Abstract
OBJECTIVE
Coronavirus disease-2019 (COVID-19) disease can cause asymptomatic and mild flu-like symptoms as well as severe symptoms ranging from respiratory failure and death. Growth hormone (GH) is produced in the anterior pituitary and plays an important role in the immune system. COVID-19 is severe in the elderly, men, obese, diabetics, and people with immune deficiency. The probability of GH deficiency is high in these patient groups. In this study, we aimed to investigate the relationship between the severity of COVID-19 infection and GH level.
METHODS
A total of 456 patients, between 45 and 80-years-old, who were hospitalized with the diagnosis of COVID-19 disease were evaluated in the study. Our study was a retrospective study. Demographic data of the patients, GH, insulin-like growth factor-I (IGF-1), and biochemical parameters and thorax tomography results were evaluated. Patients with chronic diseases that would affect GH levels and those in need of intensive care were excluded from the study.
RESULTS
456 patients were included in the study, 168 female, 288 male, mean age 67.57±12.60 years. Patients were divided into two groups according to thorax tomography findings, patients with lung involvement in Group-1:352 (77%) and those without pulmonary involvement in Group-2:104 (23%). While the GH of Group-1 was 0.125 ng/ml, the GH of Group-2 was 0.238 ng/ml, the difference between them was statistically significant (p=0.000). IGF-1 in Group-1 was: 55.05 ng/ml, while IGF-1 in Group-2 was: 104.08 ng/ml, the difference between them was statistically significant (p=0.000). In multivariate regression analysis, low IGF-1 (p=<0,01, OR:1,06 [1028–1093]) level was found to be significantly effective in lung involvement in COVID-19 disease.
CONCLUSION
In our study, we found GH and IGF-1 deficiency in COVID-19 cases with lung involvement, regardless of age and gender. We can say that COVID-19 infection progresses worse in GH and IGF-1 deficiency.
Keywords: COVID-19, growth hormone, IGF-1, lung involvement
Highlight key points
GH and IGF-1 levels of patients with lung involvement were lower than patients without lung involvement.
Lung involvement and IGF-1 deficiency had a significant effect on COVID-19 infection, while age, gender and glucose had no effect.
Growth hormone (GH) is produced in the anterior pituitary and shows its effect through insulin-like growth factor-I (IGF-I) [1]. GH and IGF1, the most important mediator of GH effects, apart their role in growth regulation during childhood, coordinate several processes throughout adult life [2]. GH stimulates protein synthesis, increases lipolysis, and potentiates the action of insulin [3]. GH and IGF-1 have an important role in the immune system. Both GH and IGF-1 stimulate the development and survival of antigen-sensitive clones of B and T cells [4]. GH has negative feedback effects on many pro-inflammatory cytokines [5].
Coronavirus disease-2019 (COVID-19) has been causing a rapidly spreading pandemic all over the world since December 2019. COVID-19 affects primarily the respiratory tract, but may also cause multi-organ dysfunction due to the widespread presence of angiotensin-converting enzyme-2 receptors, an entry point for the virus [6]. COVID-19 affects many secondary endocrine organs (thyroid, pancreas, adrenals, and gonads) are reported to be affected [7]. COVID-19 can cause asymptomatic and flu-like mild symptoms as well as severe symptoms that progress to respiratory failure and death [8]. It has a more severe course especially in the elderly, men, obese, diabetic individuals, and individuals with immune deficiency. The probability of GH deficiency is high in these patient groups. In this study, we aimed to investigate the relationship between the severity of COVID-19 infection and GH level.
MATERIALS AND METHODS
All patients hospitalized with the diagnosis of COVID-19 (verified with polymerase chain reaction; PCR) disease between June 2020 and December 2020 were evaluated restrospectively in the study. Patients with poorly controlled diabetes, patients with liver failure and renal failure, patients with a history of pituitary disease, patients treated for GH deficiency, patients who have received previous steroid therapy, and patients in need of intensive care were excluded from the study. A total of 456 patients, 168 women and 288 men, were included in the study. The patients were between the ages of 45 and 80, with a mean age of 67.57±12.60. Age, sex, weight, height, laboratory values (glucose, glycosylated hemoglobin [HbA1c], alanine amino transferase [ALT], aspartate amino transferase [AST], blood urea nitrogen [BUN], creatinine [Cr], estimated glomerular filtration rate [eGFR], c reactive protein [CRP], GH, IGF-1), and thorax tomography results were evaluated. Laboratory samples were taken at 08 a.m. on the day of hospitalization, before the start of COVID-19 treatment. GH and IGF1 were measured with the Immulite 2000 hGH and IGF1 Assays and Analyzer, Siemens Healthcare, Germany. We also normalized IGF-1 values by age/gender and expressed them as standard deviation scores. ARCHITECT 16000 integrated system (Abbott, Illinois, USA) was used for glucose, HbA1c, ALT, AST, BUN, Cr, CRP. SYSMEX XN-1000 device was used for hemogram. Non-contrast thorax CT scan for all participants was performed using a TOSHIBA AQUILLION 64 and was evaluated by a single radiologist according to the recommendation of the Radiological Society of North America Expert Consensus Document for pulmonary involvement [9]. Common imaging features of greater specificity for COVID-19 pneumonia as typical appearance, non-specific imaging features of COVID-19 as indeterminate appearance, uncommonly or not reported features of COVID-19 as atypical and the absence of findings suggestive of pneumonia were reported as negative for pneumonia. Cases with typical involvement on tomography were included as Group-1, cases without CT involvement were included as Group-2, and cases with indeterminate appearance or atypical involvement on tomography were excluded from the study.
The study was conducted according to the Declaration of Helsinki. Erzurum Regional Training and Research Hospital Ethics Committee approval was obtained (2021/02–39).
Statistical Analysis
SPSS (Version 17.0. Chicago, IL, USA) was used for statistical analyses. Numerical variables with normal distribution were shown as mean±SD. Categorical variables were presented as numbers and percentages. The Mann-Whitney test was used to compare two independent variables that did not show normal distribution. The difference between categorical variables was analyzed using Fisher’s exact test or Chi-square test. The difference between the laboratory results of the Group-1 with Group-2 was evaluated by student t-test. The differences between the CT findings recommended by the Radiological Society of North America Expert Consensus Document classification and GH/IGF-1 were evaluated by ANOVA. The relationship between GH and IGF-1 levels and lung involvement in COVID-19 infection was evaluated by regression analysis. Statistically significant p<0.005.
RESULTS
Hospitalized with the diagnosis of COVID-19 infection, 456 patients were included in the study, 168 female, 288 male, mean age 67.57±12.60. Laboratory data and demographic data are in Table 1. When the female and male patients were compared, age, BMI, lung involvement on thorax tomography, GH, IGF-1, AST, ALT, GFR, creatinine, glucose, and Hba1c, there were no statistical difference between them. The duration of hospitalization and CRP values of male patients were higher than female patients, and the difference between them was statistically significant (Table 2: Comparison of patients by gender).
Table 1.
Demographic and laboratory data of the patients
| Age | 67.57±12.60 |
|---|---|
| Gender | 168 F/288 M |
| BMI | 24.7±3.7 |
| GH (<1 ng/ml) | 0.262±0.315 |
| IGF-1(ng/ml) | 66.33±33.23 |
| AST (7–40 U/L) | 23.68±10.06 |
| ALT (7–40 U/L) | 27.58±14.28 |
| GFR | 80.56±23.05 |
| Creatinine (<1 mg/dl) | 0.95±0.33 |
| Glucose (70–100 mg/dl) | 116.0.4±20.93 |
| HBA1C (<6.5%) | 6.65±1.43 |
| CRP (<5 mg/L) | 81.59±65.42 |
| Hospital stay | 7.94±2.59 |
BMI: Body mass index; GH: Growth hormone; IGF-I: Insulin-like growth factor-I; HbA1c: Glycosylated hemoglobin; ALT: Alanine amino transferase; AST: Aspartate amino transferase; BUN: Blood urea nitrogen; eGFR: Estimated glomerular filtration rate; CRP: C reactive protein.
Table 2.
Comparison of patients by gender
| Female (n=168) | Male (n=288) | p | |
|---|---|---|---|
| Age | 69.07±12.76 | 66.69±12.51 | 0.333 |
| BMI | 24.8±3.5 | 23.6±2.7 | 0.789 |
| GH (<1 ng/ml) | 0.251±0.27 | 0.270±0.33 | 0.762 |
| IGF-1(ng/ml) | 68.20±32.01 | 65.22±34.02 | 0.647 |
| AST (7–40 U/L) | 23.23±9.84 | 25.03±10.89 | 0.604 |
| ALT (7–40 U/L) | 27.30±11.20 | 28.53±21.98 | 0.337 |
| GFR | 77.90±24.70 | 82.32±22.64 | 0.348 |
| Creatinine (<1 mg/dl) | 0.89±0.29 | 0.99±0.35 | 0.685 |
| Glucose (70–100 mg/dl) | 112.57±14.59 | 117.46±14.94 | 0.525 |
| HBA1C (<6.5%) | 6.48±1.44 | 6.73±1.45 | 0.731 |
| CRP (<5 mg/L) | 62.30±60.77 | 92.84±65.81 | 0.016 |
| Hospital stay | 6.62±1.30 | 8.90±2.91 | 0.036 |
BMI: Body mass index; GH: Growth hormone; IGF-I: Insulin-like growth factor-I; HbA1c: Glycosylated hemoglobin; ALT: Alanine amino transferase; AST: Aspartate amino transferase; BUN: Blood urea nitrogen; eGFR: Estimated glomerular filtration rate; CRP: C reactive protein.
Thorax tomography of the patients was evaluated and divided into two groups. There were 352 (77%) patients in Group-1 and 104 (23%) in Group-2. Group-1 had 132 (37.5%) female, 220 (72.5%) male, Group-2 had 36 (34.6%) female, and 68 (65.4%) male patients and there was no gender difference between the groups. While the GH of Group-1 was 0.125 ng/ml, the patients in Group-2 had a GH of 0.238 ng/ml, the difference was statistically significant (p=0.000 Figure 1: Comparison of Group-1 and Group-2 GH values). While IGF-1 of Group-1 was 55.05 ng/ml, IGF-1 of the patients in Group-2 was: 104.08 ng/ml, the difference was statistically significant (p=0.000, Figure 2 Comparison of Group-1 and Group-2 IGF-1 values). While the CRP of Group-1 was 90.72 mg/L, the CRP of the patients in Group-2 was determined to be 50 mg/L, the difference was statistically significant (p=0.006). When the patients were evaluated in terms of hospitalization days, the mean was 8.8 days for Group-1 and 4.75 days for Group-2, and the difference was statistically significant (p=0.002). There was no statistically significant difference between the two groups when BMI, AST, ALT, GFR, creatinine, glucose, and Hba1c were evaluated (Table 3: Demographic and laboratory data of the groups).
Figure 1.
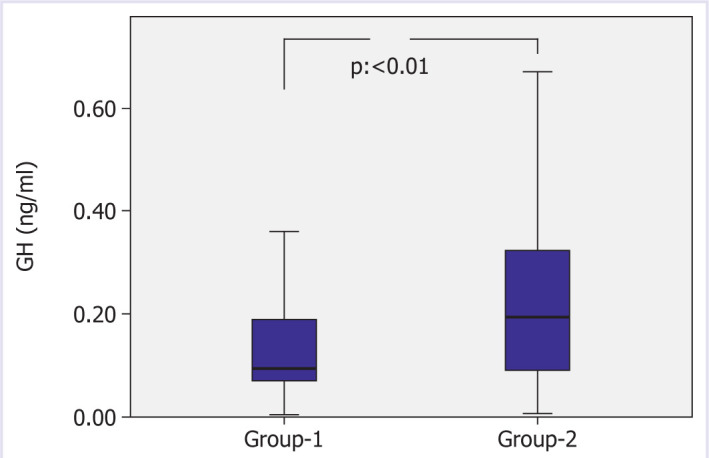
Comparison of Group-1 and Group-2 GH values.
Figure 2.
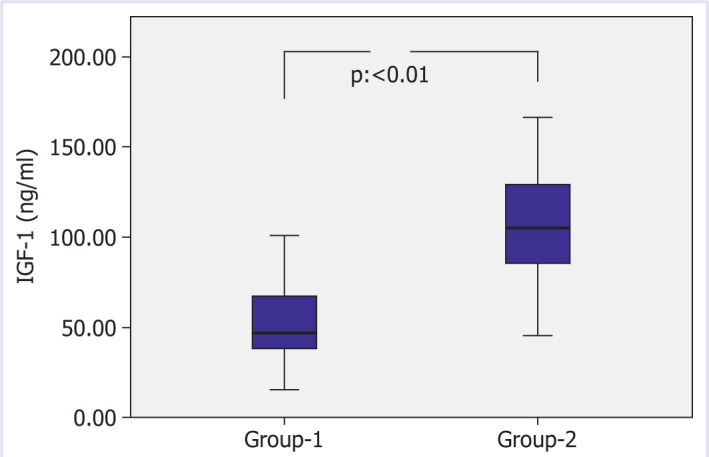
Comparison of Group-1 and Group-2 IGF-1 values.
Table 3.
Demographic and laboratory data of the groups
| Group-1 (n=352) | Group-2 (n=104) | p | |
|---|---|---|---|
| Age | 69.45±11.06 | 65.69±15.38 | 0.261 |
| Gender (%) | 0.789 | ||
| Female | 37.5 | 34.6 | |
| Male | 72.5 | 65.4 | |
| BMI | 24.2±2.75 | 23.8±2.56 | 0.856 |
| GH (<1 ng/ml) | 0.125±0.88 | 0.238±0.194 | 0.000 |
| IGF-1(ng/ml) | 55.05±23.3 | 104.08±31.46 | 0.000 |
| AST (7–40 U/L) | 23.83±9.14 | 23.03±12.89 | 0.715 |
| ALT (7–40 U/L) | 27.30±11.20 | 28.53±21.98 | 0.785 |
| GFR | 80.31±22.70 | 81.42±24.64 | 0.831 |
| Creatinine (<1 mg/dl) | 0.96±0.34 | 0.94±0.28 | 0.841 |
| Glucose (70–100 mg/dl) | 118.57±19.59 | 107.46±14.94 | 0.848 |
| HBA1C (<6.5%) | 6.75±1.46 | 6.24±0.80 | 0.880 |
| CRP (<5 mg/L) | 90.72±62.42 | 50.0±67.09 | 0.006 |
| Hospital stay | 8.80±2.14 | 4.75±1.25 | 0.002 |
BMI: Body mass index; GH: Growth hormone; IGF-I: Insulin-like growth factor-I; HbA1c: Glycosylated hemoglobin; ALT: Alanine amino transferase; AST: Aspartate amino transferase; BUN: Blood urea nitrogen; eGFR: Estimated glomerular filtration rate; CRP: C reactive protein.
In multivariate regression analysis, while IGF-1 (p=<0.01, OR: 1.06 [1028–1093]) level was found to be significantly effective in lung involvement in coronavirus 19 disease, age (p=0.939, OR: 0.997 [0.934–1.065]), crp (p=0.381, OR: 0.994 (0.981–1.008]), and glucose (p=0.619, OR: 0.995 [0.977–1.014]) were not found to be effective in lung involvement in coronavirus 19 disease (Table 4).
Table 4.
Multivariate regression analysis, Nagelkerke R Square: 0.620
| B | OR | %95 CI | p | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| IGF-1 | 0.058 | 1.060 | 1.028 | 1.093 | 0.000 |
| Age | -0.003 | 0.997 | 0.934 | 1.065 | 0.939 |
| CRP | -0.006 | 0.994 | 0.981 | 1.008 | 0.381 |
| Glucose | -0.005 | 0.995 | 0.977 | 1.014 | 0.619 |
OR: Odd ratios; CI: Confidence interval; IGF-I: Insulin-like growth factor-I; CRP: C reactive protein.
DISCUSSION
In our study, we found that GH and IGF-1 levels in patients with lung involvement in COVID-19 were lower than those without lung involvement in COVID-19. In addition, we observed that COVID-19 infection was more severe in GH and IGF-1 deficiency, regardless of age and gender.
COVID-19 disease is a pandemic problem that started in Wuhan, China and spread rapidly all over the world. The COVID-19 pandemic is still spreading rapidly around the world, causing more than 250 million cases and more than five million deaths (worldometer data). It caused the health system to collapse in many countries. Quarantine methods applied to prevent the spread of the disease negatively affected the economies of the country. Studies are ongoing in research centers around the world to create a suitable vaccine or effective medical treatment for the virus, but there is still no effective treatment for COVID-19 infection [10]. COVID-19 infection is more severe in the elderly, men and chronic diseases [11], the possibility of GH deficiency is high in this patient group. GH replacement therapy may be a promising agent in the treatment of COVID-19 infection.
COVID-19 infection is more common and more severe in men.
According to Chinese data, mortality rates are 36% in female patients and 64% in male patients [12]. According to European data, the rate of COVID-19 infection is 71% in male and 29% in female [13]. In our study, 168 (37%) of the patients were female and 288 (63%) were male. When we compared female and male, the CRP value and the number of days of hospitalization were higher in male, and the difference was statistically significant (Table 3). Consistent with the literature, we can say that COVID-19 infection is more common and more severe course in male patients.
Although IGF-1 levels are similar in adult males and females, GH secretion is higher in adult females than males [14]. Sex steroids in women increase GH secretion, GH receptor expression and IGF-1 synthesis in target tissue [14]. While the normal for GH levels is 0.4–10 ng/ml for adult men, it is 1–14 ng/ml for adult women [15]. Normal aging is associated with a gradual decline in serum GH and the decline is more rapid in men than in women [4]. In our study, IGF-1 level was higher in females than males, but there was no statistically significant difference and GH levels were similar in males and females.
GH plays an important role in the immune system, it stimulates the development of T cells by stimulating the thymus gland [16, 17]. GH stimulates the proliferation of T and B lymphocytes, the production of immunoglobulin, the development of myeloid precursor cells and their response to cytokine [18]. GH and IGF-1 stimulate the maturation of myeloid cells, stimulate phagocyte migration, increase phagocyte production of superoxide anions and cytokines, and may protect against bacterial infections. GH has negative feedback effects on many proinflammatory cytokines. In adults with GH deficiency, CRP, Interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-alpha) levels increase and decrease after GH replacement [5]. CRP level is low in patients with acromegaly, CRP increases with the treatment of acromegaly [19]. GH suppresses serum cytokine levels directly and indirectly. Estrogen inhibits receptor signals of cytokines such as IL-6, leptin and prolactin. CRP, IL-6, and TNF-alpha levels increase in both GH and estrogen deficiency [20]. IL-6 level rises in severe COVID-19 patients and as IL 6 level increases, mortality rate increases in COVID-19 patients. In our study, patients who are followed up with a diagnosis of COVID-19 infection, mean GH: 0.262 ng/ml (0–3) and IGF-1: 66.33 ng/ml (51–187) were in the normal range when evaluated by age. Pulmonary involvement in COVID-19 disease is important in both diagnosis and follow-up, as the degree of lung involvement increases, the prognosis is negatively affected [8, 21]. In our study, we found that GH and IGF-1 levels of patients with lung involvement were lower than patients without lung involvement, the difference was statistically significant (Fig. 1, 2). Patients with lung involvement had higher CRP levels and had a longer hospital stay. In the multivariate regression analysis, we found that lung involvement and IGF-1 deficiency had a significant effect on COVID-19 infection, while age, gender, and glucose had no effect. In GH and IGF-1 deficiency, we can say that COVID-19 infection progresses more severely regardless of age and gender.
The severity and mortality of COVID-19 infection increase in patients with diabetes mellitus [22]. In patients with poorly controlled diabetes, there is GH resistance in the liver due to portal insulin insufficiency. There is a decrease in the production of hepatic IGF-1 in GH resistance. Total and free IGF-1 levels decrease during insulin insufficiency [23]. In our study, although patients with poorly controlled diabetes were not included, fasting blood glucose and HbA1c values were higher in COVID-19 patients with pulmonary involvement compared to those without pulmonary involvement, but there was no statistical significance.
As a result, GH is important in the immune system, its deficiency can cause serious viral and bacterial infections. Although there are articles [10, 24–26] in the literature on GH and COVID-19 infection, our study is the first study to investigate the relationship between lung involvement and GH/IGF-1 in COVID 19 disease. In our study, we found GH and IGF-1 deficiency in COVID-19 infection with lung involvement.
Footnotes
Cite this article as: Kartal Baykan E, Baykan AR, Utlu M, Deve E, Yildiz F, Birdal C, et al. Growth hormone level in COVID-19 patients. North Clin Istanb 2022;9(5):470–475.
Ethics Committee Approval
The Erzurum Regional Training and Research Hospital Ethics Committee granted approval for this study (date: 18.01.2021, number: 2021/02-39).
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure
The authors declared that this study has received no financial support.
Authorship Contributions
Concept – EKB, ARB; Design – EKB; Supervision – KA; Materials – EKB; Data collection and/or processing – MU, ED, FY, CB, MHA; Analysis and/or interpretation – EKB, ARB; Literature review – EKB; Writing – EKB; Critical review – KA.
References
- 1.Rosenfeld RG, Hwa V. Biology of the somatotroph axis (after the pituitary) Ann Endocrinol (Paris) 2017;78:80–2. doi: 10.1016/j.ando.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijayakumar A, Yakar S, Leroith D. The intricate role of growth hormone in metabolism. Front Endocrinol (Lausanne) 2011;2:32. doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergan-Roller HE, Sheridan MA. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen Comp Endocrinol. 2018;258:119–33. doi: 10.1016/j.ygcen.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Garcia JM, Merriam GR, Kargi AY. Growth Hormone in Aging. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. Endotext [Internet] South Dartmouth (MA): MDText.com, Inc; 2000. [Google Scholar]
- 5.Serri O, St-Jacques P, Sartippour M, Renier G. Alterations of monocyte function in patients with growth hormone (GH) deficiency: effect of substitutive GH therapy. J Clin Endocrinol Metab. 1999;84:58–63. doi: 10.1210/jcem.84.1.5374. [DOI] [PubMed] [Google Scholar]
- 6.Elijah IE, Branski LK, Finnerty CC, Herndon DN. The GH/IGF-1 system in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:759–67. doi: 10.1016/j.beem.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundholm MD, Poku C, Emanuele N, Emanuele MA, Lopez N. SARS-CoV-2 (COVID-19) and the endocrine system. J Endocr Soc. 2020;4:bvaa144. doi: 10.1210/jendso/bvaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA-Secondary Publication. J Thorac Imaging. 2020;35:219–27. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkarow MH, Hamdy A. A suggested role of human growth hormone in control of the COVID-19 Pandemic. Front Endocrinol (Lausanne) 2020;11:569633. doi: 10.3389/fendo.2020.569633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–77. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 13.Bwire GM. Coronavirus: Why men are more vulnerable to Covid-19 than women? SN Compr Clin Med. 2020;2:874–6. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannoulis MG, Boroujerdi MA, Powrie J, Dall R, Napoli R, Ehrnborg C, et al. GH-2000 Study Group Gender differences in growth hormone response to exercise before and after rhGH administration and the effect of rhGH on the hormone profile of fit normal adults. Clin Endocrinol (Oxf) 2005;62:315–22. doi: 10.1111/j.1365-2265.2005.02216.x. [DOI] [PubMed] [Google Scholar]
- 15.Chernecky C, Berger B. 6th ed. St Louis: Saundres; 2013. Laboratory Tests and Diagnostic Procedures; pp. 808–9. [Google Scholar]
- 16.Tang MW, Garcia S, Gerlag DM, Tak PP, Reedquist KA. Insight into the endocrine system and the immune system: a review of the inflammatory role of prolactin in rheumatoid arthritis and psoriatic arthritis. Front Immunol. 2017;8:720. doi: 10.3389/fimmu.2017.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–91. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 18.Meazza C, Pagani S, Travaglino P, Bozzola M. Effect of growth hormone (GH) on the immune system. Pediatr Endocrinol Rev. 2004;1(Suppl 3):490–5. [PubMed] [Google Scholar]
- 19.Sesmilo G, Fairfield WP, Katznelson L, Pulaski K, Freda PU, Bonert V, et al. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J Clin Endocrinol Metab. 2002;87:1692–9. doi: 10.1210/jcem.87.4.8364. [DOI] [PubMed] [Google Scholar]
- 20.Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CK, Low TH, et al. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci U S A. 2003;100:1016–21. doi: 10.1073/pnas.0337600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Jin C, Wu CC, Liang T, Zhao H, Wang Y, et al. Association between Initial Chest CT or clinical features and clinical course in patients with coronavirus disease 2019 pneumonia. Korean J Radiol. 2020;21:736–45. doi: 10.3348/kjr.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia-A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunger DB. Insulin and insulin-like growth factors in diabetes mellitus. Arch Dis Child. 1995;72:469–71. doi: 10.1136/adc.72.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubrano C, Masi D, Risi R, Balena A, Watanabe M, Mariani S, et al. Is growth hormone insufficiency the missing link between obesity, male gender, age, and COVID-19 severity? Obesity (Silver Spring) 2020;28:2038–9. doi: 10.1002/oby.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuen KCJ. Growth hormone deficiency, acromegaly and COVID-19: Transitioning from media reports to knowledge and a growth hormone hypothesis. Growth Horm IGF Res. 2021;56:101363. doi: 10.1016/j.ghir.2020.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ilias I, Diamantopoulos A, Botoula E, Athanasiou N, Zacharis A, Tsipilis S, et al. Covid-19 and growth hormone/insulin-like growth factor 1: study in critically and non-critically ill patients. Front Endocrinol (Lausanne) 2021;12:644055. doi: 10.3389/fendo.2021.644055. [DOI] [PMC free article] [PubMed] [Google Scholar]


