Abstract
Aims
Endurance sport practice is associated with a high prevalence of atrial fibrillation (AF), which increases the risk of stroke in the general population. However, stroke risk in endurance athletes with AF is sparsely investigated. Most studies have been limited by design and are largely restricted to younger and middle-aged populations. Thus, we aimed to investigate AF and stroke risk in older athletes exposed to prolonged endurance training.
Method
During a 10-year period, 505 male athletes aged ≥65 years frequently participating in a long-distance ski race were compared with 1867 men of the same age from the general population. The main exposure was endurance sport practice with self-reported AF and stroke as outcomes. Stroke risk was further examined by joint modelling of AF and endurance practice. Statistical analysis was conducted with a modified Poisson model.
Results
Athletes (median age: 68, range: 65–90) participated in a long-distance ski race over a median of 14 years (range: 1–53). Prevalence (28.5% vs 17.8%) and adjusted risk of AF (risk ratio (RR): 1.88, 95% CI: 1.49 to 2.37) were higher in athletes compared with non-athletes, whereas the prevalence (5.4% vs 9.7%) and risk of stroke were lower (RR: 0.60, 95% CI: 0.37 to 0.95). Compared with athletes without AF, risk of stroke was twofold in athletes (RR: 2.38, 95% CI: 1.08 to 5.24) and nearly fourfold in non-athletes (RR: 3.87, 95% CI: 1.98 to 7.57) with AF.
Conclusion
Although older male endurance athletes experienced an increased risk of AF, the long-term risk of stroke was substantially reduced compared with non-athletes.
Keywords: epidemiology, atrial fibrillation, stroke
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is a high prevalence of atrial fibrillation (AF) in otherwise healthy endurance athletes.
Despite substantial benefits on traditional cardiovascular risk factors, endurance sport practice is indicated as an independent risk factor for AF.
WHAT THIS STUDY ADDS
Few studies have investigated the risk of AF and stroke among endurance athletes prospectively.
Despite the high risk of AF among older male endurance athletes, the risk of stroke was substantially lowered compared with non-athletic men of a similar age.
Our findings indicate that the risk of stroke associated with exercise-related AF might be diminished.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study adds to the existing research literature on AF and stroke risk related to endurance sport practice, and could aid physicians in nuanced counselling of endurance athletes.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide.1 AF is associated with a substantially elevated risk of cardioembolic stroke,2 particularly in the presence of concomitant cardiovascular risk factors. In contrast, there is an inverse dose-response association between physical activity (PA) and cardiovascular disease,3 4 and moderate levels of PA are also associated with a decreased risk of AF.5 6 However, high levels of vigorous PA and particularly systematic endurance exercise are associated with an increased risk of AF, even in the absence of established risk factors,7–9 resulting in a high AF prevalence in male endurance athletes.10 Although not fully understood, the risk of exercise-related AF is probably linked to cardiac remodelling caused by repetitive exposure to high blood volumes and pressures during endurance exercise.7
The majority of previous studies have been limited by retrospective or cross-sectional designs and are largely restricted to young and middle-aged male athletes, with a limited period of exposure to endurance sport practice. The long-term risk of AF and stroke in older recreational athletes exposed to prolonged endurance sport practice has been sparsely investigated. Myrstad et al 10 observed an increased risk of AF in endurance athletes aged ≥65 years in a cross-sectional investigation. However, whether the risk of AF observed in older endurance athletes translates into an increased risk of stroke remains unclear. In a cross-sectional study, AF was associated with a twofold prevalence of stroke among recreational cross-country skiers aged ≥65 years, but not in younger athletes.11 In another study that included >200 000 skiers with a mean age of 37 years, AF was associated with an increased risk of stroke during up to 9-year follow-up, but the absolute risk of stroke was very low.8
In the present study, we aimed to examine the cumulative prevalence and risk of self-reported AF and stroke related to leisure-time PA and endurance sport practice during an 8-year to 10-year follow-up among older male recreational cross-country skiers compared with men of similar age representing the general Norwegian population.
Method
Design and study population
The current study comprises two prospective cohorts, one consisting of men aged ≥65 years who participated in the Birkebeiner Ageing Study (BiAS),10 the other is a sample of men in the same age group included from the sixth wave of the population-based Tromsø Study (Tromsø6, 2007–2008).12 In total, our pooled study sample included 2372 participants.
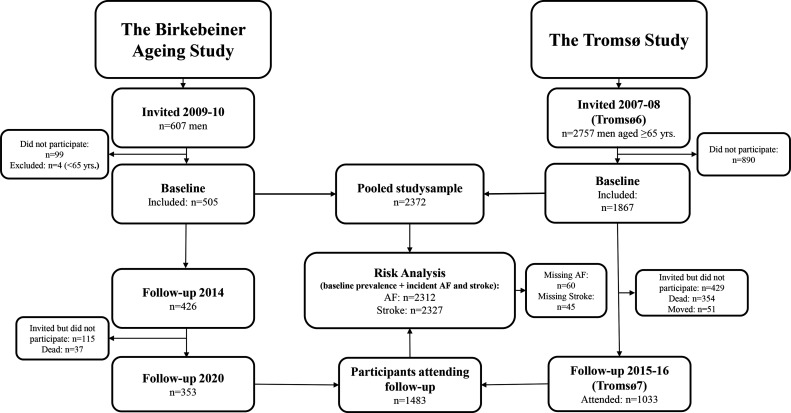
BiAS is a prospective cohort study that included participants competing in the Birkebeiner cross-country ski race, an arduous competition extending over 54 km with >1000 uphill altitude metres.10 In total, 658 (n=607 male, n=51 female) athletes who completed the race in 2009–10 and were aged ≥65 years at that time were invited to participate. In the current analyses, we included men only as there were few eligible female athlete participants. In total, 509 athletes agreed to participate, and 505 (83%) were included (figure 1). The athletes received a questionnaire that was filled out and returned by post. A follow-up questionnaire was distributed in 2014 and 2020, allowing examination of AF and stroke during a 10-year follow-up. Seventy-nine (15.6%) participants did not respond to the follow-up in 2014. Furthermore, 152 (30.1%) participants did not respond to the follow-up in 2020 (online supplemental table 1), out of which 115 did not reply, while 37 were dead. In total, 87.7% (n=443) of participants responded to follow-up either in 2014 (17.8%), 2020 (3.4%) or both (66.5%).
Figure 1.
Flow chart of participants included in the study. The Birkebeiner Ageing Study and the Tromsø Study. AF, atrial fibrillation.
openhrt-2022-002154supp001.pdf (95.8KB, pdf)
The Tromsø Study is a population-based study with repeated surveys with participants from the municipality of Tromsø, Norway.12 Participants in the Tromsø Study are considered representative of a northern European white urban population.13 Of all male inhabitants aged 65–87 years who were invited to participate in Tromsø6 (n=2757), 1867 (68%) attended and were included in the current study. Data on AF and stroke were available from the seventh Tromsø survey (Tromsø7, 2015–16), which allowed assessment of outcomes after 8 years. Out of attending participants in Tromsø6, 834 participants (44.7%) did not attend the follow-up examination in Tromsø7 (online supplemental table 1), 429 of these were invited but did not attend, 354 were dead and 51 had moved out of the municipality.
Exposures and outcomes
The main exposure was participation in the Birkebeiner race, dichotomised into athletes versus non-athletes. Secondary exposure variables were years of race participation (annual Birkebeiner races completed), medals achieved in the Birkebeiner race and self-reported leisure-time PA. A medal is achieved when a participant completes the race within the average race time +25% of the top five performers in their respective 5-year age class. In both BiAS and Tromsø6 and Tromsø7, self-reported leisure-time PA was assessed by the Saltin-Grimby Physical Activity Level Scale (SGPALS),14 which assesses PA on a 4-level scale (1=sedentary, 2=light, 3=moderate, 4=vigorous). The questionnaire has been thoroughly validated15 16 and has to a moderate extent discriminative ability in terms of an individual’s V̇O2max.14
The main outcomes were self-reported AF and stroke reported at least once either at baseline (Tromsø6 and BiAS 2009/2010) or during follow-up (Tromsø7, and BiAS 2014 and/or BiAS 2020). BiAS participants were asked “Do you have, or have you ever had atrial fibrillation?” and were given the following alternatives ‘yes, once’, ‘yes, several times’, “yes, I have it chronically” or ‘no’. All three ‘yes’ alternatives were subsequently merged into a single category before analysis. Tromsø6 participants were asked the same question with response options ‘yes’ or ‘no’. Furthermore, confirmed AF diagnoses by electrocardiography were retrieved from medical hospital records at the University Hospital of North Norway (for Tromsø Study participants only) and used in supplementary analyses. Self-reported stroke was based on the question “Do you have or have you ever had a stroke”, with either ‘yes’ or ‘no’ as possible answers. The question differed slightly at follow-up for BiAS participants as they were asked to answer if they had experienced a stroke only. Thus, in line with the skip pattern participants in BiAS with missing data for stroke at follow-up were treated as ‘no’.
Participants with missing data on AF and/or stroke at either baseline or follow-up were included in the analysis, whereas participants with missing data at all examination points were excluded from the analysis.
Covariates
In Tromsø6, height and weight were measured to the nearest centimetre and half kilo with participants wearing light clothing without shoes. In BiAS, height and weight were self-reported. Body mass index was calculated as weight divided by the square height in centimetres. All other covariates were obtained by self-report in both cohorts. Education, smoking and alcohol frequency questions differed slightly between the cohorts as there were more alternatives in the BiAS questionnaire. As such, they were collapsed into harmonised categories. Additionally, participants were asked “Do you take blood pressure lowering medication?” Coronary heart disease (CHD) was assessed by two questions: “Do you have, or have you had angina pectoris?” and “Do you have, or have you had a heart attack?” Diabetes was examined by the question “Do you have, or have you had diabetes?” The questionnaires are described in detail elsewhere.17 18
Statistics
Participants from BiAS and the Tromsø Study were analysed together in a pooled cohort. Descriptive characteristics for continuous variables are given as median (25th and 75th percentile) and categorical variables as percentages. Differences between groups for baseline covariates were assessed by independent-samples Mann-Whitney U test for continuous variables and by Pearson’s χ2 test for categorical variables. The cumulative prevalence of AF and stroke were defined as all participants still alive at follow-up who reported an outcome at least once during the study period divided by all participants who attended the baseline survey and were still alive at follow-up. We examined associations between exposures and outcomes with a Poisson loglinear regression analysis with robust SEs where parameter estimates are given as risk ratios (RR) and corresponding 95% CIs. To identify adjustment variables, directed acyclic graphs were drawn using the web application DAGitty (www.daggity.net) (online supplemental figures 1–4). In addition to an age-adjusted model, we present a total effect model (model 2) and a direct effect model (model 3). Model 2 is adjusted for age, body height, education, smoking status and frequency of alcohol consumption. Model 3 is adjusted for model 2+CHD, diabetes, body mass index and antihypertensive medication, aiming to examine exercise-related outcomes. RRs were estimated during a 10-year follow-up for AF and stroke. We present estimates for athletes compared with non-athletes as the reference and estimates for races completed and medals achieved per 10-unit increase among athletes with the lowest number of races or medals as the reference. Furthermore, AF risk for joint categories of endurance sport practice and SGPALS are presented (1=non-athletes inactive, 2=non-athletes light, 3=non-athletes moderate to vigorous, 4=athletes light, 5=athletes moderate to vigorous). In addition, we categorised participants by AF status in both cohorts and examined stroke risk in four groups (1=athletes without AF, 2=non-athletes without AF, 3=athletes with AF, 4=non-athletes with AF). The significance level was set at 0.05. All statistical analyses were performed with Stata V.17 (StataCorp, Texas, USA).
openhrt-2022-002154supp002.pdf (719KB, pdf)
openhrt-2022-002154supp003.pdf (674.3KB, pdf)
openhrt-2022-002154supp004.pdf (741.2KB, pdf)
openhrt-2022-002154supp005.pdf (1.5MB, pdf)
Sensitivity analyses
We performed sensitivity analyses by excluding participants who reported CHD and diabetes at baseline. Furthermore, to account for a larger loss to follow-up in the Tromsø Study compared with BiAS, sensitivity analyses were performed by including non-attendees of Tromsø7 (follow-up) who developed AF during the study period according to ECG confirmed AF diagnoses from the University Hospital of North Norway (online supplemental table 3). To further assess the robustness of the estimated AF risk, we removed all participants with ≥1 AF risk factor (smoking, overweight, hypertension, diabetes and CHD) and reran model 3 (online supplemental table 4). We also reanalyzed the risk of AF and stroke after exclusion of participants aged >75 years to account for possible issues related to recall bias (online supplemental table 5).
Results
Descriptive characteristics for participants at baseline are given in table 1. On average, athletes were 2.6 years younger, 4.2 cm taller, 6.7 kg lighter and more educated than non-athletes. Athletes were less often current or previous smokers and had a very low prevalence of cardiovascular risk factors and conditions, such as CHD, diabetes and use of antihypertensive medication. Athletes reported that they had participated in the Birkebeiner race over a median of 14 years (range: 1–53) and had been practising regular systematic endurance training over a median of 36 years (range: 0–67), with nearly one-fourth (24%) reporting systematic endurance training for at least 50 years. Ninety per cent (n=443) of athletes reported either moderate or vigorous PA during past 12 months. Two athletes had missing data for races, 5 were missing data for medals and 13 did not report PA. There were 245 (13.1%) non-athletes with missing data on PA.
Table 1.
Descriptive characteristics for the Tromsø Study (Tromsø6) and the Birkebeiner Ageing Study (BiAS) study participants
| Baseline characteristics | Tromsø6 (n=1867) | BiAS (n=505) | P value |
| Age (years) | 70 (67–75) | 68 (66–71) | <0.001 |
| Height (cm) | 174.5 (170.1–178.8) | 178.0 (175.0–182.0) | <0.001 |
| Weight (kg) | 81.2 (73.4–89.9) | 75.0 (70.0–80.0) | <0.001 |
| BMI (kg/m2) | 26.8 (24.4–29.1) | 23.5 (22.2–24.8) | <0.001 |
| Number of Birkebeiner races completed | – | 14 (7–27) | |
| Number of achieved Birkebeiner medals | – | 6 (0–21) | |
| Total years of systematic endurance training | – | 36 (19.0–49.0) | |
| % (n) | % (n) | ||
| Education | <0.001 | ||
| Primary/Secondary school | 35.6 (646) | 16.6 (83) | |
| High school | 35.8 (649) | 42.2 (211) | |
| College/University | 28.6 (518) | 41.2 (206) | |
| Smoking status | <0.001 | ||
| Current | 15.4 (281) | 0.8 (4) | |
| Former | 60.6 (1103) | 37.9 (191) | |
| Never | 24.0 (436) | 61.3 (309) | |
| Frequency of alcohol consumption | <0.001 | ||
| Never | 14.2 (259) | 9.4 (46) | |
| 1–4 times per month | 64.1 (1169) | 73.0 (356) | |
| 2–3 times a week | 15.6 (284) | 14.6 (71) | |
| 4 times or more per week | 6.2 (113) | 3.1 (15) | |
| Coronary heart disease | 24.3 (444) | 3.6 (18) | <0.001 |
| Diabetes | 8.5 (154) | 0.8 (4) | <0.001 |
| Currently or previously on antihypertensive medication | 40.5 (729) | 15.7 (76) | <0.001 |
| Self-reported physical activity | <0.001 | ||
| Sedentary | 19.4 (314) | 0.2 (1) | |
| Light | 58.0 (941) | 9.8 (48) | |
| Moderate | 21.9 (355) | 51.0 (251) | |
| Vigorous | 0.7 (12) | 39.0 (192) | |
| ≥1 AF risk factor present | 86.0 (1605) | 35.1 (177) | <0.001 |
Values are presented as median (25th and 75th percentile) for continuous variables and as percentages for categorical variables. AF risk factor variable: one or more of the following: BMI ≥25 kg/m2, current smoker, current use of antihypertensive medication, diabetes and coronary heart disease.
AF, atrial fibrillation; BMI, body mass index.
During the study period, 470 (20.3%) individuals reported AF. The cumulative AF prevalence was 17.8% for non-athletes and 28.5% for athletes. In the age-adjusted analysis, the RR of AF was 1.64 (95% CI: 1.38 to 1.95) for athletes compared with non-athletes. After multivariable adjustment (model 2 and 3), the RR for AF was 1.42 (95% CI: 1.17 to 1.73) and 1.88 (95% CI: 1.49 to 2.37), respectively (table 2). We did not observe any statistically significant association between AF and number of races completed or medals achieved. When modelling AF risk by joint associations of leisure-time PA during the past 12 months and years of endurance sport practice, we observed a non-linear dose-response association (figure 2, p<0.05), with the highest risk of AF observed among athletes reporting vigorous PA. In sensitivity analyses, we added incident AF cases according to hospital records and found that the cumulative AF prevalence increased to 22.5% for non-athletes, and the relative risk of AF in athletes versus non-athletes decreased (model 3—RR: 1.52, 95% CI: 1.24 to 1.88). Excluding participants with at least one risk factor for AF did not result in a meaningful change of the estimated risk of AF.
Table 2.
Risk ratio with 95% CIs for atrial fibrillation and stroke
| Exposure | N | Number of cases n (%) | Model 1 | Model 2 | Model 3 |
| Atrial fibrillation | |||||
| Non-athletes | 1807 | 325 (18.0) | 1 Reference | 1 Reference | 1 Reference |
| Athletes | 505 | 145 (28.7) | 1.64 (1.38 to 1.95) | 1.42 (1.17 to 1.73) | 1.88 (1.49 to 2.37) |
| Stroke | |||||
| Non-athletes | 1305 | 116 (8.9) | 1 Reference | 1 Reference | 1 Reference |
| Athletes | 479 | 23 (4.8) | 0.55 (0.35 to 0.85) | 0.60 (0.37 to 0.95) | 0.76 (0.47 to 1.24) |
Model 1: adjusted for age; model 2: additionally adjusted for body height, education, smoking status and frequency of alcohol intake; model 3: adjusted for model 2+coronary heart disease (atrial fibrillation only), diabetes (atrial fibrillation only), body mass index and antihypertensive medication. In the analysis of stroke, participants reporting coronary heart disease and diabetes at baseline were excluded.
Figure 2.
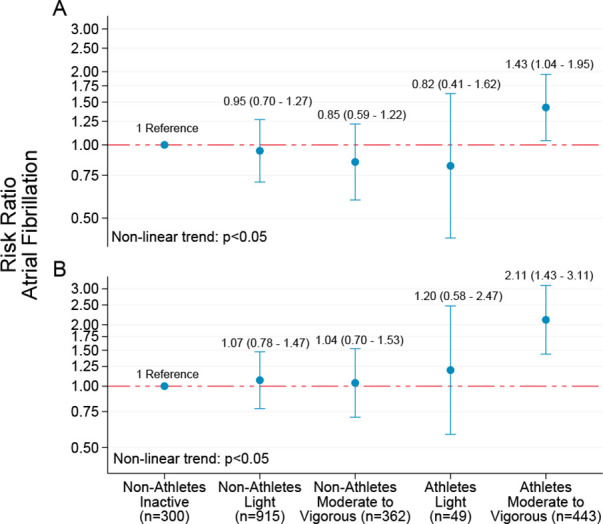
Risk ratio with 95% CIs for self-reported atrial fibrillation by joint associations of self-reported leisure-time physical activity and endurance sport practice. Non-athletes: participants from Tromsø6; athletes: participants from the Birkebeiner Ageing Study. (A) Adjusted for age, body height, education, smoking status and frequency of alcohol intake. (B) Additionally adjusted for coronary heart disease, diabetes, body mass index and antihypertensive medication. Y-axis values are given on a logarithmic scale.
In total, 211 (9.1%) participants reported a stroke, with a cumulative prevalence of 9.7% in non-athletes and 5.4% in athletes. Although the age-adjusted RR was 0.60 (95% CI: 0.41 to 0.89), the association between endurance sport practice and stroke was not significant (model 2—RR: 0.69, 95% CI: 0.45 to 1.04) after multivariable adjustment (online supplemental table 6). However, after excluding participants with CHD and diabetes at baseline the association reached statistical significance (RR: 0.60, 95% CI: 0.37 to 0.95). The estimated risk reduction was diminished after further adjustment in model 3 (RR: 0.76, 95% CI: 0.47 to 1.24). The cumulative prevalence of stroke was 8.3% in athletes with AF and 14.2% among non-athletes with AF. Compared with athletes without AF the estimated risk of stroke was increased in non-athletes without AF (RR: 1.94, 95% CI: 1.04 to 3.60), athletes with AF (RR: 2.38, 95% CI: 1.08 to 5.24) and non-athletes with AF (RR: 3.87, 95% CI: 1.98 to 7.57), respectively (figure 3).
Figure 3.
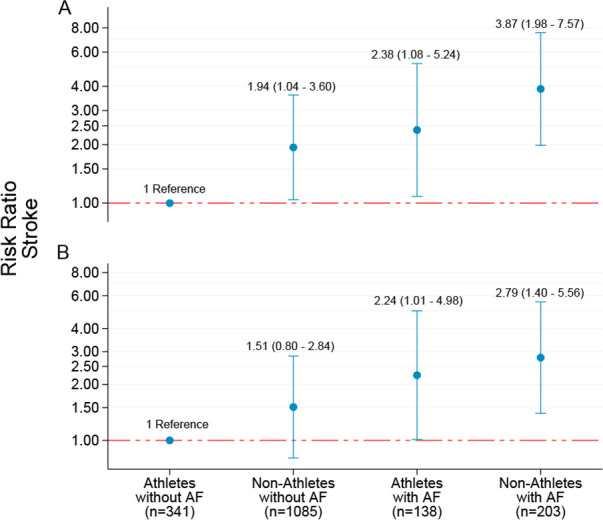
Risk ratio with 95% CI for self-reported stroke by joint associations of athlete status and participants reporting atrial fibrillation (AF) status during the study. Non-athletes: participants from Tromsø6; athletes: participants from the Birkebeiner Ageing Study. (A) Adjusted for age, body height, education, smoking status and frequency of alcohol intake. (B) Additionally adjusted for body mass index and antihypertensive medication. Participants reporting coronary heart disease and diabetes at baseline have been excluded. Y-axis is given on a logarithmic scale.
Restricting the analysis to participants ≤75 years did not change the estimated risk of AF and stroke.
Discussion
This study is to our knowledge the first to assess long-term AF and stroke risk prospectively in older men with a history of long-term endurance sport practice. Although prolonged endurance sport practice was associated with an increased risk of AF in older men during long-term follow-up, the risk of stroke was decreased. Relative to athletes who did not report AF during the study period, the risk of stroke was higher in both athletes and non-athletes reporting AF, as well as for non-athletes who did not. Furthermore, although not statistically significant, the estimated risk appeared higher in non-athletes with AF compared with athletes with AF, which may suggest that athletes with exercise-related AF are less susceptible to stroke than their non-athletic counterparts with AF.
Long-term endurance sport practice and risk of atrial fibrillation
Our results corroborate other findings reporting a high prevalence of AF among endurance athletes.10 19 AF prevalence in older male endurance athletes has previously been reported to be similar to that of the general population despite a lower overall risk of cardiovascular disease.10 20 Furthermore, Grimsmo et al 19 reported an AF prevalence of 17% in another cohort of Birkebeiner skiers aged 26–64 years during 28–30 years follow-up. However, that study had a small sample and no reference population. In comparison to the general population, our study indicates that the cumulative prevalence of AF increases at a faster rate in the older athletic population.
The accumulating prevalence of AF in older male athletes is likely due to an elevated risk of AF associated with endurance sport practice as pointed out by the majority of the research literature.7 21 However, most studies are limited by small sample sizes with few AF cases resulting in a large degree of uncertainty for the estimated risk.22 In a recent prospective study on middle-aged skiers, the AF risk increase associated with endurance sport practice was 11% for men.8 Considering that we observed a near twofold AF risk increase associated with endurance sport practice, our findings might suggest that the risk difference between athletes and the general population is magnified with age. The exercise-related cardiac remodelling claimed responsible for AF in athletes may require several years to manifest and be more pronounced in older athletes with prolonged exposure to exercise,23 and thus may explain a higher risk for athletes in the present study. This notion is further supported by a retrospective cohort study including Birkebeiner skiers, where the odds of AF increased by 16% per 10 years of regular endurance exercise, an increase that did not appear to plateau until the fourth decade of endurance training.20
Joint associations between endurance sport, physical activity and atrial fibrillation
Similar to several previous investigations, we observed a J-shaped association between levels of PA and AF.5 7 10 The benefit of moderate amounts of PA was less clear in our study compared with others.5 7 24–26 Mozaffarian et al 24 reported a >50% lower AF risk with increasing levels of PA among 5446 individuals aged ≥65 years. In contrast, a study from the third Tromsø survey observed no association between PA and AF in individuals aged >50 years.5 The same age interaction was observed in the Physicians Health Study, where no association between exercise frequency and risk of AF was found for men aged >50 years27. Notably, Drca et al 25 reported results similar to our study, indicating a 13% reduced risk of AF associated with walking/bicycling >1 hour per day in a cohort of 44 410 men. Thus, based on our findings and others, the effect of moderate PA doses on AF risk appears beneficial in older adults.
Risk of stroke
We observed a considerably lower stroke prevalence among athletes than non-athletes. This difference corresponds with but also supersedes previous results based on middle-aged endurance athletes.8 The athletes in the present study had a substantially lower prevalence of established risk factors for stroke such as hypertension, overweight, diabetes and CHD than the non-athletes, which suggests that the effect of endurance sport practice was largely but perhaps not solely mediated by its effect on these risk factors.8
Although our results alluded to a reduced risk of stroke among athletes, we observed a twofold increased stroke risk in athletes with AF compared with athletes without AF, indicating that AF increases the stroke risk also in athletes. Nevertheless, we also observed that the prevalence of stroke was nearly doubled in non-athletes with AF compared with athletes with AF, suggesting that AF is less serious in athletes. Furthermore, relative to athletes without AF, the estimated stroke risk was nearly fourfold in non-athletes with AF, further indicating that exercise mitigates the risk of stroke in patients with AF. Our results corroborate the findings of Svedberg et al,8 and extends this notion to older athletes. Collectively, these results suggest that exercise-related AF involves a lower risk of stroke than AF related to traditional cardiovascular risk factors.
Strengths and limitations
The main strength of the present study is the unique cohort of older Birkebeiner participants that should be considered representative of individuals exposed to long-term systematic endurance training. Nearly one-fourth of athletes reported systematic endurance training for more than half a century, with the most experienced athlete reporting nearly seven decades of endurance training. Furthermore, including a reference group representative of the Norwegian population enabled us to indicate risk of AF and stroke relative to the general population. Another strength is that we had multiple covariates available for adjustment. However, most of these were self-reported and residual confounding due to incomplete adjustment in addition to unobserved factors cannot be excluded.
A sizeable number of participants did not attend follow-up, and a larger proportion of athletes than non-athletes responded to the follow-up assessment, likely causing overestimation of AF and stroke risk in athletes. We attempted to remedy this in a sensitivity analysis that included incident AF cases according to hospital-confirmed AF diagnoses which were available in the Tromsø Study. This resulted in a higher AF prevalence among non-athletes comparable to the older Norwegian population.28 Then again, this analysis may conversely underestimate AF risk in athletes as we could not account for those who did not attend follow-up in BiAS in the same manner. Furthermore, such a sensitivity analysis was not possible for stroke. Another limitation is that AF and stroke were self-reported. The sensitivity for self-reported AF and stroke is approximately 50%–70%29 30 and 80% in the general population, respectively.31 Moreover, as athletes might possibly be more sensitive to symptoms related to arrhythmias, overestimation of AF risk compared with non-athletes may have occured.10 However, it has also been suggested that AF in athletes can be less symptomatic.8 Unfortunately, comparable data about management such as oral anticoagulation therapy to prevent stroke and subtypes of AF were not available. Thus, we cannot exclude that relevant differences between the two groups in terms of treatment of AF could have affected the risk of stroke.
The somewhat shorter follow-up time in the reference population may have overestimated the relative risk of AF and stroke in BiAS participants, although a small portion of participants from BiAS only had follow-up from 2009–10 until 2014. Another limitation is that we used self-reported PA as a secondary exposure. Questionnaire-based PA is prone to recall and social desirability bias,32 and therefore the effect of PA may have been underestimated.33 Consequently, the association between PA and risk of AF may have been biased towards the null.34 To mitigate the problem of missing participants at follow-up in addition to a somewhat limited sample of endurance athletes, we used RRs based on baseline prevalence in addition to incidence of outcomes at follow-up, enabling us to include all participants who gave an answer at one of the measurement points. This may increase the risk of reverse causation bias; however, as the main exposure (endurance sport practice) largely preceded the baseline assessment for the majority of athletes, we assume that the concept of temporality was of limited concern. Finally, we are limited by the inclusion of men only, as several studies have observed an interaction with gender in relation to the endurance sport practice and AF association.8 21
Conclusion
This prospective study substantiates previous research showing a high prevalence and an increased risk of AF in older men exposed to prolonged endurance sport practice. Despite a higher risk of AF, we observed that endurance athletes had a lower risk of stroke than non-athletes, and we found further indications that athletes with AF may have a lower risk of stroke than non-athletes with AF. Further prospective studies should address underlying pathophysiological mechanisms for exercise-related AF and risk of stroke related to this entity of AF.
Acknowledgments
We thank Ida Kristine Sangnes, Astrid Hylen Klippenberg and Andreas Henriksen for their invaluable contribution with collection and processing of the Birkebeiner Ageing Study data material. We thank the participants in the Birkebeiner Ageing Study and the participants in the Tromsø Study for their contribution.
Footnotes
Twitter: @KristofferRJ89, @KimAHeitmann, @MorsethBente, @MariusMyrstad
Collaborators: This work has been done within the framework of the Norwegian Exercise and Atrial Fibrillation Initiative (NEXAF) project (https://nexaf.no), which is a collaborative research initiative aiming to increase knowledge about physical activity and exercise in relation to atrial fibrillation. The NEXAF initiative: Bente Morseth1,2, Marius Myrstad3,4, Bjarne Martens Nes5, Jan Pål Loennechen5,6, Maja-Lisa Løchen7, Arnljot Tveit4,8, Turid Apelland4, Kristine Folkenborg4, Kristoffer Robin Johansen1,2, Kristin Espolin Johnson5, Jon Magne Letnes5,6, Vegard Malmo5,6, Andreas Berg Sellevold5,6, Eivind Sørensen3,4, Rune Byrkjeland3,4, Steve Enger4, Hilde Larhammer3,4, Sophia Onarheim4, Vigdis Bache-Semb4. Affiliations: 1School of Sport Sciences, Faculty of Health Sciences, UiT The Arctic University of Norway, Tromsø, Norway. 2Centre for Research and Education, University Hospital of North Norway, Tromsø, Norway. 3Department of Internal Medicine, Bærum Hospital, Vestre Viken Hospital Trust, N-1346 Gjettum, Norway. 4Department of Medical Research, Bærum Hospital, Vestre Viken Hospital Trust, N-1346 Gjettum, Norway. 5Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, NTNU, Trondheim, Norway. 6Department of Cardiology, St. Olavs Hospital, Trondheim, Norway. 7Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway, Tromsø, Norway. 8Faculty of Medicine, Institute for Clinical Medicine, University of Oslo, Oslo, Norway.
Contributors: KRJ drafted the manuscript and performed all statistical analyses. KRJ, AHR, BM and MM came up with the idea and design of the study. M-LL, AHR, ES and MM contributed to acquisition and interpretation of the data. TW provided expert advice and supervision of the statistical analyses. All authors contributed to conception of the work and made critical revision of the manuscript for key intellectual content. KRJ is responsible for the overall content as the guarantor.
Funding: KRJ was funded by the Northern Norway Regional Health Authority (grant number HNF1568-21). The Birkebeiner Ageing Study has been funded by the Kavli Research Centre for Ageing and Dementia and the Norwegian Extra Foundation.
Competing interests: M-LL has received lecture fees from Bayer, Sanofi and BMS/Pfizer not related to this study. MM has received lecture fees from Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, mSD and Pfizer not related to this work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
The NEXAF initiative:
Bente Morseth, Marius Myrstad, Bjarne Martens Nes, Jan Pål Loennechen, Maja-Lisa Løchen, Arnljot Tveit, Turid Apelland, Kristine Folkenborg, Kristoffer Robin Johansen, Kristin Espolin Johnson, Jon Magne Letnes, Vegard Malmo, Andreas Berg Sellevold, Eivind Sørensen, Rune Byrkjeland, Steve Enger, Hilde Larhammer, Sophia Onarheim, and Vigdis Bache-Semb
Data availability statement
Data are available on reasonable request. Data may be obtained from a third party and are not publicly available. The data underlying this article cannot be shared publicly due to GDPR regulations but is available on request. The legal restrictions on data availability are set by the Tromsø Study Data and Publication Committee in order to control for data sharing, including publication of datasets with the potential of reverse identification of de-identified sensitive participant information. The data can however be made available on application to the Tromsø Study Data and Publication Committee. Contact information: The Tromsø Study, Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway; e-mail: tromsous@uit.no. The datasets generated in BiAS are not publicly available as the study is not yet completed but may be available on reasonable request. The Stata code used for the main analysis is available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Norwegian Data Inspectorate and Health Research Ethics (REK: 2020-175586) and complies with Declaration of Helsinki. All participants gave an informed and written consent to participate.
References
- 1. Chugh SS, Roth GA, Gillum RF, et al. Global burden of atrial fibrillation in developed and developing nations. Glob Heart 2014;9:113–9. 10.1016/j.gheart.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 2. Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2014;45:3754–832. 10.1161/STR.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789–95. 10.1161/CIRCULATIONAHA.110.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation 2015;132:1786–94. 10.1161/CIRCULATIONAHA.115.015853 [DOI] [PubMed] [Google Scholar]
- 5. Morseth B, Graff-Iversen S, Jacobsen BK, et al. Physical activity, resting heart rate, and atrial fibrillation: the Tromsø study. Eur Heart J 2016;37:2307–13. 10.1093/eurheartj/ehw059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J 2020;41:1479–86. 10.1093/eurheartj/ehz897 [DOI] [PubMed] [Google Scholar]
- 7. Morseth B, Løchen M-L, Ariansen I, et al. The ambiguity of physical activity, exercise and atrial fibrillation. Eur J Prev Cardiol 2018;25:624–36. 10.1177/2047487318754930 [DOI] [PubMed] [Google Scholar]
- 8. Svedberg N, Sundström J, James S, et al. Long-term incidence of atrial fibrillation and stroke among cross-country skiers. Circulation 2019;140:910–20. 10.1161/CIRCULATIONAHA.118.039461 [DOI] [PubMed] [Google Scholar]
- 9. Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 2013;34:3624–31. 10.1093/eurheartj/eht188 [DOI] [PubMed] [Google Scholar]
- 10. Myrstad M, Løchen M-L, Graff-Iversen S, et al. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand J Med Sci Sports 2014;24:e238–44. 10.1111/sms.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Myrstad M, Berge T, Ihle-Hansen H, et al. Stroke in endurance athletes with atrial fibrillation. Eur J Prev Cardiol 2020;27:2123–5. 10.1177/2047487319866273 [DOI] [PubMed] [Google Scholar]
- 12. Jacobsen BK, Eggen AE, Mathiesen EB, et al. Cohort profile: the Tromso study. Int J Epidemiol 2012;41:961–7. 10.1093/ije/dyr049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eggen AE, Mathiesen EB, Wilsgaard T, et al. The sixth survey of the Tromso study (Tromso 6) in 2007-08: collaborative research in the interface between clinical medicine and epidemiology: study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand J Public Health 2013;41:65–80. 10.1177/1403494812469851 [DOI] [PubMed] [Google Scholar]
- 14. Grimby G, Börjesson M, Jonsdottir IH, et al. The "Saltin-Grimby Physical Activity Level Scale" and its application to health research. Scand J Med Sci Sports 2015;25 Suppl 4:119–25. 10.1111/sms.12611 [DOI] [PubMed] [Google Scholar]
- 15. Emaus A, Degerstrøm J, Wilsgaard T, et al. Does a variation in self-reported physical activity reflect variation in objectively measured physical activity, resting heart rate, and physical fitness? results from the Tromso study. Scand J Public Health 2010;38:105–18. 10.1177/1403494810378919 [DOI] [PubMed] [Google Scholar]
- 16. Sagelv EH, Hopstock LA, Johansson J, et al. Criterion validity of two physical activity and one sedentary time questionnaire against accelerometry in a large cohort of adults and older adults. BMJ Open Sport Exerc Med 2020;6:e000661. 10.1136/bmjsem-2019-000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UiT The Arctic University of Norway . The Tromsø study, 2022. Available: https://uit.no/research/tromsostudy [Accessed 20 June 2022].
- 18. Haraldsplass D, iakonale Sykehus . Birkebeiner Aldringstudien (bias), 2018. Available: https://www.haraldsplass.no/helsefaglig/forskningsgruppe-for-geriatri-og-demens/birkebeiner-aldringsstudien-bias#sporreskjemaer-questionnaires20 [Accessed Jun 2022].
- 19. Grimsmo J, Grundvold I, Maehlum S, et al. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: echocardiographic findings and possible predictors--a 28-30 years follow-up study. Eur J Cardiovasc Prev Rehabil 2010;17:100–5. 10.1097/HJR.0b013e32833226be [DOI] [PubMed] [Google Scholar]
- 20. Myrstad M, Nystad W, Graff-Iversen S, et al. Effect of years of endurance exercise on risk of atrial fibrillation and atrial flutter. Am J Cardiol 2014;114:1229–33. 10.1016/j.amjcard.2014.07.047 [DOI] [PubMed] [Google Scholar]
- 21. Mohanty S, Mohanty P, Tamaki M, et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol 2016;27:1021–9. 10.1111/jce.13023 [DOI] [PubMed] [Google Scholar]
- 22. Newman W, Parry-Williams G, Wiles J, et al. Risk of atrial fibrillation in athletes: a systematic review and meta-analysis. Br J Sports Med 2021;55:1233–8. 10.1136/bjsports-2021-103994 [DOI] [PubMed] [Google Scholar]
- 23. Opondo MA, Aiad N, Cain MA, et al. Does high-intensity endurance training increase the risk of atrial fibrillation? A longitudinal study of left atrial structure and function. Circ Arrhythm Electrophysiol 2018;11:e005598. 10.1161/CIRCEP.117.005598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 2008;118:800–7. 10.1161/CIRCULATIONAHA.108.785626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drca N, Wolk A, Jensen-Urstad M, et al. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart 2014;100:1037–42. 10.1136/heartjnl-2013-305304 [DOI] [PubMed] [Google Scholar]
- 26. Khurshid S, Weng L-C, Al-Alusi MA, et al. Accelerometer-derived physical activity and risk of atrial fibrillation. Eur Heart J 2021;42:2472–83. 10.1093/eurheartj/ehab250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aizer A, Gaziano JM, Cook NR, et al. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 2009;103:1572–7. 10.1016/j.amjcard.2009.01.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kjerpeseth LJ, Igland J, Selmer R, et al. Prevalence and incidence rates of atrial fibrillation in Norway 2004-2014. Heart 2021;107:201–7. 10.1136/heartjnl-2020-316624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malmo V, Langhammer A, Bønaa KH, et al. Validation of self-reported and hospital-diagnosed atrial fibrillation: the HUNT study. Clin Epidemiol 2016;8:185–93. 10.2147/CLEP.S103346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angell MS, Tiwari S, Løchen M-L. Prevalens og risikofaktorer for selvrapportert atrieflimmer hos menn og kvinner - Tromsøundersøkelsen. Hjerteforum 2015;28:36–42. [Google Scholar]
- 31. Engstad T, Bønaa KH, Viitanen M. Validity of self-reported stroke : the Tromso study. Stroke 2000;31:1602–7. 10.1161/01.str.31.7.1602 [DOI] [PubMed] [Google Scholar]
- 32. Warren JM, Ekelund U, Besson H, et al. Assessment of physical activity - a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010;17:127–39. 10.1097/HJR.0b013e32832ed875 [DOI] [PubMed] [Google Scholar]
- 33. Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 2003;37:discussion 06:197–206. 10.1136/bjsm.37.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ 2010;340:c2289. 10.1136/bmj.c2289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002154supp001.pdf (95.8KB, pdf)
openhrt-2022-002154supp002.pdf (719KB, pdf)
openhrt-2022-002154supp003.pdf (674.3KB, pdf)
openhrt-2022-002154supp004.pdf (741.2KB, pdf)
openhrt-2022-002154supp005.pdf (1.5MB, pdf)
Data Availability Statement
Data are available on reasonable request. Data may be obtained from a third party and are not publicly available. The data underlying this article cannot be shared publicly due to GDPR regulations but is available on request. The legal restrictions on data availability are set by the Tromsø Study Data and Publication Committee in order to control for data sharing, including publication of datasets with the potential of reverse identification of de-identified sensitive participant information. The data can however be made available on application to the Tromsø Study Data and Publication Committee. Contact information: The Tromsø Study, Department of Community Medicine, Faculty of Health Sciences, UiT The Arctic University of Norway; e-mail: tromsous@uit.no. The datasets generated in BiAS are not publicly available as the study is not yet completed but may be available on reasonable request. The Stata code used for the main analysis is available on reasonable request.