Abstract
SGP (for Streptococcus GTP-binding protein) is a Streptococcus mutans essential GTPase which has significant sequence identity to the previously identified Escherichia coli Era protein and to numerous other prokaryotic GTPase proteins of unknown function. Recent studies in our laboratory have addressed the possible role of SGP in the stress response of the oral pathogen S. mutans. Here we report that during growth in the early stationary phase, and in response to elevated temperatures or acidic pH, the distribution of SGP between the cytoplasm and the membranes of S. mutans cells varies. Immunoblot analysis of soluble and membrane protein fractions collected from the mid-log and early stationary growth phases of bacterial populations grown at normal temperature (37°C) and at the elevated temperature of 43°C, or at acidic pH, demonstrated that the total amount of SGP increased with the age of the bacterial culture, elevated temperature, or acidic pH. Furthermore, it was established that a substantial amount of SGP is associated with the membrane fraction under stress conditions. In order to investigate the physiological role of SGP, we constructed an S. mutans strain capable of chromosomal sgp antisense RNA expression, which interferes with the normal information processing of the sgp gene. Utilizing this strain, we determined conditions whereby the streptococcal cells can be depleted of SGP, thus avoiding the problem of constructing a conditional lethal system. From the results of measurements of the nucleotide pools extracted from the antisense strain and its isogenic counterpart, we propose that one of the physiological roles of SGP is regulation and modulation of the GTP/GDP ratio under different growth conditions. Moreover, we observed that in SGP-depleted cells the levels of glucan-binding protein A (GbpA) substantially increased, suggesting that GbpA may have stress response-related physiological functions. Finally, the potential applications of the antisense RNA approach that we employed are discussed.
Protein molecules related by their ability to bind guanine nucleotides and hydrolyze GTP (the GTPase superfamily) have been identified in organisms that belong to all three domains of life (3). A common structural design and shared molecular mechanism distinguish these proteins. Each of them is a precisely engineered molecular switch which is able to change its affinity for other macromolecules with which it is designed to interact. Activated by the binding of GTP and deactivated by hydrolysis of bound GTP to GDP, the switch mechanism is extremely versatile. It enables different GTPases to sort and amplify transmembrane signals and to direct the synthesis and translocation of proteins, and it has been shown to be involved in diverse cellular processes, including signal transduction and cell cycle regulation (3).
The sgp gene of Streptococcus mutans was discovered by sequencing of DNA downstream of the dgk (diacylglycerol kinase) gene (36). Its protein product, SGP (for Streptococcus GTP-binding protein), is a member of the GTPase superfamily (34). It has significant sequence identity to the previously identified Escherichia coli Era protein (1) and to numerous other prokaryotic proteins of unknown functions. Era and SGP have been shown to bind guanine nucleotides specifically and are able to hydrolyze GTP to GDP (6, 20, 34). Both proteins are required for viability in their respective organisms, and it was not possible to construct strains bearing lethal mutations in each gene. Moreover, the functions of these G proteins still remain to be determined. E. coli Era temperature-sensitive mutants have been described, and the pleiotropic nature of the mutants suggested that Era may regulate multiple functions in its host (13, 18). Studies with a strain from which Era could be depleted at low temperatures indicated that the cells became elongated, thus suggesting a defect in cell division (9). However, in a strain in which cells were depleted of Era at elevated temperatures, no such defects in cell division were apparent (18). Era has also been demonstrated to be autophosphorylated, and the phosphorylated species has been suggested to be its active form (32). Era has also been found to be associated with the inner membrane fraction, but the component of the membrane to which Era binds has yet to be identified (19). SGP has also been demonstrated to complement the era mutation in E. coli (26). Characterization of membrane-associated Pseudomonas aeruginosa GTP-binding protein (Pra) has been recently reported (7). Although significantly larger than the Era and SGP proteins, Pra was shown to cross-react with anti-Era antibody. Therefore, it is very likely that these bacterial G proteins play similar, if not identical, roles in their respective hosts.
Recently, we reported the utilization of an sgp antisense RNA strategy employed in order to initially examine the role of SGP in S. mutans (30). In that study a shuttle vector carrying the cloned sgp sequence in the antisense orientation downstream of the scrB promoter was utilized. However, evidence of growth inhibition caused by the vector alone was also noted. Therefore, in the present study we further developed the antisense strategy by constructing and employing S. mutans integration vectors designed to express sgp antisense RNA from the host chromosome.
Since SGP is essential for cell growth, it was of interest to further investigate its role in S. mutans physiology. Therefore, the objectives of the present work were to analyze the potential role of SGP in normal and environmentally stressed cells and to investigate its possible function(s) in the oral pathogen S. mutans.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, growth conditions, and chemicals.
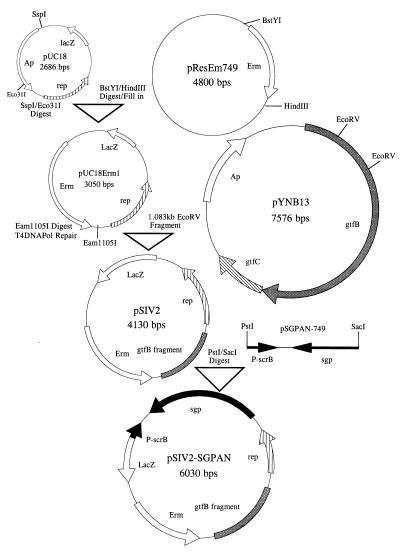
Construction of pSIV2 (Streptococcus integration vector) and pSIV2-SGPAN, which carries the sgp gene in the antisense orientation downstream of the scrB promoter, is depicted in Fig. 1 and described in Results section. Construction of the S. mutans GS5(gtfB)::pSIV2 and S. mutans GS5(gtfB)::pSIV2-SGPAN strains was carried out by transformation of the parental S. mutans GS5 strain with the respective plasmids essentially as described previously (25). Selection for the integration events was performed on mitis salivarius agar (Difco Laboratories, Detroit, Mich.). The initial experiments for studying the SGP distribution during different growth phases and under different stress conditions were performed with the isogenic S. mutans SP2 nonaggregating mutant, which has been described earlier (25). The S. mutans UA130 gbpA mutant was previously described (10) and was supplied by J. Banas (Albany Medical College, Albany, N.Y.), and S. mutans BCH 150, an NADP-dependent GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mutant (4) was from I. Hamilton (University of Manitoba, Winnipeg, Manitoba, Canada). The plasmids pUC18 and pYNB13 were propagated in E. coli JM109, while all other plasmids were maintained in E. coli DH5α (Life Technologies, Gaithersburg, Md.). Plasmid DNA of pSIV2-SGPAN was purified from cells grown on agar plates, as the E. coli strain harboring this construct was not able to grow in liquid culture. The following antibiotic concentrations were used where indicated: for E. coli, 200 μg of erythromycin per ml and 100 μg of ampicillin per ml; for S. mutans, 10 μg of erythromycin per ml for selection and routine maintenance and 1.0 μg per ml for the growth experiments. Use of the latter concentration of erythromycin was indicated by our observation that the antibiotic at concentrations above 1.0 μg per ml diminished the total amount of SGP in the streptococcal cells and exerted a negative effect on the growth rate. All experiments with S. mutans strains were performed in static Todd-Hewitt broth (THB) or SMM (defined minimal medium), supplemented with 1% glucose or 1% sucrose as the sole carbon source, at 37 or 43°C aerobically. The composition of the minimal medium was as described previously (8) except for the following modifications. Preparation of all components, as well as the final sterilizations, was carried out by filtration through 0.22-μm-pore-size, vacuum-driven disposable bottle top filters (Millipore Corp., Bedford, Mass.). The final pH of 7.0 or 5.5 was adjusted with phosphoric acid; the amount of the dibasic potassium phosphate was 2.5 g per liter, and that of folic acid was 0.1 μg per liter. E. coli strains harboring different plasmids were cultivated in 2TY medium (Bacto tryptone, 16 g; yeast extract, 10 g; NaCl, 5 g; pH 7.2) at 37°C. Mycophenolic acid was purchased from Sigma (St. Louis, Mo.), while psicofurarine was a generous gift from Pharmacia & Upjohn (Kalamazoo, Mich.).
FIG. 1.
Construction of S. mutans integration vector pSIV2. As described in the text, the final plasmid, pSIV2-SGPAN, was designed to express sgp antisense RNA under control of the scrB promoter.
DNA manipulations.
DNA isolation, endonuclease restriction, ligation, and agarose gel electrophoresis were carried out by standard techniques (29).
Preparation of streptococcal membrane and cytoplasmic protein fractions.
Routinely, cells from 1-liter cultures were harvested by centrifugation at 15,000 × g at 4°C for 5 min in a Sorvall GSA rotor. The pellet was washed three times with ice-cold water and suspended in 8 to 20 ml (depending on the amount of the cells) of ice-cold 10 mM Na-phosphate buffer (pH 7.2)–1.0 mM EDTA–0.1 mM phenylmethylsulfonyl fluoride (Na-P buffer) to obtain a homogeneous suspension. Subsequently, the cells were lysed with a French pressure cell (SLM Instruments INC., Rochester, N.Y.) at 2,000 lb/in2 at least five times. The crude cell lysate was centrifuged at 20,000 × g at 4°C for 40 min to remove cell debris and unbroken cells. The resulting clear cell lysate was collected in Beckman polycarbonate centrifuge bottles (25 by 89 mm or 16 by 76 mm) and placed in precooled Beckman 70Ti or 50Ti ultracentrifuge rotors. The clear cell lysate obtained was centrifuged at 105,000 × g at 4°C for at least 1 h to sediment the membranes. The resulting pellet was washed two times with ice cold Na-P buffer and resuspended in ice-cold Na-P buffer (typically 200 to 900 μl) supplemented with 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} and used as the membrane fraction for all subsequent experiments. The supernatant fluid was used as the cytoplasmic protein fraction. All protein determinations were carried out with the Coomassie Plus protein assay kit (Pierce, Rockford, Ill.) as described by the manufacturer. Both protein fractions were stored at −20°C for short periods or at −70°C for long-term storage. In order to confirm that the membrane fractions were not contaminated with cytoplasmic components, all preparations were assayed for lactate dehydrogenase activity (11). Typically, the membrane fractions exhibited negligible amounts of lactate dehydrogenase activity.
SDS-PAGE and immunoblotting.
The protein samples (30 μg each) were resuspended in 6× sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and subjected to SDS–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) with a Bio-Rad (Hercules, Calif.) Mini-PROTEAN II system. Following electrophoresis, the proteins from the polyacrylamide gels were transferred to 0.2-μm-pore-size Immun-Blot polyvinylidene difluoride (PVDF) membranes (Bio-Rad) by using a TE series Transphor electrophoresis unit (Hoefer Scientific Instruments, San Francisco, Calif.) in a transfer buffer containing 10% methanol and 2.2 g of CHAPS per liter at pH 11 for 3 h at 40 V or overnight at 14 V in a cold room (4°C). After transfer, one membrane was stained for 5 min in a solution containing 0.1% Coomassie blue and 50% methanol. Subsequently, it was destained for about 15 min in a solution consisting of 50% methanol and 10% acetic acid. This membrane was used as a control, or, when needed, specific protein bands were cut out and used for amino-terminal sequencing (Pro Seg, Salem, Mass.). The second membrane was washed with TBS (100 mM Tris-HCl [pH 7.5], 0.9% NaCl) containing 1% (wt/vol) nonfat dry milk (blotting grade; Bio-Rad) for 5 min. The membranes were then treated with the primary antibodies in the same buffer and maintained at 4°C overnight. During the course of these experiments, we found that the PVDF membranes did not require nonspecific blocking. Following two washes of 5 min each with TTBS (TBS containing 0.1% [vol/vol] Tween 20), the membranes were treated with the respective secondary antibodies at room temperature for 30 min. The membranes were then washed twice for 5 min each with TTBS. The HRP Conjugate Substrate Kit (Bio-Rad) was employed to detect the positions of the antigenic bands of interest. These data were then quantitated by densitometric analysis of the respective immunoblots with a GS300 scanning densitometer (Hoefer). All immunoblotting was performed three to five times from as many different cell preparations. Data obtained with bacterial cultures grown in THB, THB supplemented with 0.1% sucrose (where applicable), or SMM were essentially identical. In this work we report data obtained with bacterial cultures grown in SMM.
Antibodies. (i) Primary antibodies.
Anti-SGP polyclonal antibody induced by a purified maltose-binding protein–SGP fusion protein was described previously (34). Anti-Hsp60 was purchased from StressGen Biotechnologies Corp. (Victoria, British Columbia, Canada); anti-S. mutans DnaK antibody was a gift from Jose Lemos (Rochester University, Rochester, N.Y.). Anti-GbpA and anti-Gbp59 antibodies were kindly supplied by J. Banas (Albany Medical College, Albany, N.Y.) and D. Smith (Forsyth Dental Center, Boston, Mass.), respectively.
(ii) Secondary antibodies.
Goat anti-rabbit immunoglobulin G–horseradish peroxidase (HRP) was obtained from Bio-Rad. Goat anti-rat HRP-conjugated antibody was purchased from Chemicon International Inc. (Temecula, Calif.).
Assay of nucleotide pools.
The nucleotide pool assays were based on the method described by Ochi (22). Samples of culture (100 ml) were filtered through 90-mm-diameter filters (Millipore; 0.45-μm pore size). Nucleotides were extracted with 15 ml of ice-cold 1 M formic acid for 1 h and centrifuged for 10 min at 6000 × g, and the supernatants were filtered through a nitrocellulose filter (Gelman; 0.45-μm pore size). The filtrates were freeze-dried and resuspended in 400 μl of ultrapure water. Intracellular concentrations of nucleotides were determined by high-performance liquid chromatography on a Partisil 10 SAX column (Whatman). Buffers used were 7 mM KH2PO4 (pH 4.0) (buffer A) and 0.5 M KH2PO4–0.5 M Na2SO4 (pH 5.4) (buffer B). The gradient was 100 to 53% buffer B over 50 min and 100% buffer A over 25 min, with a flow rate of 1.5 ml/min. Nucleotides were detected at 254 nm, and concentrations were expressed relative to optical density at 600 nm (OD600). GDP and GTP standards were from Sigma. Pure samples of ppGpp and pppGpp were obtained from Mercian Corporation, Tokyo, Japan.
RESULTS
Construction of a chromosomal sgp antisense RNA-expressing strain.
The basic strategy initially involved constructing an S. mutans integration vector (Fig. 1) containing a replicon which cannot be maintained in S. mutans. Upon transformation, this plasmid would integrate via a single crossover event into the S. mutans chromosome at a predetermined site, the gtfB gene. The plasmid pUC18 (37) was double digested with SspI and Eco311, and the fragment bearing the rep region was ligated to a BstYI/HindIII filled-in fragment carrying an erythromycin resistance marker from pResEm749 (31) to yield pUC18Erm1. Following Eam11051 digestion and T4 DNA polymerase repair, the latter plasmid was ligated to the gtfB 1.083-kb EcoRV fragment derived from pYNB13 (21a). The resulting plasmid was designated pSIV2 (Streptococcus integration vector). Following PstI and SacI digestion of pSIV2, the plasmid was ligated to a PstI/SacI fragment derived from pSGPAN749 carrying the sgp gene in an antisense orientation downstream of the scrB promoter (30). We reported earlier the expression of sgp antisense RNA from this fragment (30). The resultant construct was designated pSIV2-SGPAN. Subsequently, pSIV2 and pSIV2-SGPAN were transformed into S. mutans GS5, and the cells were plated on mitis salivarius agar plates. This allowed for convenient detection of the integration event, since disruption of the gtfB gene results in colonies which appeared smooth in contrast to the rough wild-type phenotype. The efficiency of transformation and integration was 1,600 to 2,000 erythromycin-resistant colonies per μg of plasmid DNA. That the vectors were indeed integrated into gtfB gene was confirmed by Southern blot analysis (data not shown). The two newly constructed strains were designated S. mutans GS5(gtfB)::pSIV2 for the strain having integrated the vector alone and S. mutans GS5(gtfB)::pSIV2-SGPAN for the strain expressing antisense sgp RNA from the host chromosome. In addition, a strain designated S. mutans GS5(gtfB)::pSIV2-SGP, which expresses sgp mRNA from the scrB promoter, was constructed by the same strategy (data not shown). The growth rates of this strain, S. mutans GS5(gtfB)::pSIV2, the parental S. mutans GS5, and S. mutans SP2 were similar, although we observed that the strain carrying the second sgp copy grows better than the other strains, especially at 43°C.
Stress-induced membrane association of SGP.
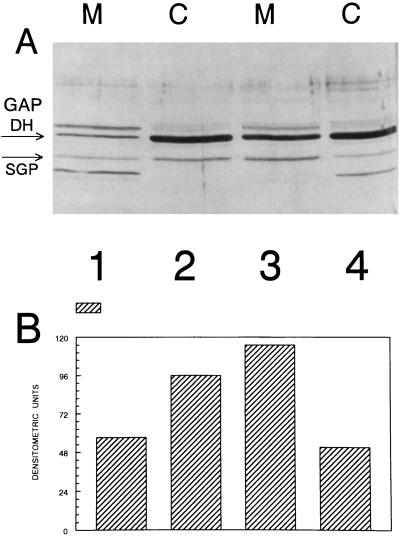
Both of the G proteins E. coli Era and P. aeruginosa Pra were reported to be in part associated with the membrane fractions derived from their respective hosts (7, 19). Therefore, it was of interest to examine the distribution of SGP between the cytoplasm and the cell membranes of the streptococcal cells under different growth and stress conditions. All experiments were carried out with S. mutans SP2 grown in SMM–1% glucose in a final volume of 1 liter. Figure 2 depicts an anti-SGP immunoblot of the membrane and cytoplasmic fractions derived from cells grown at 37°C to mid-log and stationary phases. Several unidentified protein bands in addition to SGP were detected by the maltose-binding protein–SGP antibody, which may have resulted from contamination of the antigen preparation (34). It is evident that with increasing age of the bacterial culture, SGP is more readily associated with the cell membrane. By contrast, when cells are grown at 43°C, even in mid-log growth a substantial portion of SGP is associated with the cell membrane (Table 1). When the cells were grown under acidic conditions (pH 5.5), both the SGP pool size and its relative association with membranes appeared to increase (Table 1) relative to those for cells grown at pH 7.0. Taken together, these results suggest increased association of SGP with the membrane fraction under stress conditions (elevated temperature, acidic pH, or stationary-phase growth).
FIG. 2.
(A) Immunoblot of membrane (M) and cytoplasmic (C) fractions from S. mutans SP2 grown in SMM with 1% glucose as the sole carbon source at 37°C to the mid-log growth phase (lanes 1 and 2) and to stationary phase (lanes 3 and 4). The bands corresponding to SGP and GAPDH are indicated with arrows. (B) Densitometric quantitation of the relative amounts of SGP in the membrane and cytoplasmic fractions.
TABLE 1.
Effects of the environment on the cellular distribution of SGP
| Growth conditionsa | SGP, DUb (%)
|
||
|---|---|---|---|
| Total | Membrane associated | Cytoplasmic | |
| 43°C, mid-log phase | 368 ± 16 | 239 ± 9 (65) | 128 ± 24 (35) |
| 43°C, stationary phase | 408 ± 36 | 244 ± 10 (60) | 164 ± 26 (40) |
| 37°C, mid-log phase | 150 ± 15 | 33 ± 20 (28) | 117 ± 26 (72) |
| 37°C, pH 5.5, mid-log phase | 390 ± 23 | 244 ± 25 (63) | 146 ± 29 (37) |
| 37°C, mid-log phase, 50 μM mycophenolic acid | 263 ± 19 | 128 ± 5 (49) | 135 ± 23 (51) |
| 37°C, mid-log phase, 250 μM psicofurarine | 252 ± 15 | 127 ± 3 (51) | 125 ± 14 (49) |
Growth was in SMM–1% glucose at pH 7.0 unless indicated otherwise.
Means and standard deviations from three independent experiments are shown. The values (arbitrary DU) were obtained by scanning the immunoblots as shown in Fig. 2.
Recently, the Pra protein of P. aeruginosa was reported to be involved in modulating the activity of the membrane-bound nucleoside diphosphate kinase (Ndk) during stationary-phase growth, resulting in alterations in the synthesis of GTP (7). Likewise, membrane association between Ndk and G proteins is well documented (15, 16). Therefore, in order to examine the relationship of SGP to GTP synthesis, we employed inhibitors of de novo guanosine nucleotide biosynthesis, i.e., mycophenolic acid and psicofurarine (23, 28, 33, 35). IMP dehydrogenase is the specific target of mycophenolic acid, while psicofurarine inhibits XMP aminase. S. mutans SP2 cultures were grown in SMM–1% glucose supplemented with 50 μM mycophenolic acid or 250 μM psicofurarine, which did not substantially inhibit cell growth (Table 1). For both antibiotics the relative proportions of SGP associated with the membrane fractions increased compared with those in the untreated cells (Fig. 2, lanes 1 and 2). Therefore, under conditions where guanosine nucleotide synthesis can be only marginally affected, increased association of SGP with the membranes is observed.
During these experiments, we noted a prominent immunopositive band at approximately 38 kDa (Fig. 2). We observed that the intensity of this band, especially associated with the membrane fractions, increased with the age of the bacterial culture or under stress conditions and correlated with the membrane association of SGP. Therefore, we eluted this protein band from Coomassie blue-stained control PVDF membranes and obtained an unambiguous amino-terminal sequence for this protein. Database searches showed that the protein of interest appeared to be the glycolytic enzyme GAPDH (EC 1.2.1.12), since the determined amino sequence (the 10 N-terminal amino acid residues) was 100% identical to the corresponding sequences of the enzymes from Streptococcus equisimilis (accession no. Q59906), Streptococcus pyogenes (accession no. P50467), and Lactococcus lactis subsp. lactis (Streptococcus lactis) (accession no. P52987) and to four other eukaryotic sequences. The relationship between changes in the distribution of GAPDH and SGP remains to be determined.
Effects of chromosomal sgp antisense RNA expression in S. mutans GS5(gtfB)::pSIV2-SGPAN.
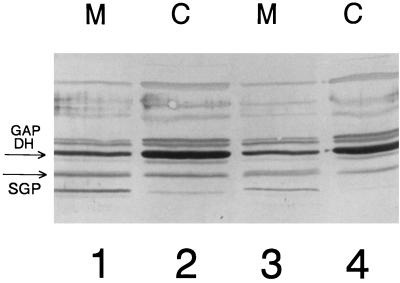
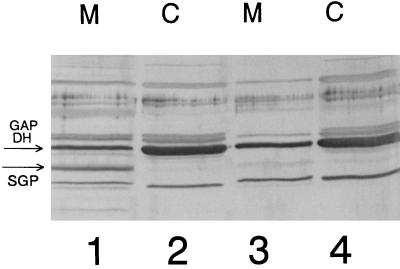
The sgp antisense RNA-producing strain (referred to below as the antisense strain) grew normally at 37°C in THB or in SMM–1% glucose. The growth curves did not significantly differ from those obtained with the strain which had only the vector integrated into the gtfB gene. However, in both THB and SMM–1% glucose media at 43°C at a low initial inoculum density (OD600 of 0.01), the antisense strain needed 4 to 5 days to reach the stationary growth phase, in contrast to the S. mutans GS5(gtfB)::pSIV2 control strain, which reached stationary phase within 24 h. When a high-density inoculum (0.2 to 0.3 OD600 units) was used, the antisense strain grew at 43°C nearly as well as the control strain. In minimal medium supplemented with 1% glucose at pH 5.5, the antisense strain grew but autoaggregated to the bottoms of the growth bottles, leaving a clear supernatant. In contrast, the control strain formed uniform turbid cultures. Immunoblot analysis (Fig. 3) revealed that the amounts of SGP associated with the membrane and cytoplasmic fractions at pH 5.5 (77 densitometric units [DU] [lane 3] and 38 DU [lane 4], respectively) in the antisense strain were smaller than those in the control strain (153 DU [lane 1] and 102 DU [lane 2]). However, when the antisense strain was grown in minimal medium supplemented with 1% sucrose, it grew slowly, leaving a clear supernatant, and aggregated at the bottoms of the growth bottles at 37°C. Immunoblot analysis of this experiment revealed nearly complete depletion of SGP from the cytoplasmic and membrane protein fractions of the antisense strain (Fig. 4, lanes 3 and 4) in the stationary growth phase. It is likely that this occurs because the scrB promoter is more active when sucrose is present than with glucose alone or in THB (12). The antisense strain did not grow at all in minimal medium–1% sucrose at 43°C, at 37°C at pH 5.5, or under the same conditions in THB supplemented with 0.1% sucrose, with either low- or high-density initial inocula. Therefore, the efficiency of antisense interference with sgp expression is very high.
FIG. 3.
Immunoblot of membrane (M) and cytoplasmic (C) fractions from S. mutans GS5(gtfB)::pSIV2 (lanes 1 and 2) and S. mutans GS5(gtfB)::pSIV2-SGPAN (lanes 3 and 4) grown in SMM–1% glucose at pH 5.50 and 37°C to the early stationary growth phase. The bands corresponding to SGP and GAPDH are indicated with arrows.
FIG. 4.
Immunoblot of membrane (M) and cytoplasmic (C) fractions from S. mutans GS5(gtfB)::pSIV2 (lanes 1 and 2) and S. mutans GS5(gtfB)::pSIV2-SGPAN (lanes 3 and 4) grown in SMM with 1% sucrose as the sole carbon source at 37°C to the early stationary growth phase. The bands corresponding to SGP and GAPDH are indicated with arrows.
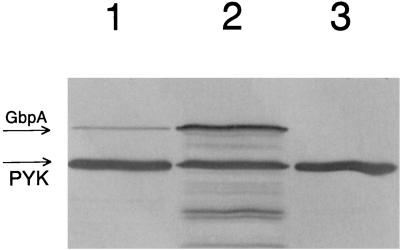
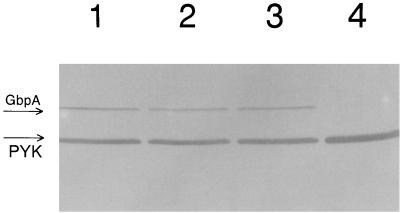
It was also of interest to determine if the depletion of SGP resulted in alteration of the general stress response of S. mutans. Immunoblot analysis of membrane and cytoplasmic fractions of the antisense strain depleted of SGP was done but did not show any significant differences in the content of the stress chaperone Hsp 60 or DnaK (Hsp 70). Since sucrose-dependent aggregation of S. mutans cells can be mediated by glucan-binding proteins as well as glucosyltransferase (Gtf) enzymes (17), the levels of several of these proteins in the SGP-depleted strain were compared. No differences in the Gtf activities or in the levels of the glucan-binding protein Gbp59 (determined by immunoblotting) were detected between the SGP-depleted strain and the control (data not shown). However, the use of anti-GbpA antibodies which react with glucan-binding protein A revealed that the total amount of GbpA in the antisense strain depleted of SGP was much larger than that in the strain with normal SGP content (Fig. 5). These results indicated that the level of GbpA in the control strain (38 DU [Fig. 5, lane 1]) was significantly lower than that in the antisense strain (192 DU [lane 2]). As an additional control, the S. mutans UA130 gbpA mutant (10) was also examined. The larger amount of GbpA in the SGP-depleted antisense strain was likely due to the stress conditions caused by an SGP deficiency. Therefore, GbpA does not appear to be a general stress response protein but may be induced under conditions of SGP depletion. Immunoblot analysis of total protein samples of bacterial antisense strain cultures grown in SMM–1% sucrose (Fig. 6, lane 1), SMM–1% sucrose–0.1% glucose (lane 2), SMM–1% sucrose–0.4% glucose (lane 3), and SMM–1% glucose (lane 4) revealed that growth in the presence of glucose alone did not yield detectable GbpA. Furthermore, glucose did not appear to suppress sucrose activation of the scrB promoter. Immunoblot analysis of the same protein samples with anti-SGP antibodies showed that SGP was present only in the protein sample derived from bacteria grown in SMM–1% glucose, i.e., when the bacterial cells are not depleted of SGP (data not shown). Performing the same experiments depicted in Fig. 6 with the control (nonantisense) strain revealed that GbpA was not detectable (data not shown). Anti-GbpA also detected a 52-kDa immunopositive protein band (Fig. 5 and 6) in these experiments. We also noticed that this band was more strongly associated with the membranes of the control strain than with those of the antisense strain (data not shown). In order to identify this protein, the 52-kDa protein band was eluted and its N-terminal amino acid sequence was determined. The results suggested that the band was comprised of two proteins, pyruvate kinase (EC 2.7.1.40) and NADP-dependent GAPDH (EC 1.2.1.9), since the determined amino acid sequences were 90 and 100% identical to the corresponding sequences from L. lactis subsp. lactis (accession no. Q07637) and S. mutans (accession no. Q59931), respectively. In an attempt to determine which of these two S. mutans proteins was reacting with anti-GbpA, the total protein extract from S. mutans BCH 150, which is NADP-dependent GAPDH deficient (4), was probed with anti-GbpA. The intensity of the 52-kDa band was identical to that observed in the previous experiments, suggesting that the immunopositive protein was pyruvate kinase.
FIG. 5.
Immunoblot of the total protein extracts from S. mutans GS5(gtfB)::pSIV2 (lane 1), S. mutans GS5(gtfB)::pSIV2-SGPAN (lane 2), and S. mutans BCH 150 (lane 3) grown in SMM–1% sucrose at 37°C to the early stationary growth phase. The bands corresponding to glucan-binding protein A (GbpA) and pyruvate kinase (PYK) are indicated with arrows.
FIG. 6.
Immunoblot of total protein extracts from S. mutans GS5(gtfB)::pSIV2-SGPAN grown in 5 ml of SMM with 1% sucrose (lane 1), 1% sucrose plus 0.1% glucose (lane 2), 1% sucrose plus 0.4% glucose (lane 3), and 1% glucose (lane 4) at 37°C to the early stationary growth phase. The bands corresponding to glucan-binding protein A (GbpA) and pyruvate kinase (PYK) are indicated with arrows.
Assay of guanine nucleotide pools.
In order to further examine whether SGP is involved in regulating the GTP/GDP ratio, we analyzed the intracellular guanine nucleotide pools during growth of the S. mutans strains in minimal medium by high-performance liquid chromatography analysis. The results presented in Table 2 showed that during the growth of S. mutans GS5(gtfB)::pSIV2, which contains a functional sgp gene, intracellular GDP pools could not be detected and the major guanine nucleotide was GTP. The GTP pool increased threefold between mid-log phase and early stationary phase, concomitant with a decrease in ppGpp levels.
TABLE 2.
Intracellular levels of guanine nucleotides during growth of S. mutans in SMM–1% glucose at 37°C
| S. mutans strain and growth phase | pmol/A600 unita
|
|||
|---|---|---|---|---|
| GDP | GTP | ppGpp | pppGpp | |
| GS5(gtfB)::pSIV2 | ||||
| Mid-log | 0 | 250 ± 22 | 131 ± 9 | 0 |
| Early stationary | 0 | 749 ± 21 | 7 ± 0.43 | 0 |
| GS5(gtfB)::pSIV2-SGPAN | ||||
| Mid-log | 505 ± 26 | 246 ± 10 | 90.5 ± 4 | 39.5 ± 2 |
| Early stationary | 1,509 ± 82 | 865 ± 35 | 40 ± 1.0 | 0 |
Means and standard deviations from duplicate experiments.
In S. mutans GS5(gtfB)::pSIV2-SGPAN, which is attenuated in SGP expression, the GTP/GDP ratio at mid-log and early stationary phases is vastly different from that in the control strain. Although the levels of GTP in the two strains are comparable at each growth phase, it can be seen that GDP was the major guanine nucleotide, with levels approximately twice those of the GTP pool in the antisense RNA-producing strain (Table 2). Interestingly, pppGpp and ppGpp were detected at mid-logarithmic growth phase, but only ppGpp was observed at early stationary phase in this strain.
DISCUSSION
Previously, by employing immunogold labeling, we demonstrated that SGP was associated with both the cytoplasmic membrane and the cytoplasm of S. mutans (34). Recent studies (19) have also indicated that the Era protein specifically binds to E. coli membranes, although the site of interaction was not identified. Purification and identification of the Pra protein from membrane fractions of P. aeruginosa were also described (7). Therefore, in this work we examined in detail the distribution of SGP between the membrane and cytoplasmic fractions derived from cells at different growth stages and under stress conditions. From the present results it is evident that during different growth phases, at elevated temperatures, and at acidic pH, the distribution of SGP varies between the cytoplasm and the membranes of the streptococcal cells. Immunoblot analysis of cytoplasmic and membrane protein fractions isolated from mid-log and stationary growth stages of bacterial populations grown at normal (37°C) and at elevated (43°C) temperatures or at acidic pH (pH 5.5) demonstrated that the total amount of SGP increased with the age of the bacterial culture, elevated temperatures, or acidic pH. Further, it was demonstrated that a substantial portion of SGP is associated with the membrane fraction with increasing age of the bacterial culture as well as under stress conditions. The actual amounts of cytoplasmic SGP change, although not in the range of the membrane-associated SGP (Table 1). That the expression of SGP is subject to upregulation when it is needed, i.e., under stress conditions, is a reasonable assumption. Furthermore the antimetabolites mycophenolic acid and psicofurarine, which are known to alter nucleotide pools, particularly the level of GTP (23, 28, 33, 35), also increased the total cellular amounts and the relative amounts of SGP associated with the membrane fraction. In addition, these experiments demonstrate that the association of SGP with the cellular membrane is likely due to the inhibitory action of these antimetabolites exerted on key enzymes involved in de novo guanine nucleotide biosynthesis. This finding, as well as PAGE experiments performed under nondenaturating conditions (data not shown), ruled out the possibility that the association of the SGP with the cellular membrane might be due to artifacts caused by autoaggregation of SGP molecules under stress conditions, resulting in cosedimentation of SGP with membranes. Additionally, we performed two sets of experiments that demonstrate that the binding of SGP to the cellular membranes is direct and specific. Binding of SGP to membranes immobilized on microtiter plates as well as immunoprecipitation experiments demonstrate such binding and will be reported elsewhere.
Experiments with eukaryotic GTP-binding proteins have pointed out that these proteins may form complexes with multiple protein species and modulate their activities (27). The P. aeruginosa Pra protein has recently been proposed to regulate GTP levels in the stationary growth phase (7). Direct interaction between membrane-associated nucleoside diphosphate kinase and a GTP-binding protein (Gs) has also been reported for rat liver plasma membrane (15). Based on our results and the published data, it was suggested that the membrane association of SGP during nutrient depletion or under stress conditions could be a physiologically relevant process. This may result from a role for SGP in the regulation of the intracellular GTP/GDP ratio. In order to examine this possibility, a strain capable of expressing sgp antisense RNA from the host chromosome was constructed. This antisense RNA was transcribed under the direction of the scrB promoter. Since recent results from this laboratory (12) suggested that sucrose increases transcription from the scrB promoter in S. mutans, this disaccharide could be used to alter SGP levels in the cells. When grown in THB containing 0.1% sucrose or in SMM supplemented with 1% sucrose as a sole carbon source, the antisense strain was nearly completely depleted of SGP (Fig. 4). Under these conditions, this strain strongly autoaggregated, which did not allow for an accurate assessment of the growth rate relative to that of the control strain S. mutans GS5(gtfB)::pSIV2.
Measurement of intracellular guanine nucleotide pools has indicated that the presence of a functional sgp gene may enable S. mutans to maintain high-energy GTP as the major guanine nucleotide. It is known that GTP is required in many cellular functions during rapid growth and also under stress conditions. Cells producing low levels of SGP are no longer able to maintain GTP as the major guanine nucleotide, and increased levels of GDP are observed. It is known that physiological levels of GDP are normally low compared with the levels of GTP (5). As the only difference between the isogenic strains is apparently the levels of SGP, it is suggested that SGP is involved in regulating the intracellular GTP/GDP ratio. In addition, the absolute level of the guanosine nucleotides is markedly increased during attenuation of SGP expression (Table 2). However, the molecular basis for such increases still remains to be determined. In enteric bacteria during the stress response (stringent response), GTP is the immediate precursor for pppGpp, whereas GDP can be converted directly into ppGpp via a RelA-dependent pathway (5). However, previous work with other streptococci (21) has shown that GTP is the acceptor nucleotide required for ppGpp synthesis. This suggests that the increase in the GTP pools at early stationary phase is in part due to a lowered requirement for ppGpp synthesis, although ppGpp was obviously required by the antisense strain during the early stationary growth phase (Table 2). However, we cannot formally rule out the possibility that the alterations in the guanosine nucleotide pools are not direct effects of altered SGP levels but may be an indirect response to general stress conditions resulting from SGP depletion.
If SGP is able to regulate Ndk activity, we might expect to see not only differences in the GDP/GTP ratio between S. mutans GS5(gtfB)::pSIV2 and S. mutans GS5(gtfB)::pSIV2-SGPAN but also a difference in the levels of ppGpp and pppGpp. Indeed, there is a notable difference between the two strains. At the mid-log phase in S. mutans GS5(gtfB)::pSIV2, ppGpp (131 pmol/A600 unit) is the sole highly phosphorylated stress response nucleotide detected. However, in GS5(gtfB)::pSIV2-SGPAN, the normally short-lived pppGpp can also be detected (39.5 pmol/A600 unit). It is also likely that depletion of SGP in S. mutans GS5(gtfB)::pSIV2-SGPAN may result in a general stress response, as the levels of ppGpp (40 pmol/A600 unit) were considerably higher in the early stationary phase than the levels (7 pmol/A600 unit) in S. mutans GS5(gtfB)::pSIV2; i.e., there appears to be a prolonged production of ppGpp suggestive of a prolonged stress response.
Glucan-binding protein A is thought to contribute to sucrose-dependent adherence of the mutans streptococci to hard surfaces and thus play a role in tooth colonization and caries formation (2, 10). However, it has recently been reported that a gbpA mutant strain actually displayed enhanced sucrose-dependent adherence in vitro and increased cariogenicity in vivo (10). Like the glucosyltransferases, GbpA is a secreted protein found both in association with the cell surface and in the extracellular fluids. Immunoblot analysis carried out with total protein samples (Fig. 5 and 6) as well as cytoplasmic and membrane fractions (data not shown) revealed high intracellular levels of GbpA. Moreover, upon SGP depletion, the amount of GbpA in the antisense sgp strain markedly increased. It is unlikely that this was due to the presence of sucrose in the growth medium, since the level of GbpA in the control strain grown under the same conditions was significantly lower. Furthermore, it was shown previously that the regulation of gbpA expression was not affected by sucrose (2). The same authors suggested that GbpA might have a function for S. mutans in addition to, but independent of, plaque formation (2). Therefore, it was rather surprising to find that in the SGP-depleted strain the amount of GbpA was much higher than that in a strain with normal SGP content. Thus, GbpA appears to be stress-related protein. The autoaggregation of the cells in the presence of sucrose under stress conditions may have unknown beneficial properties for S. mutans. In addition, we observed higher levels of GbpA in strains grown at pH 5.5 versus pH 7.0 (data not shown).
Recently, by employing an E. coli-Staphylococcus aureus shuttle vector, expression of an antisense hla fragment in S. aureus was shown to reduce alpha-toxin production in vitro (14). Antisense vectors have also been used to develop a conditional mutagenesis system in mycobacteria (24). In developing our system, we constructed and employed a vector which upon integration into a predetermined target sequence could render chromosomal expression of antisense RNA designed to specifically interfere with information processing of an essential gene. However, we did not determine the precise site of this alteration. This could occur at the level of transcription, as well as at a posttranscriptional step. Theoretically, integration vectors bearing potential for antisense RNA expression can be constructed to study any desirable gene or product function in a biological system.
The present results suggest that SGP plays a role in the environmental stress response of S. mutans. This is likely mediated by the association of the protein with the cytoplasmic membrane. Membrane-bound SGP complexes could be crucial for maintaining the intracellular pools of GTP required for many diverse cellular functions. One consequence of the interference with the maintenance of sufficient SGP concentrations in S. mutans is the induction of glucan-binding protein A. However, it is not clear how increased levels of GbpA would enhance the ability of the organism to survive under relatively harsh environmental conditions. Therefore, additional approaches will be required to further understand the complex stress response of these organisms.
ACKNOWLEDGMENT
These studies were supported in part by National Institutes of Health grant DE 10711.
REFERENCES
- 1.Ahnn J, March P E, Takiff H E, Inouye M. A GTP-binding protein of Escherichia coli has homology to yeast RAS proteins. Proc Natl Acad Sci USA. 1986;83:8849–8853. doi: 10.1073/pnas.83.23.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas J A, Potvin H C, Singh R N. The regulation of Streptococcus mutans glucan-binding protein A expression. FEMS Microbiol Lett. 1997;154:289–292. doi: 10.1111/j.1574-6968.1997.tb12658.x. [DOI] [PubMed] [Google Scholar]
- 3.Bourne H R, Sanders D A, McCormick F. The GTPase super-family: conserved structure and molecular mechanism. Nature (London) 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 4.Boyd D A, Cvitkovitch D G, Hamilton I R. Sequence, expression, and function of the gene for the nonphosphorylating, NADP-dependent glyceraldehyde-3-phosphate dehydrogenase of Streptococcus mutans. J Bacteriol. 1995;177:2622–2627. doi: 10.1128/jb.177.10.2622-2627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashel M, Gentry D R, Hernandez J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 1458–1496. [Google Scholar]
- 6.Chen S-M, Takiff H E, Barber A M, Dubois G C, Bardwell J C A, Court D I. Expression and characterization of RNaseIII and Era proteins. J Biol Chem. 1990;265:2888–2895. [PubMed] [Google Scholar]
- 7.Chopade B A, Shankar S, Sundin G W, Mukhopadhyay S, Chakrabarty A M. Characterization of membrane-associated Pseudomonas aeruginosa Ras-like protein Pra, a GTP-binding protein that forms complexes with truncated nucleoside diphosphate kinase and pyruvate kinase to modulate GTP synthesis. J Bacteriol. 1997;179:2181–2188. doi: 10.1128/jb.179.7.2181-2188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara S, Kobayashi S, Nakayama H. Development of a minimal medium for Streptococcus mutans. Arch Oral Biol. 1978;23:601–602. doi: 10.1016/0003-9969(78)90280-7. [DOI] [PubMed] [Google Scholar]
- 9.Gollop N, March P E. A GTP-binding protein (Era) has an essential role in growth rate and cell cycle control in Escherichia coli. J Bacteriol. 1991;173:2265–2270. doi: 10.1128/jb.173.7.2265-2270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazlett K R O, Michalek S M, Banas J A. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect Immun. 1998;66:2180–2185. doi: 10.1128/iai.66.5.2180-2185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillman J D, Duncan M J, Stashenko K P. Cloning and expression of the gene encoding the fructose-1,6-diphosphate-dependent l-(+)-lactate dehydrogenase of Streptococcus mutans. Infect Immun. 1990;58:1290–1295. doi: 10.1128/iai.58.5.1290-1295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiratsuka K, Wang B, Sato Y, Kuramitsu H K. Regulation of sucrose-6-phosphate hydrolase activity in Streptococcus mutans: characterization of the scrR gene. Infect Immun. 1998;66:3736–3743. doi: 10.1128/iai.66.8.3736-3743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inada T, Kawakami K, Chen S, Takiff H E, Court D I, Nakamura Y. Temperature-sensitive lethal mutant of Era, a G protein in Escherichia coli. J Bacteriol. 1989;171:5017–5024. doi: 10.1128/jb.171.9.5017-5024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kernodle D S, Voladri R K R, Menzies B E, Hager C C, Edwards K M. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect Immun. 1997;65:179–184. doi: 10.1128/iai.65.1.179-184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura N, Shimada N. Direct interaction between membrane-associated nucleoside diphosphate kinase and GTP-binding protein (Gs), and its regulation by hormones and guanine nucleotides. Biochem Biophys Res Commun. 1988;151:248–256. doi: 10.1016/0006-291x(88)90586-4. [DOI] [PubMed] [Google Scholar]
- 16.Kimura N, Shimada N. Evidence for complex formation between GTP-binding protein (Gs) and membrane-associated nucleoside diphosphate kinase. Biochem Biophys Res Commun. 1990;168:99–106. doi: 10.1016/0006-291x(90)91680-q. [DOI] [PubMed] [Google Scholar]
- 17.Kuramitsu H K. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993;42:159–176. doi: 10.1177/10454411930040020201. [DOI] [PubMed] [Google Scholar]
- 18.Lerner C G, Inouye M. Pleiotropic changes resulting from depletion of Era, an essential GTP-binding protein in Escherichia coli. Mol Microbiol. 1991;5:951–957. doi: 10.1111/j.1365-2958.1991.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y P, Sharer J D, March P E. GTPase-dependent signaling in bacteria: characterization of a membrane-binding site for Era in Escherichia coli. J Bacteriol. 1994;176:44–49. doi: 10.1128/jb.176.1.44-49.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.March P E, Lerner C G, Ahnn J, Cui X, Inouye M. The Escherichia coli Ras-like protein (Era) has GTPase activity and is essential for cell growth. Oncogene. 1988;2:539–544. [PubMed] [Google Scholar]
- 21.Mechold U, Malke H. Characterization of the stringent and relaxed responses of Streptococcus equisimilis. J Bacteriol. 1997;179:2658–2667. doi: 10.1128/jb.179.8.2658-2667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Nakano, Y. J., and H. K. Kuramitsu. Unpublished results.
- 22.Ochi K. Occurrence of the stringent response in Streptomyces sp. and its significance for the initiation of morphological and physiological differentiation. J Gen Microbiol. 1986;132:2621–2631. doi: 10.1099/00221287-132-9-2621. [DOI] [PubMed] [Google Scholar]
- 23.Ochi K, Kandala J, Freese E. Evidence that Bacillus subtilis sporulation induced by the stringent response is caused by the decrease in GTP or GDP. J Bacteriol. 1982;151:1062–1065. doi: 10.1128/jb.151.2.1062-1065.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parish T, Stoker N G. Development and use of a conditional antisense mutagenesis system in mycobacteria. FEMS Microbiol Lett. 1997;154:151–157. doi: 10.1111/j.1574-6968.1997.tb12637.x. [DOI] [PubMed] [Google Scholar]
- 25.Perry D, Wondrack L M, Kuramitsu H K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983;41:722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillutla R C, Sharer J D, Gulati P S, Wu E, Yamashita Y, Lerner C G, Inouye M, March P E. Cross-species complementation of the indispensable Escherichia coli era gene highlights amino acid regions essential for activity. J Bacteriol. 1995;177:2194–2196. doi: 10.1128/jb.177.8.2194-2196.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randazzo P A, Northup J K, Kahn R A. Activation of a small GTP-binding protein by nucleoside diphosphate kinase. Science. 1991;254:850–853. doi: 10.1126/science.1658935. [DOI] [PubMed] [Google Scholar]
- 28.Rohlman C E, Matthews R G. Role of purine biosynthetic intermediates in response to folate stress in Escherichia coli. J Bacteriol. 1990;172:7200–7210. doi: 10.1128/jb.172.12.7200-7210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30.Sato T, Wu J, Kuramitsu H K. The sgp gene modulates stress responses of Streptococcus mutans: utilization of an antisense RNA strategy to investigate essential gene functions. FEMS Microbiol Lett. 1998;159:241–245. doi: 10.1111/j.1574-6968.1998.tb12867.x. [DOI] [PubMed] [Google Scholar]
- 31.Shiroza T, Kuramitsu H K. Construction of a model secretion system for oral streptococci. Infect Immun. 1993;61:3745–3755. doi: 10.1128/iai.61.9.3745-3755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood P, Lerner C G, Shimamoto T, Lu Q, Inouye M. Characterization of the autophosphorylation of Era, an essential GTPase in Escherichia coli. Mol Microbiol. 1994;12:201–208. doi: 10.1111/j.1365-2958.1994.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 33.Udaka S, Moyed H S. Inhibition of parental and mutant xanthosine 5′-phosphate aminase by psicofurarine. J Biol Chem. 1963;238:2797–2803. [PubMed] [Google Scholar]
- 34.Wu J, Cho M-I, Kuramutsu H K. Expression, purification, and characterization of a novel G protein, SGP, from Streptococcus mutans. Infect Immun. 1995;63:2516–2521. doi: 10.1128/iai.63.7.2516-2521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu T-W, Scrimgeour K G. Properties of inosinic acid dehydrogenase from Bacillus subtilis. II. Kinetic properties. Can J Biochem. 1973;51:1391–1398. doi: 10.1139/o73-182. [DOI] [PubMed] [Google Scholar]
- 36.Yamashita Y, Takehara T, Kuramitsu H K. Molecular characterization of a Streptococcus mutans mutant altered in environmental stress response. J Bacteriol. 1993;175:6220–6228. doi: 10.1128/jb.175.19.6220-6228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]