Summary
Background
The aim of the study is to estimate the incidence of pancreatic cancer among individuals with new-onset type 2 Diabetes (T2DM) and evaluate the relationship of pancreatic cancer risk with age at diabetes onset and diabetes duration.
Methods
This longitudinal cohort study included 428,362 new-onset T2DM patients in Shanghai and Mendelian randomization (MR) in the east-Asian population were used to investigate the association. Incidence rates of pancreatic cancer in all patients and by subgroups were calculated and compared to the general population.
Findings
A total of 1056 incident pancreatic cancer cases were identified during eight consecutive years of follow-up. The overall pancreatic cancer annual incidence rate was 55·28/100,000 person years in T2DM patients, higher than that in the general population, with a standardized incidence ratio (SIR) of 1·54 (95% confidence interval [CI], 1·45–1·64). The incidence of pancreatic cancer increased with age and a significantly higher incidence was observed in the older groups with T2DM. However, the relative pancreatic cancer risk was inversely related to age of T2DM onset, and a higher SIR of 5·73 (95%CI, 4·49–7·22) was observed in the 20–54 years old group. The risk of pancreatic cancer was elevated at any diabetes duration. Fasting blood glucose ≥10·0 mmol/L was associated with increased risk of pancreatic cancer. MR analysis indicated a positive association between T2DM and pancreatic cancer risk.
Interpretation
Efforts toward early and close follow-up programs, especially in individuals with young-onset T2DM, and the improvement of glucose control might represent effective strategies for improving the detection and results of treatment of pancreatic cancer.
Funding
Chinese National Natural Science Foundation.
Keywords: Type 2 diabetes mellitus, Pancreatic cancer, Onset age, Diabetes duration, Mendelian randomization
Research in context.
Evidence before this study
We searched PubMed for articles published in English up to December 31, 2021, using the terms “diabetes”, “new-onset diabetes”, “pancreatic cancer”. The association between T2DM and the development of pancreatic cancer has been recognized for more than one century and diabetes is potentially a modifiable risk factor for pancreatic cancer. However, these studies included relatively small sample sizes of diabetes. Furthermore, few studies have investigated age of diabetes onset and diabetes duration in relation to the incidence of pancreatic cancer across a large population. In 2020, The Lancet Oncology, The Lancet Gastroenterology & Hepatology, and EBioMedicine presented a cross-journal series of four reviews highlighting the progress being made in all areas of pancreatic cancer research and emphasized that in order to work together to reduce the burden of pancreatic cancer, effective strategies for improving the detection and results of treatment of pancreatic cancer needed to be explored in new clinical studies.
Added value of this study
The present study is the largest study in China to investigate the association of pancreatic cancer and new-onset T2DM. The key findings can be summarized as three points: (1) Patients with new-onset T2DM have a higher risk of pancreatic cancer in both males and females among Chinese adults when compared to those in the general population, where causality was suggested by MR analysis in an East-Asian population; (2) Our study investigated both the absolute and relative risk of pancreatic cancer across different age groups and found for the first time, to our knowledge, that although the absolute risk of pancreatic cancer increased with age, the relative risk of pancreatic cancer was inversely related to age at onset of T2DM; (3) This is the first report of pancreatic cancer and diabetes duration in a large-scale T2DM study. Patients with T2DM diagnosed more than 5 years and those with higher FBG levels had increased risk of pancreatic cancer.
Implications of all the available evidence
New-onset T2DM had a significantly association with a higher risk of pancreatic cancer among East-Asian adults. Young-onset T2DM had notably higher relative risk of pancreatic cancer. Patients with T2DM diagnosed more than 5 years and with elevated FBG levels had increased risk of pancreatic cancer. Efforts toward early and close follow-up programs, especially in individuals with young-onset T2DM, and the improvement of glucose control might represent effective strategies for improving the detection and results of treatment of pancreatic cancer.
Alt-text: Unlabelled box
Introduction
Pancreatic cancer is one of the leading causes of cancer mortality around the world and in China.1 The prognosis of pancreatic cancer is typically worse than that of most other tumors, and the 5-year survival rate is less than 5%. In 2020, The Lancet Oncology, The Lancet Gastroenterology & Hepatology, and EBioMedicine presented a cross-journal series of four reviews highlighting the progress being made in all areas of pancreatic cancer research and emphasized the need to work together to reduce the burden of pancreatic cancer. Previous studies showed that early diagnosis and treatment can improve the overall prognosis of pancreatic cancer.2,3 However, in the general population, screening of large groups is not considered feasible to detect the disease at its early stage. The key to diagnosis and treatment is to identify the population at high-risk of pancreatic cancer as early as possible.4,5
A number of modifiable risk factors have been identified for pancreatic cancer, including smoking, obesity, and alcohol use, while age and familial cancer syndromes are regarded as nonmodifiable risk factors for the disease. Diabetes is potentially a modifiable risk factor for pancreatic cancer in populations of the Asia-Pacific region.6 The global prevalence of T2DM is growing and the increase of T2DM in Asia is predicted to be higher and faster than other continents. Due to the rapid development of the economy and dramatic change of lifestyle, the prevalence of T2DM was reported to be 12·8% in China, revealing a rapid increase over the past 30 years.7,8 Impaired glucose metabolism is associated with adverse macro- and micro- vascular outcomes, and with a higher incidence of cancers.9,10 The association between T2DM and the development of pancreatic cancer has indeed been recognized for more than one century.11 Convincing evidence has indicated that T2DM is associated with an increased risk for pancreatic cancer and that T2DM can worsen cancer stage and increase cancer-related mortality.12,13
However, few studies have investigated age of diabetes onset and diabetes duration in relation to the incidence of pancreatic cancer across a large population. Further, little is known about the effect of various levels of fasting blood glucose (FBG) on the risk of pancreatic cancer among patients with new-onset T2DM.14 In the present analysis, we studied the association between age of diabetes onset and diabetes duration with pancreatic cancer, investigated the relationship of various levels of FBG to the risk of pancreatic cancer, and used two-sample Mendelian randomization (MR) to investigate additional evidence supporting a causal relationship between type 2 diabetes and pancreatic cancer in a separate east Asian population (Figure 1).
Figure 1.
Association and causal effect of T2DM and pancreatic cancer.
Methods
Study design and population
We performed a longitudinal cohort study to assess the association of type 2 diabetes with the risk of pancreatic cancer. Analysis was performed on data collected from the Shanghai Standardized Diabetes Management System (SSDMS), operated by the Shanghai Municipal Center for Disease Control and Prevention (SCDC). The system was initiated in 2004 and well-established, covering overall 241 community health service centers in 16 districts of Shanghai after the launch of the National Basic Public Health Service Program (NBPHSP) in 2009.15 According to the requirement of the NBPHSP in Shanghai, community health centers (CHCs) are responsible for providing management for T2DM patients and for uploading electronic records to SSDMS. All diagnosed cases of T2DM in Shanghai were required to register in the system, including newly diagnosed cases through community-based screenings or physical examinations and recently diagnosed cases through routine outpatient visits.16 In order to ensure the accuracy and reliability of registration management information, the SCDC organized for the 16 district CDC to randomly select a proportion of patients with type 2 diabetes for quality control in the form of annual telephone and face-to-face investigation. T1DM patients were not included in the system. Baseline information for each case of T2DM in the SSDMS, including height, weight, blood pressure and blood glucose were collected during an initial assessment at registration. Medication use, smoking history, alcohol consumption, and physical activity were not available from all patients in the current system and were not included in the present analysis.
A malignant tumor registration and reporting system was established in Shanghai in 1963. In 2002, according to the requirement of the Shanghai malignant tumor reporting measures, the SCDC was fully responsible for the malignant tumor registry, which established a population-based tumor registration and reporting information system. The malignant tumor registry covered all new cases of malignant tumors, tumor deaths and survival data in Shanghai's residents. In accordance with the requirements of the measures of Shanghai Municipality on the reporting of malignant tumors, all medical institutions in Shanghai report new cases of malignant tumors which were found during outpatient, emergency and inpatient treatment. The medical staff responsible for cancer diagnosis and treatment completes the “Shanghai tumor case report card” following diagnosis to register the information into the malignant tumor registry. The relevant technical standards issued by the International Agency for Research on Cancer (IARC) and the relevant technical requirements issued by the National Office of Cancer Prevention and Treatment were used for tumor registration.17 In order to ensure the integrity of the reported information, the SCDC regularly organized for the 16 district centers to carry out missing report investigations at relevant medical institutions every year. The prevalence of missing reports was found to be 0.5%. The classification of tumor location was coded according to the ICD-10 codes. Pancreatic cancers were defined based on ICD-10 codes C25.
From January 2011 to the end of 2018, a total of 428,568 new-onset T2DM patients had been registered in the SSDMS. The median time from the diagnosis of T2DM to registration was 8.6 months. New cases of pancreatic cancer were identified through the malignant tumor registry system during an 8-year period. The geographic distribution of both registries are for exactly the same regions including all 16 districts of Shanghai. From January 2011, the ID card number was matched between the diabetes management system and the malignant tumor registry system, allowing investigation of the relationship between tumors and diabetes mellitus.
In order to eliminate T2DM caused by islet destruction by pancreatic cancer, we excluded 206 T2DM patients in whom the pancreatic cancer was diagnosed before or at the same time of the confirmation of T2DM (Supplemental Figure 1 and Supplemental Table 1). Patients were censored if they were lost to follow-up, died of other causes, moved out of Shanghai or reached the end of the study.
This study was approved by the Ethical Review Committee of Shanghai Municipal Center for Disease Control and Prevention, and the requirement for informed consent was exempted.
Related definitions
Participants were identified as having T2DM if FBG level ≥ 7·0 mmol/L and/or a 2-hour glucose (after 75 g oral glucose tolerance test) level ≥ 11·1 mmol/L or use of antidiabetic medication according to the WHO criteria.18 The first fasting glucose value when patients registered in the SSDMS was considered in ascertaining the relationship between glucose and pancreatic cancer risk. The following subgroups of FBG were used for certain analyses: <6·0 mmol/L, 6·0–7·9 mmol/L, 8·0–9·9 mmol/L and ≥ 10·0 mmol/L according to the Chinese Expert Consensus on Management of Diabetes in Chinese Adults.19 The patients with T2DM were classified into four age groups: 20–54 years old, 55–64 years old, 65–74 years old and ≥ 75 years old. BMI was calculated as weight (kg) divided by the square of height (m).
Statistical analyses
Baseline characteristics were summarized by pancreatic cancer status and by sex, categorical data were presented as number and percentage, and continuous variables presented as mean and standard deviation. The age-standardized rates (ASRs; per 100,000 person years) were calculated using the direct method, based on the sixth nationwide population census by age groups and age-specific incidence. Person-years (PYs) of follow-up were calculated from the date when T2DM was first diagnosed to the date of diagnosis with pancreatic cancer, the date of loss to follow-up, or the cutoff date of the study, December 31, 2018. The standardized incidence ratio (SIR) was calculated as the ratio between the observed and the expected number of pancreatic cancers in each year. The expected number was defined by multiplying the accumulated PYs of follow-up in each year by cancer incidence rate in the Shanghai tumor registry system. The annual incidence in the general population was calculated by dividing the number of incident pancreatic cancers by the number of adults aged 20 years or older in the Shanghai general population from 2011 to 2015. When we prepared the data until Dec, 31 2018, the Shanghai Cancer Report only updated to 2015. So, we used the general denominator data from 2011 to 2015. The 95% confidence intervals (95% CI) for the SIR were based on the assumption that the number of observed cases followed a Poisson distribution. The overall or group-specific incidence rate of pancreatic cancer was calculated by dividing the number of incident pancreatic cancer cases by person-years of follow-up.
A log-rank test was used to examine the difference in pancreatic cancer incidence rates across age at diagnosis, diabetes duration, FBG and BMI subgroups and Kaplan-Meier survival curves were plotted by FBG category. Cox proportional hazards regression models were applied to assess the relationship of FBG levels to the risk of pancreatic cancer. FBG levels were used as continuous variables and confounding variables including age, sex and SBP were adjusted for in the model. Analyses were undertaken in participants overall and by sex. Statistical analyses were performed using the SAS software (version 9.4; SAS Institute, Inc., Cary, North Carolina). P values < 0·05 were considered statistically significant.
Mendelian randomization
To identify potential causal links between FBG level, T2DM and pancreatic cancer, we performed two-sample MR analyses using the TwoSampleMR (v.0.5.5) and MRPRESSO (v1.0) package.20,21 We used T2DM and FBG (in non-diabetic people) as exposures in our MR. The Asian Genetic Epidemiology Network performed a large genome-wide association study (GWAS) of T2DM in the east Asian population.22 The Meta-Analyses of Glucose and Insulin-related traits Consortium provided Asia-ancestry GWAS summary statistics of FBG level in participants without T2DM.23 The GWAS of pancreatic cancer was extracted from the biobank in east-Asian population, the Biobank of Japan (BBJ).24
For MR analyses, we selected independent single nucleotide polymorphism (SNP) associated with T2DM or FBG at the genome-wide significant level (P<5 × 10−8) and linkage disequilibrium based on r2<0.1. When no shared SNPs were available between exposures and outcome, proxies with r2>0.8 were added. Finally, the remained SNPs were considered as valid instruments variants (IVs) used in MR.
MR causality tests were performed using the inverse-variance weighted method by meta-analyzing the effect of each IV from Wald ratio. We also estimated the causality using additional methods: Weighted median, MR-Egger, and MR-PRESSO. Heterogeneity of the results was assessed using Cochran's Q statistic and leave-one-out analyses. Pleiotropy was assessed using MR-Egger intercept and MR-PRESSO outliers-adjusted test.
Role of the funding source
The study funder had no role in the study design; collection, analysis, and interpretation of data; or report writing. The corresponding authors had full access to the data and have final responsibility for publication.
Results
Baseline characteristics
A total of 428,362 patients (mean age, 63·81±11·10 years) with new-onset T2DM were investigated in this study, including 205,694 males (48·02%) and 222,668 females (51·98%). Baseline characteristics are shown by pancreatic cancer status in Table 1 and by sex in Supplemental Table 2. Compared to males with T2DM, females were older (64·61±10·87 vs. 62·94±11·27 years, P<0·0001) and had lower FBG (7·18±1·61 vs. 7·34±1·81, P<0·0001). Compared to patients without pancreatic cancer, patients with pancreatic cancer were older (69·71±9·93 vs. 63·80±11·09, P<0·0001), had lower BMI (23·80±3·24 vs. 24·43±3·11 kg/m2, P<0·0001) and had higher FBG (7·69±2·46 vs. 7·26±1·71 mmol/L, P<0·0001) respectively. Patients with pancreatic cancer diagnosed within 12 months after the diagnosis of T2DM had lower BMI (23·13±3·08 kg/m2) and higher glucose (8·24±3·13 mmol/L) at baseline than the respective levels in those diagnosed more than 12 months after diagnosis of diabetes (Supplemental Table 3).
Table 1.
Baseline characteristics of individuals with and without pancreatic cancer in the cohort of new-onset T2DM.
| No pancreatic cancer | Pancreatic cancer | P value | |
|---|---|---|---|
| Total, n | 427306 | 1056 | .. |
| Age, years | 63·80 (52·71–74·89) | 69·71 (59·78–79·64) | <0·0001 |
| Age, years (category) | .. | .. | <0·0001 |
| 20–54 | 83742 (19·60) | 72 (6·82) | .. |
| 55–64 | 159443 (37·31) | 296 (28·03) | .. |
| 65–74 | 112435 (26·31) | 324 (30·68) | .. |
| 75– | 71686 (16·78) | 364 (34·47) | .. |
| BMI, Kg/m2 | 24·43 (21·32–27·54) | 23·80 (20·56–27·04) | <0·0001 |
| FPG, mmol/L | 7·26 (5·55–8·97) | 7·69 (5·23–10·15) | <0·0001 |
| FPG, mmol/L (category) | <0·0001 | ||
| <6·0 | 43410 (11·00) | 103 (10·96) | .. |
| 6·0–7·9 | 269591 (68·34) | 572 (60·85) | .. |
| 8·0–9·9 | 57870 (14·67) | 158 (16·81) | .. |
| ≥10 | 23595 (5·98) | 107 (11·38) | .. |
| Male, n | 205155 | 539 | .. |
| Age, years | 62·93 (51·66–74·20) | 68·58 (58·73–78·43) | <0·0001 |
| Age, years (category) | .. | .. | <0·0001 |
| 20–54 | 45117 (21·99) | 47 (8·72) | .. |
| 55–64 | 75886 (36·99) | 159 (29·50) | .. |
| 65–74 | 53664 (26·16) | 174 (32·28) | .. |
| 75– | 30488 (14·86) | 159 (29·50) | .. |
| BMI, Kg/m2 | 24·41 (21·50–27·32) | 23·80 (20·92–26·68) | <0·0001 |
| FPG, mmol/L | 7·34 (5·53–9·15) | 7·77 (5·17–10·37) | <0·0001 |
| FPG, mmol/L (category) | .. | .. | <0·0001 |
| <6·0 | 19944 (10·54) | 58 (12·08) | .. |
| 6·0–7·9 | 127010 (67·09) | 283 (58·96) | .. |
| 8·0–9·9 | 29254 (15·45) | 78 (16·25) | .. |
| ≥10 | 13092 (6·92) | 61 (12·71) | .. |
| Female, n | 222151 | 517 | .. |
| Age, years | 64·60 (53·73–75·47) | 70·88 (60·99–80·77) | <0·0001 |
| Age, years (category) | .. | .. | <0·0001 |
| 20–54 | 38625 (17·39) | 25 (4·84) | .. |
| 55–64 | 83557 (37·61) | 137 (26·50) | .. |
| 65–74 | 58771 (26·46) | 150 (29·01) | .. |
| 75– | 41198 (18·55) | 205 (39·65) | .. |
| BMI, Kg/m2 | 24·45 (21·17–27·73) | 24·23 (20·66–27·80) | <0·0001 |
| FPG, mmol/L | 7·18 (5·58–8·78) | 7·60 (5·29–9·91) | <0·0001 |
| FPG, mmol/L (category) | .. | .. | <0·0001 |
| <6·0 | 23466 (11·44) | 45 (9·78) | .. |
| 6·0–7·9 | 142581 (69·50) | 289 (62·83) | .. |
| 8·0–9·9 | 28616 (13·95) | 80 (17·39) | .. |
| ≥10 | 10503 (5·12) | 46 (10·00) | .. |
The incidence rate of pancreatic cancer
A total of 1,056 incident pancreatic cancer cases were identified during the study period in patients followed from the time of diagnosis of T2DM, with a mean follow-up of 4·5±2·2 years. The crude incidence rates of pancreatic cancer were 55·28 per 100.000 PYs (95%CI, 51·99–58·69) in all T2DM patients, and 59·61 (95%CI, 54·68–64·80) and 51·38 (95%CI, 47·05–55·96) per 100,000 PYs in males and females, respectively. Overall, the ASR of pancreatic cancer in patients with T2DM was 20·75/100,000 PYs (95%CI, 18·95–22·54). The SIRs of pancreatic cancer in T2DM vs general population were 1·54 (95%CI, 1·45–1·64) in all T2DM patients, 1·54 (95%CI, 1·41–1·67) in males and 1·57(95%CI, 1·44–1·71) in females (Table 2).
Table 2.
Incidence of pancreatic cancer in general population of Shanghai and the cohort of new-onset T2DM across sex and age at onset of diabetes groups.
| General population |
DM population |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population at risk | Number of pancreatic cancer cases | Annual incidence per 100,000 person years |
Population at risk | Number of pancreatic cancer cases | Annual incidence per 100,000 person years |
Standardized incidence ratios |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |||||
| Total, year ages | 62639100 | 13119 | 20·94 | 20·59–21·31 | 428362 | 1056 | 55·28 | 51·99–58·69 | 1·54 | 1·45–1·64 |
| 20–54 | 35565903 | 1093 | 3·07 | 2·89–3·26 | 83814 | 72 | 17·62 | 13·79–22·05 | 5·73 | 4·49–7·22 |
| 55–64 | 14405920 | 3078 | 21·37 | 20·62–22·13 | 159739 | 296 | 40·76 | 36·25–45·61 | 1·91 | 1·70–2·14 |
| 65–74 | 6692647 | 3145 | 46·99 | 45·36–48·66 | 112759 | 324 | 68·45 | 61·20–76·1 | 1·46 | 1·30–1·62 |
| 75– | 5974630 | 5803 | 97·13 | 94·64–99·66 | 72050 | 364 | 120·45 | 108·39–133·31 | 1·24 | 1·12–1·37 |
| Male, year ages | 31034169 | 7070 | 22·78 | 22·25–23·32 | 205694 | 539 | 59·61 | 54·68–64·80 | 1·54 | 1·41–1·67 |
| 20–54 | 17970992 | 730 | 4·06 | 3·77–4·37 | 45164 | 47 | 21·75 | 15·98–28·66 | 5·36 | 3·93–7·12 |
| 55–64 | 7192641 | 1913 | 26·60 | 25·42–27·82 | 76045 | 159 | 46·77 | 39·78–54·47 | 1·76 | 1·50–2·05 |
| 65–74 | 3354683 | 1840 | 54·85 | 52·37–57·41 | 53838 | 174 | 78·14 | 66·96–90·41 | 1·42 | 1·22–1·65 |
| 75– | 2515853 | 2587 | 102·83 | 98·90–106·87 | 30647 | 159 | 126·68 | 107·75–147·54 | 1·23 | 1·05–1·44 |
| Female, year ages | 31604931 | 6049 | 19·14 | 18·66–19·63 | 222668 | 517 | 51·38 | 47·05–55·6 | 1·57 | 1·44–1·71 |
| 20–54 | 17594911 | 363 | 2·06 | 1·86–2·29 | 38650 | 25 | 12·98 | 8·40–18·85 | 6·29 | 4·07–9·29 |
| 55–64 | 7213279 | 1165 | 16·15 | 15·24–17·11 | 83694 | 137 | 35·47 | 29·78–41·80 | 2·20 | 1·84–2·60 |
| 65–74 | 3337964 | 1305 | 39·10 | 37·00–41·28 | 58921 | 150 | 59·84 | 50·64–70·00 | 1·53 | 1·30–1·80 |
| 75– | 3458777 | 3216 | 92·98 | 89·79–96·25 | 41403 | 205 | 116·03 | 100·69–132·74 | 1·25 | 1·08–1·43 |
The absolute and relative risk of pancreatic cancer across different ages at onset
The annual incidence of pancreatic cancer increased from 17·62 per 100,000 PYs in those with age of diagnosis of T2DM 20–54 years to 40·76 in patients aged 55–64 years, 68·45 in those aged 65–74 years and 120·45 per 100,000 PYs in patients ≥75 years old. A significantly higher incidence of pancreatic cancer was observed in the older groups with T2DM. The relative pancreatic cancer risk was presented as the SIR, which was inversely related to age at onset of T2DM. A significantly higher SIR of 5·73 (95%CI, 4·49–7·22) was observed in those with age of diagnosis of T2DM 20–54 years, while SIRs were 1·91(95%CI, 1·70–2·14) in patients aged 55–64 years, 1·46 (95%CI, 1·30–1·62) in those aged 65–74 years and 1·24(95%CI, 1·12–1·37) in those new-onset T2DM group aged ≥75 years old. There were similar patterns in males and females analyzed separately (Table 2 and Figure 2). After excluding patients in whom the pancreatic cancer was diagnosed before or within six months after confirmation of T2DM, the incidence of pancreatic cancer in the cohort across sex and age groups presented similar patterns as shown in Supplemental Table 6.
Figure 2.
The absolute and relative risk of pancreatic cancer with new-onset T2DM patients across different age groups (A: all T2DM patients; B: males; C: females).
The association of diabetes duration and the risk of pancreatic cancer
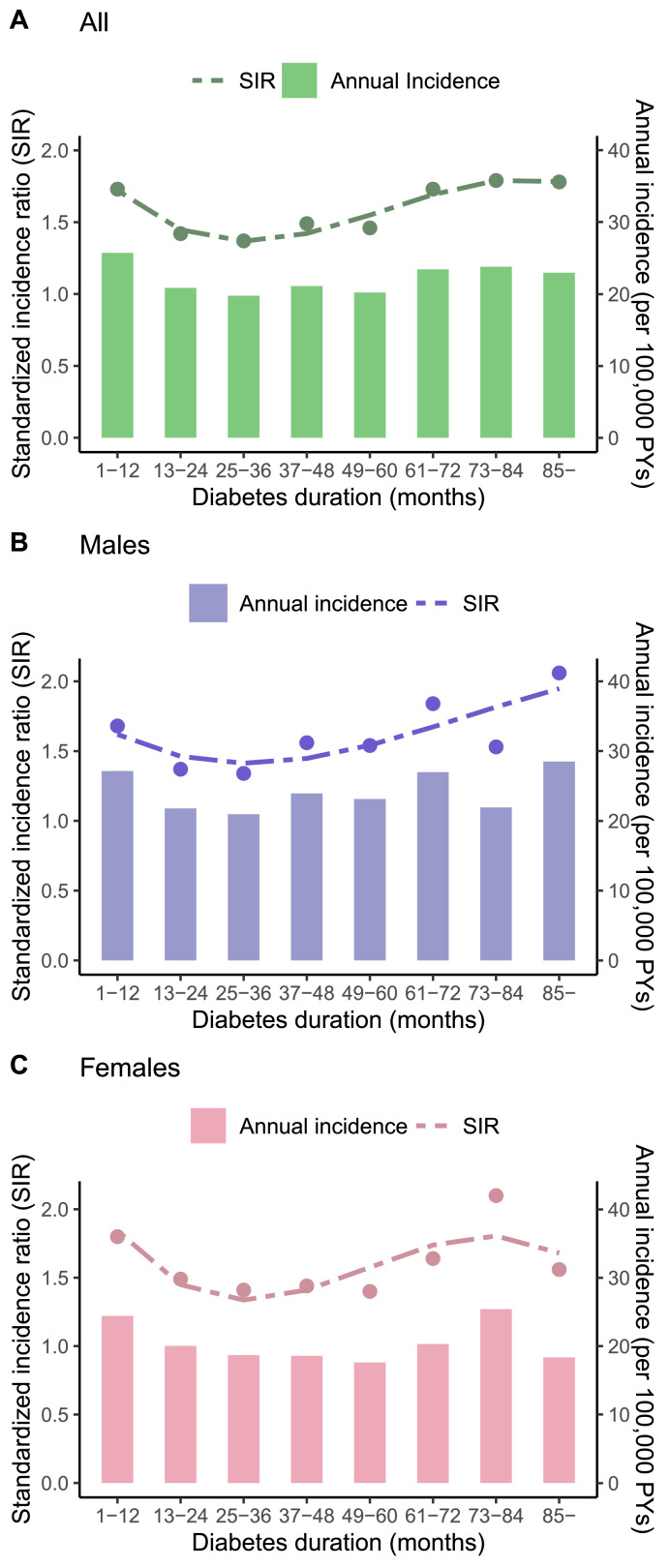
We investigated the association of pancreatic cancer and T2DM duration by every12 months. As shown in Figure 3 and Supplemental Table 4, new-onset T2DM was significantly associated with elevated risk of pancreatic cancer in all eight years follow-up, with the higher crude rate and SIR among T2DM diagnosed within ≤ 1 year (64·31; 1·73), within the 6-year (58·55;1·73), the 7-year (59·47; 1·79) and more than 7 years (57·42; 1·78). Similar patterns were seen in males and females analyzed separately.
Figure 3.
The absolute and relative risk of pancreatic cancer with new-onset T2DM patients across different diabetes durations groups (A: all T2DM patients; B: males; C: females).
The association of FBG levels and the risk of pancreatic cancer
We studied the relationship of various FBG levels to the risk of pancreatic cancer among patients with new-onset T2DM. In categorical analysis, the group with FBG ≥ 10·0 mmol/L has significantly higher pancreatic cancer risk than that of the groups with lower FBG. The hazard ratio for pancreatic cancer among patients with FBG ≥ 10·0 mmol/L was 2·35 (95% CI; 1·77–3·13) compared to those with FBG <6·0 mmol/L (Table 3 and Supplemental Figure 2). After adjustment for sex, age, BMI, and SBP, spline curves demonstrated a log linear relationship between FBG and the risk of pancreatic cancer in all subjects. The similar association pattern was seen in males and females after adjustment for age, BMI, and SBP (Table 3 and Supplemental Figure 3, P<0·0001). After excluding patients in whom pancreatic cancer was diagnosed before or within six months after confirmation of T2DM, the association of pancreatic cancer and FBG levels in patients with new-onset T2DM showed similar patterns (Supplemental Table 7).
Table 3.
Pancreatic cancer in relation to FBG levels in patients with new-onset T2DM.
| No. of cases | No. at risk | Rate/100,000 person years | Adjusted HR (95% CI)* | |
|---|---|---|---|---|
| Total | ||||
| <6·0 | 103 | 43513 | 52·70 | 1 (ref) |
| 6·0–7·9 | 572 | 270163 | 47·40 | 0·90 (0·73–1·12) |
| 8·0–9·9 | 158 | 58028 | 62·47 | 1·29 (0·99–1·66) |
| ≥10 | 107 | 23702 | 107·01 | 2·35 (1·77–3·13) |
| Male | ||||
| <6·0 | 58 | 20002 | 65·30 | 1 (ref) |
| 6·0–7·9 | 283 | 127293 | 50·43 | 0·82 (0·61–1·11) |
| 8·0–9·9 | 78 | 29332 | 62·03 | 1·13 (0·79–1·62) |
| ≥10 | 61 | 13153 | 112·36 | 2·25 (1·54–3·29) |
| Female | ||||
| <6·0 | 45 | 23511 | 42·21 | 1 (ref) |
| 6·0–7·9 | 289 | 142870 | 44·76 | 0·99 (0·72–1·37) |
| 8·0–9·9 | 80 | 28696 | 62·91 | 1·46 (1·00–2·13) |
| ≥10 | 46 | 10549 | 100·67 | 2·41 (1·56–3·72) |
*Adjusted for age, sex, BMI and SBP in all subjects and adjusted for age, BMI and SBP in males and females.
The association of combined age at onset and FBG levels with the risk of pancreatic cancer
We assessed the effects on the risk of pancreatic cancer among patients stratified according to four age at onset categories and two FBG categories combined. The highest incidence of pancreatic cancer (250·25 per 100.000 PYs) was observed in those aged ≥75 years old having FBG ≥ 10·0 mmol/L. The highest SIR of 16·73 (95%CI, 9·37–27·59) was observed in those with age of diagnosis of T2DM 20–54 years having FBG ≥ 10·0 mmol/L (Supplemental Tables 5).
Causal relationship between T2DM and pancreatic cancer
T2DM is suggested to be associated with a 24% increase of pancreatic cancer risk (OR: 1.24, 95% CI: 1.09–1.41, p = 0.001) in the east Asian population. However, increased FBG level in the normal range is not causally associated with pancreatic cancer risk (p= 0.577). Sensitivity analyses from Weighted median, MR-Egger and MR-PRESSO methods are similar to the primary results (Figure 4).
Figure 4.
MR analysis shows the causal effect of T2DM and FBP on pancreatic cancer in an east-Asian population.
Discussion
In the present study, we collected information on new-onset T2DM and pancreatic cancer over eight consecutive years. The key findings can be summarized as three points: (1) Patients with new-onset T2DM have a higher risk of pancreatic cancer in both males and females among Chinese adults when compared to those in the general population, where causality was suggested by MR analysis in an east-Asian population; (2) Our study investigated both the absolute and relative risk of pancreatic cancer across different age groups and found for the first time, to our knowledge, that although the absolute risk of pancreatic cancer increased with age, the relative risk of pancreatic cancer was inversely related to age at onset of T2DM, with a significantly higher SIR of 5·73 observed in those aged 20–54 years at onset of T2DM; (3) We firstly reported the risk of pancreatic cancer and overall diabetes duration in a large-scale T2DM study. Patients with diabetes diagnosed more than 5 years and those with higher FBG levels had increased risk of pancreatic cancer, with the risk of 2·35 times among those with FBG ≥ 10·0 mmol/L compared to those with FBG < 6·0 mmol/L.
The association between T2DM and the development of pancreatic cancer has been investigated for more than one century.11,25,26 However, the observational results varied across different races and regions. In a US cohort from female participants in the Nurses' Health Study and male participants in the Health Professionals Follow-Up Study, recent-onset diabetes had 2·97-fold increased risk of pancreatic cancer and long-standing diabetes had 2·16 -fold increased risk of pancreatic cancer when compared with no diabetes.27 A recent meta-analysis of 26 case-control studies in China involving 7,702 pancreatic cancer cases and 10,186 controls produced pooled results showing that patients with T2DM had an overall 3·69-fold (95% CI; 3·12–4·37) increased risk of pancreatic cancer compared to the risk in the general population.28 The present study was consistent with the studies mentioned above and identified positive association between T2DM and pancreatic cancer where we compared the absolute and relative incidence of pancreatic cancer in T2DM with the general population. MR analysis further established the potential causality under the positive association between T2DM and pancreatic risk, especially in the east-Asian patients. To the best of our knowledge, this study was the first to investigate both the absolute and relative pancreatic cancer risk across different age groups. Similar to findings shown in a US cohort,27 the absolute pancreatic cancer risk increased with age in patients with T2DM in the present study. However, the SIRs of pancreatic cancer were inversely related to age at onset of T2DM and a significantly higher SIR of 5·73 was observed in those with onset between 20 and 54 years of age. Cancer trends in these young patients with early onset of T2DM reflect recent changes,29 which could foreshadow the future overall disease burden. Young-onset T2DM might be an important risk factor for pancreatic cancer and this suggests that close follow-up program for pancreatic cancer should be implemented in this group with early onset of T2DM.
In the cohort of persons with T2DM observed from shortly after the time of diagnosis, there is increased risk of pancreatic cancer during the initial year and then again subsequent to more than 5 years after diagnosis. In a meta-analysis with thirty-five cohort studies, the highest risk of pancreatic cancer was found among patients diagnosed within less than 1 year.30 In our large-scale study, we found T2DM patients in the first 1-year duration had lower body mass index and higher glucose levels compared to those in other groups. The relationship between T2DM and pancreatic cancer are complex,31 while diabetes of ≤ 1 year duration is associated with pancreatic cancer, this could be an early manifestation of pancreatic cancer. In a retrospective cohort study using the UK General Practice Research Database, the incidence of pancreatic cancer was highest in patients with ≥ 5 years duration of T2DM.32 In our study, we also found T2DM patients diagnosed more than 5 years had the higher risk. Individuals with a long duration of T2DM also have a significantly increased risk of pancreatic cancer, which supports the likelihood of a causal relationship between diabetes and pancreatic cancer. To the best of our knowledge, this is the first report between the risk of pancreatic cancer and diabetes duration in a large-scale T2DM study.
Prospective studies have indicated that elevated blood glucose levels may be linked with increased cancer risk, but the strength of the association is unclear. We found that the risk of pancreatic cancer among patients with FBG ≥ 10·0 mmol/L was 2·35 (95% CI; 1·77-3·13) times greater than in all T2DM patients, 2·25 (95% CI; 1·54–3·29) times in males, and 2·41 (95% CI; 1·56–3·72) times in females respectively, compared to that among those with FBG <6·0 mmol/L. Thus, along with T2DM diagnosed more than 5 years, higher FBG should be considered a risk marker for pancreatic cancer. After assessing the association between FBG categories and risk of pancreatic cancer, we continued to analyze the risk by FBG categories and age at onset categories combined. As expected, we found that the highest incidence of pancreatic cancer (250·25 per 100.000 PYs) was observed in those aged ≥75 years old having FBG ≥ 10·0 mmol/L. The highest SIR of 16·73 (95%CI, 9·37–27·59) was observed in those with age of diagnosis of T2DM 20–54 years having FBG ≥ 10·0 mmol/L. The MR analysis showed increased FBG in the normal range might not increase the risk of pancreatic cancer (p = 0.577). However, when the FBG approached pathological level, which was diagnosed as T2DM, it could significantly worsen the status of pancreas neoplasm (p = 0.001).
This stronger association with highest glucose levels held up over many years and this association reflects a greater severity of metabolic syndrome which would be expected to have some dose relationship with pancreatic cancer risk.14 Metabolic syndrome and its components have long been investigated as risk factors for pancreatic cancer. Recently, Park et al investigated the association between metabolic syndrome and pancreatic cancer risk in a nationwide Korean cohort from the Korean National Health Insurance System.33 In this study, recovery from metabolic syndrome was associated with a reduced risk of pancreatic cancer compared with persistent metabolic syndrome, suggesting that pancreatic cancer risk can be altered by changes in metabolic syndrome status. No significant differences by sex or obesity status were observed. The findings from this study highlight the importance of considering both the presence and the changes in the metabolic syndrome when evaluating pancreatic cancer risk. We have attempted to investigate some of these potential mechanisms via multivariate analysis adjusting for age, SBP and BMI, such that FBG ≥10·0 mmol/L continued to be significantly associated with a higher risk of pancreatic cancer than FBG <6·0 mmol/L. The relationship of pancreatic cancer and poor glucose control in T2DM has important implications. Efforts toward early detection and the improvement of glucose metabolism might represent an effective strategy to slow the increasing trend of pancreatic cancer.
The relationship of pancreatic cancer to long diabetes duration and poor glucose control in T2DM has important implications. Efforts toward early detection and the improvement of glucose metabolism might represent an effective strategy to slow the increasing trend of pancreatic cancer. Antidiabetic drugs have been found to have various effects on pancreatic cancer in previous studies. Use of metformin was associated with a significantly decreased risk, while long-term insulin use among patients was associated with a moderately higher risk of pancreatic cancer.34 Present data do not suggest any association of DPP4i with pancreatic cancer, although they are insufficient to draw definitive conclusions.35 T2DM is considered a modifiable risk factor for pancreatic cancer in populations of the Asia-Pacific region.6 With rapid industrialization, urbanization, and westernization of lifestyle, T2DM has increased rapidly in the Chinese population. China has been among the countries with the highest prevalence of T2DM in Asia and has the largest absolute number of T2DM around the world.7,8 The high prevalence and accelerated epidemic of T2DM has become a major public health challenge and undoubtedly increases the risk of pancreatic cancer. Risk reducing activities could be expected to lead to a significant reduction of this cancer, particularly in China with its very large population.
Our study should be interpreted within the context of its limitations. First, potential risk factors related to pancreatic cancer incidence, such as medication use, smoking history, alcohol consumption, and physical activity were not available from all patients in the current system and were not included in the present analysis, which might exert a bias on the statistical analysis. Second, although all diagnosed cases of T2DM in Shanghai were required to register in the management system, selection bias might exist because the registered T2DM cases were mainly identified through screenings and outpatient visits to community health centers. Elderly people tended to participate in screenings and visit community health centers, while young and middle-aged adults preferred the academic hospitals. However, for total risk evaluation, we used age-standardized rates of pancreatic cancer to avoid the influence of age. Moreover, we used Mendelian randomization (MR) to further validate the results from our observational cohort. This type of study that combines both observational and clinical research with MR has been widely accepted to mutually verify the results.36,37 However, few previous studies compared the causal effect of fasting plasma glucose (normal range) and type 2 diabetes (abnormal range) on pancreatic cancer, especially in east Asian population.38,39 Therefore, another novelty of our study is that we firstly extracted instrument variables of fasting plasma glucose and type 2 diabetes from the latest and largest released Asian genome-wide association studies to support our observational results.22,23
To the best of our knowledge, this is the largest study in China to investigate the incidence of pancreatic cancer in new-onset T2DM. New-onset T2DM was significantly associated with a higher risk of pancreatic cancer among Chinese adults, which was supported by MR in an east-Asian ancestry population. Young-onset T2DM had notably higher relative risk of pancreatic cancer. Patients with T2DM diagnosed more than 5 years and with elevated FBG levels had increased risk of pancreatic cancer. Efforts toward early and close follow-up programs, especially in individuals with young-onset T2DM, and the improvement of glucose control might represent effective strategies for improving the detection and results of treatment of pancreatic cancer.
Contributors
B. S., J. T. and Y. S. contributed to the study design. Y. L. and L. L. have accessed and verified the data. T. H. contributed to the Mendelian randomization analysis. C-S.S. and J.T. wrote the manuscript. T. H., Y. P., K. G., C.W., S.S. and X. L. contributed to the discussion of the project. J. C., Y.S., K. H., Z. T. B. and B. S. reviewed and edited the manuscript. All authors participated in critical revision of the manuscript for important intellectual content. B. S., J.T., J. C. and Y. S. were responsible for the decision to submit the manuscript.
Data sharing statement
The data analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of interests
None.
Acknowledgments
Acknowledgments
The investigators acknowledge and thank all investigators for collecting data in the study.
Funding
This work was supported by the Chinese National Natural Science Foundation (81871906, 81770418, 81400346 and 81270935), the Foundation of National Facility for Translational Medicine (Shanghai)(TMSK-2021-506) and Three-year Action Plan of Shanghai Public Health. (GWV-7).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100596.
Contributor Information
Baiyong Shen, Email: shenby@shsmu.edu.cn.
Jingyan Tian, Email: tianjypaper@163.com.
Yan Shi, Email: shiyan@scdc.sh.cn.
Appendix. Supplementary materials
References
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.Landman A, Feetham L, Stuckey D. Working together to reduce the burden of pancreatic cancer. Lancet Oncol. 2020;21(3):334–335. doi: 10.1016/S1470-2045(20)30088-7. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Jin J, Qiu W, et al. Short-term outcomes after robot-assisted vs open pancreaticoduodenectomy after the learning curve. JAMA Surg. 2020;155(5):389–394. doi: 10.1001/jamasurg.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. 2019;156(7):2024–2040. doi: 10.1053/j.gastro.2019.01.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansary-Moghaddam A, Huxley R, Barzi F, et al. The effect of modifiable risk factors on pancreatic cancer mortality in populations of the Asia-Pacific region. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2435–2440. doi: 10.1158/1055-9965.EPI-06-0368. [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian J, Sheng CS, Sun W, et al. Effects of high blood pressure on cardiovascular disease events among Chinese adults with different glucose metabolism. Diabetes Care. 2018;41(9):1895–1900. doi: 10.2337/dc18-0918. [DOI] [PubMed] [Google Scholar]
- 10.Yuan C, Rubinson DA, Qian ZR, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol. 2015;33(1):29–35. doi: 10.1200/JCO.2014.57.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bright R. Cases and observations connected with disease of the pancreas and duodenum. Med Chir Trans. 1833;18(Pt 1):1–56. doi: 10.1177/09595287330180p102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. a meta-analysis. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 13.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ. 2015;350:g7371. doi: 10.1136/bmj.g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Lu J, Hu S, et al. The primary health-care system in China. Lancet. 2017;390(10112):2584–2594. doi: 10.1016/S0140-6736(17)33109-4. [DOI] [PubMed] [Google Scholar]
- 16.Ruan Y, Yan QH, Xu JY, et al. Epidemiology of diabetes in adults aged 35 and older from Shanghai, China. Biomed Environ Sci. 2016;29(6):408–416. doi: 10.3967/bes2016.053. [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Zeng H, Zheng R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–e349. doi: 10.1016/S1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442–458. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spracklen CN, Horikoshi M, Kim YJ, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582(7811):240–245. doi: 10.1038/s41586-020-2263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Spracklen CN, Marenne G, et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. 2021;53(6):840–860. doi: 10.1038/s41588-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishigaki K, Akiyama M, Kanai M, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet. 2020;52(7):669–679. doi: 10.1038/s41588-020-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi J, He P, Yao H, et al. Cancer risk among patients with type 2 diabetes: a real-world study in Shanghai, China. J Diabetes. 2019;11(11):878–883. doi: 10.1111/1753-0407.12926. [DOI] [PubMed] [Google Scholar]
- 26.Pang Y, Kartsonaki C, Guo Y, et al. Diabetes, plasma glucose and incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int J Cancer. 2017;140(8):1781–1788. doi: 10.1002/ijc.30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan C, Babic A, Khalaf N, et al. Diabetes, weight change, and pancreatic cancer risk. JAMA Oncol. 2020;6(10) doi: 10.1001/jamaoncol.2020.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JJ, Jia JP, Shao Q, Wang YK. Diabetes mellitus and risk of pancreatic cancer in China: a meta-analysis based on 26 case-control studies. Prim Care Diabetes. 2019;13(3):276–282. doi: 10.1016/j.pcd.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137–e147. doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 30.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47(13):1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Molina-Montes E, Coscia C, Gomez-Rubio P, et al. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut. 2021;70(2):319–329. doi: 10.1136/gutjnl-2019-319990. [DOI] [PubMed] [Google Scholar]
- 32.Brodovicz KG, Kou TD, Alexander CM, et al. Impact of diabetes duration and chronic pancreatitis on the association between type 2 diabetes and pancreatic cancer risk. Diabetes Obes Metab. 2012;14(12):1123–1128. doi: 10.1111/j.1463-1326.2012.01667.x. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Han K, Hong JY, et al. Changes in metabolic syndrome status are associated with altered risk of pancreatic cancer: a nationwide cohort study. Gastroenterology. 2022;162(2) doi: 10.1053/j.gastro.2021.09.070. 509-20 e7. [DOI] [PubMed] [Google Scholar]
- 34.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137(2):482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dicembrini I, Montereggi C, Nreu B, Mannucci E, Monami M. Pancreatitis and pancreatic cancer in patientes treated with dipeptidyl peptidase-4 inhibitors: an extensive and updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2020;159 doi: 10.1016/j.diabres.2019.107981. [DOI] [PubMed] [Google Scholar]
- 36.Kim MS, Kim WJ, Khera AV, et al. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and Mendelian randomization studies. Eur Heart J. 2021;42(34):3388–3403. doi: 10.1093/eurheartj/ehab454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emerging Risk Factors Collaboration E-CVDVDSC Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021;9(12):837–846. doi: 10.1016/S2213-8587(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Goto A, Yamaji T, Sawada N, et al. Diabetes and cancer risk: a Mendelian randomization study. Int J Cancer. 2020;146(3):712–719. doi: 10.1002/ijc.32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson-Stuttard J, Papadimitriou N, Markozannes G, et al. Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1218–1228. doi: 10.1158/1055-9965.EPI-20-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.