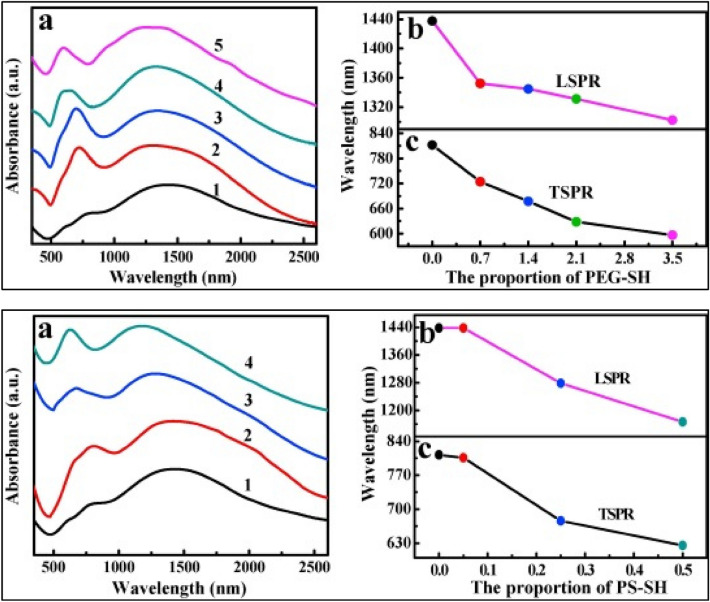
Xiaowei et al. (2019) reported the synthesis of gold nanorods (GNRs) Janus membranes functionalized with polyethylene glycol (PEG-SH) and thiol-terminated polystyrene (PS-SH), at the oil/water interface. The Raman enhancement factor (EF) of the PS-GNRs-PEG Janus membrane was found to be 6.41 × 108 which is higher than the conventional gold nanorods. It was observed that the thiol-terminated polymers enhance the SPR and SERS activity. On addition of PEG-SH or PS-SH, the PS-GNRs-PEG Janus membrane displayed two distinct SPR peaks along with a continuous blue shift which otherwise showed a significant red shift, compared with the corresponding GNRs colloid (Fig. 12). Overall, the sensor shows greater reproducibility and enhanced SERS activity compared to the conventional gold nanorods [58].
Fig. 12.
A (a) Absorption spectra of PEG-GNRs membranes coated on slide glass with the varying proportion of PEG-SH (1: 0 mg/ml, 2: 0.7 mg/ml, 3: 1.4 mg/ml, 4: 2.1 mg/ml, 5: 3.5 mg/ml), (b) the peak position of LSPR of the PEG-GNRs membranes as a function of the proportion of PEG-SH, (c) the peak position of TSPR of the PEG-GNRs membranes as a function of the proportion of PEG-SH. B (a) Absorption spectra of PS-GNRs Janus membranes coated on slide glass with the varying proportion of PS-SH (1: 0 mg/ml, 2: 0.05 mg/ml, 3: 0.25 mg/ml, 4: 0.5 mg/ml), (b) the peak position of LSPR of the PS-GNRs Janus membranes as a function of the proportion of PS-SH, (c) the peak position of TSPR of the PS-GNRs Janus membranes as a function of the proportion of PS-SH. Reprinted figure with permission from Zhang X, Zhao Z, Liu L, Li Y (2019) Design of Gold nanorods Janus membrane for efficient and high-sensitive surface-enhanced Raman scattering and tunable surface plasmon resonance. Chemical Physics Letters.
Copyright © 2019 Elsevier B.V. All rights reserved
The reports hitherto suggest that the aggregation of Janus particles results in an enhanced plasmonic coupling effect [59]. This Plasmon coupling effect between nanoparticles is used in SERS to achieve ultralow detection limit. The droplet-based microfluidic method was used for the synthesis of Au nanorod (NR)@Ag)-polyaniline (PANI) Janus nanoparticles (JNPs) as Surface-enhanced Raman scattering (SERS) sensors for the detection of Hg2+ ions in river water (Fig. 4). The sensor was highly selective due to the strong binding affinity between PANI and Hg2+ ions leading to the high Raman intensity of PANI. The higher concentration of the Hg2+ ions within a certain range result in a stronger SERS signal. The detection limit of Hg2+ ions concentration was 0.97 nM with a linear relationship of Raman intensity increment of PANI and Hg2+ ions concentration in the range of 1–150 nM [43].
Similarly, Janus nanoparticles were utilized as Raman signal enhancer for detection of Rhodamine 6G (R6G) in aqueous solution at a concentration of 1 fM. They integrated the superhydrophobic surface design and SERS substrate design together to construct a highly roughened surface using noble metal nanostructure arrays termed as superhydrophobic surface-enhanced Raman scattering (SSERS). The presence of copious “hot spots” or SERS sensitive sites present in the roughened PS-Ag Janus particle array led to their high SERS sensitivity of Rhodamine 6G (R6G) aqueous solution. This technique was also used for the low concentration and high sensitivity detection of 1 nm of poliovirus (PV) RNA-dependent RNA polymerase (RdRp) and a virus PV type I Sabin of about a 1 fM solution. This discovery is capable of detecting minuscule quantities of analyte present in a highly diluted solution, for example upto femtomolar level. This SSER technique has abundant applications in the biosensing field for the fabrication of accurate and highly sensitive biosensors [56].
These reports imply that Janus nanoparticles-based biosensors exert/have plasmonic coupling effect resulting in higher signal response. The anisotropy of these nanoparticles enhances this effect sequentially leading to higher detection limit of upto 1 fM.
Janus particle-based optical-motile biosensors
The Janus particles, owing to their superior chemistry have been utilized for development of motile biosensors aimed for detection of biomolecules in body fluids. These are particles in the micro or nano dimensions with an ability to convert chemical fuel or external energy (light, sound and chemical energy, etc.) into mechanical motion [60]. The application of these particles in biosensing can be conceded to fabricate a motile biosensor eventually capable of detecting or sensing biomolecules in vivo by travelling from one system to another, which will prove to be superior to the conventional immobile biosensors (Fig. 13).
Fig. 13.
Janus particle-based optical-motile, in-vivo biosensors travelling from one system to another for the selective detection of biomarkers in the body for disease detection. Created with BioRender.com
The above-mentioned particles have one side coated with a material that offers a self-propulsion due to a bubble thrust, or a diffusio- or electrophoretic mechanism. This particle is capable of moving in the desired direction with respect to the certain stimuli-responsive site [27]. The principles behind such biosensors are the bio-recognition reaction that can result in the change of velocity of the particles and this change can be transformed into signals based on the trajectories of the collection of particles and calculating their average velocity.
Nickle coated Silicon dioxide particles after deposition of a platinum layer, form SiO2–Ni-Pt Janus particles which could be propelled in H2O2 and controlled by an external magnetic field [38]. This feature allows these particles to be utilized for targeted drug delivery and controlled cargo delivery [61]. This can also fabricate the development of a self-propelled Janus nanomotor-based in-vivo biosensor, the motion of which can be manoeuvred by an external magnetic field without using hazardous chemical fuels.
Displacement or movement of particles or a colour change offers more distinct sensing signals to a biosensor, which are easily distinguishable compared to the sensors that continuously emanate light or are stationary. A novel motion-to-colour signal generation mechanism using Janus particles was proposed by Russell et al. (2019) to quantify the concentration of the protein Procalcitonin (PCT), the presence of which in high concentration in blood, is an indication of sepsis (Fig. 10). The biorecognition reaction leads to the propulsion of the Janus particle as detectable by a colour change. Thus, the propulsion and colour intensity of the spot gives the concentration of PCT generating signals within seconds that are read in realtime using a simple mobile application [27].
Wang et al. (2020) demonstrated the fluorescent-based nanomotors biosensors for the detection of 2,4,6-trinitrophenol (TNP), an explosive in aqueous media. It is composed of Py-Azine COF (Covalent organic frameworks) based micromotors along with MnO2 and Fe3O4 particles located asymmetrically on one side of the micromotors for propulsion of spherical micromotors in the desired direction. COFs (Covalent organic frameworks) were distributed throughout the micromotors as they were miscible with PCL (Polycaprolactone), brightening the micromotors with fluorescence. The principle of the detection was based on the rapid “On–Off” fluorescent detection of TNP in aqueous media. The COF has the ability to fluoresce when excited. This fluorescence would be turned off due to the interaction with TNP by hydrogen bonding. Also, The MnO2 microparticles catalyse the decomposition of hydrogen peroxide which permits the movement of the Janus micromotors. Besides, the Fe3O4 NPs allow the movement of the particles in the desired direction, the absence of which would lead to random circular or linear motion of the particles. Also, this sensor was inexpensive due to the replacement of Platinum with MnO2 as a catalyst. Furthermore, urchin-like MnO2 microspheres were synthesized to increase the surface area for efficient catalysis. This biosensor offers the detection of TNP at the ppm level within minutes, offering a rapid, cost-effective, and sensitive detection [40].
Yuan et al. (2020) demonstrated the ON–OFF fluorescent biosensing (using fluorescence-labelled affinity peptide) of Cholera Toxin B by competitive ligand binding process, combined with 2D-nanomaterials (graphdiyne oxide (GDYO)-, graphene oxide (GO)-, and black phosphorous (BP)) wrapped Janus micromotors. It was observed that a rapid fluorescence quenching was obtained with moving micromotors, whereas; negligible quenching is obtained with static micromotors. This phenomenon has been observed in all experiments following a similar approach, indicating the importance of micromotor movement for biosensing. It was also noted that the fluorescence of the Janus particles increased, as the concentration of Cholera Toxin B increased. This was due to availability more analyte to compete with the micromotors for peptide release thusly indicating the presence of the toxins. Moreover, GO micromotors induced the highest fluorescence enhancement which was followed by GDYO and BP micromotors. The limit of detection of BP micromotor for cholera B toxin was reported to be 0.015 μg/mL with linearity in the range of 0.05 − 10, 0.003 μg/mL. Whereas the detection limit of GO and GDYO micromotors were found to be 0.003 μg/mL and 0.002 μg/mL respectively. It was also observed that GDYO and GO micromotors displayed fivefold greater sensing efficiency as compared to that of BP micromotors due to their distinct surface properties influencing the loading and release capacity of the peptide which is important for the signal generation [62].
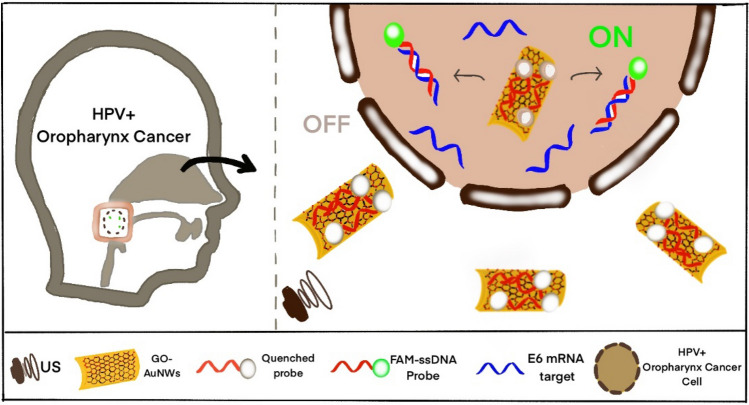
Beltrán-Gastélum et al. (2019) designed and fabricated a nanomotor-based biosensor comprising of a fluorescent-labelled aptamer that selectively binds to the target AIB1 protein (biomarker for breast cancer) immobilized on graphene oxide (GO) coated gold nanowires (AuNWs) (Fig. 14). These particles permitted the propulsion of nanomotors towards the breast cancer cells and get internalized in them for rapid detection of intracellular AIB1 protein. The FAM-AIB1- aptamer led to quenching of the fluorescence of dye due to the FRET between the fluorophore (FAM) and GO. When this nanomotor was internalized, the aptamer was displaced by the AIB1 protein resulting in the recovery of fluorescence, indicating the presence of protein. The propulsion of the nanomotors was based on acoustic waves, i.e. ultrasound (US) waves [60]. A similar approach was used by Qualliotine JR et al. (2019) for the intracellular detection of Human Papillomavirus (HPV16) [63]. However, the in-vivo toxicity of these sensors is unexplored and should be investigated further for their clinical application.
Fig. 14.
OFF-ON detection system for intracellular HPV16 (human papillomavirus) E6 mRNA transcripts. GO-AuNWs (Graphene oxide-gold nanowire) are modified with 6-carboxyfluorescein FAM-ssDNA probes reverse complementary to HPV16 E6 transcript expressed within HPV-positive OPC (oropharyngeal cancer) cells. Nanomotors quench probe fluorescence and can penetrate cell membranes when powered by ultrasound. Annealing of target E6 mRNA liberates the probe from the nanomotor and restores the probe’s fluorescent signal. Recreated and Reprinted figure with permission from Qualliotine JR, Bolat G, Beltrán-Gastélum M, de Ávila BEF, Wang J, Califano JA. Acoustic Nanomotors for Detection of Human Papillomavirus–Associated Head and Neck Cancer. Otolaryngol - Head Neck Surg (United States). 2019. Copyright Copyright © 2019, © SAGE Publications
These studies propose that optical-motile Janus nanoparticles can be utilized for in-vivo biosensing. These motile nanoparticles travel through the medium owing to their anisotropic behaviour and reach to the target site via different propulsion mechanisms like diffusion or electrophoretic propulsion. The Janus nanoparticles functionalized with bio-recognition elements bind to the target sites resulting in revival of fluorescence. However, very few reports provide quantitative data, toxicological studies and discuss limitations related to fluorescence signal through the skin during in-vivo sensing.
Conclusion and future prospects
This review was aimed to study the different Janus nanoparticles, their properties, factors affecting the properties and their role in sensing applications. Based on the reviewed literature, it can be concluded that factors like pH, temperature, method of synthesis influence the overall properties of Janus nanoparticles. The pH of the medium plays a crucial role in determining the morphological features of the Janus nanoparticles. The Janus particles fabricated/ produced by controlling pH can be utilized for developing target oriented in-vivo biosensors. Temperature is another factor that can be utilized to selectively modify the faces of the Janus nanoparticles thus contributing in synthesizing Janus particles for bio-sensing applications. Different strategies for synthesizing Janus particles also play a major role in determining the properties of Janus nanoparticles. The most efficient of the reviewed methods is the droplet-based microfluidic method, as it results in formation of uniform Janus particles along with easy manipulation of every single droplet formed to produce the Janus particles.
In conjunction with this, the review has also focussed on understanding the utilization of the Janus nanoparticles for bio-sensing applications. Among the different types of sensors reviewed, electrochemical sensors developed using Janus nanoparticles showed better overall performance as compared to optical sensors. This type of sensor demonstrated the enhanced sensitivity with limit of detection in the range of pM. Unlike colorimetric and fluorescence-based sensors, electrochemical sensors are immune to problems like reduced accuracy due to aggregation or auto fluorescence of Janus particles. Raman based sensors developed using Janus nanoparticles also showed higher sensitivity upto fM level however, the higher instrumentation cost and portability of these sensors can limit their use as point of care (POC) devices. The toxicity of Janus nanoparticles is still an under-explored area and hence more research needs to be carried out in understanding the toxicity of these particles, synergistic toxicity of different faces for in-vivo application. Approaches in countering the possible toxic nature of Janus nanoparticles for example, utilizing biocompatible materials as the faces of Janus nanoparticles can be an area of future research.
Author contributions
All authors have equal contribution in the work.
Funding
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
No conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Srushti Karadkar, Email: srushtikaradkar17@gmail.com.
Abhishekh Tiwari, Email: abhishekh_ac@nano.mu.ac.in.
Atul Changdev Chaskar, Email: achaskar@nano.mu.ac.in.
References
- 1.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi Ziarani G, Malmir M, Lashgari N, Badiei A. The role of hollow magnetic nanoparticles in drug delivery. RSC Adv. 2019;9(43):25094–25106. doi: 10.1039/c9ra01589b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Glackin CA, Horwitz MA, Zink JI. Nanomachines and other caps on mesoporous silica nanoparticles for drug delivery. Acc. Chem. Res. 2019;52(6):1531–1542. doi: 10.1021/acs.accounts.9b00116. [DOI] [PubMed] [Google Scholar]
- 4.Yin XT, et al. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloys Compd. 2019;805:229–236. doi: 10.1016/j.jallcom.2019.07.081. [DOI] [Google Scholar]
- 5.Gloag L, Mehdipour M, Chen D, Tilley RD, Gooding JJ. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019;31(48):1–26. doi: 10.1002/adma.201904385. [DOI] [PubMed] [Google Scholar]
- 6.Chu HW, et al. Nanoparticle-based LDI-MS immunoassay for the multiple diagnosis of viral infections. ACS Sens. 2019;4(6):1543–1551. doi: 10.1021/acssensors.9b00054. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Ke H, Wang Y, Li P, Huang C, Jia N. 3D carbon nanosphere and gold nanoparticle-based voltammetric cytosensor for cell line A549 and for early diagnosis of non-small cell lung cancer cells. Microchim. Acta. 2019;186(1):2–8. doi: 10.1007/s00604-018-3160-4. [DOI] [PubMed] [Google Scholar]
- 8.Dissanayake NM, Arachchilage JS, Samuels TA, Obare SO. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta. 2019;200:218–227. doi: 10.1016/j.talanta.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Rahman MM, Alam MM, Asiri AM. Development of an efficient phenolic sensor based on facile Ag2O/Sb2O3 nanoparticles for environmental safety. Nanoscale Adv. 2019;1(2):696–705. doi: 10.1039/c8na00034d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, et al. Surface-imprinted gold nanoparticle-based surface-enhanced raman scattering for sensitive and specific detection of patulin in food samples. Food Anal. Methods. 2019;12(7):1648–1657. doi: 10.1007/s12161-019-01498-4. [DOI] [Google Scholar]
- 11.Li Y, Wang Z, Sun L, Liu L, Xu C, Kuang H. Nanoparticle-based sensors for food contaminants. TrAC Trends Anal. Chem. 2019;113:74–83. doi: 10.1016/j.trac.2019.01.012. [DOI] [Google Scholar]
- 12.Ular N, Üzer A, Durmazel S, Erçaǧ E, Apak R. Diaminocyclohexane-functionalized/thioglycolic acid-modified gold nanoparticle-based colorimetric sensing of trinitrotoluene and tetryl. ACS Sens. 2018;3(11):2335–2342. doi: 10.1021/acssensors.8b00709. [DOI] [PubMed] [Google Scholar]
- 13.Üzer A, Can Z, Akin I, Erçaǧ E, Apak R. 4-aminothiophenol functionalized gold nanoparticle-based colorimetric sensor for the determination of nitramine energetic materials. Anal. Chem. 2014;86(1):351–356. doi: 10.1021/ac4032725. [DOI] [PubMed] [Google Scholar]
- 14.Gui Y, Xie C, Xu J, Wang G. Detection and discrimination of low concentration explosives using MOS nanoparticle sensors. J. Haz. Mater. 2009;164(2–3):1030–1035. doi: 10.1016/j.jhazmat.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Charbgoo F, Ramezani M, Darroudi M. Bio-sensing applications of cerium oxide nanoparticles: advantages and disadvantages. Biosens. Bioelectron. 2017;96:33–43. doi: 10.1016/j.bios.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Su H, Hurd Price CA, Jing L, Tian Q, Liu J, Qian K. Janus particles: design, preparation, and biomedical applications. Mater Today Bio. 2019;4:100033. doi: 10.1016/j.mtbio.2019.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun XT, Zhang Y, Zheng DH, Yue S, Yang CG, Xu ZR. Multitarget sensing of glucose and cholesterol based on Janus hydrogel microparticles. Biosens. Bioelectron. 2017;92(February):81–86. doi: 10.1016/j.bios.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, She ZG, Wang S, Sharma G, Smith JW. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir. 2012;28(9):4459–4463. doi: 10.1021/la2042185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safaie N, Ferrier RC. Janus nanoparticle synthesis: overview, recent developments, and applications. J. Appl. Phys. 2020 doi: 10.1063/5.0003329. [DOI] [Google Scholar]
- 20.Jiang S, Granick S. Janus balance of amphiphilic colloidal particles. J. Chem. Phys. 2007 doi: 10.1063/1.2803420. [DOI] [PubMed] [Google Scholar]
- 21.Walther A, Müller AHE. Janus particles. Soft Matter. 2008;4(4):663–668. doi: 10.1039/b718131k. [DOI] [PubMed] [Google Scholar]
- 22.Gheisari F, et al. Janus nanoparticles: an efficient intelligent modern nanostructure for eradicating cancer. Drug Metab. Rev. 2021;53(4):592–603. doi: 10.1080/03602532.2021.1878530. [DOI] [PubMed] [Google Scholar]
- 23.Lattuada M, Hatton TA. Synthesis, properties and applications of Janus nanoparticles. Nano Today. 2011;6(3):286–308. doi: 10.1016/j.nantod.2011.04.008. [DOI] [Google Scholar]
- 24.Lazarides AA, Lance Kelly K, Jensen TR, Schatz GC. Optical properties of metal nanoparticles and nanoparticle aggregates important in biosensors. J. Mol. Struct. THEOCHEM. 2000;529(1–3):59–63. doi: 10.1016/S0166-1280(00)00532-7. [DOI] [Google Scholar]
- 25.Anker JN, Behrend C, Kopelman R. Aspherical magnetically modulated optical nanoprobes (MagMOONs) J. Appl. Phys. 2003;93(102):6698–6700. doi: 10.1063/1.1556926. [DOI] [Google Scholar]
- 26.Xing Y, et al. Construction strategy for ratiometric fluorescent probe based on Janus silica nanoparticles as a platform toward intracellular pH detection. Talanta. 2019;205(May):120021. doi: 10.1016/j.talanta.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Russell SM, Alba-Patiño A, Borges M, de la Rica R. Multifunctional motion-to-color janus transducers for the rapid detection of sepsis biomarkers in whole blood. Biosens. Bioelectron. 2019;140:111346. doi: 10.1016/j.bios.2019.111346. [DOI] [PubMed] [Google Scholar]
- 28.Soylemez S, Udum YA, Kesik M, Gündoʇdu Hizliateş C, Ergun Y, Toppare L. Electrochemical and optical properties of a conducting polymer and its use in a novel biosensor for the detection of cholesterol. Sens. Actu. B Chem. 2015;212:425–433. doi: 10.1016/j.snb.2015.02.045. [DOI] [Google Scholar]
- 29.Zhang G, et al. A fast and general approach to produce a carbon coated Janus metal/oxide hybrid for catalytic water splitting. J. Mater. Chem. A. 2021;9(12):7606–7616. doi: 10.1039/d0ta12021a. [DOI] [Google Scholar]
- 30.Iqbal MZ, et al. A facile fabrication route for binary transition metal oxide-based Janus nanoparticles for cancer theranostic applications. Nano Res. 2018;11(10):5735–5750. doi: 10.1007/s12274-017-1628-x. [DOI] [Google Scholar]
- 31.Sanchez-Vazquez B, Amaral AJR, Yu DG, Pasparakis G, Williams GR. Electrosprayed Janus particles for combined photo-chemotherapy. AAPS PharmSciTech. 2017;18(5):1460–1468. doi: 10.1208/s12249-016-0638-4. [DOI] [PubMed] [Google Scholar]
- 32.Karshalev E, et al. Micromotors for active delivery of minerals toward the treatment of iron deficiency anemia. Nano Lett. 2019;19(11):7816–7826. doi: 10.1021/acs.nanolett.9b02832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng ZQ, et al. Magnetic Janus particles as a multifunctional drug delivery system for paclitaxel in efficient cancer treatment. Mater. Sci. Eng. C. 2019;104(June):110001. doi: 10.1016/j.msec.2019.110001. [DOI] [PubMed] [Google Scholar]
- 34.Le TC, Zhai J, Chiu WH, Tran PA, Tran N. Janus particles: recent advances in the biomedical applications. Int. J. Nanomed. 2019;14:6749–6777. doi: 10.2147/IJN.S169030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, et al. Click-grafting of cardanol onto mesoporous silica/silver Janus particles for enhanced hemostatic and antibacterial performance. ACS Appl. Bio Mater. 2020;3(12):9054–9064. doi: 10.1021/acsabm.0c01267. [DOI] [PubMed] [Google Scholar]
- 36.Dehghani E, Barzgari-Mazgar T, Salami-Kalajahi M, Kahaie-Khosrowshahi A. A pH-controlled approach to fabricate electrolyte/non-electrolyte janus particles with low cytotoxicity as carriers of DOX. Mater. Chem. Phys. 2020;249(March):123000. doi: 10.1016/j.matchemphys.2020.123000. [DOI] [Google Scholar]
- 37.Li X, et al. Degradation-restructuring induced anisotropic epitaxial growth for fabrication of asymmetric diblock and triblock mesoporous nanocomposites. Adv. Mater. 2017;29(30):1–8. doi: 10.1002/adma.201701652. [DOI] [PubMed] [Google Scholar]
- 38.Ye Y, et al. Fabrication of self-propelled micro- and nanomotors based on Janus structures. Chem. A Eur. J. 2019;25(37):8663–8680. doi: 10.1002/chem.201900840. [DOI] [PubMed] [Google Scholar]
- 39.Tan JSJ, Chen Z. Mask-less preparation of Janus particles through ultraviolet irradiation on hydrophobic particles assembled at the air-water interface. J. Colloid Interface Sci. 2019;546:285–292. doi: 10.1016/j.jcis.2019.03.081. [DOI] [PubMed] [Google Scholar]
- 40.Wang K, Wang W, Pan S, Fu Y, Dong B, Wang H. Fluorescent self-propelled covalent organic framework as a microsensor for nitro explosive detection. Appl. Mater. Today. 2020;19:100550. doi: 10.1016/j.apmt.2019.100550. [DOI] [Google Scholar]
- 41.Sun XT, Yang CG, Xu ZR. Controlled production of size-tunable Janus droplets for submicron particle synthesis using an electrospray microfluidic chip. RSC Adv. 2016;6(15):12042–12047. doi: 10.1039/c5ra24531a. [DOI] [Google Scholar]
- 42.Mou F, Chen C, Guan J, Chen DR, Jing H. Oppositely charged twin-head electrospray: a general strategy for building Janus particles with controlled structures. Nanoscale. 2013;5(5):2055–2064. doi: 10.1039/c2nr33523a. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Shang M, Wang Y, Xu Z. Droplet-based microfluidic synthesis of (Au nanorod@Ag)-polyaniline Janus nanoparticles and their application as a surface-enhanced Raman scattering nanosensor for mercury detection. Anal. Methods. 2019;11(31):3966–3973. doi: 10.1039/c9ay01213c. [DOI] [Google Scholar]
- 44.Kumar S. Structural evolution of iron–copper (Fe–Cu) bimetallic Janus nanoparticles during solidification: an atomistic investigation. J. Phys. Chem. C. 2019 doi: 10.1021/acs.jpcc.9b08411. [DOI] [Google Scholar]
- 45.Jiang S, Chen Q, Tripathy M, Luijten E, Schweizer KS, Granick S. Janus particle synthesis and assembly. Adv. Mater. 2010;22(10):1060–1071. doi: 10.1002/adma.200904094. [DOI] [PubMed] [Google Scholar]
- 46.Paniagua G, et al. Amperometric aptasensor for carcinoembryonic antigen based on the use of bifunctionalized Janus nanoparticles as biorecognition-signaling element. Anal. Chim. Acta. 2019;1061:84–91. doi: 10.1016/j.aca.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Yang YJ, et al. A Label-free aptasensor based on Aptamer/NH2 Janus particles for ultrasensitive electrochemical detection of Ochratoxin A. Talanta. 2019;199:310–316. doi: 10.1016/j.talanta.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, et al. Electrochemical sensor for determination of ractopamine based on aptamer/octadecanethiol Janus particles. Sens. Actu. B Chem. 2018;276:204–210. doi: 10.1016/j.snb.2018.08.110. [DOI] [Google Scholar]
- 49.Kong L, Rohaizad N, Nasir MZM, Guan J, Pumera M. Micromotor-assisted human serum glucose biosensing. Anal. Chem. 2019;91(9):5660–5666. doi: 10.1021/acs.analchem.8b05464. [DOI] [PubMed] [Google Scholar]
- 50.Pacheco M, Jurado-Sánchez B, Escarpa A. Sensitive monitoring of enterobacterial contamination of food using self-propelled Janus microsensors. Anal. Chem. 2018;90(4):2912–2917. doi: 10.1021/acs.analchem.7b05209. [DOI] [PubMed] [Google Scholar]
- 51.Pacheco M, de la Asunción-Nadal V, Jurado-Sánchez B, Escarpa A. Engineering Janus micromotors with WS2 and affinity peptides for turn-on fluorescent sensing of bacterial lipopolysaccharides. Biosens. Bioelectron. 2020;165:112286. doi: 10.1016/j.bios.2020.112286. [DOI] [PubMed] [Google Scholar]
- 52.de La Asunción-Nadal V, Pacheco M, Jurado-Sánchez B, Escarpa A. Chalcogenides-based tubular micromotors in fluorescent assays. Anal. Chem. 2020;92(13):9188–9193. doi: 10.1021/acs.analchem.0c01541. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Concellón A, Yoshinaga K, Nelson Z, He Q, Swager TM. Janus emulsion biosensors for Anti-SARS-CoV-2 spike antibody. ACS Cent. Sci. 2021;7(7):1166–1175. doi: 10.1021/acscentsci.1c00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Savagatrup S, Nelson Z, Yoshinaga K, Swager TM. Fluorescent Janus emulsions for biosensing of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA. 2020;117(22):1–8. doi: 10.1073/pnas.2002623117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue Chen X, Tian Ma R, Ha W, Ping Shi Y. Direct colorimetric detection of aspartic acid in rat brain based on oriented aggregation of Janus gold nanoparticle. Sens. Actu. B Chem. 2018;274:668–675. doi: 10.1016/j.snb.2018.08.008. [DOI] [Google Scholar]
- 56.Yang S, et al. Superhydrophobic surface enhanced Raman scattering sensing using Janus particle arrays realized by site-specific electrochemical growth. J. Mater. Chem. C. 2014;2(3):542–547. doi: 10.1039/c3tc31635a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petry R, Schmitt M, Popp J. Raman spectroscopy—a prospective tool in the life sciences. ChemPhysChem. 2003;4(1):14–30. doi: 10.1002/cphc.200390004. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Zhao Z, Liu L, Li Y. Design of Gold nanorods Janus membrane for efficient and high-sensitive surface-enhanced Raman scattering and tunable surface plasmon resonance. Chem. Phys. Lett. 2019;721(February):117–122. doi: 10.1016/j.cplett.2019.02.035. [DOI] [Google Scholar]
- 59.Park JH, Dumani DS, Arsiwala A, Emelianov S, Kane RS. Tunable aggregation of gold-silica janus nanoparticles to enable contrast-enhanced multiwavelength photoacoustic imaging in vivo. Nanoscale. 2018;10(32):15365–15370. doi: 10.1039/c8nr03973a. [DOI] [PubMed] [Google Scholar]
- 60.Beltrán-Gastélum M, et al. Rapid detection of AIB1 in breast cancer cells based on aptamer-functionalized nanomotors. ChemPhysChem. 2019;20(23):3177–3180. doi: 10.1002/cphc.201900844. [DOI] [PubMed] [Google Scholar]
- 61.Demirörs AF, Akan MT, Poloni E, Studart AR. Active cargo transport with Janus colloidal shuttles using electric and magnetic fields. Soft Matter. 2018;14(23):4741–4749. doi: 10.1039/c8sm00513c. [DOI] [PubMed] [Google Scholar]
- 62.Yuan K, López MÁ, Jurado-Sánchez B, Escarpa A. Janus micromotors coated with 2D nanomaterials as dynamic interfaces for (Bio)-sensing. ACS Appl. Mater. Interfaces. 2020 doi: 10.1021/acsami.0c15389. [DOI] [PubMed] [Google Scholar]
- 63.Qualliotine JR, Bolat G, Beltrán-Gastélum M, de Ávila BEF, Wang J, Califano JA. Acoustic nanomotors for detection of human papillomavirus-associated head and neck cancer. Otolaryngol. Head Neck Surg. (USA) 2019;161(5):814–822. doi: 10.1177/0194599819866407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.