Abstract
OBJECTIVE
Various techniques, needle types, and additional methods such as on-site pathological evaluation (ROSE) are used to increase the sensitivity of endoscopic ultrasound-fine needle aspiration (EUS-FNA), which is used in the diagnosis of pancreatic solid lesions. In this study, diagnosticity of the lesions according to the regions of the pancreas with EUS-FNA and ROSE performed with the slow pull technique using a 22 G needle will be evaluated.
METHODS
A total of 82 patients who underwent EUS-FNA between January 2, 2015, and March 14, 2020, were included in the study. General and clinical information of the patients were recorded retrospectively. The patients were diagnosed according to The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology Classification. If the diagnosis could not be made with EUS-FNA and ROSE, the diagnosis was made with alternative methods of surgery or percutaneous biopsy. Patients diagnosed as benign with EUS-FNA and ROSE were followed for at least 1 year and were accepted as benign.
RESULTS
The mean age of the patients was 63.2±10.5 years and 54 (69.6%) of them were male. The mean lesion size was 36.8 mm and the number of needle passes was 2.87. The overall sensitivity was 82.9% and the specificity was 100%. The sensitivity of EUS-FNA and ROSE in solid lesions in the head and body of the pancreas was higher than in lesions in the tail region (p=0.024).
CONCLUSION
EUS-FNA and ROSE are an effective method in the diagnosis of pancreatic solid lesions. The use of a 22 G needle may be more diagnostic in the head and body of the pancreas than in the tail region.
Keywords: Endoscopic ultrasound, fine needle aspiration, pancreas, rapid on-site cytopathologic examination, slow pull technique
Highlight key points
EUS-FNA and ROSE are an effective method in the diagnosis of solid lesions of the pancreas.
The risk of complications is low with the slow pull technique and 22G needle use in EUS-FNA procedure.
EUS-FNA performed with a 22 G needle may make more effective diagnosis lesions in the head and body of the pancreas than lesions in the tail region.
Pancreatic cancer (PC) is the seventh leading cause of death from cancer worldwide. The 5-year survival rate for PC is about 6% [1]. A good prognosis is achieved by early diagnosis and surgical resection, especially for tumors smaller than 1 cm. Eight in ten cases have a 5-year survival rate [2]. Abdominal ultrasonography, computed tomography, magnetic resonance imaging, endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP) are used to diagnose PC. Advances in technology have paved the way for more precise imaging methods. EUS allows us to image the pancreas at high resolution. EUS has a sensitivity of 94% for the diagnosis of PC [3].
EUS-guided fine-needle aspiration (EUS-FNA) has been used since 1992 to sample pancreatobiliary tissues. It allows us to conduct a biopsy on EUS target lesions. It has been a popular method since fine-needle aspiration cytology/biopsy devices started to be used [4]. EUS-FNA may not always allow us to collect sufficient tissue from biopsy samples for pathological diagnosis. False-negative and non-diagnostic results can cause delays in treatment. Researchers have developed needles in different sizes and shapes and different techniques (suction, slow-pull, and aeration techniques) and methods (rapid on-site evaluation [ROSE]) to improve the diagnostic performance of EUS-FNA [5–9]. In this study, EUS-FNA was performed using a 22 gauge (G) calibrated needle and slow-pull technique, and aspirates were examined with ROSE. The diagnostic efficacy in solitary lesions according to the regions of the pancreas was analyzed.
MATERIALS AND METHODS
This study was conducted at the Gastroenterology Clinic of Health Sciences University Umraniye Training and Research Hospital between January 2, 2015, and March 14, 2020. The study population consisted of 145 patients who underwent EUS FNA and ROSE. The sample consisted of 82 patients with solid lesion of the pancreas. Figure 1 shows the inclusion and exclusion criteria.
Figure 1.
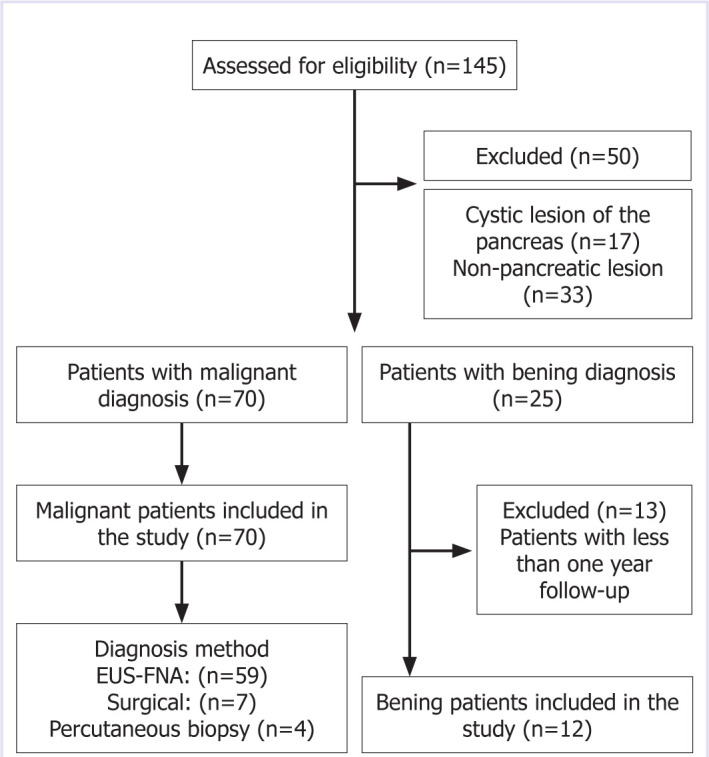
Inclusion and exclusion criteria.
Informed consent was obtained from participants. Each participant was sedated with 2 mg/kg propofol by an anesthesiologist. EUS for guided puncture of the lesion was conducted using a Fujinon (Fujifilm, Tokyo, Japan, VP-4450 HD) EG 580UT gastroscope. Fine-needle aspiration was performed through a transgastric approach if the lesion involved the body and the tail. It was achieved through a transduodenal approach for lesions in the head and uncinate process. A 22 G needle was used for fine-needle aspiration. The slow-pull technique was used to collect samples.
In the ROSE examination, two primary staining methods are used to perform basic cytology. After aspiration, one or two smears were prepared immediately and stained with diff-quick stain for a pathologist to evaluate adequacy on-site. The others were fixed with alcohol and stained with Papanicolaou staining in a pathology laboratory. The aspiration needle was further washed in 10% formol in test tubes for cell block preparation. A pathologist evaluated the smears and sections of the cell block to confirm the on-site diagnosis and render the final diagnosis, which was then recorded according to the classification of the Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology (PSCPC). According this system, category I is non-diagnostic, II is negative (for malignancy), III is atypical, IV is neoplastic: benign or other, V is suspicious for malignancy, and VI is positive/malignant. A PSCPC category of V or VI was regarded as malign [10].
Age, sex, hospital admission symptom, location of the lesion in the pancreas, EUS lesion size, CA 19.9 levels at diagnosis, and diagnosis of malignant patients were recorded retrospectively.
The study endpoints were tissue diagnosis with EUS-FNA and ROSE in malignant patients and diagnosis by percutaneous biopsy or surgery in cases tissue could not be identified through EUS-FNA and ROSE. A lesion was benign if it did not change in size at a 1-year follow-up. This study was conducted following the principles of the Helsinki Declarations revised in 2013; designed retrospectively and approved by the ethics committee of Health Sciences University Umraniye Training and Research Hospital (date: 27.05.2021, no:B.10.1.TKH.4.34.H.GP.0.01/167).
Statistical Analysis
All statistical procedures were performed using SPSS software (version 25.0, SPSS Inc., Chicago, IL, institutionally registered software). The Shapiro–Wilk test was used for normality testing. Median, minimum, maximum, and frequency were used for descriptive statistics. The Kruskal–Wallis H test was used for nonparametric data. The Chi-square (Fisher’s Exact) test was used to analyze categorical data. P<0.05 was considered significant.
RESULTS
Eighty-two patients underwent EUS-FNA due to solid lesions in the pancreas. Participants had a mean age of 63.2±10.5 years. More than half the participants were men (69.6%; n=54). Table 1 shows the patients general characteristics, clinical symptoms, and diagnostic methods.
Table 1.
General characteristics, clinical symptoms, and diagnostic methods
| Parameters | |
|---|---|
| Age± | 63.2±10.5 |
| Gender (%) | |
| Female | 30.4 |
| Male | 69.6 |
| Symptom at admission (%) | |
| Icterus | 41.4 |
| Abdominal pain | 26.8 |
| Weight loss | 12.1 |
| Weakness | 8.5 |
| Other | 10.9 |
| Lesion size mm | 36.8 (14–80) |
| Lesion location (%) | |
| Head | 67.1 |
| Body | 23.2 |
| Tail | 9.8 |
| Malignant patient (%) | 85.4 |
| Benign patient (%) | 14.6 |
| Diagnostic method | |
| EUS-FNA (%) | 86.6 |
| The average number of needle passes | 2.87 |
| *Surgical (%) | 8.5 |
| *Percutaneous biopsy (%) | 4.9 |
: Patients with clinical suspicion of severe malignancy who could not be diagnosed with the EUS-FNA method were diagnosed with alternative methods.
Gender, lesion size, CA 19.9 and diagnosis, PSCPC categories, sensitivity, and specificity values of patients with malignant lesions according to the regions of the pancreas are shown in Table 2. EUS-FNA and ROSE were found to have higher diagnostic sensitivity in solid lesions in the head and body of the pancreas than in the tail region (p=0.024). Eleven patients with PSCPC Category I and III were diagnosed with adenocarcinoma by alternative diagnostic methods. In addition, all remaining patients could be diagnosed with EUS-FNA and ROSE method. A total of three patients were diagnosed with neuroendocrine tumor (NET) by EUS-FNA and ROSE. One patient was diagnosed with a well-differentiated NET, so it was considered category IV. The other two patients with NET were considered category V because they contained solid-cellular clearly neoplastic epithelial proliferation [10].
Table 2.
Evaluation of lesion characteristics and diagnosticity by pancreatic regions
| Parameters | Head | Body | Tail | P |
|---|---|---|---|---|
| Malignant patients (%) | 68.6 | 21.4 | 10 | |
| Gender (%) | ||||
| Male | 67.4 | 25.6 | 7 | |
| Female | 70.4 | 14.8 | 14.8 | |
| Lesion size mm | 35 (14–65)* | 40 (30–60)* | 42 (32–80)* | 0.130† |
| Malignant diagnoses (%) | ||||
| Adenocarcinoma | 62.9 | 21.4 | 8.6 | |
| SCC | 1.4 | – | – | |
| RCC metastasis | 1.4 | – | – | |
| NET | 2.9 | – | 1.4 | |
| Benign (%) | 58.3 | 33.3 | 8.3 | |
| CA 19.9 pg/mL‡ | 780 (2–176170)* | 385 (31–1834)* | 278 (24–1642)* | 0.300† |
| PSCPC category (%) | ||||
| I | 6.3 | 6.7 | 0.0 | |
| III | 4.2 | 13.3 | 42.9 | |
| IV | 2.1 | 0.0 | 0.0 | 0.024§ |
| V | 27.1 | 20.0 | 42.9 | |
| VI | 60.4 | 60.0 | 14.3 | |
| Sensitivity (%) | 87.5 | 80 | 57.1 | |
| Specificity (%) | 100 | 100 | 100 | |
| Total sensitivity (%) | 82.9 | |||
| Total specificity (%) | 100 |
PSCPS: Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology; SCC: Squamoz cell carcinoma; RCC: Renal cell carcinoma; NET: Neuroendocrine tumor; *: Minimum maximum value; †: Kruskal Wallis test; §: Fisher’s exact test; ‡: CA 19.9 level was calculated in patients with adenocarcinoma.
DISCUSSION
The latest international guidelines on PC stipulate that pancreatic carcinoma should be diagnosed pathologically before chemotherapy. Therefore, EUS-guided sampling is recommended for locally advanced patients, who are candidates for neoadjuvant therapy, and those with metastatic disease. These two groups account for 80–85% of all PC patients [11, 12]. EUS-FNA can diagnose pancreatic lesions with high sensitivity and specificity through cytological and/or histological samples [13]. Fine-needle biopsy (FNB) needles have been developed to increase diagnostic sensitivity in solid pancreatic lesions. However, most research shows no difference in sensitivity between FNB and FNA [14–17]. We think that EUS-FNA and ROSE are an effective method in solid lesions of the pancreas. In the evaluation of a patient of our study; EUS image of a lesion in the head region of the pancreas is shown in Figure 2. Diagnosis of adenocarcinoma with the ROSE is shown in Figure 3. This study reported the results of patients who underwent EUS-FNA and ROSE.
Figure 2.
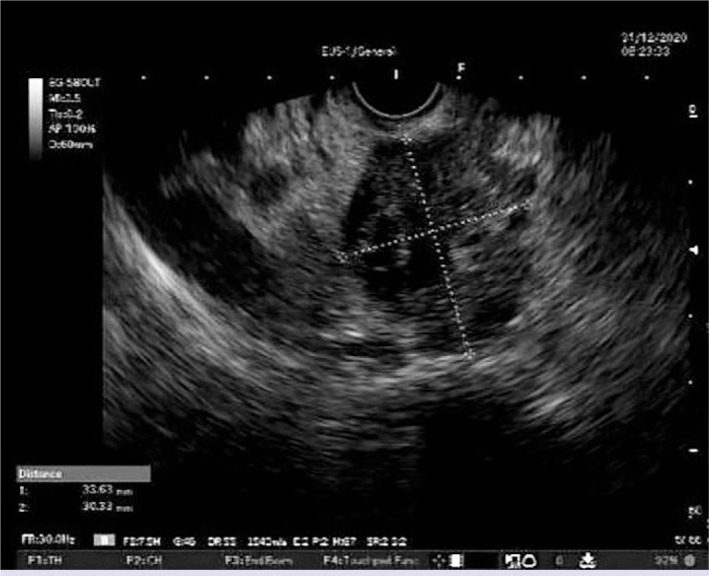
At the head of the pancreas 33×30 mm in size hypoechoic irregularly circumscribed lesion.
Figure 3.
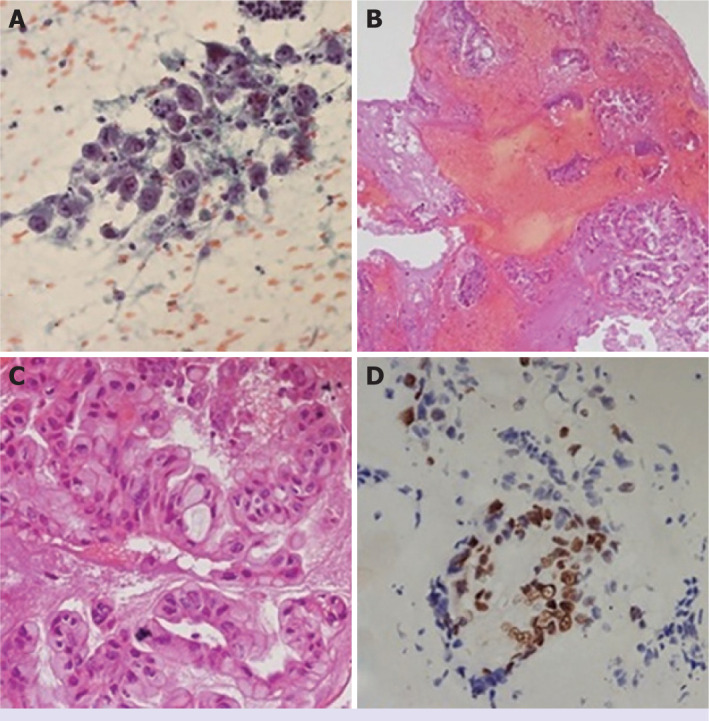
Cytopathological evaluation. (A) Atypical pancreatic ductal cells (B, C) Mucinous tumor aggregates on cell block (×20 HPF, ×40 HPF) (D) Strong, diffuse p53 staining (×40 HPF). HPF: High power field.
In the patients in this study; first three symptoms at admission were icterus (41.4%), abdominal pain (26.8%), and weight loss (12.1%). Most patients with malign lesions were diagnosed with adenocarcinoma (92.9%). More than half the lesions were at the head of the pancreas (68.6%). Less than a quarter of the lesions were at the body of the pancreas (21.4%). The remaining lesions were at the tail of the pancreas (10%). The symptoms at admission and lesion locations and diagnosis were consistent with the literature [18–20]. In general, due to presenting with early symptoms, head lesions have been diagnosed as smaller than the other localization of the pancreas, at diagnosis [21]. However, in our study, we found no statistical difference regarding lesion diameter among sites of pancreatic lesions. We think that it may be associated with, most of our patients, first diagnostic workout done in many different centers and referred to our tertiary referral center.
Hewitt et al. [22] conducted a meta-analysis on 33 studies with a total sample size of 4984 patients and reported 85% sensitivity (95CI%: 84–86%) and 98% specificity (95CI%: 97–99%) for EUS-FNA malignant cytology. We also reported 82.9% sensitivity and 100% specificity. Our results are consistent with the literature [22–24]. The number of needle passes is another factor affecting diagnosis in EUS FNA. LeBlanc et al. [25] reported that the ideal number of EUS-FNA needle passes to obtain a diagnosis ranged from 2 to 6. Fewer needle passes mean shorter operation time, reducing anesthesia time, medical costs, and adverse events [26]. EUS-FNA-related major complications are bleeding, perforation, infection, and acute pancreatitis [27]. 19G–25G caliber needles are generally used for the EUS-FNA examination of solid pancreatic lesions, and ROSE examination is recommended to increase diagnostic efficiency [28, 29]. However, research shows no difference in sensitivity and complication development between 22 G and 25 G needles in the EUS-FNA procedure of solid pancreatic lesions [23, 30]. Nakai et al. [31] found that the slow pull technique had better diagnostic efficiency than the suction technique. We used 22 G needles to perform the slow pull technique. The mean number of needle passes was 2.87. We performed ROSE on all patients. None of the patients developed complications. EUS-FNA showed higher sensitivity in malignant lesions at the head and body of the pancreas than in those at the tail of the pancreas (p=0.024). There was no significant difference in lesion sizes (p=0.130). This may be because trans-gastric passage is more difficult than trans-duodenal passage and imaging of pancreatic tail lesions is more suboptimal than pancreatic head and body lesions. To the best of our knowledge, this is the first study with PSCPC category to compare the sensitivity of EUS-FNA by the regions of the pancreas. This study had two limitations. First, it was a retrospective study. Second, it was conducted in one center.
Conclusion
EUS-FNA and ROSE are effective methods to diagnose solid pancreatic lesions. 22 G needles may be better at diagnosing lesions at the head and body of the pancreas than those at the tail. ROSE may be associated with a decrease in the number of needle passes. EUS-FNA with ROSE may be associated with a reduced risk of complications, regardless of needle diameter.
Footnotes
Cite this article as: Ak C, Sayar S, Tarikci Kilic E, Kahraman R, Ozturk O, Zemheri IE, et al. EUS-FNA and ROSE in solid lesions of the pancreas; have the same diagnostic efficacy compared to pancreatic sites? North Clin Istanb 2022;9(5):464–469.
Ethics Committee Approval
The Health Sciences University Umraniye Training and Research Hospital Clinical Research Ethics Committee granted approval for this study (date: 27.05.2021, number: B.10.1.TKH.4.34.H.GP.0.01/167).
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure
The authors declared that this study has received no financial support.
Authorship Contributions
Concept – CA; Design – SS, CA; Supervision – RK, OO; Fundings – OO, KO; Materials – SS, KO; Data collection and/or processing – CA; Analysis and/or interpretation – ETK; Literature review – CA, RK; Writing – CA; Critical review – IEZ, KO.
References
- 1.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egawa S, Toma H, Ohigashi H, Okusaka T, Nakao A, Hatori T, et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985–92. doi: 10.1097/MPA.0b013e318258055c. [DOI] [PubMed] [Google Scholar]
- 3.Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19–32. doi: 10.1007/s00535-018-1519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 5.Jani BS, Rzouq F, Saligram S, Lim D, Rastogi A, Bonino J, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: a systematic review of technical and procedural variables. N Am J Med Sci. 2016;8:1–11. doi: 10.4103/1947-2714.175185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014;46:1056–62. doi: 10.1055/s-0034-1377558. [DOI] [PubMed] [Google Scholar]
- 7.Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, et al. Japan EUS-FNA Negative Pressure Suction Study Group High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–7. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitman MB, Centeno BA, Ali SZ, Genevay M, Stelow E, Mino-Kenudson M, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 2014;11:3. doi: 10.4103/1742-6413.133343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempero MA, Malafa MP, Chiorean EG, Czito B, Scaife C, Narang AK, et al. Pancreatic Adenocarcinoma, version 1. 2019. J Natl Compr Canc Netw. 2019;17:202–10. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 12.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. ESMO Guidelines Committee Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015 Sep;26(Suppl 5):v56–68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias García J, Domínguez-Muñoz JE. Endoscopic ultrasound-guided biopsy for the evaluation of pancreatic tumors [Article in Spanish] Gastroenterol Hepatol. 2007;30:597–601. doi: 10.1157/13112588. [DOI] [PubMed] [Google Scholar]
- 14.Khan MA, Grimm IS, Ali B, Nollan R, Tombazzi C, Ismail MK, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh HC, Kang H, Lee JY, Choi GJ, Choi JS. Diagnostic accuracy of 22/25-gauge core needle in endoscopic ultrasound-guided sampling: systematic review and meta-analysis. Korean J Intern Med. 2016;31:1073–83. doi: 10.3904/kjim.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 17.Nagula S, Pourmand K, Aslanian H, Bucobo JC, Gonda TA, Gonzalez S, et al. New York Endoscopic Research Outcomes Group (NYERO) Comparison of endoscopic ultrasound-fine-needle aspiration and endoscopic ultrasound-fine-needle biopsy for solid lesions in a multicenter, randomized trial. Clin Gastroenterol Hepatol. 2018;16:1307–13. doi: 10.1016/j.cgh.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224–32. doi: 10.1007/s00534-006-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchini C, Capelli P, Scarpa A. Pancreatic ductal adenocarcinoma and its variants. Surg Pathol Clin. 2016;9:547–60. doi: 10.1016/j.path.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 21.De La Cruz MS, Young AP, Ruffin MT. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89:626–32. [PubMed] [Google Scholar]
- 22.Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Iwai T, Kida M, Yamauchi H, Okuwaki K, Imaizumi H, et al. Analysis of the diagnostic yield of endoscopic ultrasonography-guided fine-needle aspiration in patients with a suspected pancreatic malignancy. Rev Esp Enferm Dig. 2018;110:544–50. doi: 10.17235/reed.2018.5455/2017. [DOI] [PubMed] [Google Scholar]
- 24.Tian G, Bao H, Li J, Jiang T. Systematic review and meta-analysis of diagnostic accuracy of endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) using 22-gauge and 25-gauge needles for pancreatic masses. Med Sci Monit. 2018;24:8333–41. doi: 10.12659/MSM.911405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: A meta-analysis. Medicine (Baltimore) 2017;96:e7452. doi: 10.1097/MD.0000000000007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuide M, Ryozawa S, Fujita A, Ogawa T, Katsuda H, Suzuki M, et al. Complications of endoscopic ultrasound-guided fine needle aspiration: a narrative review. Diagnostics (Basel) 2020;10:964. doi: 10.3390/diagnostics10110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–90. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, Domínguez-Muñoz JE. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451–7. doi: 10.3748/wjg.v20.i28.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–7. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Nakai Y, Isayama H, Chang KJ, Yamamoto N, Hamada T, Uchino R, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]


