Abstract
Coronavirus disease 2019 (COVID-19) and COVID-19 vaccination may cause splanchnic vein thrombosis (SVT), which is potentially fatal. The present study aims to pool the incidence and outcomes of SVT patients with COVID-19 or having received COVID-19 vaccines. The PubMed, EMBASE, and Cochrane databases were searched. Based on the data from cohort studies, meta-analyses were performed to evaluate the incidence of SVT in COVID-19 patients or people having received COVID-19 vaccines. Pooled proportions were calculated. Based on the individual data from case reports, logistic regression analyses were performed to identify factors associated with death in SVT patients. Odds ratios (ORs) were calculated. Among 654 papers initially identified, 135 were included. Based on 12 cohort studies, the pooled incidence of SVT in COVID-19 patients was 0.6%. Data were insufficient to estimate the incidence of SVT after COVID-19 vaccination. Based on 123 case reports, the mortality was 14% (9/64) in SVT patients with COVID-19 and 25% (15/59) in those who received COVID-19 vaccines. Univariate analyses demonstrated that age (OR = 1.061; p = 0.017), diabetes mellitus (OR = 14.00; p = 0.002), anticoagulation (OR = 0.098; p = 0.004), and bowel resection (OR = 16.00; p = 0.001) were significantly associated with death in SVT patients with COVID-19; and anticoagulation (OR = 0.025; p = 0.003) and intravenous immunoglobulin (OR = 0.175; p = 0.046) were significantly associated with death in SVT patients who received COVID-19 vaccines. Multivariate analyses did not identify any independent factor for death in both patients. SVT in COVID-19 patients and in subjects who received COVID-19 vaccines carries a high mortality, but may be improved by anticoagulation.
PROSPERO Identifier CRD42022315254.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-022-02732-3.
Keywords: Splanchnic vein, Portal vein, Thrombosis, COVID-19, COVID-19 vaccines
Highlights
Until now, no study has reported the pooled incidence and outcomes of splanchnic vein thrombosis (SVT) in patients with COVID-19 or subjects who received COVID-19 vaccines.
We performed a systematic review and meta-analysis to evaluate the incidence, characteristics, treatments, and outcomes of SVT in patients with COVID-19 or in subjects who received COVID-19 vaccines.
SVT is a rare complication in COVID-19 patients. SVT may lead to a high mortality in patients with COVID-19 or having received COVID-19 vaccines.
Anticoagulation may improve the outcomes of SVT patients with COVID-19 and those who received COVID-19 vaccines.
Introduction
Coronavirus disease 2019 (COVID-19), an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], has resulted in more than 500 million cases and over 6 million deaths worldwide till April 17, 2022, and poses a catastrophic threat to global public health [2]. Most COVID-19 patients are asymptomatic or only experience mild infection, but others may develop severe pneumonia and even multiple organ failure [3]. COVID-19 patients, especially those admitted to the intensive care unit, frequently have coagulation activation, thereby increasing the risk of venous thromboembolism (VTE) [4–6]. A recent meta-analysis showed that deep vein thrombosis (DVT) and pulmonary embolism (PE) developed in 16.5% and 14.8% of COVID-19 patients, respectively, and significantly increased the risk of death [7]. Splanchnic vein thrombosis (SVT) is an unusual type of VTE, including portal vein thrombosis (PVT), mesenteric vein thrombosis (MVT), splenic vein thrombosis (SpVT), and Budd-Chiari syndrome (BCS) [8], which can cause life-threatening complications, such as intestinal ischemia or necrosis and gastrointestinal bleeding [8, 9]. Differently from usual site VTE, no study has reported the pooled incidence and outcomes of SVT in COVID-19 patients.
COVID-19 vaccines have been rapidly developed and used to prevent from the transmissions of SARS-CoV-2 and decrease the severity of this disease [10], and 11 billion doses of COVID-19 vaccines have already been administered till April 17, 2022 [11]. Scattered studies have suggested that SVT may occur as a complication after the administration of some COVID-19 vaccines [12, 13].
Hence, we performed a systematic review and meta-analysis to evaluate the incidence, characteristics, treatments, and outcomes of SVT in patients with COVID-19 or in subjects who received COVID-19 vaccines.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. The PRISMA checklist was shown in Supplementary Materials.
Literature search
The PubMed, EMBASE, and Cochrane library databases were searched. We also manually identified the relevant studies. The last search was performed on July 27, 2022. Search terms were as follows: ((COVID-19 (all fields)) OR (coronavirus disease 2019 (all fields)) OR (2019 novel coronavirus (all fields)) OR (SARS-CoV-2 (all fields)) OR (2019-nCov (all fields)) OR (2019 novel coronavirus-infected pneumonia (all fields)))AND ((splanchnic vein (all fields)) OR (splanchnic venous (all fields)) OR (portal vein (all fields)) OR (portal venous (all fields)) OR (mesenteric vein (all fields)) OR (mesenteric venous (all fields)) OR (splenic vein (all fields)) OR (splenic venous (all fields)) OR (hepatic vein (all fields)) OR (hepatic venous (all fields)) OR (Budd-Chiari syndrome (all fields))).
Selection criteria
Eligible studies should have included patients who developed SVT after they were diagnosed with COVID-19 or had received COVID-19 vaccines. The exclusion criteria were as follows: (1) duplicated papers; (2) consensus, comments, editorials, notes, or letters; (3) reviews or meta-analyses; (4) experimental or animal studies; (5) patients without a diagnosis of COVID-19 or those who had not received COVID-19 vaccines; (6) all patients were not diagnosed with SVT; (7) all patients were younger than 18 years old; (8) overlapping data; and (9) the number, characteristics, treatments, and outcomes of SVT among patients with COVID-19 or people having received COVID-19 vaccines were lacking. Publication language was not restricted.
Data extraction
Two reviewers (XZ and YM) independently extracted the data from the finally included cohort studies and case reports according to the predesigned extraction table, and any disagreement was resolved by discussion or consultation with a third reviewer (XQ). We extracted the following data from cohort studies: first author, publication year, country, study design, target population, sample size, and number of SVT patients with COVID-19 or who had received COVID-19 vaccines.
We also extracted the following data from case reports: first author, publication year, country, age, sex, comorbidity, type of COVID-19 vaccines, diagnosis of inherited or acquired thrombophilia, diagnostic tests for COVID-19, anti-platelet factor 4 (PF4) antibody test, symptoms/signs of SVT, interval from the onset of COVID-19 or COVID-19 vaccination to symptoms/signs of SVT, imaging tests for SVT diagnosis, extension of SVT, concomitant thrombosis developing at other locations, and laboratory tests (i.e., D-dimer, C-reactive protein, white blood cell, platelet count, alanine aminotransferase, aspartate aminotransferase, prothrombin time, and activated partial thromboplastin time), type and duration of anticoagulants, other treatment, outcome, and follow-up duration.
Outcomes
The primary outcome was the incidence of SVT in patients with COVID-19 or in subjects who received COVID-19 vaccines. The secondary outcomes were characteristics and outcomes of SVT patients who were diagnosed with COVID-19 or received COVID-19 vaccines.
Study quality assessment
Two reviewers (XZ and FG) independently evaluated quality of included cohort studies and case reports, and any disagreement was resolved by discussion or consultation with a third reviewer (XQ). The quality of cohort studies was evaluated by the Newcastle–Ottawa Scale (NOS), which includes three parts (i.e., selection, comparability, and outcomes) and eight questions. The highest NOS score is nine points, and a NOS score of above six points is considered high quality.
The quality of case reports was evaluated by the scale developed by the Joanna Briggs Institute (JBI) evidence-based health care centers. The scale includes eight items and answers with “yes”, “no”, “unclear”, and “not available”. According to the number of answers with “yes”, they can be divided into high quality (≥ 6 “yes”), moderate quality (4 or 5 “yes”), and low quality (< 4 “yes”).
Statistical analyses
StatsDirect version 2.8.0 (StatsDirect Ltd, Cheshire, UK) and Stata version 12.0 (StataCorp LP, College Station, TX, USA) were employed to pool the incidence of SVT after a diagnosis of COVID-19 based on the data from cohort studies. A random-effects model was employed. Heterogeneity was evaluated by the I2 statistics and the Cochran Q test. I2 > 50% or p < 0.1 was considered as statistically significant heterogeneity. The sources of heterogeneity were explored by using subgroup and meta-regression analyses. RStudio version 1.4.1717 (R Foundation for Statistical Computing, Vienna, Austria) was emplyed to test the interaction between subgroups. P < 0.1 was considered as a statistically significant interaction. They were performed according to the region (Europe vs. USA vs. Other), publication year (2020 vs. 2021 vs. 2022), type of study design (retrospective vs. prospective), type of publication (abstract vs. full text), target population (specific patients with COVID-19 vs. non-specific patients with COVID-19), sample size (≥ 1000 vs. < 1000), and NOS (≥ 6 vs. < 6). The presence of publication bias was evaluated by the Egger test, and p < 0.1 was considered as statistically significant publication bias.
The statistical software IBM SPSS version 20.0 (International Business Machines Corp, Armonk, NY, USA) was employed to conduct statistical analyses based on the data from every case with SVT. Continuous variables were presented as mean (range), and categorical variables as frequency (percentage). Univariate and multivariate logistic regression analyses were performed to identify the risk factors for death among SVT patients. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A two-tailed p value < 0.05 was considered statistically significant.
Results
Study selection
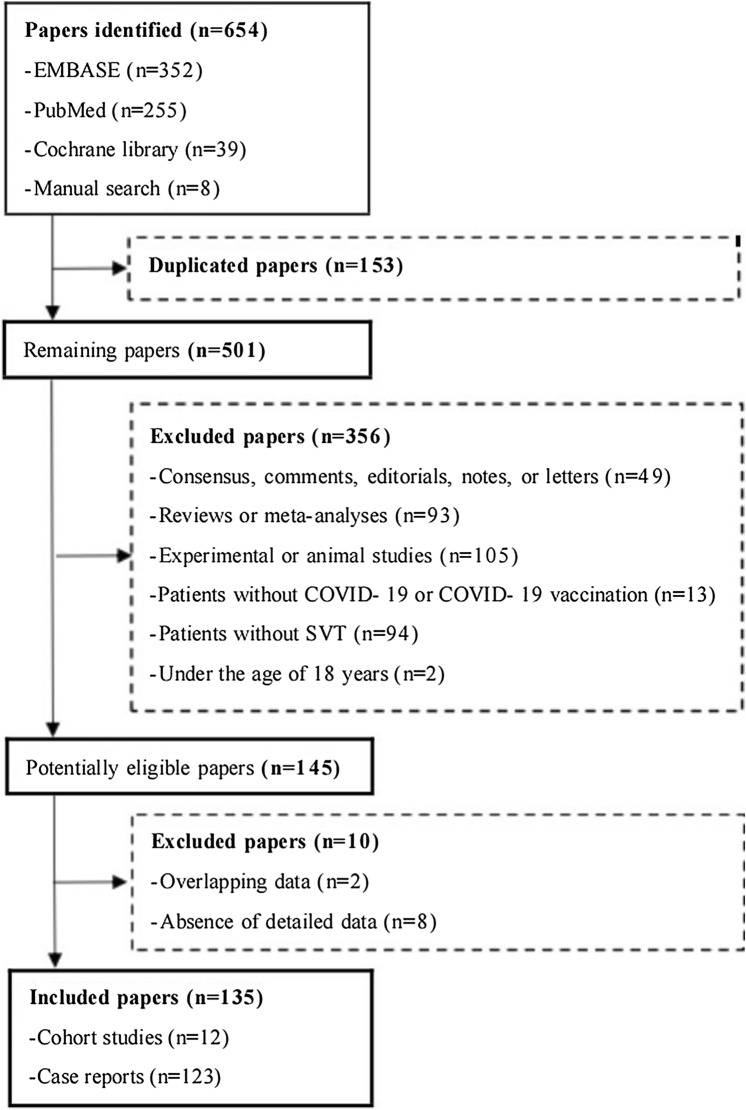
Overall, 646 papers were identified in the three electronic databases, and eight papers were identified after manual search. Finally, 135 studies were eligible (Fig. 1). They included 12 cohort studies reporting the incidence of SVT among COVID-19 patients and 123 case reports reporting the characteristics, treatments, and outcomes of SVT patients with COVID-19 or those having received COVID-19 vaccines. Among these case reports, 77 COVID-19 patients and 61 patients having received COVID-19 vaccines developed SVT.
Fig. 1.
Flow chart of study selection. COVID-19 coronavirus disease 2019, SVT splanchnic vein thrombosis
Incidence of SVT in COVID-19 patients
Among the 12 cohort studies, four were conducted in USA [14–17], five in Europe [18–22], one in Asia [23], and two in Africa [24, 25]; two studies were prospective cohort studies [14, 24], and 10 were retrospective cohort studies [15–23, 25]; three studies were published as abstracts [17, 19, 20], and nine as full texts [14–16, 18, 21–25] (Table 1). Six studies were of high quality [14, 15, 17, 22, 24, 25], and six were of low quality [16, 18–21, 23] (Supplementary Table 1).
Table 1.
Characteristics of studies regarding incidence of SVT in COVID-19 patients
| First author (year) | Country | Study design | Type of publication | Target population | No. Pts COVID-19 | Extension of SVT |
|---|---|---|---|---|---|---|
| Filippidis (2020) [19] | UK | Retrospective study | Abstract | Patients with COVID-19 | 450 | PVT (n = 1) |
| Li (2021) [15] | USA | Retrospective study | Full text | Adult patients with COVID-19 | 2832 | PVT (n = 2) |
| Arachchillage (2021) [18] | UK | Retrospective study | Full text | Patients with COVID-19 | 171 | PVT (n = 1) |
| Elbadry (2021) [24] | Egypt | Prospective study | Full text | Young adult non-critically ill patients with COVID-19 | 1564 | SVT (n = 11) |
| Patel (2021) [23] | India | Retrospective study | Full text | Patients with COVID-19 who underwent CT | 93 | MVT (n = 2) |
| Gardner (2021) [20] | UK | Retrospective study | Abstract | Critically ill patients with COVID-19 | 34 | PVT (n = 2) |
| Taya (2021) [16] | USA | Retrospective study | Full text | Patients with COVID-19 using CE-CT | 63 | PVT (n = 1) |
| Purroy (2021) [22] | Spain | Retrospective study | Full text | Patients with COVID-19 | 1737 | PVT (n = 1) |
| Kapoor (2021) [14] | USA | Prospective study | Full text | Adult patients with COVID-19 admitted to ICU | 107 | PVT (n = 1) |
| Zhang (2021) [17] | USA | Retrospective study | Abstract | Adult patients with COVID-19 | 55 | SpVT (n = 1) |
| Norsa (2021) [21] | Italy | Retrospective study | Full text | Patients with COVID-19 | 2929 | SVT (n = 3) |
| Hassnine (2022) [25] | Egypt | Retrospective study | Full text | Patients with liver cirrhosis infected with COVID-19 | 28 | PVT (n = 3) |
COVID-19 coronavirus disease 2019, SVT splanchnic vein thrombosis, PVT portal vein thrombosis, MVT mesenteric vein thrombosis, SpVT splenic vein thrombosis, CE-CT contrast-enhanced computed tomography
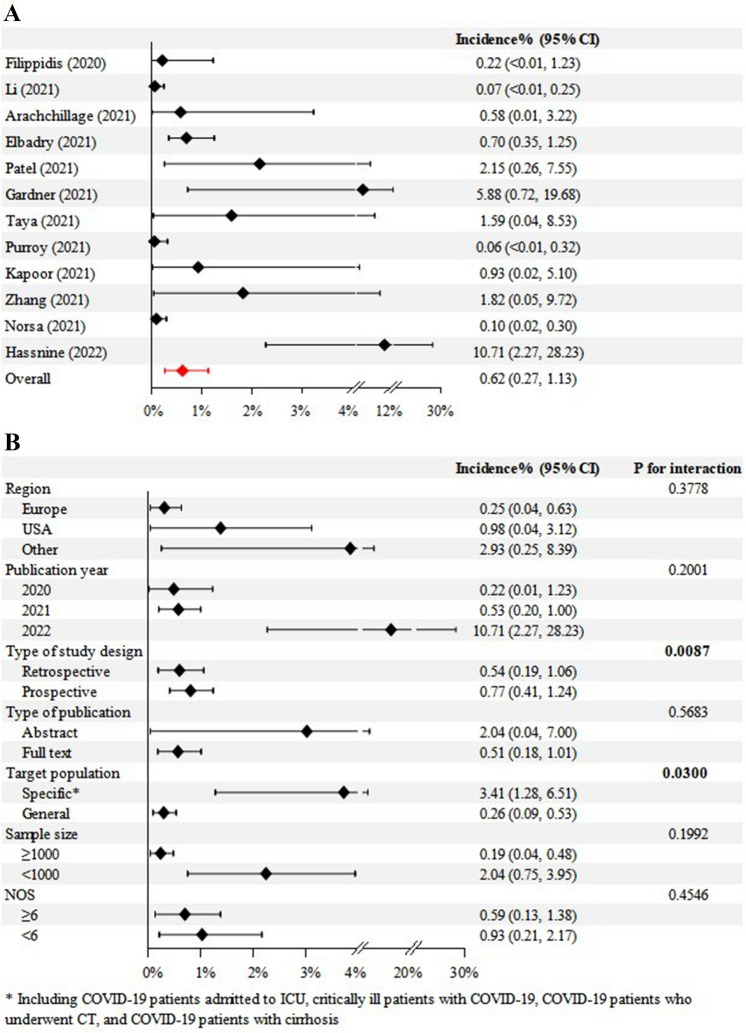
Meta-analysis demonstrated that the pooled incidence of SVT in COVID-19 patients was 0.6% (95% CI = 0.3% to 1.1%) with significant heterogeneity (I2 = 77.7%; p < 0.0001) (Fig. 2A).
Fig. 2.
Overall (A) and subgroup (B) analyses regarding pooled incidence of SVT in COVID-19 patients. COVID-19 coronavirus disease 2019, CIs confidence intervals, NOS Newcastle–Ottawa scale, SVT splanchnic vein thrombosis
Subgroup analyses demonstrated that the pooled incidence of SVT in COVID-19 patients was 0.2% in Europe, 1.0% in North America, and 2.9% in other countries; 0.2% in studies published in 2020, 0.5% in 2021, and 10.7% in 2022; 0.5% in retrospective studies and 0.8% in prospective studies; 2.0% in studies published as abstracts and 0.5% in studies published as full texts; 3.4% in studies involving specific patients with COVID-19 and 0.3% in studies involving non-specific patients with COVID-19; 0.2% in studies with a sample size of ≥ 1000 and 2.0% in studies with a sample size of < 1000; and 0.6% in high-quality studies and 0.9% in low-quality studies. The interaction between subgroups was significant in the subgroup analysis according to the type of study design (p = 0.0087) and target population (p = 0.03), but not in others (Fig. 2B).
Meta-regression analyses demonstrated that region and type of study design might be potential sources of heterogeneity, but not publication year, type of publication, sample size, or study quality (Supplementary Table 2).
Publication bias was significant (p = 0.0015).
Characteristics, treatments, and outcomes of SVT patients with COVID-19
Among the selected case reports, 24 were conducted in North America, 20 in Europe, five in South America, 26 in Asia, and two in Africa. Sixty case reports were of high quality, 12 were of moderate quality, and five were of low quality (Supplementary Table 3).
The mean age of the patients was 45.7 years (range: 20–88). There were 46 males and 27 females. Comorbidities mainly included diabetes mellitus (n = 12), obesity (n = 6), hypertension (n = 9), and liver-related diseases (n = 10). Eleven patients had inherited or acquired thrombophilia, and 28 had negative thrombophilia tests. COVID-19 was mainly diagnosed based on positive RT-PCR on nasopharynx swab (n = 34). The average interval from the onset of COVID-19 to symptoms/signs of SVT was 12 days (range: 0–90). The site of SVT included PVT (n = 55), MVT (n = 41), SpVT (n = 19), and BCS (n = 8). Meanwhile, concomitant thrombosis developing at other locations mainly included abdominal arterial thrombosis (n = 7) and pulmonary embolism (n = 4). The main symptoms were abdominal pain (n = 63), followed by fever (n = 28), vomiting or nausea (n = 13), and dyspnea (n = 12) (Table 2). The laboratory tests were shown in Supplementary Table 4.
Table 2.
Characteristics, treatments, and outcomes of SVT patients with COVID-19
| Variables | No. Pts evaluated | Frequency (percentage) or Mean ± SD (range) |
|---|---|---|
| Age (years) | 75 | 45.73 ± 15.83 (20–88) |
| Male | 73 | 46 (63.01%) |
| Comorbidity | 63 | 40 (63.49%) |
| Obesity | 63 | 6 (9.52%) |
| Diabetes mellitus | 63 | 12 (19.05%) |
| Hypertension | 63 | 9 (14.29%) |
| Liver diseases | 63 | 10 (15.87%) |
| Alcohol liver cirrhosis | 63 | 5 (7.94%) |
| Non-alcoholic fatty liver disease | 63 | 2 (3.17%) |
| Hepatitis B virus | 63 | 2 (3.17%) |
| Hepatitis C virus | 63 | 1 (1.59%) |
| Diagnosis of inherited or acquired thrombophilia | 39 | 11 (28.21%) |
| Positive lupus anticoagulant | 39 | 5 (12.82%) |
| Positive JAK2 V617F mutation alone | 39 | 1 (2.56%) |
| Decreased protein C and S | 39 | 2 (5.13%) |
| Essential thrombocythemia | 39 | 2 (5.13%) |
| Positive AT-III | 39 | 1 (2.56%) |
| Diagnostic tests for COVID-19 | ||
| Positive RT-PCR on nasopharynx swab | 48 | 35 (72.92%) |
| Positive SARS-CoV-2 antibody | 48 | 10 (20.83%) |
| Chest CT | 48 | 2 (4.17%) |
| RNA in situ hybridization technique | 48 | 1 (2.08%) |
| Imaging tests for SVT diagnosis | ||
| CT scans | 69 | 62 (89.86%) |
| Contrast-enhanced CT scans specified | 69 | 55 (79.71%) |
| Ultrasound | 69 | 2 (2.90%) |
| Others | 69 | 5 (7.25%) |
| Symptoms | ||
| Abdominal pain | 75 | 63 (84.00%) |
| Fever | 75 | 28 (37.33%) |
| Abdominal distension | 75 | 5 (6.67%) |
| Vomiting, nausea | 75 | 13 (17.33%) |
| Dyspnea | 75 | 12 (16.00%) |
| Fatigue, weakness | 75 | 5 (6.67%) |
| Interval from the onset of COVID-19 to symptoms/signs of SVT (days) | 44 | 12 ± 19 (0–90) |
| Extension of SVT | ||
| Portal vein thrombosis | 77 | 55 (71.43%) |
| Splenic vein thrombosis | 77 | 19 (24.68%) |
| Mesenteric vein thrombosis | 77 | 41 (53.25%) |
| Budd-Chiari syndrome | 77 | 8 (10.39%) |
| Concomitant thrombosis developing at other locations | 77 | 12 (15.58%) |
| Pulmonary embolism | 77 | 4 (5.19%) |
| Deep venous thrombosis | 77 | 1 (1.30%) |
| Abdominal artery thrombosis | 77 | 7 (9.10%) |
| Anticoagulant therapy | 72 | 60 (83.33%) |
| Type of anticoagulants | ||
| Heparins | 48 | 38 (79.17%) |
| Unfractionated heparin | 48 | 3 (6.25%) |
| Low molecular weight heparin | 48 | 27 (56.25%) |
| Enoxaparin | 48 | 16 (33.33%) |
| Dalteparin | 48 | 2 (4.17%) |
| Vitamin K antagonists | 48 | 9 (18.75%) |
| Warfarin | 48 | 8 (16.67%) |
| Direct oral anticoagulants | 48 | 12 (25.00%) |
| Rivaroxaban | 48 | 4 (8.33%) |
| Apixaban | 48 | 8 (16.67%) |
| Bowel resection | 72 | 11 (15.28%) |
| After anticoagulation failed | 72 | 5 (6.94%) |
| Without anticoagulation | 72 | 6 (8.33%) |
| Intravenous immunoglobulin | 72 | 2 (2.78%) |
| Antibiotic therapy | 72 | 19 (26.39%) |
| Duration of anticoagulation ≥ 6 months | 21 | 16 (76.19%) |
| Improvement of clinical symptoms | 64 | 55 (85.94%) |
| After anticoagulant therapy | 60 | 47 (78.33%) |
| Death | 64 | 9 (14.01%) |
| Bowel ischemia/necrosis | 9 | 5 (55.56%) |
| Septic shock | 9 | 4 (44.44%) |
| Follow-up duration ≥ 1 month | 20 | 17 (85.00%) |
COVID-19 coronavirus disease 2019, SVT splanchnic vein thrombosis, CT computed tomography
Treatments were reported for 72 patients. Sixty patients received anticoagulants, six underwent immediate bowel resection without anticoagulation, five underwent bowel resection after anticoagulation failed, 19 received antibiotic therapy, and two received intravenous immunoglobulin (IVIG). The most common type of anticoagulants used was heparins (n = 38), especially low molecular weight heparin (LMWH) (n = 27), followed by direct oral anticoagulants (DOACs) (n = 12) and vitamin K antagonists (n = 9). The duration of anticoagulation was ≥ 6 months in 16 of 21 patients for whom this information was available (Table 2).
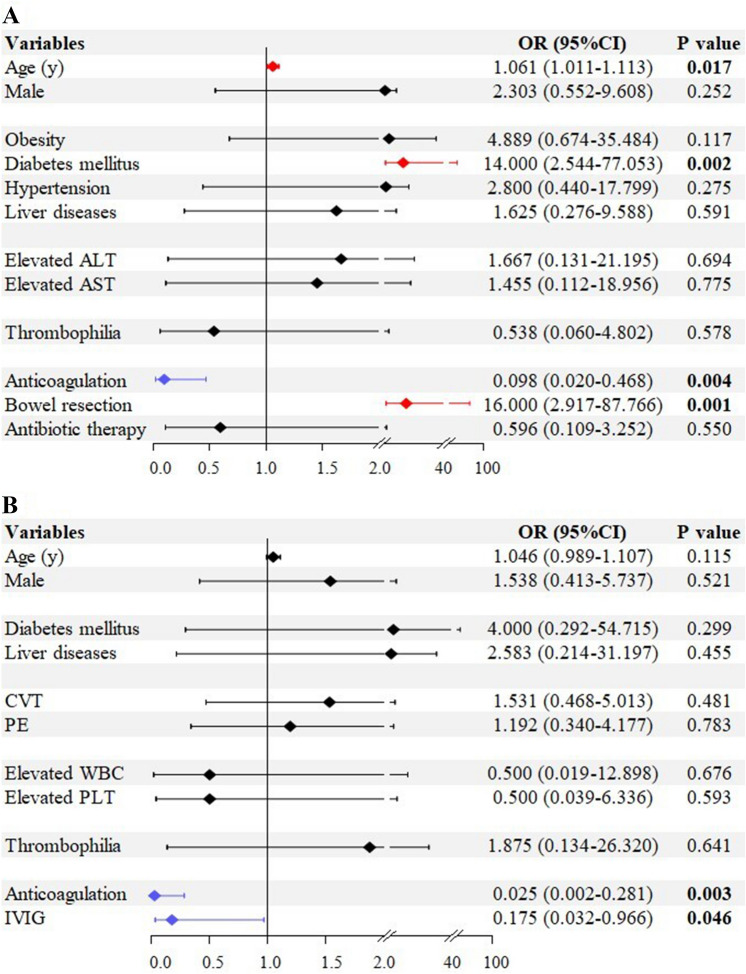
Follow-up duration was ≥ 1 month in 17 patients, < 1 month in 3 patients, and unknown in 57 patients. Outcomes were clearly reported in 64 patients. The rate of improvement of clinical symptoms was 86% (55/64). The mortality was 14% (9/64). Their causes of death included intestinal ischemia/necrosis or perforation (n = 5) and septic shock (n = 4) (Table 2). Univariate logistic regression analyses showed that age (OR = 1.061; 95% CI = 1.011–1.113; p = 0.017), diabetes mellitus (OR = 14.000; 95% CI = 2.544–77.053; p = 0.002), anticoagulant therapy (OR = 0.098; 95% CI = 0.020–0.468; p = 0.004), and bowel resection (OR = 16.000; 95% CI = 2.917–87.766; p = 0.001) were significantly associated with death among SVT patients with COVID-19 (Fig. 3A). Multivariate logistic regression analyses did not identify any independent factor for death among SVT patients with COVID-19.
Fig. 3.
Risk factors for death among SVT patients with COVID-19 (A) or who received COVID-19 vaccines (B). ALT alanine aminotransferase, AST aspartate aminotransferase, APTT activated partial thromboplastin time, COVID-19 coronavirus disease 2019, CIs confidence intervals, CRP C-reactive protein, CVT cerebrovascular thrombosis, IVIG intravenous immunoglobulin, OR odds ratios, PLT platelet, PT prothrombin time, PE pulmonary embolism, SVT splanchnic vein thrombosis, WBC white blood cell
Characteristics, treatments, and outcomes of SVT patients who received COVID-19 vaccines
Among the selected case reports, six were conducted in South America, 43 in Europe, 11 in Asia, and one in Oceania. Forty case reports were of high quality, 13 were of moderate quality, and eight were of low quality (Supplementary Table 3).
The mean age of the patients was 43.9 years (range: 23–68). There were 20 males and 37 females. Comorbidities mainly included diabetes mellitus (n = 3), hypertension (n = 4), and liver-related diseases (n = 4). Fifty-two patients had received ChAdOx1 nCoV-19 vaccines (AstraZeneca), five had received Ad26.COV2.S vaccines (Johnson & Johnson/Janssen), and one had received a BNT162b2 mRNA vaccine (BioNTech/Pfizer). Five patients had inherited or acquired thrombophilia and 18 had negative thrombophilia tests. Thirty-four patients tested positive for anti-PF4 antibody. The average interval from COVID-19 vaccination to the onset of symptoms/signs of SVT was 15 days (range: 1–110). The site of SVT included PVT (n = 52), MVT (n = 23), SpVT (n = 25), and BCS (n = 11). Concomitant thrombosis developing at other locations mainly included cerebrovascular thrombosis (n = 25) and pulmonary embolism (n = 19). The main symptoms were abdominal pain (n = 36), followed by headache (n = 19), vomiting or nausea (n = 15), and fever (n = 8) (Table 3). The laboratory tests were shown in Supplementary Table 5.
Table 3.
Characteristics, treatments, and outcomes of SVT patients having received COVID-19 vaccines
| Variables | No. Pts evaluated | Frequency (percentage) or Mean ± SD (range) |
|---|---|---|
| Age (years) | 57 | 43.86 ± 11.53 (23–68) |
| Male | 57 | 20 (35.09%) |
| Comorbidity | 39 | 18 (46.15%) |
| Obesity | 39 | 5 (12.82%) |
| Diabetes mellitus | 39 | 3 (7.69%) |
| Hypertension | 39 | 4 (10.26%) |
| Liver diseases | 39 | 4 (10.26%) |
| Alcohol liver cirrhosis | 39 | 1 (2.56%) |
| Non-alcoholic fatty liver disease | 39 | 1 (2.56%) |
| Hepatitis C virus | 39 | 1 (2.56%) |
| Chronic Budd-Chiari syndrome | 39 | 1 (2.56%) |
| Type of COVID-19 vaccines | ||
| ChAdOx1 nCoV-19 vaccine (AstraZeneca) | 58 | 52 (89.66%) |
| Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) | 58 | 5 (8.62%) |
| BNT162b2 mRNA vaccine (BioNTech/Pfizer) | 58 | 1 (1.72%) |
| Diagnosis of inherited or acquired thrombophilia | 23 | 5 (21.74%) |
| Positive lupus anticoagulant | 23 | 1 (4.35%) |
| Von Willebrand disease | 23 | 2 (8.70%) |
| Positive JAK2 V617F mutation | 23 | 1 (4.35%) |
| C667T methylenetetrahydrofolate reductase polymorphism | 23 | 1 (4.35%) |
| Positive anti-platelet factor 4 antibody test | 38 | 34 (89.47%) |
| Imaging tests for SVT diagnosis | ||
| CT scans | 47 | 43 (91.49%) |
| Contrast-enhanced CT scans specified | 47 | 36 (76.60%) |
| Ultrasound | 47 | 4 (8.51%) |
| Symptoms | ||
| Abdominal pain | 52 | 36 (69.23%) |
| Fever | 52 | 8 (15.38%) |
| Vomiting, nausea | 52 | 15 (28.85%) |
| Dyspnea | 52 | 3 (5.77%) |
| Headache | 52 | 19 (36.54%) |
| Interval from COVID-19 vaccination to symptoms/signs of SVT (days) | 51 | 15 ± 18 (1–110) |
| Extension of SVT | ||
| Portal vein thrombosis | 61 | 52 (85.25%) |
| Splenic vein thrombosis | 61 | 25 (40.98%) |
| Mesenteric vein thrombosis | 61 | 23 (37.70%) |
| Budd-Chiari syndrome | 61 | 11 (18.03%) |
| Concomitant thrombosis developing at other locations | 61 | 37 (60.66%) |
| Cerebrovascular thrombosis | 37 | 25 (67.57%) |
| Pulmonary embolism | 37 | 19 (51.35%) |
| Abdominal artery thrombosis | 37 | 6 (16.22%) |
| Anticoagulant therapy | 50 | 45 (90.00%) |
| Type of anticoagulants | ||
| Unfractionated heparin | 41 | 4 (9.76%) |
| Low molecular weight heparin | 41 | 8 (19.51%) |
| Enoxaparin | 41 | 4 (9.76%) |
| Fondaparinux (i.v.) | 41 | 15 (36.59%) |
| Direct oral anticoagulants | 41 | 16 (39.02%) |
| Rivaroxaban | 41 | 2 (4.88%) |
| Apixaban | 41 | 9 (21.95%) |
| Dabigatran | 41 | 3 (7.32%) |
| Argatroban (i.v.) | 41 | 12 (29.27%) |
| Bowel resection after anticoagulation failed | 50 | 2 (4.00%) |
| Intravenous immunoglobulin | 50 | 33 (66.00%) |
| Antibiotic therapy | 50 | 2 (4.00%) |
| Duration of anticoagulation ≥ 3 months | 5 | 5 (100%) |
| Improvement of clinical symptoms | 59 | 38 (64.41%) |
| After anticoagulant therapy | 45 | 35 (77.78%) |
| Death | 59 | 15 (25.42%) |
| Multidistrict thrombosis | 15 | 2 (13.33%) |
| Multiple organ failure | 15 | 3 (20.00%) |
| Septic shock | 15 | 1 (6.67%) |
| Causes of death were not specified | 15 | 9 (60.00%) |
| Follow-up duration ≥ 1 month | 10 | 7 (70.00%) |
COVID-19 coronavirus disease 2019, SVT splanchnic vein thrombosis, CT computed tomography
Treatments were reported for 50 patients. Forty-five patients received anticoagulants, 33 received IVIG, two received antibiotics, and two underwent bowel resection after anticoagulation failed. The most common type of anticoagulant used was fondaparinux (n = 15), followed by DOACs (n = 16) and argatroban (n = 12). The duration of anticoagulation was ≥ 3 months in five patients (Table 3).
Follow-up duration was ≥ 1 month in seven patients, < 1 month in three patients, and unknown in 51 patients. Outcomes were clearly reported in 59 patients. The rate of improvement of clinical symptoms was 64.4% (38/59). The mortality was 25.4% (15/59). Causes of death were clearly reported in only five patients, which included multidistrict thrombosis (n = 2), multiple organ failure (n = 3), and septic shock (n = 1) (Table 3). Univariate logistic regression analyses showed that anticoagulant therapy (OR = 0.025; 95% CI = 0.002–0.281; p = 0.003) and IVIG (OR = 0.175; 95% CI = 0.032–0.966; p = 0.046) were significantly associated with death among SVT patients having received COVID-19 vaccines (Fig. 3B). Multivariate logistic regression analyses did not identify any independent factor for death among SVT patients having received COVID-19 vaccines.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis that has reported the incidence and described baseline characteristics, management, and outcomes of SVT associated with COVID-19 or COVID-19 vaccines. Based on our findings, 0.6% of COVID-19 patients developed SVT, an incidence that is much lower than that of PE and DVT [7]. However, unlike PE and DVT, SVT is a rare disease, and has not been routinely screened in COVID-19 patients, probably underestimating its true occurrence. Meanwhile, a large epidemiologic study showed that the incidence of PVT was 3.78 and 1.73 per 100,000 inhabitants in males and females, respectively, and that of BCS was 2.0 and 2.2 per 1,000,000 inhabitants in males and females, respectively [26], which were much lower than the pooled incidence of SVT in COVID-19 patients. Therefore, we can assume that SARS-CoV-2 infection may be a potential risk factor for SVT. Until now, no specific mechanism has been described to explain the onset of SVT in COVID-19 patients. However, the mechanisms to explain how SARS-CoV-2 induces the development of thrombosis have been explored and described. The spike protein of SARS-CoV-2 can bind to angiotensin-converting enzyme 2 receptors which are abundantly expressed on the endothelium, thereby resulting in infection and subsequent vascular injury, vascular endothelium dysfunction, and endotheliitis [27]. In addition, SARS-CoV-2 infection activates immune response and increases inflammatory cytokines, such as interleukin-6, interleukin-10, and tumor necrosis factor-α, which may be involved in the pathogenesis of thrombosis, including SVT, in COVID-19 patients [28].
We identified 77 COVID-19 patients who developed SVT, of whom about 83% were treated with anticoagulants, and nearly half received LMWH. Notably, anticoagulant therapy was effective for improving clinical symptoms in about 78.3% of COVID-19 patients with SVT, and it might be a significant factor for decreasing their risk of death. The fact that LMWH is more commonly used is probably due to its lower risk of interaction with antiviral therapy compared with DOACs and a higher stability compared with unfractionated heparin, especially during the cytokine storm phase [9, 29]. In addition, the use of DOACs is currently considered off label in patients with SVT since they were not included in the pivotal phase III trials. The reported duration of anticoagulation was at least 6 months in most patients.
We identified 61 patients developing SVT after receiving COVID-19 vaccines. COVID-19 vaccines, especially ChAdOx1 nCov-19 vaccines (AstraZeneca) [30], may cause an immune-mediated reduction of platelets, also named as vaccine induced thrombosis thrombocytopenia (VITT), which is similar to heparin-induced thrombocytopenia (HIT) [30, 31]. The currently recognized pathogenesis of VITT is that anti-PF4 antibodies bind to a specific site on PF4, producing immune complexes to activate massive platelets through the Fc receptor, and then developing thrombosis with over-consumption of platelets and thrombocytopenia [32]. Of interest, 96.2% (50/52) of our included patients had decreased platelets count, whereas only 3.8% (2/52) had elevated platelets count which may be secondary to inflammation, haematological malignancy, or other causes [33]. Collectively, a combination of thrombocytopenia and positive anti-PF4 antibody with negative platelet activation test should be considered highly suspicious for the development of SVT after receiving COVID-19 vaccines [34, 35].
In our study, approximately 90% of patients who developed SVT after receiving COVID-19 vaccines were treated with anticoagulants. Like for COVID-19 patients with SVT, anticoagulant therapy was effective for improving clinical symptoms in about 77.8% of patients with COVID-19 vaccination associated SVT, and might be a significant protective factor for their survival. Notably, fondaparinux, argatroban, and DOACs were the most commonly used anticoagulants in such patients, as suggested by clinical guidance documents to prevent the risk of further immune response to heparin [36]. However, if anti-PF4 test is negative, LWMH and unfractionated heparin can be considered [37]. In addition, IVIG can potentially inhibit platelet activation secondary to anti-PF4 antibodies [36, 38]. Further, we found that IVIG might be another significant protective factor for the survival in patients who developed SVT after receiving COVID-19 vaccines.
Mortality was substantial in SVT patients with COVID-19, and even more in those who received COVID-19 vaccines. Specifically, the mortality would be up to 14% and 25%, respectively. Notably, the distribution in the causes of death was different between the two groups. Intestinal ischemia or necrosis secondary to SVT was the main cause of death in COVID-19 patients with SVT, and bowel resection was identified as a potential risk factor of death. By comparison, cerebrovascular thrombosis and PE, which are more lethal than SVT, were the primary causes of death in patients having received COVID-19 vaccines. This is probably because COVID-19 vaccine-induced thrombosis is more prone to develop at multiple districts. Indeed, our study demonstrated that concomitant thrombosis at other locations were observed in 61% of patients with COVID-19 vaccination associated SVT, but in only 16% of patients with COVID-19 associated SVT.
Our study has several limitations. First, COVID-19 patients admitted to ICU, critically ill patients with COVID-19, COVID-19 patients who underwent CT, and COVID-19 patients with cirrhosis, who are more likely to develop SVT, were selected in these cohort studies [14, 16, 20, 23, 25], which could overestimate the pooled incidence of SVT in the general population. Thus, we performed a subgroup analysis and found that in the specific and non-specific patients with COVID-19, the incidence of SVT was 3.4% and 0.3%, respectively, and the interaction was significant (p = 0.03). Second, the incidence of SVT after receiving COVID-19 vaccines could not be evaluated due to the absence of relevant data. Third, the information on clinical manifestations, treatments, and outcomes of COVID-19 patients with SVT was often missing in cohort studies. However, we summarized and analyzed the relevant data from individual case reports and provided more accurate description of this population. Fourth, some patients having received COVID-19 vaccines might not perform anti-PF4 antibody test, so that it was hard to distinguish coincidental thrombotic events after vaccination versus vaccine associated SVT. Fifth, the quality of reporting was unsatisfactory in some studies, which potentially influences the reliability of our results. Finally, the statistical significance of anticoagulant therapy for survival benefits disappeared in multivariate analyses, which made it less convincing. However, given the rarity of SVT, it should be very difficult to organize a sufficiently powered study with adequate sample size, anticoagulant users, and death events in SVT patients with COVID-19 or having received COVID-19 vaccines.
In conclusion, based on our systematic review and meta-analysis, SVT is a rare complication in COVID-19 patients. Development of SVT predicts poor outcomes in both patients with COVID-19 and subjects who received COVID-19 vaccines, but likely benefits from anticoagulant therapy in terms of clinical symptoms and survival.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- BCS
Budd-Chiari syndrome
- COVID-19
Coronavirus disease 2019
- CIs
Confidence intervals
- DVT
Deep vein thrombosis
- DOACs
Direct oral anticoagulants
- HIT
Heparin-induced thrombocytopenia
- IVIG
Intravenous immunoglobulin
- JBI
Joanna Briggs Institute
- LMWH
Low molecular weight heparin
- MVT
Mesenteric vein thrombosis
- NOS
Newcastle–Ottawa Scale
- OR
Odds ratios
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PE
Pulmonary embolism
- PVT
Portal vein thrombosis
- PF4
Platelet factor 4
- SVT
Splanchnic vein thrombosis
- SpVT
Splenic vein thrombosis
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- VTE
Venous thromboembolism
- VITT
Vaccine induced thrombosis thrombocytopenia
Author contributions
Conceptualization: XQ; Methodology: XZ, FG, LW, and XQ; Data curation: XZ, FG, LW, YM, and XQ; Formal analysis and data interpretation: XZ, FG, LW, YM, WA, and XQ; Writing-original draft: XZ and XQ; Writing-review and editing: XZ, FG, LW, YM, WA, and XQ; Supervision: XQ. All authors have made an intellectual contribution to the manuscript and approved the submission.
Funding
None.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaojie Zheng, Fangbo Gao and Le Wang have contributed equally to this work.
References
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation report. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-april-2022 Accessed 20 April 2022
- 3.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. 2020;136(4):381–383. doi: 10.1182/blood.2020007335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The LH. COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7(6):e425. doi: 10.1016/S2352-3026(20)30151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kollias A, Kyriakoulis KG, Lagou S, Kontopantelis E, Stergiou GS, Syrigos K. Venous thromboembolism in COVID-19: a systematic review and meta-analysis. Vasc Med. 2021;26(4):415–425. doi: 10.1177/1358863X21995566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298(2):E70–e80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EASL Clinical Practice Guidelines Vascular diseases of the liver. J Hepatol. 2016;64(1):179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Valeriani E, Di Nisio M, Riva N, et al. Anticoagulant therapy for splanchnic vein thrombosis: a systematic review and meta-analysis. Blood. 2021;137(9):1233–1240. doi: 10.1182/blood.2020006827. [DOI] [PubMed] [Google Scholar]
- 10.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Coronavirus disease 2019 (COVID-19) vaccines. https://covid19.who.int/. [PubMed]
- 12.Bogovic N, Doenecke A, Hart C, et al. Covid19 vaccination-associated portal vein thrombosis—an interdisciplinary clinical challenge. Clin Res Hepatol Gastroenterol. 2022;2022:101932. doi: 10.1016/j.clinre.2022.101932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbasi S, Alsermani A, Alsegayyir A, Altahan T, Alsermani M, Almustanyir S. Vaccine-induced thrombotic thrombocytopenia: a case of splanchnic veins thrombosis. Cureus. 2022;14(3):e23507. doi: 10.7759/cureus.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor S, Chand S, Dieiev V, et al. Thromboembolic events and role of point of care ultrasound in hospitalized covid-19 Patients needing intensive care unit admission. J Intensive Care Med. 2021;36(12):1483–1490. doi: 10.1177/0885066620964392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Zhao W, Kaatz S, Latack K, Schultz L, Poisson L. Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw Open. 2021;4(11):e2135397. doi: 10.1001/jamanetworkopen.2021.35397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taya M, Paroder V, Redelman-Sidi G, et al. Abdominal imaging findings on computed tomography in patients acutely infected with SARS-CoV-2: what are the findings? Emerg Radiol. 2021;28(6):1087–1096. doi: 10.1007/s10140-021-01986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Vissichelli N, De Carvalho H, Rocawich K, Grossman C, Kashiouris M. Thromboelastography (TEG) informs anticoagulation management in hospitalized patients with COVID-19. Crit Care Med. 2021;49(1 SUPPL 1):73. doi: 10.1097/01.ccm.0000726588.80278.32. [DOI] [Google Scholar]
- 18.Arachchillage DRJ, Shi C, Saliu D, et al. Efficacy and safety of D-dimer, weight, and renal function-adjusted thromboprophylaxis in patients with coronavirus disease 2019 (COVID-19) Semin Thromb Hemost. 2021;47(4):436–441. doi: 10.1055/s-0040-1722309. [DOI] [PubMed] [Google Scholar]
- 19.Filippidis P, Kampouri E, Viala B, et al. Reduction of venous thromboembolic events in hospitalized patients with coronavirus disease 2019 after intensification of thromboprophylaxis. Res Practice Thrombosis Haemost. 2020;4(SUPPL 2):18–19. [Google Scholar]
- 20.Gardner L, Kelly D, Smith K, et al. Optimising COVID-19 thromboprophylaxis within the critical care unit of a large UK-based teaching hospital. Br J Haematol. 2021;193(SUPPL 1):211–212. [Google Scholar]
- 21.Norsa L, Bonaffini PA, Caldato M, et al. Intestinal ischemic manifestations of SARS-CoV-2: results from the ABDOCOVID multicentre study. World J Gastroenterol. 2021;27(32):5448–5459. doi: 10.3748/wjg.v27.i32.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purroy F, Arqué G. Influence of thromboembolic events in the prognosis of COVID-19 hospitalized patients. Results from a cross sectional study. PLoS ONE. 2021;16(6):e0252351. doi: 10.1371/journal.pone.0252351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel PS, Patel S, Shah V, Aswani V, Narwaria M. Early experience of high-dose intravenous mycobacterium w in critically ill patients of covid-19. Indian J Crit Care Med. 2021;25(9):1066–1068. doi: 10.5005/jp-journals-10071-23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elbadry MI, Tawfeek A, Abdellatif MG, et al. Unusual pattern of thrombotic events in young adult non-critically ill patients with COVID-19 may result from an undiagnosed inherited and acquired form of thrombophilia. Br J Haematol. 2022;196(4):902–922. doi: 10.1111/bjh.17986. [DOI] [PubMed] [Google Scholar]
- 25.Hassnine AA, Elsayed AM. COVID-19 in cirrhotic patients: is portal vein thrombosis a potential complication? Can J Gastroenterol Hepatol. 2022;2022:5900468. doi: 10.1155/2022/5900468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ageno W, Dentali F, Pomero F, et al. Incidence rates and case fatality rates of portal vein thrombosis and Budd-Chiari syndrome. Thromb Haemost. 2017;117(4):794–800. doi: 10.1160/TH16-10-0781. [DOI] [PubMed] [Google Scholar]
- 27.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergamaschi L, Mescia F, Turner L, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. 2021;54(6):1257–75.e8. doi: 10.1016/j.immuni.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buso G, Becchetti C, Berzigotti A. Acute splanchnic vein thrombosis in patients with COVID-19: a systematic review. Dig Liver Dis. 2021;53(8):937–949. doi: 10.1016/j.dld.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 33.Graça LL, Amaral MJ, Serôdio M, Costa B. Extensive thrombosis after COVID-19 vaccine: cause or coincidence? BMJ Case Rep. 2021;14(8):e244878. doi: 10.1136/bcr-2021-244878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arepally GM, Ortel TL. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021;138(4):293–298. doi: 10.1182/blood.2021012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavord S, Hunt BJ, Horner D, Bewley S, Karpusheff J. Vaccine induced immune thrombocytopenia and thrombosis: summary of NICE guidance. BMJ. 2021;375:n2195. doi: 10.1136/bmj.n2195. [DOI] [PubMed] [Google Scholar]
- 37.Gresele P, Marietta M, Ageno W, et al. Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): a position statement from the Italian society for the study of haemostasis and thrombosis (SISET) Blood Transfus. 2021;19(4):281–283. doi: 10.2450/2021.0117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385(8):720–728. doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.