Abstract
Objectives
Accumulating evidence suggested that the laminin γ2 (LAMC2) expression level was upregulated in various cancers. However, the potential prognostic value of LAMC2 in cancers remains unclear. We conducted a meta-analysis to clarify the association of LAMC2 expression with prognosis.
Design
We searched Embase, Web of Science and PubMed (up to 25 November 2021) to collect all eligible studies, and meta-analysis was performed to interpret the association of LAMC2 expression with clinicopathological parameters, overall survival (OS), disease-specific survival (DSS) and progression-free survival (PFS).
Eligibility criteria for including studies
We included studies that investigate the relationship between LAMC2 and prognosis of cancers, patients were divided into two groups, and associations of LAMC2 expression with clinicopathological features were described.
Results
Seven studies were finally included. We found that increased LAMC2 expression was significantly associated with lymph node metastasis (log OR 0.88, 95% CI 0.38 to 1.38, p<0.001), tumour-node-metastasis stages (log OR: 0.95, 95% CI 0.39 to 1.50, p<0.001) and tumour status (log OR 1.26, 95% CI 0.84 to 1.68, p<0.001), but not with age (log OR −0.05, 95% CI −0.37 to 0.27, p=0.75) or gender (log OR −0.07, 95% CI −0.52 to 0.38, p=0.75). In addition, higher LAMC2 expression was found to be significantly associated with OS/PFS/DSS (HR 1.85, 95% CI 1.31 to 2.40, p<0.001). A similar result was found in The Cancer Genome Atlas database. High LAMC2 expression was significantly associated with OS in lung adenocarcinoma, mesothelioma, skin cutaneous melanoma, neck squamous cell carcinoma and brain lower grade glioma.
Conclusion
Our results suggested that higher LAMC2 expression was correlated with worse survival, lymph node metastasis, tumour-node-metastasis stages and tumour status. This study was subject to inherent limitations, but the results presented here provide insights regarding the potential use of LAMC2 as a biomarker for human cancer.
Study registration
researchregistry.com (researchregistry1319).
Keywords: ONCOLOGY, Adult oncology, Oncogenes
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This systematic review and meta-analysis provide comprehensive literature published up to November 2021 was performed in Embase, Web of Science and PubMed.
This study adheres to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Additional data sources, such as grey literature, were not searched.
Introduction
Laminins are trimeric proteins composed of α, β and γ chains, which are the main component of basement membranes.1 Mammalian genome encodes five α chains, four β chains and three γ chains.2 Loss-of-function studies show that most laminin mutants are embryonic lethal.3 Laminins are involved in various biological processes, including cellular phenotype maintenance, adhesion, migration, growth and differentiation in vivo and in vitro.4 5
In recent years, laminin γ2 (LAMC2) has attracted increasingly attractive because of the aberrant expression of LAMC2 in various cancer. The LAMC2 expression was significantly upregulated in colorectal cancer tissues compared with adjacent normal tissues, and high LAMC2 expression is known to be associated with poor patient prognosis.6 Furthermore, overexpression of LAMC2 has been reported in pancreatic ductal adenocarcinoma,7–11 non-small cell lung cancer,12 penile squamous cell carcinoma,13 ovarian cancer,14 oral tongue squamous cell carcinoma,15 cholangiocarcinoma16 and oesophageal squamous cell carcinoma,17 leading to poor clinicopathological features and short survival time.
However, individual studies may be inadequate and inaccurate due to their small sample and study design. To date, there was no meta-analysis has been performed to investigate the relationship between LAMC2 and the prognostic value. Therefore, we performed a comprehensive meta-analysis to assess the correlation between LAMC2 and survival outcomes and clinicopathological features in human cancers.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines18 (online supplemental table S1).
bmjopen-2022-063682supp002.pdf (4KB, pdf)
Patient and public involvement
No patient was involved.
Search strategy
A literature search was conducted in Embase, Web of Science and PubMed (up to 25 November 2021). The keywords were (laminin C2 OR LAMC2 OR laminin subunit gamma 2) AND (prognosis OR prognostic OR survival). The detailed search strategy is in online supplemental file 1. The reference lists and citation sections of relevant studies were also screened for additional eligible studies.
bmjopen-2022-063682supp001.pdf (65KB, pdf)
Study selection
Studies that met the following criteria were included in the meta-analysis: (1) study of the relationship between LAMC2 and prognosis of cancers, (2) patients were divided into two groups: high LAMC2 expression and low LAM2 expression group, and (3) associations of LAMC2 expression with overall survival (OS) and clinicopathological features were described. The exclusion criteria in our meta-analysis were as follows: (1) case reports, letters, reviews, editorials and expert opinions, (2) studies published non-English language, (3) non-human studies and (4) studies without sufficient available data.
Quality assessment
The Newcastle-Ottawa Scale (NOS) criteria were used to assess the quality of the eligible studies.19 The NOS contains nine items that include selection, comparability and outcome for studies. When the NOS score was ≥6, the study was considered high quality.
Data extraction
Two authors (TF and ZY) performed the data extraction independently. Disagreements were resolved by discussion and consensus with the third author (JX). The following information from the included studies was collected: the first author’s name, publication year, country, number of cases, cancer type, the detection method of LAMC2, clinicopathological features and survival outcome. When multivariate and univariate analyses were simultaneously reported, only the former was extracted. If studies only provided Kaplan-Meier curves, the survival data were extracted from the graphical curve and calculated HR and 95% CI were reckoned using the published method.20
Public data and tools
The web-based tool named Gene Expression Profiling Interactive Analysis (GEPIA) was used to analyse associations between LAMC2 and clinical outcomes.21
Statistical analysis
We used Stata MP V.16 software (Stata) to perform statistical analysis. Log OR and 95% CI were calculated for the association of LAMC2 expression and clinicopathological characteristics. The prognostic role of LAMC2 expression in OS, disease-specific survival (DSS) and progression-free survival (PFS) was evaluated through HR with 95% CI. The statistical heterogeneity among the studies was analysed by using I2 test and Q test. When significant heterogeneity (I2≥50%, p<0.05) was observed, the random-effects model was chosen. Otherwise, the fixed-effects model was used. The Egger’s test and Begg’s test were used to assess the potential publication bias. We conducted a sensitivity analysis to explore the stability of the overall meta-analysis results. p<0.05 was considered as statistically significant.
Results
Study identification and characteristics
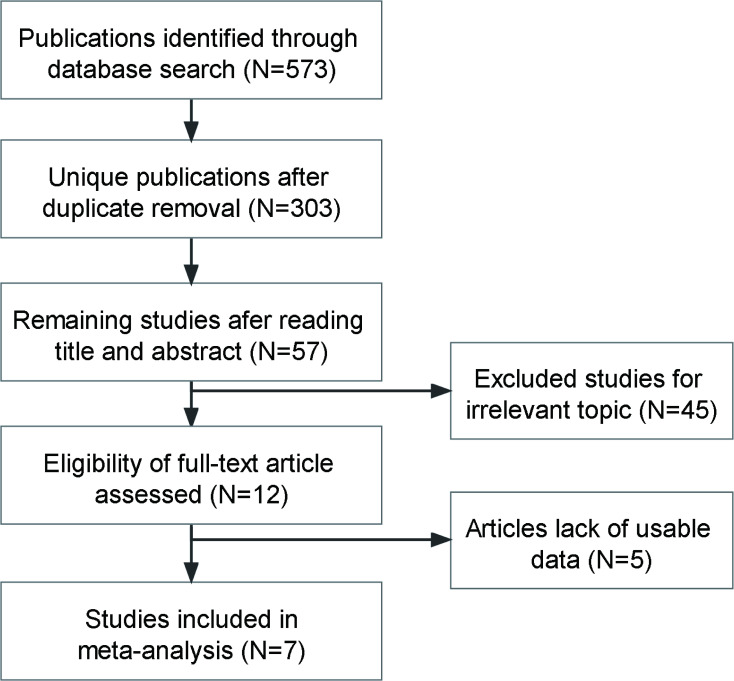
As is shown in figure 1, a total of 7 studies with 1056 patients with cancer were included in this meta-analysis satisfied the inclusion criteria.6 7 13 16 17 22 23 The publication period ranged from 2017 to 2021. All of our included studies had high quality with NOS ≥6. The included studies addressed six different cancer types: oesophageal squamous cell carcinoma (n=1), pancreatic ductal adenocarcinoma (n=2), cholangiocarcinoma (n=1), papillary thyroid cancer (n=1), colorectal cancer (n=1) and penile squamous cell carcinoma (n=1). The main characteristics of the seven studies are summarised in table 1.
Figure 1.
Flow diagram of the literature search and selection.
Table 1.
Characteristics of studies included in this meta-analysis
| Study | Year | Region | Sample | Cancer | Method | Outcome | NOS score |
| Okada et al7 | 2021 | Japan | 114 | Pancreatic ductal adenocarcinoma | qRT-PCR | OS | 8 |
| Okada et al22 | 2021 | Japan | 121 | Pancreatic ductal adenocarcinoma | qRT-PCR | OS | 8 |
| Pei et al16 | 2019 | China | 121 | Cholangiocarcinoma | IHC | – | 7 |
| Liang et al17 | 2018 | China | 64 | Oesophageal squamous cell carcinoma | qRT-PCR | OS | 7 |
| Zhan et al23 | 2019 | China | 473 | Papillary thyroid cancer | RNA-seq | PFS | 6 |
| Zhou et al13 | 2018 | China | 114 | Penile squamous cell carcinoma | IHC | DSS | 7 |
| Huang et al6 | 2017 | China | 49 | Colorectal cancer | IHC | – | 6 |
DSS, disease-specific survival; IHC, immunocytochemistry; NOS, Newcastle-Ottawa scale; OS, overall survival; PFS, progression-free survival; qRT-PCR, quantitative real-time PCR.
Relationship between LAMC2 expression and clinicopathological parameters
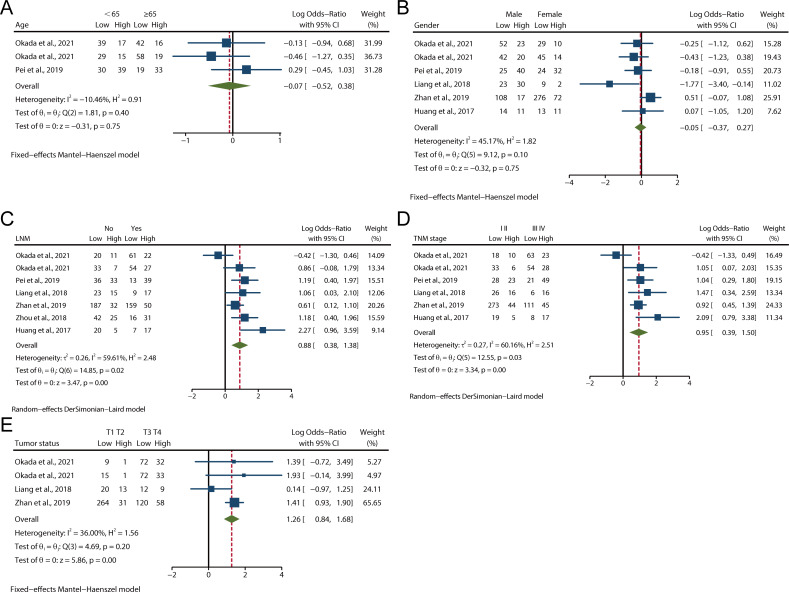
We evaluated the relationship between LAMC2 expression and clinicopathological parameters in various cancers. As shown in figure 2A, B, there were three and six studies describing patients of age and gender, respectively. The pooled analysis demonstrated that there was no significant association between LAMC2 expression and age or gender. High expression of LAMC2 was significantly associated with lymph node metastasis (LNM) (log OR 0.88, 95% CI 0.38 to 1.38, p<0.001, figure 2C) and tumour-node-metastasis (TNM) stage (log OR: 0.95, 95% CI 0.39 to 1.50, p<0.001, figure 2D). There was significant heterogeneity among these studies (I2=59.61%, p=0.02; I2=60.16%, p=0.03), and thus the random-effects DerSimonian-Laird model was adopted. A total of four studies evaluated tumour status according to LAMC2 expression. No statistically significant heterogeneity was found among the studies (I2=36.00%, p=0.20); thus, the fixed-effect Mantel-Haenszel model was adopted. As shown in figure 2E, high expression of LAMC2 was significantly associated with tumour status (log OR 1.26, 95% CI 0.84 to 1.68, p<0.001). This finding suggests that high LAMC2 expression is associated with clinicopathological features.
Figure 2.
Forest plots of studies evaluating the relationship between LAMC2 expression and clinicopathological features. (A) LNM, (B) TNM stage, (C) Tumour status, (D) Gender, (E) Age. LNM, lymph node metastasis; TNM, tumour-node-metastasis.
Relationship between LAMC2 expression and OS/PFS/DSS
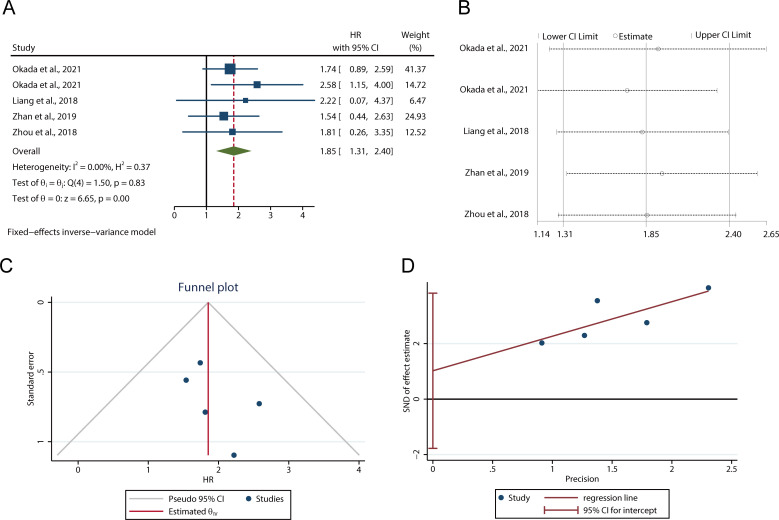
There were 5 studies including 872 patients presenting the relationship between LAMC2 and OS/PFS/DSS. Due to no significant heterogeneity being found among studies (I2=0.00%, p=0.83), the fixed-effect inverse-variance model was adopted to estimate the pooled HR and 95% CI. The pooled HR indicated that the expression of LAMC2 was negatively associated with OS/PFS/DSS (HR 1.85, 95% CI 1.31 to 2.40, p<0.001, figure 3A), which demonstrated that LAMC2 was a risk factor for the prognosis of patients with cancer. In addition, sensitivity analysis was performed to determine the effect of individual studies on the OS/PFS/DSS. It revealed that no single study altered the pooled LAMC2 HR result significantly (figure 3B). This suggested that the result of the meta-analysis was stable.
Figure 3.
The relationship between LAMC2 expression and OS/PFS/DSS. (A) Forest plot for the meta-analysis of OS/PFS/DSS. (B) Sensitivity analysis for LAMC2 expression with OS/PFS/DSS. (C) Funnel plot for the meta-analysis of OS/PFS/DSS. (D) Egger’s graph for analysing publication bias. DSS, disease-specific survival; OS, overall survival; PFS, progression-free survival.
Analysis of publication bias
Funnel plot (figure 3C), Begg’s test and Egger’s linear regression test (figure 3D) were used to assess publication bias. The results showed that the funnel plots scatter symmetrically. The statistical tests showed p values were greater than 0.05 (Begg’s test: p=0.4624; Egger’s test: p=0.329). Thus, there was no obvious publication bias in the prognostic meta-analysis.
Validation of TCGA data set results
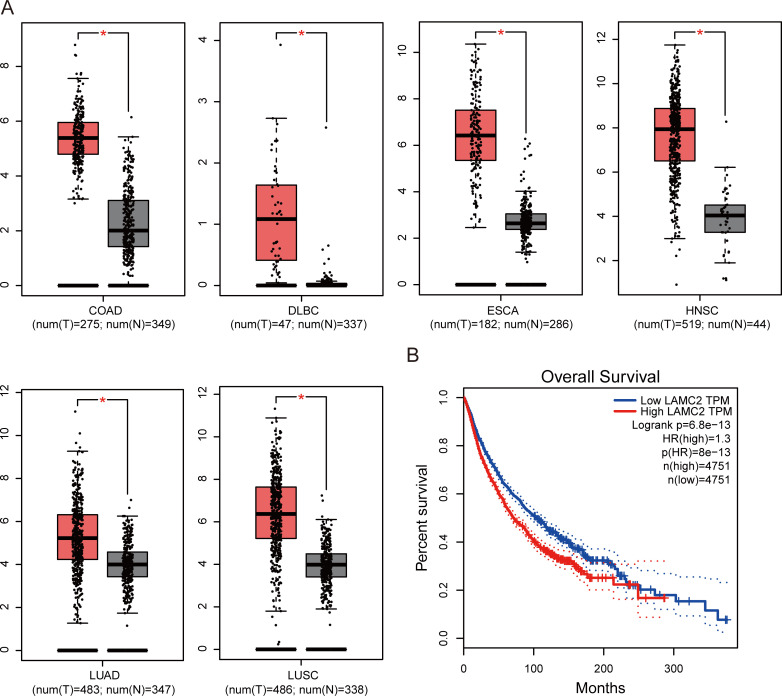
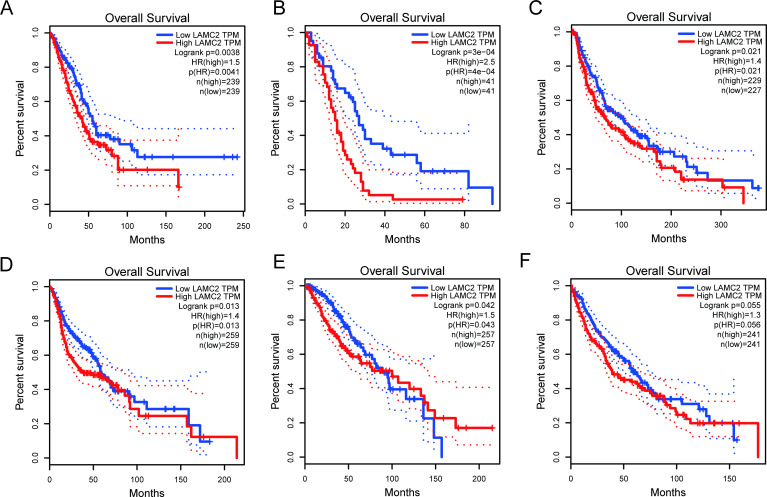
To validate our result, we retrieved LAMC2 expression data and clinical data from the TCGA dataset. As shown in figure 4A, LAMC2 was increased in colon adenocarcinoma (COAD), lymphoid neoplasm diffuse large B-cell lymphoma, oesophageal carcinoma, head and neck squamous cell carcinoma (HNSC), lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC), determined using a log2FC cut-off of 1 and a p value cut-off of 0.01. A total of 9502 patients with urinary, digestive, female reproductive, respiratory and blood systems cancers were included in the survival analysis from the TCGA database. According to the expression of LAMC2, the patients were divided into high and low groups using the median score as the cut-off by GEPIA.21 The results showed that higher LAMC2 expression was correlated with worse survival (figure 4B). We also explored the prognostic role of LAMC2 in different cancer types. As shown in figure 5, LAMC2 expression was significantly associated with OS in LUAD (figure 5A), mesothelioma (MESO, figure 5B), skin cutaneous melanoma (SKCM, figure 5C), HNSC (figure 5D) and brain lower grade glioma (LGG, figure 5E). However, LAMC2 expression was not related to OS in LUSC (figure 5F).
Figure 4.
The expression of LAMC2 in TCGA database. (A) LAMC2 expression in colon adenocarcinoma (COAD), diffuse large B-cell lymphoma (DLBC), oesophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC). *p<0.01. (B) OS rate of LAMC2 expression in TCGA database (n=9502). Grey boxes indicate normal, and red boxes indicate tumour. N, normal; OS, overall survival; T, tumour; TCGA, The Cancer Genome Atlas.
Figure 5.
Kaplan-Meier curves showing the prognostic value of LAMC2 in the TCGA database. (A) LUAD, (B) MESO, (C) SKCM, (D) HNSC, (E) LGG, (F) LUSC. P values were calculated using the log-rank test. HNSC, head and neck squamous cell carcinoma; LGG, lower grade glioma; LUAD, lung adenocarcinoma; MESO, mesothelioma; SKCM, skin cutaneous melanoma.
Discussion
Laminins are involved in various cancer development and prognosis.24 25 Increasing evidence suggests that the various laminin isoforms could be useful biomarkers of cancer diagnosis and might be potential therapeutic targets for cancers treatment, such as LAMA5,14 LAMB126 and LAMC1,27 LAMC2 is encoding by Laminin γ2 and has been confirmed as a therapeutic target for cancers. Here we performed a meta-analysis of seven studies to achieve a comprehensive evaluation between higher LAMC2 expression and clinicopathological characteristics in various cancer types. Our results showed that higher LAMC2 expression was significantly associated with LNM, TNM stage and tumour status. However, there was no significant association between LAMC2 expression and age or gender. Simultaneously, we also found that OS/PFS/DSS was higher in patients with low LAMC2 expression. Publication bias analysis demonstrated that no publication bias was observed among the included studies. These results suggested that LAMC2 might be a valuable biomarker for predicting prognosis in patients with cancer. Steyerberg et al provide the prognostic model that can provide effective solving discrimination and predictiveness measures.28 We will plan to investigate this in a later study.
Several kinds of researches have shown that LAMC2 promotes cancer cells proliferation, motility and invasion.29–33 However, the specific mechanisms are not particularly well understood. There is a study indicating that ZNF750 inhibited the migration of oesophageal squamous cancer cells by inhibiting the LAMC2 transactivation.29 In hepatocellular carcinoma (HCC), LAMC2 has been found to be regulated by miR-548c-3p and inhibited the epithelial-mesenchymal transition in HCC.30 In pancreatic cancer cells, LAMC2 promoted Akt-Ser473 phosphorylation and increased expression and cell membrane accumulation of NHE1, promoting cell migration and invasion.31 A study by Wu et al showed that the LAMP3-LAMC2-TNC signal regulated the efficacy of radiation exposure in laryngeal squamous cell carcinoma.32 High-throughput sequencing results showed that miR-338-5 p/3p targets LAMC2 to suppress invasion in salivary adenoid cystic carcinoma cells.33 LAMC2 was found to promote tumour progression by EGFR signalling.34 35 The latest research showed that overexpression of LAMC2 enhanced pancreatic ductal adenocarcinoma metastasis and tumourigenesis through the EGFR/ERK1/2/AKT/mTOR signalling pathway.11 These findings suggested that LAMC2 might play as an oncogene and predict prognosis in patients with cancer.
Recent researches have also investigated LAMC2 as a valuable biomarker of cancer diagnosis.36 37 Due to a limitation of the small sample size, we explored the expression of LAMC2 in various cancer types using (The Cancer Genome Atlas) TCGA database. The results showed that LAMC2 was upregulated in tumours and might be used as a biomarker for a variety of tumour types. Moreover, we explored the survival analysis from the TCGA database. The results demonstrated that higher LAMC2 expression was associated with poor OS in 9502 patients. We also explored the prognostic role of LAMC2 in different types of cancer. LAMC2 expression was significantly associated with OS in LUAD, MESO, SKCM, HNSC and LGG, but not in LUSC. This deserves further investigation.
Some potential limitations of our study should be noted. First, only seven articles were included in our meta-analysis, the limited number of studies might influence the reliability of the results. Of the seven included studies, five were from China and two were from Japan. So, our results may only be applicable to the Asian population. Although we determined data from the TCGA database, future studies from non-Asian populations are needed to confirm our findings. Second, there was no consensus on a cut-off value for higher LAMC2 expression. Third, in some researches, the data for HR and 95% CI value was not provided. Although we tried our best to extract the HR and 95% CI value from the Kaplan-Meier curve, some errors are inevitable. Fourth, the numbers of patients and tumour types included in this meta-analysis were still limited. So, our results may exaggerate the prognostic value of LAMC2.
Conclusions
In conclusion, LAMC2 may be a valuable biomarker for cancer diagnosis, and upregulation of LAMC2 is associated with a poor prognosis in patients with cancer. And increased LAMC2 expression is significantly associated with bad tumour status, TNM stages and LNM. For future clinical applications, more high-quality studies with large sample sizes are needed to confirm the role of LAMC2 in various cancers and regions.
Supplementary Material
Acknowledgments
We sincerely thank Dr. Yang Wu for her support in this study.
Footnotes
Contributors: TF and ZY designed the study; TF, ZY and JX searched the literature and extracted data; ZY and TF analysed data; ZY prepared the figures; ZY, J-XL and ZG wrote the manuscript. All authors gave the final approval for the paper to be published. ZY and TF act as guarantors.
Funding: This study was supported by the Chongqing Science and Health Joint Project (2021MSXM108).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Aumailley M. The laminin family. Cell Adh Migr 2013;7:48–55. 10.4161/cam.22826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hohenester E. Structural biology of laminins. Essays Biochem 2019;63:285–95. 10.1042/EBC20180075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y. Laminin: loss-of-function studies. Cell Mol Life Sci 2017;74:1095–115. 10.1007/s00018-016-2381-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap L, Tay HG, Nguyen MTX, et al. Laminins in cellular differentiation. Trends Cell Biol 2019;29:987–1000. 10.1016/j.tcb.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Durbeej M. Laminins. Cell Tissue Res 2010;339:259–68. 10.1007/s00441-009-0838-2 [DOI] [PubMed] [Google Scholar]
- 6.Huang D, Du C, Ji D, et al. Overexpression of LAMC2 predicts poor prognosis in colorectal cancer patients and promotes cancer cell proliferation, migration, and invasion. Tumour Biol 2017;39:101042831770584. 10.1177/1010428317705849 [DOI] [PubMed] [Google Scholar]
- 7.Okada Y, Takahashi N, Takayama T, et al. LAMC2 promotes cancer progression and gemcitabine resistance through modulation of EMT and ATP-binding cassette transporters in pancreatic ductal adenocarcinoma. Carcinogenesis 2021;42:546–56. 10.1093/carcin/bgab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C, Liu Z, Zeng X, et al. Evaluation of the diagnostic ability of laminin gene family for pancreatic ductal adenocarcinoma. Aging 2019;11:3679–703. 10.18632/aging.102007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam S, Kitagawa T, Baron B, et al. ITGA2, LAMB3, and LAMC2 may be the potential therapeutic targets in pancreatic ductal adenocarcinoma: an integrated bioinformatics analysis. Sci Rep 2021;11:10563. 10.1038/s41598-021-90077-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin G, Ruan Q, Shangguan F, et al. Runx2 and LAMC2: promising pancreatic cancer biomarkers identified by an integrative data mining of pancreatic adenocarcinoma tissues. Aging 2021;13:22963–84. 10.18632/aging.203589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirtonia A, Pandey AK, Ramachandran B, et al. Overexpression of laminin-5 gamma-2 promotes tumorigenesis of pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR cascade. Cell Mol Life Sci 2022;79:362. 10.1007/s00018-022-04392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Cai R, Wang T, et al. LAMC2 promotes the proliferation of cancer cells and induce infiltration of macrophages in non-small cell lung cancer. Ann Transl Med 2021;9:1392. 10.21037/atm-21-4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q-H, Deng C-Z, Chen J-P, et al. Elevated serum LAMC2 is associated with lymph node metastasis and predicts poor prognosis in penile squamous cell carcinoma. Cancer Manag Res 2018;10:2983–95. 10.2147/CMAR.S171912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao B, Yang P. Comprehensive analysis of the expression and prognosis for laminin genes in ovarian cancer. Pathol Oncol Res 2021;27:1609855. 10.3389/pore.2021.1609855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thangaraj SV, Shyamsundar V, Krishnamurthy A, et al. Deregulation of extracellular matrix modeling with molecular prognostic markers revealed by transcriptome sequencing and validations in oral tongue squamous cell carcinoma. Sci Rep 2021;11:250. 10.1038/s41598-020-78624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei Y-F, Liu J, Cheng J, et al. Silencing of LAMC2 reverses epithelial-mesenchymal transition and inhibits angiogenesis in cholangiocarcinoma via inactivation of the epidermal growth factor receptor signaling pathway. Am J Pathol 2019;189:1637–53. 10.1016/j.ajpath.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Chen X, Wu Y, et al. LNCRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ 2018;25:1980–95. 10.1038/s41418-018-0084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 20.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98–102. 10.1093/nar/gkx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada Y, Nishiwada S, Yamamura K, et al. Identification of laminin γ2 as a prognostic and predictive biomarker for determining response to gemcitabine-based therapy in pancreatic ductal adenocarcinoma. Eur J Cancer 2021;146:125–34. 10.1016/j.ejca.2020.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan S, Wang T, Wang M, et al. In-depth proteomics analysis to identify biomarkers of papillary thyroid cancer patients older than 45 years with different degrees of lymph node metastases. Proteomics Clin Appl 2019;13:e1900030. 10.1002/prca.201900030 [DOI] [PubMed] [Google Scholar]
- 24.Garg M, Braunstein G, Koeffler HP. LAMC2 as a therapeutic target for cancers. Expert Opin Ther Targets 2014;18:979–82. 10.1517/14728222.2014.934814 [DOI] [PubMed] [Google Scholar]
- 25.Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells. Cancer Lett 2005;223:1–10. 10.1016/j.canlet.2004.08.030 [DOI] [PubMed] [Google Scholar]
- 26.Ran T, Chen Z, Zhao L, et al. LAMB1 is related to the T stage and indicates poor prognosis in gastric cancer. Technol Cancer Res Treat 2021;20:153303382110049. 10.1177/15330338211004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashima H, Wu R-C, Wang Y, et al. Laminin C1 expression by uterine carcinoma cells is associated with tumor progression. Gynecol Oncol 2015;139:338–44. 10.1016/j.ygyno.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–38. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazawa M, Lin D-C, Handral H, et al. ZNF750 is a lineage-specific tumour suppressor in squamous cell carcinoma. Oncogene 2017;36:2243–54. 10.1038/onc.2016.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin J, Liu H, Jin M, et al. Silencing of hsa_circ_0101145 reverses the epithelial-mesenchymal transition in hepatocellular carcinoma via regulation of the miR-548c-3p/LAMC2 axis. Aging 2020;12:11623–35. 10.18632/aging.103324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Cai J, Du S, et al. LAMC2 modulates the acidity of microenvironments to promote invasion and migration of pancreatic cancer cells via regulating Akt-dependent NHE1 activity. Exp Cell Res 2020;391:111984. 10.1016/j.yexcr.2020.111984 [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Li J, Chen J, et al. Efficacy of radiation exposure in laryngeal squamous cell carcinoma is mediated by the LAMP3/LAMC2/tenascin-C pathway. Exp Biol Med 2019;244:1070–80. 10.1177/1535370219867643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Zhang L, Shi P, et al. Genome-wide profiles of metastasis-associated mRNAs and microRNAs in salivary adenoid cystic carcinoma. Biochem Biophys Res Commun 2018;500:632–8. 10.1016/j.bbrc.2018.04.122 [DOI] [PubMed] [Google Scholar]
- 34.Garg M, Kanojia D, Okamoto R, et al. Laminin-5γ-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J Clin Endocrinol Metab 2014;99:E62–72. 10.1210/jc.2013-2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daisuke H, Kato H, Fukumura K, et al. Novel LAMC2 fusion protein has tumor-promoting properties in ovarian carcinoma. Cancer Sci 2021;112:4957–67. 10.1111/cas.15149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shokati Eshkiki Z, Khayer N, Talebi A, et al. Novel insight into pancreatic adenocarcinoma pathogenesis using liquid association analysis. BMC Med Genomics 2022;15:30. 10.1186/s12920-022-01174-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You G-R, Cheng A-J, Lee L-Y, et al. Prognostic signature associated with radioresistance in head and neck cancer via transcriptomic and bioinformatic analyses. BMC Cancer 2019;19:64. 10.1186/s12885-018-5243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063682supp002.pdf (4KB, pdf)
bmjopen-2022-063682supp001.pdf (65KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.