Abstract
Background
The relationship between childhood adversity and inflammation is well-established. Examination of positive experiences can provide a more complete understanding of intervention opportunities. We investigated associations of adverse and positive experiences, and their intersection, with inflammation in children and adolescents.
Methods
Data sources: Longitudinal Study of Australian Children (LSAC; N = 1237) and Avon Longitudinal Study of Parents and Children (ALSPAC; N = 3488). Exposures: Adverse and positive experiences assessed repeatedly (LSAC: 0–11 years; ALSPAC: 0–15 years). Outcomes: Inflammation quantified by high sensitivity C-reactive protein (hsCRP) and glycoprotein acetyls (GlycA) (LSAC: 11–12 years; ALSPAC: 15.5 years). Analyses: Linear regression on the log-transformed outcomes estimated the relative difference in inflammatory markers with adverse/positive experiences, adjusting for socio-demographics and concurrent positive/adverse experiences, respectively.
Results
Most associations were in the expected direction but differed in magnitude by exposure, outcome and cohort. Across both cohorts, adverse experiences were associated with up to 7.3% higher hsCRP (95% CI: −18.6%, 33.2%) and up to 2.0% higher GlycA (95% CI: 0.5%, 3.5%); while positive experiences were associated with up to 22.1% lower hsCRP (95% CI: −49.0%, 4.7%) and 1.3% lower GlycA (95% CI: −2.7%, 0.2%). In LSAC, the beneficial effect of positive experiences on inflammation was more pronounced among those with fewer concurrent adverse experiences.
Conclusion
Across two cohorts, we found small but directionally consistent associations between adverse experiences and higher inflammation, and positive experiences and lower inflammation, particularly for GlycA. Future research should give further consideration to positive experiences to complement the current focus on adversity and inform the design and evaluation of early life interventions.
Keywords: Adversity, Positive experiences, Inflammation, Longitudinal, LSAC, ALSPAC
Highlights
-
•
Adverse and positive childhood experiences both had small effects on inflammation.
-
•
Adverse experiences reduced the beneficial effect of positive experiences on inflammation.
-
•
The magnitude of effects varied depending on exposures, outcomes, and contexts.
-
•
Increased attention is needed to positive experiences within child health research.
1. Introduction
Childhood adversity refers to a wide range of negative events or circumstances that may result in harm to a child's physical or psychological health and well-being (Burgermeister, 2007). A large body of life course research shows that exposure to childhood adversity, such as experiences of violence, parent imprisonment, household member mental illness or substance use, is a major risk factor for many health conditions, including non-communicable diseases (NCDs) and mental illness in later life (Kuhlman et al., 2020; Hughes et al., 2017). Globally, a pooled prevalence of 18.7%–35.0% of individuals experience at least two childhood adversities (e.g., 52.8% by 10–11 years in Australia (O'Connor et al., 2020a), 74.6% by 16 years in the UK (Houtepen et al., 2018)) (Bellis et al., 2019). The long-term health and economic consequences are substantial, with an estimated cost of $24 billion in Australia and $78.6 billion in the UK annually (Hughes et al., 2021; DCKHossack et al., 2015).
Inflammation is a common mechanism in the pathogenesis of many NCDs (Connelly et al., 2017). Empirical research shows that levels of inflammatory markers (e.g., interleukin-6 (IL-6), high sensitivity C-reactive protein (hsCRP), glycoprotein acetyls (GlycA)) are higher among those exposed to early life adversity, suggesting inflammation is a key pathway linking childhood adversity to adverse health outcomes, including NCDs (Kuhlman et al., 2020; Connelly et al., 2017; Russell et al., 2019). The allostatic load model suggests that exposure to childhood adversity can lead to chronic activation and dysregulation of immune, neuroendocrine, and metabolic systems, which in turn result in chronic inflammation and subsequent NCDs (Danese and McEwen, 2012). Associations between exposure to childhood adversity and inflammatory markers have been reported among both adults and children (Kuhlman et al., 2020; Chen and Lacey, 2018; Slopen et al., 2013). Intervening to prevent adversity in early life is therefore proposed as a strategy to reduce the later burden of NCD (Suglia et al., 2020), and examination of inflammation is a key way to investigate contributors to NCDs before disease emerges.
While prior research has shown the relationship between childhood adversity and inflammation (Kuhlman et al., 2020; Chen and Lacey, 2018; Slopen et al., 2013), the role of positive experiences has largely been overlooked. The bioecological model suggests that the context of child development is a system with multiple interacting factors at multiple levels (e.g., child, family, community) (Bronfenbrenner and Evans, 2000; Bronfenbrenner and Ceci, 1994). Within this complex social environment, families living in poor socioeconomic conditions, and those from racialised ethnic groups and who are Indigenous face structural barriers related to poverty, racism and colonisation, and migration experiences, that impact access to resources and opportunities required for health, including health services, education, and employment. This in turn leads to inequitable patterns of exposure to both adverse and positive experiences (e.g., warm parenting, social support, and school enjoyment) (O'Connor et al., 2020a; Weyers et al., 2008; Kiernan and Mensah, 2011). Critically, positive experiences do not simply reflect the absence of adversities; rather they are independent attributes or assets that enhance health and resilience over time (Narayan et al., 2018; Powell et al., 2021; Crandall et al., 2019). During childhood, exposure to positive experiences such as stimulating home learning environments and safe, stable, and nurturing relationships are important factors for optimal physical, emotional, cognitive and social development (Sege and Browne, 2017).
Emerging evidence suggests that positive experiences, although variably defined, are associated with lower levels of inflammation and better adult cardiovascular health (Appleton et al., 2013; Slopen et al., 2016, 2017). For example, using data from the United States, Slopen et al. (2016) found that high emotional and instrumental support in childhood were associated with lower levels of inflammation (e.g., IL-6, hsCRP, fibrinogen) in midlife. A recent review examining positive aspects of parent-child relationships (e.g., responsiveness), which are one aspect of positive childhood experiences, in relation to inflammatory markers (e.g., IL-6, hsCRP, tumour necrosis factor α (TNF-α)) assessed in childhood and adulthood also found inverse associations in six of the ten studies identified (O'Brien et al., 2021).
Previous studies showed that positive experiences had a reduced beneficial effect on adult health (e.g., psychological health, body mass index, physical activity) among those with four or more adversities compared to those with fewer than four adversities (Crandall et al., 2019). However, the association between positive experiences and inflammation among children and adolescents in the presence of concurrent adverse experiences remains unknown, particularly from a life course perspective. Further investigation of the extent to which positive childhood experiences may uniquely contribute to shaping the inflammatory pathway is therefore required.
Investigating the intersection between adverse and positive childhood experiences is potentially important for understanding pathways to later disease risk via inflammation. Examining both adverse and positive experiences also aligns with calls to shift child health research away from overly deficit-focused and deterministic perspectives (VanderWeele et al., 2020). In the present study, we aimed to answer three interrelated research questions:
-
1.
To what extent do adverse experiences increase inflammation in late childhood and mid-adolescence, adjusting for concurrent positive experiences?
-
2.
To what extent do positive experiences reduce inflammation in late childhood and mid-adolescence, adjusting for concurrent adverse experiences?
-
3.
As a starting point for understanding potential effect modification, to what extent do adverse experiences modify the relationship between positive experiences and inflammation?
2. Material and methods
2.1. Data sources
We drew on high-quality prospective data from the Longitudinal Study of Australian Children (LSAC) and Avon Longitudinal Study of Parents and Children (ALSPAC), allowing the investigation of common associations across different settings and cohort designs (O'Connor et al., 2022a). Rather than undertaking identical analyses, we aimed to address these questions as robustly as possible within each cohort separately, given their available data and specific study features (Downes et al., 2022). Differences between the two cohorts include settings (geographic location, calendar period, source population), sampling methods, measurement of key variables, and follow-up periods. The available data allowed us, for example, to examine a more extended period in ALSPAC (0–15.5 years) as compared to LSAC (0–12 years). We did not hypothesize any country-specific differences a priori, however given the systematic differences in study design described above, results should be interpreted within the context of each cohort.
Longitudinal Study of Australian Children (LSAC). LSAC is a nationally representative sample of two cohorts of Australian children: a birth cohort of 5107 infants (B cohort); and a kindergarten cohort of 4983 four-year-olds (K cohort). Families were recruited between March and November in 2004. A complex survey design was used to select a sample that is broadly representative of all Australian children, with the exception of children living in highly remote geographic areas (Soloff et al., 2005). This paper draws on data from the B-cohort, collecting information on adverse and positive experiences from 0 –1 year to 10–11 years (Waves 1–6). In 2015, a comprehensive, Australia-wide, cross-sectional physical health and biomarker module, the Child Health CheckPoint, was conducted for the B cohort between LSAC Waves 6 and 7, when children were 11–12 years of age (i.e. late childhood) (Clifford et al., 2019). Approximately half (53%, N = 1874 families) of the Wave 6 sample agreed to participate in the Child Health CheckPoint, and N = 1237 children had blood collected, with inflammatory markers available for the current study. The LSAC website contains a searchable data dictionary (https://growingupinaustralia.gov.au/data-and-documentation). The LSAC (ID 13–04) and CheckPoint (ID 14–26) methodologies were approved by the Australian Institute of Family Studies Human Research Ethics Review Board, and the CheckPoint additionally by The Royal Children's Hospital Melbourne Human Research Ethics Committee (33225D).
Avon Longitudinal Study of Parents and Children (ALSPAC). ALSPAC is a prospective prenatal cohort from the Avon region of South-West England (Boyd et al., 2013; Fraser et al., 2013). This study recruited 14541 women during pregnancy with expected delivery dates of April 1, 1991 to December 31, 1992. The sample was boosted when the cohort children were approximately 7 years old with children with eligible birth dates who were not previously included in the study, resulting in a total of 15454 pregnancies and 15589 fetuses. Of these, 14901 children were alive at 1 year of age. Due to the demographic profile of the catchment area population and differential attrition, the most disadvantaged groups and ethnic minority groups are under-represented in ALSPAC. Adverse and positive experiences were measured from birth to 15 years, and inflammatory markers were available for N = 3488 children at 15.5 years (i.e. mid-adolescence). The study website contains a searchable data dictionary (http://www.bristol.ac.uk/alspac/researchers/our-data/). Consent for biological samples was collected in accordance with the Human Tissue Act (2004). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
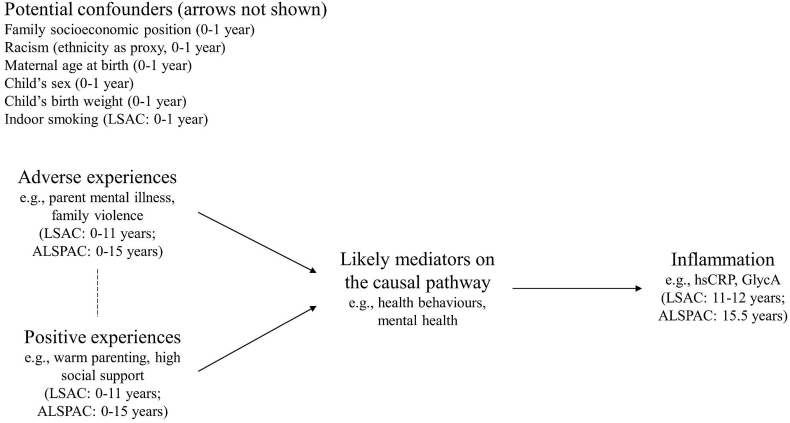
2.2. Conceptual model
Our conceptual model shown in Fig. 1 visually represents the hypothesized relationships between adverse/positive experiences and inflammation, informed by current knowledge. Fig. 1 was used to guide the selection of measures and inform the analytic approach.
Fig. 1.
Conceptual model for the proposed relationships between adverse and positive experiences and inflammation, adapted from Suglia. This model assumes that adverse and positive experiences are correlated with each other (indicated by the dashed line), but does not make assumptions about directionality. Potential confounders have arrows to all other nodes, omitted for simplicity.
2.3. Measures
2.3.1. Outcome: inflammatory biomarkers
Two inflammatory markers (hsCRP and GlycA) were measured in serum samples at 11–12 years in LSAC (n = 1237) and plasma samples at 15.5 years in ALSPAC (n = 3488). Compared to hsCRP, an acute phase reactant, GlycA is a biomarker of chronic and cumulative inflammation with low intra-individual variability (Connelly et al., 2017). hsCRP (mg/L) was determined using Roche/Hitachi Cobas c311 and GlycA (mmol/L) was quantified by the high-throughput proton NMR metabolomics (Nightingale Health, Helsinki, Finland) (Clifford et al., 2019). hsCRP measurements that were below the detection limit (LSAC: n = 261; ALSPAC: n = 0) were assigned a value equal to 50% of the detection limit (LSAC: 0.01 mg/L). As the distributions of hsCRP and GlycA were skewed, log-transformed hsCRP and GlycA values were used in data analysis, with higher values indicating increased inflammation.
2.3.2. Exposure: adverse childhood experiences
Informed by the work of O'Connor et al. (O'Connor et al., 2020a), we examined adversities that: 1) have been consistently measured in the childhood adversity literature, and 2) had repeated assessments available from birth to late childhood/mid-adolescence in both cohorts. Nine types of adverse experiences met these criteria: parent legal problems, family violence (interparental violence and physical abuse towards children), household member mental illness, household member substance abuse, harsh parenting, parental separation/divorce, unsafe neighbourhood, family member death, and bullying victimization.
For interpretability within the current analysis, we created binary indicators of presence of each adversity type (yes/no) at each wave based on dichotomization of each measure (Appendix 1). When measuring multiple adverse experiences at each wave and over the follow-up period, we first calculated a count of the number of adversities present at each wave. Then we dichotomized this count at ‘two or more’ at each wave and over the follow-up period (e.g., in the case of children who experienced the same type of adversity at two waves, they were counted as “two”) to create measures capturing a clustering of adverse experiences (i.e., multiple adverse experiences) likely to take a cumulative toll on health (O'Connor et al., 2020a). While blunt, this approach was considered appropriate for the specific study aim because it could be consistently implemented across cohorts, and we aimed to assess the presence versus absence of a high level of childhood adversity rather than other features of exposure such as chronicity, type, or co-occurrence of specific adversities.
2.3.3. Exposure: positive childhood experiences
Similarly, we used prospectively collected data on positive childhood experiences. Informed by the Health Outcomes from Positive Experiences (HOPE) framework (Sege and Browne, 2017), and previous operationalization and validation of this model within LSAC (Guo et al., 2022), we examined four types of positive experiences (positive parenting practice, trusting and supportive relationships, supportive neighbourhood and home learning environments, social engagement and enjoyment) that had repeated assessments available from birth to late childhood/mid-adolescence. We selected 17 indicators (continuous scores, see Appendix 1 for detailed measures), each mapping to one of the four types of positive experiences listed above, that represented the presence of assets rather than the absence of risk factors (e.g., high levels of warm parenting but not low levels of harsh parenting).
For interpretability, we dichotomized each of the 17 indicators using the top quartile to indicate exposure to a positive experience at each wave (yes/no, see Appendix 1 for details). When measuring multiple positive experiences at each wave and over the follow-up period, we first summed the number of relevant exposure indicators for each type of positive experience (positive parenting practice, trusting and supportive relationships, supportive neighbourhood and home learning environments, social engagement and enjoyment) and dichotomized it using ‘two or more’ as the cut-point at each wave. Then we summed exposure across the four types of positive experiences at each wave and at any wave over the follow-up period, again using ‘two or more’ (i.e., 0–1 versus 2–4) as the cut-point to capture a clustering of positive experiences (i.e., multiple positive experiences) that is likely to have a cumulative benefit on health. In the absence of clear guidance in the literature, the cut-point of ‘two or more’ was selected to ensure adequate sample size (especially for children who were exposed to both “two or more” adverse experiences and “two or more” positive experiences) to be able to generate usefully precise estimates.
2.3.4. Covariates
Following our conceptual model (Fig. 1) and recommendations for confounder selection (VanderWeele, 2019), we posited six potential confounders: child's sex (female/male), family socioeconomic position in infancy (LSAC: measured by a composite z-score of each parent's annual income, education and occupation level, defined as the bottom 25%/top 75%; ALSPAC: measured by each parent's education and occupation), maternal age at birth (continuous), child's birth weight z-scores (continuous), ethnicity (LSAC: Anglo or European/Ethnic minority/Indigenous; ALSPAC: White/non-White) and indoor smoking of household members in infancy (yes/no; LSAC only). We also adjusted for child's age at the outcome assessment (continuous) in LSAC due to data availability to increase the precision of estimates.
2.4. Statistical analysis
Analytic samples consisted of all children who had blood samples (LSAC: N = 1237; ALSPAC: N = 3488). All analyses were conducted using Stata 16.1 (StataCorp, 2017). Participant characteristics were summarized using descriptive statistics based on the available data. The proportions of children with adverse and positive experiences (each type and multiple) were examined over the follow-up period. The distribution of each inflammatory marker was examined according to whether children were exposed to adverse and positive experiences (each type and multiple) over the follow-up period. Multiple imputation was performed to handle missing data in regression analyses across all analysis variables in both cohorts (see Appendix 2 and next section).
For each inflammatory biomarker, we conducted a series of multivariable linear regression models to estimate the effect of exposure to multiple adverse/positive experiences in late childhood and mid-adolescence. Model 1 showed unadjusted estimates, Model 2 was adjusted for all potential confounders, and Model 3 was additionally adjusted for the other exposure (e.g., adjusting for positive experiences when examining the relationship between adverse experiences and inflammation). Finally, subgroup analyses were conducted to examine effect modification, that is, whether the effect of positive experiences on inflammation differed between those exposed to adverse experiences and those not. All estimates from regression models were expressed as percentage differences in geometric means for hsCRP and GlycA to aid interpretation in the original scale, given analyses were performed on the log-transformed inflammatory biomarkers.
2.5. Multiple imputation
The proportion of complete cases in LSAC and ALSPAC analytic samples was 70.8% and 28.0% (with high missing data on child's birth weight in ALSPAC, see Appendix 2 for details), respectively. To handle missing data in study variables for regression estimation of the effects of interest, multiple imputation by chained equations was conducted for each cohort separately (White et al., 2011), producing 30 and 70 imputed datasets in LSAC and ALSPAC, respectively, according to different levels of missingness in each cohort (White et al., 2011). We imputed continuous variables using linear regression models and binary variables using logistic regression models. The imputation model included all study variables, three auxiliary variables at baseline (gestation age in weeks, family financial difficulties, parents' medical conditions) as well as all two-way interactions amongst exposures and outcomes to ensure compatibility with the most complex model (Model 3). Results across imputed datasets were combined using Rubin's rules.
2.6. Sensitivity analysis for potential bias
To examine the potential impact of the choice of missing data approach on our findings, we repeated all regression analyses using the complete case dataset (see Appendix 3). In addition, we also examined the individual effect of each type of adverse and positive experience over the follow-up period to check whether results are consistent with those using a binary exposure of multiple adverse and positive experiences (Appendix 3). To understand the potential impact on results of our decision to impose a cut point of “two or more” to define multiple adverse and positive experiences, we conducted additional analyses using the count of adverse and positive experiences at each wave and summed over the follow-up period (Appendix 4). This is in recognition that there are no standard approaches to defining exposure to multiple adverse and positive experiences.
3. Results
3.1. Participant characteristics
There was an even distribution of males and females in both cohorts (LSAC: 48.9% male; ALSPAC: 48.7% male; see Table 1). The majority of the LSAC children were Anglo-European (89.0%), 9.7% of children were from ethnic minority backgrounds, and a smaller proportion of children (1.3%) came from Indigenous families. Around 4.1% of the ALSPAC children came from non-White backgrounds.
Table 1.
Socio-demographic characteristics of LSAC and ALSPAC children. Observed data are shown.
| Variable |
LSAC (N = 1237) |
ALSPAC (N = 3488) |
||
|---|---|---|---|---|
| N |
Frequency (%)/Mean (SD) |
N |
Frequency (%)/Mean (SD) |
|
| Child's sex | 1237 | 3485 | ||
| Female | 632 (51.1) | 1787 (51.3) | ||
| Male | 605 (48.9) | 1698 (48.7) | ||
| Family socioeconomic position | 1165 | ˆ | ||
| Top 75% | 873 (74.9) | ˆ | ||
| Bottom 25% | 292 (25.1) | ˆ | ||
| Household social class | * | 3132 | ||
| HSP 1 Professional | * | 402 (12.8) | ||
| Managerial and technical | * | 1360 (43.4) | ||
| Skilled non-manual | * | 859 (27.4) | ||
| Skilled manual | * | 315 (10.1) | ||
| Partly skilled | * | 160 (5.1) | ||
| Unskilled | * | 36 (1.2) | ||
| Household education | * | 3198 | ||
| CSE/Vocational/Ordinary level | * | 2069 (64.7) | ||
| Advanced level | * | 699 (21.9) | ||
| Degree | * | 430 (13.5) | ||
| Ethnicity | 1237 | 3150 | ||
| Anglo/European or White | 1101 (89.0) | 3021 (95.9) | ||
| Ethnic minority or non-White | 120 (9.7) | 129 (4.1) | ||
| Indigenous | 16 (1.3) | ˆ | ||
| Maternal age at birth | 1237 | 32.21 (4.76) | 3105 | 29.56 (4.57) |
| Child's birth weight (gm) | 1233 | 3462.83 (557.95) | 1281 | 3415.68 (554.97) |
| Child's birth weight z-scores | 1233 | 0 (1) | 1281 | 0 (1) |
| Child's age at the outcome assessment | 1237 | 11.95 (0.39) | ˆ | ˆ |
| Indoor smoking | 1149 | ˆ | ||
| No | 1086 (94.5) | ˆ | ||
| Yes | 63 (5.5) | ˆ | ||
| Inflammatory markers | ||||
| hsCRP# (mg/L) | 1116 | 0.14 (0.02, 0.50) | 3488 | 0.39 (0.22, 0.91) |
| GlycA# (mmol/L) | 1180 | 0.96 (0.91, 1.04) | 3363 | 1.19 (1.12, 1.28) |
| hsCRP (log-transformed) | 1116 | −2.256 (2.080) | 3488 | −0.692 (1.106) |
| GlycA (log-transformed) | 1180 | −0.020 (0.118) | 3363 | 0.185 (0.103) |
ALSPAC, Avon Longitudinal Study of Parents and Children; hsCRP, high-sensitivity C-reactive protein; GlycA, glycoprotein acetyls; LSAC, Longitudinal Study of Australian Children; ˆ Not assessed; * Not applicable as the education and occupation systems are different in the Australian context. SD, standard deviation. # Median and interquartile range are shown.
3.2. Proportion of children exposed to multiple adverse and positive experiences
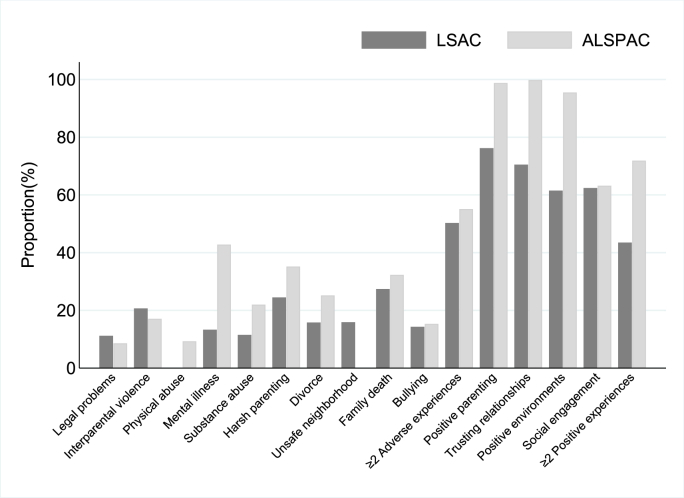
As shown in Fig. 2, over the follow-up period, the most common adverse experience to which children had been exposed was family member death (27.4%) in LSAC and household member mental illness (42.8%) in ALSPAC. The most common positive experience over the follow-up period was positive parenting practices (76.2%) in LSAC and trusting and supportive relationships (99.8%) in ALSPAC.
Fig. 2.
Observed proportions of children exposed to adverse and positive experiences in LSAC and ALSPAC across childhood.
Almost half of LSAC children had been exposed to multiple adverse experiences (50.3%) and multiple positive experiences (43.5%) by the end of childhood. More than half of ALSPAC children had been exposed to multiple adverse experiences (55.1%) and multiple positive experiences (71.9%) by mid-adolescence, perhaps reflecting the longer period captured.
When cross-tabulating exposure to multiple adverse experiences and exposure to multiple positive experiences in each cohort using the observed data, we found that 30.6% and 71.3% of children were exposed to both multiple adverse and multiple positive experiences in LSAC and ALSPAC, respectively (Appendix 5).
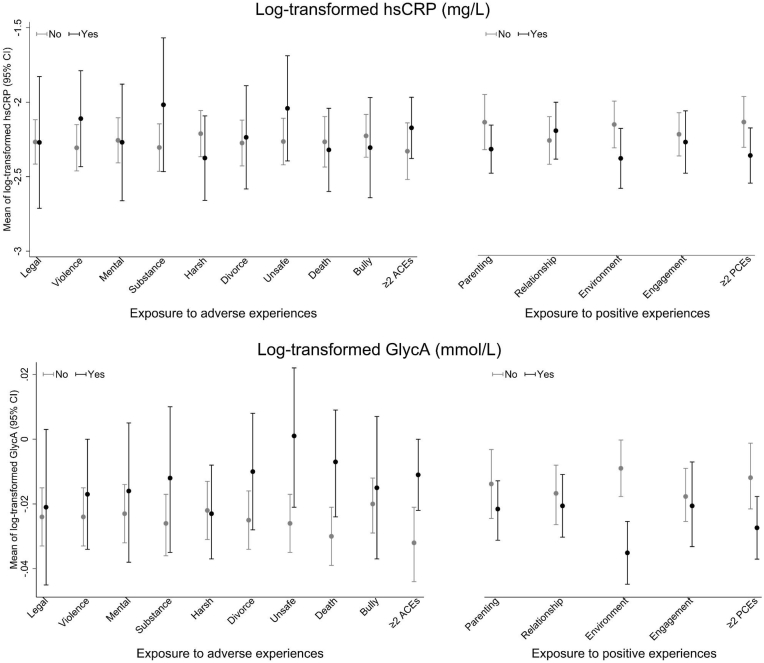
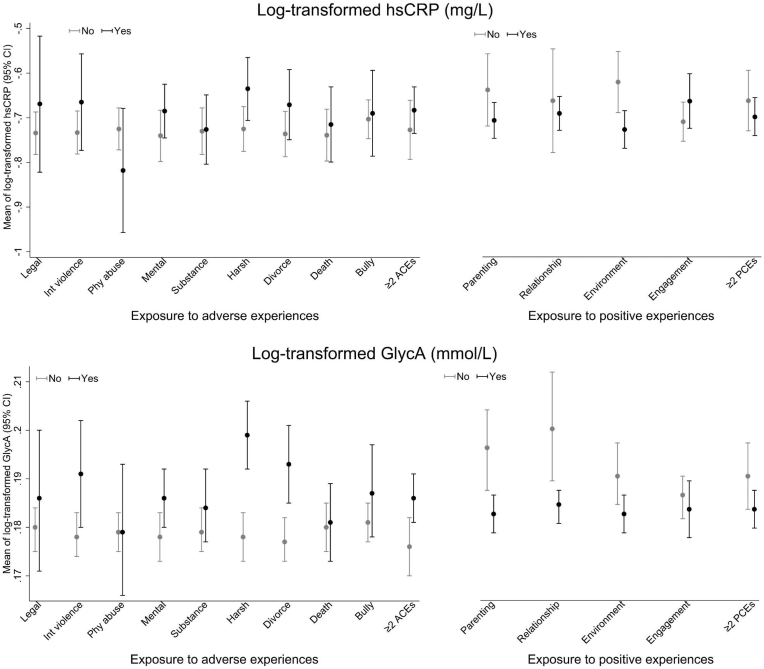
3.3. Distribution of inflammatory markers by adverse and positive experiences
Observed distributions of hsCRP and GlycA were examined according to whether children were exposed to adverse and positive experiences (each type and multiple) over the follow-up period, respectively. Estimates of biomarker means are shown for each cohort separately (Fig. 3, Fig. 4). The distributions of hsCRP and GlycA differed between the two cohorts, with higher means in LSAC than in ALSPAC hence different scales are used for the vertical axes in these figures. In both cohorts, children exposed to adverse experiences typically showed higher levels of inflammation. Conversely, children exposed to positive experiences on average tended to have lower levels of inflammation. In addition, all differences appeared to be more pronounced for GlycA relative to hsCRP.
Fig. 3.
Observed distributions of hsCRP and GlycA (on log-transformed scale) at 11–12 years by groups of exposure (Yes/No) to adverse and positive experiences in LSAC children.
Fig. 4.
Observed distributions of hsCRP and GlycA (on log-transformed scale) at 15.5 years by groups of exposure (Yes/No) to adverse and positive experiences in ALSPAC children.
3.4. The effect of adverse childhood experiences on inflammation after adjusting for concurrent positive experiences
While associations between exposure to multiple adverse experiences and higher levels of hsCRP were in the expected direction in LSAC children (Model 3 results in Table 2), confidence intervals were wide (7.3% higher, 95% CI: −18.6%, 33.2%). When each type of adversity was examined individually, results were inconsistent with small effects observed in both directions (Appendix 3). A similar pattern of results was observed in ALSPAC children at 15.5 years (2.5% higher, 95% CI: −6.2%, 11.3%), with estimates again directionally inconsistent for specific types (Appendix 3). Compared to hsCRP, the estimated association between exposure to multiple adverse experiences and inflammation as measured by GlycA was smaller in magnitude but more precisely estimated in LSAC children at 11–12 years (2.0% higher, 95% CI: 0.5%, 3.5%) and in ALSPAC children at 15.5 years (0.5% higher, 95% CI: −0.3%, 1.3%), with narrower confidence intervals and greater consistency in estimates when examined by type. The clinical relevance of these small differences in inflammatory markers remains unknown.
Table 2.
The estimated effect (represented as a % difference in geometric mean) of multiple adverse and positive experiences on inflammation in late childhood and mid-adolescence, obtained using multiple imputation for handling missing data (N = 1237 for LSAC and 3488 for ALSPAC).
| Exposure | Outcomes (log-transformed) |
||||||
|---|---|---|---|---|---|---|---|
| hsCRP |
GlycA |
||||||
| % difference (95% CI) |
% difference (95% CI) |
||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | ||
|
To what extent do adverse experiences increase inflammation in late childhood and mid-adolescence? | |||||||
| Fewer adverse experiences (<2) | Ref | Ref | Ref | Ref | Ref | Ref | |
| LSAC | Multiple adverse experiences (≥2) |
15.7 (−9.5, 40.9) | 12.3 (−13.1, 37.7) | 7.3 (−18.6, 33.2) | 2.3 (0.8, 3.7) | 2.3 (0.8, 3.8) | 2.0 (0.5, 3.5) |
| ALSPAC |
5.2 (−3.3, 13.7) |
2.5 (−6.1, 11.0) |
2.5 (−6.2, 11.3) |
1.0 (0.2, 1.8) |
0.5 (−0.2, 1.3) |
0.5 (−0.3, 1.3) |
|
| To what extent do positive experiences reduce inflammation in late childhood and mid-adolescence? | |||||||
| Fewer positive experiences (<2) |
Ref |
Ref |
Ref |
Ref |
Ref |
Ref |
|
| LSAC | Multiple positive experiences (≥2) | −25.2 (−51.4, 1.0) | −23.8 (−50.1, 2.5) | −22.1 (−49.0, 4.7) | −1.6 (−3.1, −0.2) | −1.7 (−3.1, −0.3) | −1.3 (−2.7, 0.2) |
| ALSPAC | −3.7 (−11.8, 4.5) | −0.3 (−8.6, 8.0) | 0.2 (−8.3, 8.6) | −0.6 (−1.4, 0.2) | −0.4 (−1.2, 0.3) | −0.4 (−1.1, 0.4) | |
Ref = reference group. Model 1: Unadjusted; Model 2: Adjusted for covariates of child's sex, early life socioeconomic position, maternal age at birth, child's birth weight z-scores, ethnicity, child's age at the outcome assessment (LSAC only), and indoor smoking (LSAC only); Model 3: Model 2 + additionally adjusted for positive experiences when examining the effect of adverse experiences on inflammation, or Model 2 + additionally adjusted for adverse experiences when examining the effect of positive experiences on inflammation.
3.5. The effect of positive childhood experiences on inflammation after adjusting for concurrent adverse experiences
Associations between exposure to multiple positive experiences and lower levels of hsCRP were observed in the expected direction in LSAC children at 11–12 years (22.1% lower, 95% CI: −49.0%–4.7%), but confidence intervals were wide (Model 3 results in Table 2) and estimates were directionally inconsistent for specific types of positive experiences (Appendix 3). Differences in hsCRP by multiple positive experience exposure were negligible in ALSPAC children at 15.5 years (0.2% higher, 95% CI: −8.3%, 8.6%), with estimates again directionally inconsistent for specific types (Appendix 3). Compared to hsCRP, the estimated association between exposure to multiple positive experiences and GlycA was smaller in magnitude but more precisely estimated in LSAC children at 11–12 years (1.3% lower, 95% CI: −2.7%, 0.2%) and in ALSPAC children at 15.5 years (0.4% lower, 95% CI: −1.1%, 0.4%), which was also found when looking by specific types. Again, the clinical relevance of these differences in inflammatory markers remains unknown.
3.6. Effect modification by multiple adverse childhood experiences
When analyses of the effect of positive experiences were stratified by multiple adverse experiences (Table 3), we found that the reduction in inflammation linked to multiple positive experiences in LSAC children at 11–12 years was more pronounced among those with fewer concurrent adverse experiences (hsCRP: 37.7% lower, 95% CI: −76.1%, 0.7%; GlycA: 1.6% lower, 95% CI: −3.6%, 0.4%) than those with multiple adverse experiences (hsCRP: 5.7% lower, 95% CI: −48.5%, 37.1%; GlycA: 0.9% lower, 95% CI: −3.3%, 1.5%), albeit these were imprecisely estimated. This may suggest that exposure to multiple adverse experiences could undermine the benefit of positive experiences on inflammation in late childhood. However, the extent of effect modification was negligible in ALSPAC children at 15.5 years.
Table 3.
Subgroup analyses examining effect modification by multiple adverse experiences of the effect of multiple positive experiences on inflammation in late childhood and mid-adolescence (represented as a % difference in geometric mean), obtained using multiple imputation for handling missing data (N = 1237 for LSAC and 3488 for ALSPAC).
| Cohort | Exposure | Outcomes (log-transformed) |
|
|---|---|---|---|
| hsCRP |
GlycA |
||
| % difference (95% CI) | % difference (95% CI) | ||
| LSAC | Fewer adverse experiences (<2) | ||
| Fewer positive experiences (<2) | Ref | Ref | |
| Multiple positive experiences (≥2) | −37.7 (−76.1, 0.7) | −1.6 (−3.6, 0.4) | |
| Multiple adverse experiences (≥2) | |||
| Fewer positive experiences (<2) | Ref | Ref | |
| Multiple positive experiences (≥2) | −5.7 (−48.5, 37.1) | −0.9 (−3.3, 1.5) | |
| ALSPAC | Fewer adverse experiences (<2) | ||
| Fewer positive experiences (<2) | Ref | Ref | |
| Multiple positive experiences (≥2) | 3.6 (−16.0, 23.1) | 0.2 (−1.6, 2.0) | |
| Multiple adverse experiences (≥2) | |||
| Fewer positive experiences (<2) | Ref | Ref | |
| Multiple positive experiences (≥2) | −0.6 (−10.8, 9.6) | −0.5 (−1.5, 0.4) | |
Ref = reference group. Models are fully adjusted for child's sex, early life socioeconomic position, maternal age at birth, child's birth weight z-scores, ethnicity, child's age at the outcome assessment (LSAC only), and indoor smoking (LSAC only).
3.7. Sensitivity analyses
When using the complete case data, we found results were broadly consistent (Appendix 3), including the extent of effect modification by multiple adverse experiences in LSAC. When considering a cumulative scoring approach for defining multiple adverse and positive experiences, the estimated effects were smaller in magnitude (Appendix 4).
4. Discussion
4.1. Summary of key findings
This study investigated the effects of adverse and positive experiences on two inflammatory markers in late childhood and mid-adolescence across two cohorts. We found most associations were in the expected direction but differed in magnitude by exposure, outcome and cohort. Consistent detrimental effects of multiple adverse experiences on inflammation, after accounting for concurrent positive experiences, were observed in both cohorts, each of which measured outcomes at different ages and in different socio-cultural contexts. Small beneficial effects of multiple positive experiences on inflammation were consistently observed in both cohorts, even in the presence of concurrent adverse experiences. Our findings also suggested that when multiple adverse experiences were present, the reduction in inflammation associated with exposure to multiple positive experiences was smaller in magnitude than when not exposed to adverse experiences in Australian children at 11–12 years.
While associations between multiple adverse experiences and higher levels of hsCRP were in the expected direction in both cohorts, aligning with previous research (Kuhlman et al., 2020; Slopen et al., 2013; O'Connor et al., 2020b), there were wide confidence intervals. In addition, associations were not coherent with the hypotheses and underlying theoretical model when examined at the level of specific adversity type (O'Connor et al., 2020a). As well as the large variance of hsCRP in the original data, another possible reason is that hsCRP, an acute phase reactant, is a poorer biomarker for chronic inflammation in childhood than GlycA (Chiesa et al., 2022). Our findings using GlycA as an inflammatory marker were more consistent at both the level of overall exposure and when individual types were examined, thus aligning more coherently with the underlying theoretical model (O'Connor et al., 2020a). They also align with two recent studies in LSAC and ALSPAC (O'Connor et al., 2020b; Crick et al., 2022), showing that children with multiple adverse experiences were more likely to have higher levels of inflammation (i.e., three or more adverse experiences resulted in 3.2% higher GlycA in LSAC children aged 11–12 years (O'Connor et al., 2020b); cumulative scores of adverse experiences resulted in 1.0% higher GlycA in ALSPAC children aged 18 years (Crick et al., 2022)). We extend these recent studies by adding evidence that adverse experiences have an independent and detrimental effect on inflammation, beyond the presence or absence of positive experiences. The underlying mechanism is potentially related to multiple biological, psychological, and behavioral factors, which may affect the biological pathways linking adverse experiences to inflammation in children and adolescents (Suglia et al., 2018; Bujtor et al., 2021). Exposure to these adverse experiences can have negative and wide-ranging effects on neural, endocrine, immune, and metabolic physiology that increase the lifetime risk of NCDs (Berens et al., 2017). For example, among those exposed to early life adverse experiences, a ‘pro-inflammatory phenotype’ is characterized by exaggerated cytokine response to bacterial challenge and progressive glucocorticoid receptor desensitization (Miller and Chen, 2010). Other genome-wide analyses and epigenetic studies have also shown altered expression of genes controlling the developmental programming of monocytes for excessive inflammatory responses (Essex et al., 2013; Powell et al., 2013).
The present study also extends our current understanding by adding evidence of an association between positive experiences and inflammation. While parallel estimates for hsCRP were inconsistent across cohorts, perhaps due to their different ages at outcome assessment, most associations observed here align with prior research (Slopen et al., 2016; Stellar et al., 2015; Sin et al., 2015), showing an inverse relationship between positive experiences and inflammation. We found modest evidence that multiple positive experiences reduced the levels of GlycA in late childhood and mid-adolescence, even in the presence of concurrent adverse experiences. This may suggest positive experiences are not just a correlate of the absence of adverse experiences but have their own unique contribution to shaping the inflammatory pathway. One possible mechanism linking positive experiences to inflammation is through emotional, social and behavioral factors such as trait positive affect, higher education attainment, and healthier dietary patterns (Appleton et al., 2013; Bujtor et al., 2021; Sin et al., 2015). Prior research suggested positive psychological resources such as social support and connections were associated with health behaviours that were consequential for inflammation in adolescence. The pathways that confer lower inflammation following positive experiences are also likely through positive biological responses (e.g., cellular, molecular, hormonal) and trained innate immunity that reduce physiological reactivity to stressful life events and promote resiliency (Appleton et al., 2013; Slopen et al., 2016; Salam et al., 2018).
We found that the reduction in inflammation linked to multiple positive experiences in LSAC children aged 11–12 years was more pronounced among those with fewer concurrent adverse experiences than those with multiple adverse experiences. Due to the small effect sizes and wide confidence intervals observed here, further investigation is needed to investigate how adverse and positive experiences interact with each other to contribute to inflammation in children and adolescents.
Overall, the estimated effects were larger in magnitude in LSAC compared to ALSPAC. Discrepant findings across the two cohorts may result from country-specific differences as well as systematic differences such as in the measurement of adverse and positive experiences, follow-up periods and outcome measurement timing. For example, ALSPAC children reported more positive experiences than LSAC children, which is likely to be largely due to the different ways positive experiences were captured between the two cohorts. To further investigate heterogeneity in effects across cohorts, future studies are encouraged to use the recently proposed “target trial” approach as a useful and powerful tool for the planning and interpretation of causal analyses in multi-cohort studies (Downes et al., 2022). Using the target trial framework can help to minimise biases within each cohort as well as aid in outlining possible remaining biases, thus contributing to meaningful and informative estimates within the study context.
4.2. Strengths and limitations
Data were analyzed from two large-scale samples of children (a national-level sample in Australia and a county-level sample in the UK), thus increasing the generalizability of our findings. Nevertheless, the most disadvantaged families (i.e., those in extreme poverty) in both cohorts are likely under-represented due to difficulties accessing and engaging these families, and more disadvantaged families who were recruited have been disproportionately lost to follow up. In addition, the boost sample (n = 913) in the total ALSPAC sample (N = 15589) did not have exposure measures between birth and seven years old. Only a small number of children in the boost sample were included in our analytic sample, which consisted of all children with blood samples. We used multiple imputation in the analytic sample to reduce the potential for selection bias arising from missing data due to attrition for the samples with blood. We used prospectively collected data on adverse and positive experiences across childhood, thereby avoiding the potential for recall bias inherent to retrospective designs. We also used both traditional and novel biomarkers to measure inflammation in late childhood and mid-adolescence, thus providing a more comprehensive understanding of the impact of early life experiences on inflammation.
The dichotomization approach to defining multiple adverse and positive experiences is blunt and not designed to be informative for understanding issues such as dose-response, onset, chronicity, or mechanisms of influence. We used this dichotomization approach because it was appropriate for the current research questions which focused on the presence or absence of multiple adversity/positive experiences. Future research should also focus on selecting measurement approaches well aligned to their purpose and aims (Bethell et al., 2019a), which may differ to that herein. For example, other research questions may necessitate consideration of specific types of experiences, different periods of exposure, and chronic exposure over time.
Third, LSAC and ALSPAC are not purpose-designed studies of adverse and positive experiences. Not all types of adverse and positive experiences were captured or available (e.g., racial discrimination, family cohesion). Therefore, we selected the most appropriate available indicators based on our knowledge and from a content validity perspective. In some cases, a proxy indicator was sometimes used (e.g., harsh parenting in the absence of direct indicators of child maltreatment). Self-report bias may also exist. Reporting on questions about parenting and other adversities can be influenced by feelings of guilt, shame and embarrassment, and the desire to portray oneself in a positive light.
Fourth, the possibility of residual confounding can never be fully eliminated. We cannot rule out the possibility that such factors exist and influence the association between exposure and outcome. Intervention studies such as quasi-experimental trials would help to continue progressing our understanding and strengthening evidence as to whether the observed effects are causal, or due to factors such as unmeasured confounding. While it would be unethical to randomize adverse or positive experiences themselves, examining inflammation as a secondary outcome of interventions that do achieve improvements in each of these (reduced adverse experiences, increased positive experiences) would be extremely valuable. The integration of trials into large-scale cohorts could present a viable platform for such investigations (Wake et al., 2022), given that cohorts do tend to collect data on relevant bio-samples.
4.3. Implications for future research and practice
This study is the first to examine the relationship between positive experiences and inflammation in children and adolescents in the presence of adverse experiences. Similar to the mental health research field where the dual continuum model has come to prominence (O'Connor et al., 2022b; Masten and Curtis, 2000), there has been increasing attention on positive childhood experiences, showing how these early life experiences contribute to adult physical and mental health (e.g., body mass index, depression, stress) (Narayan et al., 2018; Crandall et al., 2019; Bethell et al., 2019b). These emerging interests in positive experiences have shifted the research paradigm from a deficit-focused perspective (i.e., only focusing on adverse experiences) to a strengths-based perspective. A sole focus on reducing adverse experiences is not sufficient to achieve optimal academic, physical and mental health outcomes in children and adolescents (Sege and Browne, 2017). Future research is needed to include positive experiences as an integral part of understanding individual and population health in its fullest sense.
To further extend our current understanding in the role of positive experiences, it will be valuable for future research to investigate their effects on positive development outcomes (e.g., mental health competence and academic success) in different cohorts and populations outside Australia and the UK, in order to promote the positive epidemiology to understand the full range of health assets (VanderWeele et al., 2020). It is also worthwhile for future research to explore potential effect modifiers such as socioeconomic status and gender in the associations between adverse/positive childhood experiences and inflammation. Prior research indicated both socioeconomic position and gender as modifiers of the relationship between adverse experiences and health outcomes (Currie et al., 2021; Lacey et al., 2020). Further investigation will be helpful to understand the mechanisms linking these early life experiences and physiological dysfunction and to inform upstream intervention opportunities in practice.
We investigated inflammation using two biomarkers at a single time point (late childhood in LSAC and mid-adolescence in ALSPAC). Both inflammatory markers in our samples had lower mean estimates in LSAC than in ALSPAC. While the source of inflammatory biomarkers was different (serum in LSAC and plasma in ALSPAC), previous studies showed high correlations between these two sources (Brindle et al., 2010). One possible reason for the higher levels of inflammation in ALSPAC could be that the level of systematic inflammation increases with age (Chung et al., 2019). Future research may consider longitudinally examining whether inflammation as measured using multiple biomarkers changes with age for children and adolescents. The variations in estimates by biomarkers used to indicate inflammation may also reflect the nuanced difference in the outcome measurement. As suggested by previous research (O'Connor et al., 2020b; Collier et al., 2019), GlycA captured chronic inflammation whereas hsCRP more likely reflected acute inflammation. Compared to hsCRP, GlycA showed a greater within-subject correlation over a long period of follow-up in children (Chiesa et al., 2020). Most clinical studies suggest that correlations between GlycA and hsCRP are low or medium (in our samples, LSAC: 0.40; ALSPAC: 0.32) (Connelly et al., 2017), indicating GlycA and hsCRP likely capture different inflammatory pathways. Future research is needed to explore these nuanced differences using multiple markers in a single study to reflect the inflammatory status, and prioritizing the exploration of clinical meaning. Further omics data analyses (e.g., genomic, transcriptomic and proteomic) are beyond the scope of the current study but would be essential in future studies to understand the underlying mechanisms and identify potential targets for intervention.
Our results highlight the potential differential benefit of positive experiences on reducing inflammation, depending on concurrent exposure to adverse experiences. This suggests that optimal policy and practice opportunities should focus on intervening to both promote positive experiences and prevent and address adversities. Aligning with the bioecological model (Bronfenbrenner and Evans, 2000; Bronfenbrenner and Ceci, 1994) both adverse and positive experience should be targeted with multi-collaborations between families, schools and communities. For example, strengths-based mental health initiatives such as Within My Reach in the USA and Be You in Australia have multi-sector collaborations and engagement with families, schools, communities, and health systems that aim to target both adverse experiences and positive experiences, and have shown promising results (Sterrett-Hong et al., 2018; Hoare et al., 2020).
5. Conclusions
We found that children exposed to multiple adverse experiences had higher levels of inflammation, whereas children exposed to multiple positive experiences had lower levels of inflammation, across two cohorts with differing ages and socio-cultural contexts. Associations with these early experiences were smaller but more precisely estimated for GlycA than hsCRP. The beneficial effect of positive experiences on inflammation at 11–12 years was more pronounced among those with fewer concurrent adverse experiences than those with more adverse experiences. Positive experiences are a crucial part of children's early life experiences. There is a need for future research and public health practice that includes positive experiences in addition to adverse experiences to inform intervention opportunities to improve life course health outcomes. Public health and social policy efforts are also needed to address structural and systemic factors (e.g., poverty, racism) that drive inequitable distribution of adverse and positive experiences, which in turn lead to child health inequities.
Funding statement
The UK Medical Research Council (MRC) and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Prof Priest and Dr Lacey will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/Alspac/external/documents/grant-acknowledgements.pdf); This research was specifically funded by the National Institute of Health (NIH) (Grant ref: DK077659), Wellcome Trust and MRC (Grant ref: 07467/Z/05/Z). Access to ALSPAC data was supported by a University College London Global Engagement Award. This work was also supported by the Victorian Government's Operational Infrastructure Support Program. Prof Priest was supported by a NHMRC Career Development Fellowship (APP1123677). Dr Lacey and Dr Gondek's time on this study was supported by a UK Economic and Social Research Council grant (Grant ref: ES/P010229/1). Dr Slopen is supported by the National Institutes of Health Grant (1R01HL151848-01). Dr Moreno-Betancur is supported by Australian Research Council Discovery Early Career Award (DE190101326). Prof Goldfeld is supported by Australian National Health and Medical Research Council (NHMRC) Practitioner Fellowship (1155290). Prof Burgner is supported by an NHMRC Investigator Grant (APP1175744). Dr O'Connor is supported by the Melbourne Children's LifeCourse initiative, funded by a Royal Children's Hospital Foundation Grant (2018–984).
Role of the funder
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing statement
All data used in this manuscript are available from the Longitudinal Study of Australian Children (https://dataverse.ada.edu.au/dataverse/lsac) and Avon Longitudinal Study of Parents and Children (http://www.bristol.ac.uk/alspac/).
Coding sharing statement
The coding used in the data analysis is available online (https://osf.io/24dqy/).
Declaration of competing interest
None.
Acknowledgments
This paper uses unit record data from Growing Up in Australia, the Longitudinal Study of Australian Children (LSAC). LSAC is conducted by the Australian Government Department of Social Services (DSS). The findings and views reported in this paper, however, are those of the authors and should not be attributed to the Australian Government DSS or any of DSS′ contractors or partners. DOI: 10.26193/F2YRL5. We are also extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bbih.2022.100550.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Data availability
I have shared the link to my data code in the manuscript.
References
- Appleton A.A., Buka S.L., Loucks E.B., Rimm E.B., Martin L.T., Kubzansky L.D. A prospective study of positive early-life psychosocial factors and favorable cardiovascular risk in adulthood. Circulation. 2013;127(8):905–912. doi: 10.1161/CIRCULATIONAHA.112.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis M.A., Hughes K., Ford K., Rodriguez G.R., Sethi D., Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4(10):e517–e528. doi: 10.1016/S2468-2667(19)30145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens A.E., Jensen S.K.G., Nelson C.A., III Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15:1–12. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell C., Jones J., Gombojav N., Linkenbach J., Sege R. Positive childhood experiences and adult mental and relational health in a statewide sample: associations across adverse childhood experiences levels. JAMA Pediatr. 2019;173(11) doi: 10.1001/jamapediatrics.2019.3007. e193007-e193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell C., Jones J., Gombojav N., Linkenbach J., Sege R. Positive childhood experiences and adult mental and relational health in a statewide sample: associations across adverse childhood experiences levels. JAMA Pediatr. 2019;173(11) doi: 10.1001/jamapediatrics.2019.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., et al. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon longitudinal study of parents and children. Int. J. Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle E., Fujita M., Shofer J., O'Connor K.A. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J. Immunol. Methods. 2010;362(1–2):112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U., Ceci S.J. Nature-nuture reconceptualized in developmental perspective: a bioecological model. Psychol. Rev. 1994;101(4):568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U., Evans G.W. Developmental science in the 21st century: emerging questions, theoretical models, research designs and empirical findings. Soc. Dev. 2000;9(1):115–125. [Google Scholar]
- Bujtor M., Turner A.I., Torres S.J., Esteban-Gonzalo L., Pariante C.M., Borsini A. Associations of dietary intake on biological markers of inflammation in children and adolescents: a systematic review. Nutrients. 2021;13(2):356. doi: 10.3390/nu13020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister D. Childhood adversity: a review of measurement instruments. J. Nurs. Meas. 2007;15(3):163–176. doi: 10.1891/106137407783095766. [DOI] [PubMed] [Google Scholar]
- Chen M., Lacey R.E. Adverse childhood experiences and adult inflammation: findings from the 1958 British birth cohort. Brain Behav. Immun. 2018;69:582–590. doi: 10.1016/j.bbi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Chiesa S.T., Charakida M., Georgiopoulos G., et al. medRxiv; 2020. Elevated Glycoprotein Acetyl Levels in Adolescence and Early Adulthood Predict Adverse Cardiometabolic Profiles and Risk of Metabolic Syndrome in up to 10 Year Follow-Up. [Google Scholar]
- Chiesa S.A.-O., Charakida M., Georgiopoulos G., et al. Glycoprotein acetyls: a novel inflammatory biomarker of early cardiovascular risk in the young. J. Am. Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024380. 2047-9980 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.Y., Kim D.H., Lee E.K., et al. Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging and Disease. 2019;10(2):367–382. doi: 10.14336/AD.2018.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S.A., Davies S., Wake M. Child health CheckPoint: cohort summary and methodology of a physical health and biospecimen module for the longitudinal study of Australian children. BMJ Open. 2019;9(Suppl. 3):3–22. doi: 10.1136/bmjopen-2017-020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier F., Ellul S., Juonala M., et al. Glycoprotein acetyls (GlycA) at 12 months are associated with high-sensitivity C-reactive protein and early life inflammatory immune measures. Pediatr. Res. 2019;85(5):584–585. doi: 10.1038/s41390-019-0307-x. [DOI] [PubMed] [Google Scholar]
- Connelly M.A., Otvos J.D., Shalaurova I., Playford M.P., Mehta N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017;15(1):219. doi: 10.1186/s12967-017-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall A., Miller J.R., Cheung A., et al. ACEs and counter-ACEs: how positive and negative childhood experiences influence adult health. Child Abuse Neglect. 2019;96 doi: 10.1016/j.chiabu.2019.104089. [DOI] [PubMed] [Google Scholar]
- Crick D.C.P., Halligan S.L., Howe L.D., et al. Associations between Adverse Childhood Experiences and the novel inflammatory marker glycoprotein acetyls in two generations of the Avon Longitudinal Study of Parents and Children birth cohort. Brain Behav. Immun. 2022;100:112–120. doi: 10.1016/j.bbi.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.L., Higa E.K., Swanepoel L.-M. Socioeconomic status moderates the impact of emotional but not physical childhood abuse on women’s sleep. Adversity and Resilience Science. 2021;2:169–179. doi: 10.1007/s42844-021-00035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., McEwen B. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- DCK Am, Hossack N., Pam D.R., Stavropoulos P.H.D., Burley P.I.P. Adults Surviving Child Abuse and Pegasus Economics; Sydney, Australia: 2015. The Cost of Unresolved Childhood Trauma and Abuse in Adults in Australia. [Google Scholar]
- Essex M.J., Thomas Boyce W., Hertzman C., et al. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Dev. 2013;84(1):58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Macdonald-Wallis C., Tilling K., et al. Cohort profile: the Avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., O'Connor M., Mensah F., et al. Measuring positive childhood experiences: testing the structural and predictive validity of the health outcomes from positive experiences (HOPE) framework. Academic Pediatrics. 2022;22(6):942–951. doi: 10.1016/j.acap.2021.11.003. [DOI] [PubMed] [Google Scholar]
- Hoare E., Thorp A., Bartholomeusz-Raymond N., McCoy A., Butler H., Berk M. Be You: a national education initiative to support the mental health of Australian children and young people. Aust. N. Z. J. Psychiatr. 2020;54(11):1061–1066. doi: 10.1177/0004867420946840. [DOI] [PubMed] [Google Scholar]
- Houtepen L.C., Heron J., Suderman M.J., Tilling K., Howe L.D. Adverse childhood experiences in the children of the Avon longitudinal study of parents and children (ALSPAC) Wellcome open research. 2018;3:106. doi: 10.12688/wellcomeopenres.14716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K., Bellis M.A., Hardcastle K.A., et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- Hughes K., Ford K., Bellis M.A., Glendinning F., Harrison E., Passmore J. Health and financial costs of adverse childhood experiences in 28 European countries: a systematic review and meta-analysis. Lancet Public Health. 2021;6(11):e848–e857. doi: 10.1016/S2468-2667(21)00232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan K.E., Mensah F.K. Poverty, family resources and children's early educational attainment: the mediating role of parenting. Br. Educ. Res. J. 2011;37(2):317–336. [Google Scholar]
- Kuhlman K.R., Horn S.R., Chiang J.J., Bower J.E. Early life adversity exposure and circulating markers of inflammation in children and adolescents: a systematic review and meta-analysis. Brain Behav. Immun. 2020;86:30–42. doi: 10.1016/j.bbi.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R.E., Bartley M., Kelly-Irving M., et al. Adverse childhood experiences and early life inflammation in the Avon longitudinal study of parents and children. Psychoneuroendocrinology. 2020;122 doi: 10.1016/j.psyneuen.2020.104914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten A.S., Curtis W.J. Integrating competence and psychopathology: pathways toward a comprehensive science of adaptation in development. Dev. Psychopathol. 2000;12(3):529–550. doi: 10.1017/s095457940000314x. [DOI] [PubMed] [Google Scholar]
- Miller G.E., Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan A.J., Rivera L.M., Bernstein R.E., Harris W.W., Lieberman A.F. Positive childhood experiences predict less psychopathology and stress in pregnant women with childhood adversity: a pilot study of the benevolent childhood experiences (BCEs) scale. Child Abuse Neglect. 2018;78:19–30. doi: 10.1016/j.chiabu.2017.09.022. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Slopen N., Becares L., Burgner D., Williams D.R., Priest N. Inequalities in the distribution of childhood adversity from birth to 11 years. Academic Pediatrics. 2020;20(5):609–618. doi: 10.1016/j.acap.2019.12.004. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Ponsonby A.-L., Collier F., et al. Exposure to adversity and inflammatory outcomes in mid and late childhood. Brain, Behavior, & Immunity - Health. 2020;9 doi: 10.1016/j.bbih.2020.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J.R., Loi E.C., Byrne M.L., Zalewski M., Casement M.D. Child Psychiatry & Human Development; 2021. The Link between Positive and Negative Parenting Behaviors and Child Inflammation: A Systematic Review; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M., Spry E., Patton G., et al. Better together: advancing life course research through multi-cohort analytic approaches. Adv. Life Course Res. 2022;53 doi: 10.1016/j.alcr.2022.100499. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Guo S., Letcher P., Sanson A., Goldfeld S., Olsson C.A. Developmental relationships between socio-economic disadvantage and mental health across the first 30 years of life. Longitudinal and Life Course Studies. 2022:1–22. doi: 10.1332/175795921X16459587898770. [DOI] [PubMed] [Google Scholar]
- Powell K.M., Rahm-Knigge R.L., Conner B.T. Resilience protective factors checklist (RPFC): buffering childhood adversity and promoting positive outcomes. Psychol. Rep. 2021;124(4):1437–1461. doi: 10.1177/0033294120950288. [DOI] [PubMed] [Google Scholar]
- Powell N.D., Sloan E.K., Bailey M.T., et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.E., Heron J., Gunnell D., et al. Pathways between early-life adversity and adolescent self-harm: the mediating role of inflammation in the Avon Longitudinal Study of Parents and Children. J. Child Psychol. Psychiatry Allied Discip. 2019;60(10):1094–1103. doi: 10.1111/jcpp.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam A.P., Borsini A., Zunszain P.A. Trained innate immunity: a salient factor in the pathogenesis of neuroimmune psychiatric disorders. Mol. Psychiatr. 2018;23(2):170–176. doi: 10.1038/mp.2017.186. [DOI] [PubMed] [Google Scholar]
- Sege R.D., Browne C.H. Responding to ACEs with HOPE: health outcomes from positive experiences. Academic Pediatrics. 2017;17(7):S79–S85. doi: 10.1016/j.acap.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Sin N.L., Graham-Engeland J.E., Almeida D.M. Daily positive events and inflammation: findings from the national study of daily experiences. Brain Behav. Immun. 2015;43:130–138. doi: 10.1016/j.bbi.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., Kubzansky L.D., McLaughlin K.A., Koenen K.C. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38(2):188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., Chen Y., Priest N., Albert M.A., Williams D.R. Emotional and instrumental support during childhood and biological dysregulation in midlife. Prev. Med. 2016;84:90–96. doi: 10.1016/j.ypmed.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., Chen Y., Guida J.L., Albert M.A., Williams D.R. Positive childhood experiences and ideal cardiovascular health in midlife: associations and mediators. Prev. Med. 2017;97:72–79. doi: 10.1016/j.ypmed.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff C., Lawrence D., Johnstone R. Australian Institute of Family Studies; Melbourne, Australia: 2005. LSAC Technical Paper No. 1. Sample Design. [Google Scholar]
- StataCorp . StataCorp LLC; College Station, TX: 2017. Stata Statistical Software: Release 15. [Google Scholar]
- Stellar J.E., John-Henderson N., Anderson C.L., Gordon A.M., McNeil G.D., Keltner D. Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 2015;15(2):129–133. doi: 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Sterrett-Hong E., Antle B., Nalley B., Adams M. Changes in couple relationship dynamics among low-income parents in a relationship education program are associated with decreases in their children's mental health symptoms. Children. 2018;5(7):90. doi: 10.3390/children5070090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S.F., Koenen K.C., Boynton-Jarrett R., et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation. 2018;137(5):e15–e28. doi: 10.1161/CIR.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia S.F., Campo R.A., Brown A.G.M., et al. Social determinants of cardiovascular health: early life adversity as a contributor to disparities in cardiovascular diseases. J. Pediatr. 2020;219:267–273. doi: 10.1016/j.jpeds.2019.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019;34:211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T.J., Chen Y., Long K., Kim E.S., Trudel-Fitzgerald C., Kubzansky L.D. Positive epidemiology? Epidemiology. 2020;31(2):189–193. doi: 10.1097/EDE.0000000000001147. [DOI] [PubMed] [Google Scholar]
- Wake M., Goldfeld S., Davidson A. Embedding life course interventions in longitudinal cohort studies: Australia's GenV opportunity. Pediatrics. 2022;149(Suppl. 5) doi: 10.1542/peds.2021-053509R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers S., Dragano N., Möbus S., et al. Low socio-economic position is associated with poor social networks and social support: results from the Heinz Nixdorf Recall Study. Int. J. Equity Health. 2008;7:13. doi: 10.1186/1475-9276-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Downes M, O'Connor M, Olsson CA, et al. 2022. Causal Inference in Multi-Cohort Studies Using the Target Trial Approach. arXiv preprint arXiv:220611117.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
I have shared the link to my data code in the manuscript.