Summary
Background
Intensive lifestyle modification showed variable success in the prevention of major clinical events and mortality among people with prediabetes. We propose that age may partly explain the heterogeneity and that health hazards related to prediabetes are age-specific.
Methods
We conducted a retrospective analysis of a territory-wide diabetes surveillance dataset from the Hong Kong Hospital Authority between 2000 and 2019. Prediabetes was defined according to the American Diabetes Association criteria. Proportional Cox regression was performed, stratified by baseline age categories (20-39, 40-59, 60-79 and ≥80 years).
Findings
1,630,942 individuals were included in the analysis. Compared with normoglycaemia, prediabetes was associated with greater hazards for cardiovascular disease (CVD) and all-cause mortality in most age groups but the effect size attenuated with ascending age (p value for trend <0·05). In the youngest and in the oldest age categories, the respective hazard ratios (95% confidence interval) of prediabetes vs normoglycaemia were 1·79 (1·59, 2·01) and 1·00 (0·95, 1·05) for CVD, and 1·36 (1·20, 1·55) and 0·99 (0·97, 1·02) for all-cause mortality. Similar associations were found for chronic kidney disease, end-stage kidney disease, all-site cancer, all-site infection, subtypes of CVD, and cause-specific mortality. The associations became attenuated but remained after excluding people who later developed diabetes and adjusting for metabolic factors. Similar associations were observed in prediabetes defined by impaired fasting glucose, but not HbA1c.
Interpretation
Prediabetes is associated with higher risk of major clinical events, even excluding subsequent development of diabetes and adjusting for metabolic factors. The risk relationships are stronger in young than older people.
Funding
This study did not receive any specific funding.
Keywords: Prediabetes, Cardiovascular disease, Mortality, Kidney disease, Cancer, Infection, Ageing
Research in context.
Evidence before this study
We searched PubMed from the inception of the database to April 27, 2022, using the search terms “age-specific association” AND (“prediabetes” OR “impaired fasting glucose” OR “elevated HbA1c” OR “impaired glucose tolerance”) AND (“cardiovascular disease” OR “mortality” OR “kidney disease” OR “cancer” OR “infection”). A meta-analysis on the association between prediabetes and mortality showed larger hazard ratios in the group with mean age below 60 years compared to those aged above. However, the age-specific association between prediabetes and other outcomes have not been examined and population-based cohort data are lacking.
Added value of this study
Based on a retrospective analysis of a territory-wide database of two million people living in Hong Kong, we found prediabetes is associated with higher risk of major clinical events including cardiovascular diseases, end-stage kidney disease, all-site cancer, all-site infection, and all-cause mortality. The risk associations are stronger in young than older people, even excluding subsequent development of diabetes and adjusting for metabolic factors.
Implications of all the available evidence
This study provides a clue to the reasons of heterogeneous mortality benefits in different diabetes prevention programs. This study emphasizes the importance of age in identifying and managing high-risk population with prediabetes and calls for age-stratified preventive strategies, especially in young people who have the potential to benefit more from the prevention efforts.
Alt-text: Unlabelled box
Introduction
Prediabetes is a metabolic state characterised by blood glucose levels higher than normal but lower than diabetes thresholds.1 In 2021, 541 million and 319 million adults, or 11·4% and 6·9% of the whole adult population worldwide, are living with impaired glucose tolerance (IGT) and impaired fasting glucose (IFG).2 Prediabetes confers a high lifetime risk of progression to diabetes.3,4 It is also linked to premature mortality and higher risks of cardiovascular disease (CVD), chronic kidney disease (CKD), cancer and other adverse outcomes.5 Intensive lifestyle modification and pharmacotherapy have been shown to prevent or delay the onset of diabetes, but have variable success in the prevention of major clinical events and mortality.6,7 In the era of precision prevention, implementation of health programmes should consider individual factors that may influence responses to preventive strategies and target intervention to those who are going to derive the most benefits.
Only few studies have examined age-specific associations of fasting plasma glucose (FPG) or glycated haemoglobin (HbA1c) with incident CVD and all-cause mortality.8, 9, 10, 11, 12 A meta-analysis on the association between prediabetes and mortality showed that the strength of the association was larger in the group with mean age below 60 years compared to those aged above,13 although these results might be limited by the heterogeneity of the included studies. We propose that people with prediabetes will have more adverse health outcomes when prediabetes occurs at a younger than older age. In this context, we aimed to evaluate the age-stratified associations of prediabetes with incident major clinical events and mortality in Hong Kong Chinese, using data from the territory-wide Hong Kong Diabetes Surveillance Database (HKDSD). In addition, we examined whether these associations are robust to different definitions of prediabetes.
Methods
Study population
The Hong Kong Hospital Authority (HA) governs all public hospitals and the majority of community-based primary care clinics to provide about 90% of healthcare services for Hong Kong residents.14 In 2000, the Hong Kong HA adopted an electronic medical record system that captures diagnostic and procedure codes, laboratory tests, medication prescription and linked vital status longitudinally. The HKDSD comprises clinical information of all individuals who have ever had at least one glycaemic measurement including FPG, random plasma glucose, HbA1c, and 2 h oral glucose tolerance test (2-h OGTT) at any health facilities within the Hong Kong HA. All individuals who attended routine screening for diabetes or had opportunistic testing during clinical encounters were included. Detailed information has been reported elsewhere.15
From January 2000 to December 2019, 2,692,880 individuals had measured FPG, HbA1c, and/or 2-h OGTT for at least once in the HKDSD. To avoid misclassification of stress-induced and gestational hyperglycaemia, all records of FPG during hospital admissions and all glycaemic measurements within 24-40 gestational week were excluded from the analysis. The highest 1% and the lowest 1% records of FPG, HbA1c, and 2-h OGTT were removed to avoid the effect of extreme values. We excluded individuals who were aged < 20 years or had diabetes at baseline as identified by diagnosis codes, prescription of glucose-lowering drugs, FPG ≥ 7·0 mmol/L, HbA1c ≥ 6·5%, or 2-h OGTT ≥ 11·1 mmol/L. Finally, 2,003,361 individuals were included in this analysis. This study has received approval by the local clinical research ethics committee.
Definition of prediabetes and baseline characteristics
We defined prediabetes and normoglycaemia based on glycaemic measurements during the first 3 months from the first record in HKDSD. People who met any of the following criteria during the first 3 months according to the American Diabetes Association (ADA)16 were defined as prediabetes: IFG (FPG 5·6-6·9 mmol/L), HbA1c 5·7-6·5%, and/or IGT (2-h OGTT 7·8-11·0 mmol/L), in any one available measurement. People were considered to have normoglycaemia if their glycaemic measurements did not fulfil the diagnostic criteria of prediabetes or diabetes during the first 3 months. The end of the first 3 months was set as baseline.
We obtained the following baseline clinical information: age, sex, total cholesterol, low-density lipoprotein cholesterol (LDL-C), ratio of triglyceride to high-density lipoprotein-cholesterol (triglyceride/HDL-C), albumin, haemoglobin, and use of lipid-regulating drugs and blood pressure-lowering drugs. Past medical conditions including history of CVD, end-stage kidney disease (ESKD), cancer and infection were obtained using ICD-9 codes recorded at or before baseline. Information including smoking status, alcohol consumption, socioeconomic status, body mass index and blood pressures were not available for most people in the database as the data were not routinely captured in a structured manner in the electronic health record.
Outcome ascertainment
All individuals were observed for outcome events until death or December 2019. Outcome events of interest included incident CVD, ESKD, all-site cancer, all-site infection, and all-cause mortality. We also explored CKD and individual CVD outcomes including coronary heart disease (CHD), haemorrhagic stroke, ischemic stroke, congestive heart failure (CHF), and peripheral vascular disease (PVD). Cause-specific mortality including mortality from CVD, cancer, kidney disease, infection, and other causes were examined. We used laboratory tests and international classification of disease (ICD) codes to define these outcomes, detailed definitions are shown in Table S1.
Statistical analyses
Continuous variables were expressed as mean (standard deviation) or median (inter-quartile range), as appropriate, and categorical variables as number (percentages). Between-group comparison was conducted by χ2 test for categorical variables, Student's t-test for normally distributed continuous variables, and Kruskal-Wallis test for continuous variables with skewed distribution. Incidence rates expressed as per 1000 person-years were calculated as the total number of new cases of a corresponding event divided by the sum of the person-time of the at-risk population.
We used proportional Cox hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CI) of prediabetes versus normoglycaemia for each of the outcome event, stratified by four age groups: 20-39, 40-59, 60-79, and ≥80 years. For each outcome, we excluded individuals with histories of CVD, CKD, CHF, cancer, or corresponding events at baseline. To avoid the effect of reverse causality, people developing the corresponding event in the first year of follow-up were also excluded. To minimise the effect of competing risk of death, we censored death to calculate cause-specific hazards. Trend for associations between prediabetes and outcome events of interest across age groups was examined by adding an interaction term in the models. The interaction of age was examined by likelihood ratio test. Model 1 was adjusted for age, sex, and calendar year at baseline. Model 2 excluded people who developed diabetes after baseline and before the event or before the end of follow-up, in addition to model 1. Similar to model 2, model 3 also excluded people who developed diabetes and was further adjusted for baseline LDL-C, triglyceride/HDL-C ratio, haemoglobin, albumin, use of lipid-regulating drugs and blood pressure-lower drugs. We applied a fully-adjusted additive hazard model to estimate the absolute risk differences (ARDs, additional cases per 1,000 person-years) of each outcome associated with prediabetes. To depict the age-related trends in associations between prediabetes and outcome events of interest, we estimated HRs with 95% CI at each age from 40 to 80 years, based on which, we fitted the mixed-effect meta-regression using the restricted cubic spline with three knots located at the 5th, 50th and 95th percentiles of the age range.
We repeated the above analyses re-defining prediabetes based on either FPG (5·6-6·9 mmol/L) or HbA1c (5·7-6·4%) criteria of ADA, using available measurement during the first 3 months from the first record in HKDSD, respectively. We defined people with normoglycaemia as those not meeting the diagnostic criteria of prediabetes based on each definition. As only 1·4% of people in the HKDSD had 2 h OGTT, we did not address IGT as a separate definition of prediabetes. We also evaluated the association between one-unit increase in glycaemic biomarkers and major health outcomes.
We performed several sensitivity analyses. Firstly, we used the Fine-Gray model to account for the competing risk of death for primary outcomes including incident CVD, ESKD, all-site cancer, and all-site infection. Secondly, we estimated the E-value to quantify the evidence strength for the association in the possible presence of unmeasured confounding.17 The E-value provides a risk ratio for the minimum association that an unmeasured confounder would need to have with both the outcome and the exposure, independently of all measured covariates, to nullify the observed results. A small E-value close to 1 implies little unmeasured confounding would be needed to explain away an effect estimate, a large E-value implies that considerable unmeasured confounding would be needed to explain away an effect estimate. We estimated the E-values both for point estimates and confidence interval. Thirdly, as only 35·4% of people had complete data on metabolic factors in model 3, we repeated model 1 in people with complete data and compared results with model 1 in the full cohort to examine potential bias introduced by missing data. Two-sided p value < 0·05 was regarded as statistically significant. All analyses were conducted using R software, version 4·1·2 (R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
The study received no funding. The corresponding authors had full access to study data and are responsible for the decision to submit for publication.
Results
Compared to people with normoglycaemia, those with prediabetes were older, had a preponderance of men, higher levels of total cholesterol, LDL-C, triglyceride/HDL-C, haemoglobin and a lower albumin level. The frequencies of pre-existing CVD, CKD, CHF and cancer, and use of lipid-regulating drugs and blood pressure-lowering drugs, were higher in people with prediabetes (Table 1).
Table 1.
Clinical characteristics in people with normoglycaemia and prediabetes at baseline.
| Normoglycaemia | Prediabetes | |
|---|---|---|
| Number | 1,474,413 | 528,948 |
| Age, years | 55·0 (44·0, 65·0) | 61·0 (52·0, 71·0) |
| Men, n (%) | 603,274 (40·9) | 271,198 (51·3) |
| Total cholesterol, mmol/L | 5·1 (4·5, 5·8) | 5·2 (4·5, 5·9) |
| LDL-C, mmol/L | 3·0 (2·5, 3·6) | 3·1 (2·5, 3·7) |
| Triglyceride/HDL-C | 0·8 (0·5, 1·3) | 1·0 (0·6, 1·6) |
| Haemoglobin, g/dL | 13·5 (12·4, 14·5) | 13·7 (12·6, 14·7) |
| Albumin, g/L | 42·3 (40·0, 45·0) | 42·0 (39·0, 44·6) |
| History of CVD, n (%)a | 85,328 (5·8) | 54,660 (10·3) |
| History of CKD, n (%)a | 151,076 (10·2) | 84,606 (16·0) |
| History of CHF, n (%)a | 15,881 (1·1) | 11,367 (2·1) |
| History of cancer, n (%)a | 40,069 (2·7) | 20,031 (3·8) |
| Use of blood pressure medications, n (%) | 3,807 (0·3) | 2,696 (0·5) |
| Use of lipid-regulating drugs, n (%) | 113,582 (7·7) | 73,769 (13·9) |
History of a disease refers to a person met criteria for these morbidities from 1st January 2000 until baseline. CHF: Congestive heart failure; CKD: chronic kidney disease; CVD: cardiovascular disease; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
Prediabetes and risks of outcome events
After excluding 372,419 individuals with history of CVD, CKD, CHF or cancer at baseline, 1,630,942 individuals were observed for outcome events. Over a median period of 7·8 (3·8, 11·2) years, 81,933 people developed CVD, 18,278 developed ESKD, 77,016 developed all-site cancer, 110,748 developed all-site infection, and 97,528 people died. The incidence rates of outcome events were higher in people with prediabetes than those with normoglycaemia (Table S2).
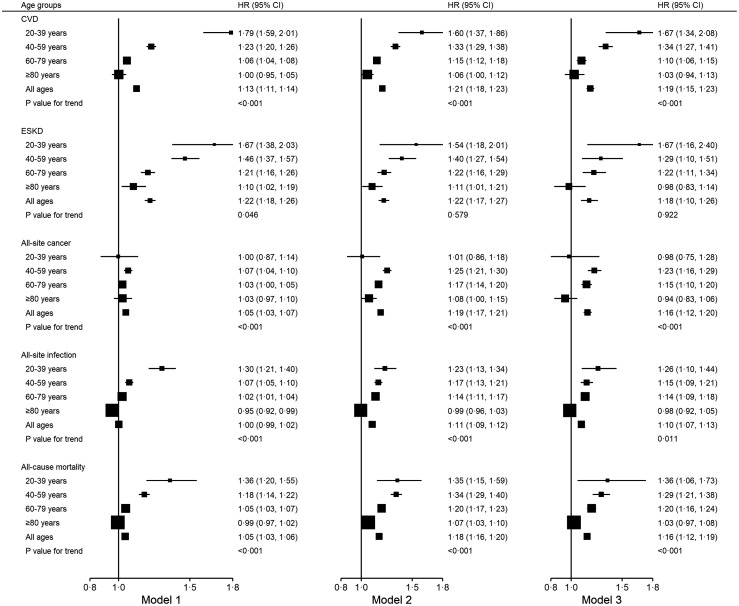
Prediabetes was associated with increased age- and sex-adjusted hazards of outcome events of interest (Figure 1) but for most events, the effect sizes diminished with ascending age (p value for trend <0·05 for model 1). In the youngest and in the oldest age categories, the respective HRs (95% CI) of prediabetes vs normoglycaemia were 1·79 (1·59, 2·01) and 1·00 (0·95, 1·05) for CVD, 1·67 (1·38, 2·03) and 1·10 (1·02, 1·19) for ESKD, 1·30 (1·21, 1·40) and 0·95 (0·92, 0·99) for all-site infection, and, 1·36 (1·20, 1·55) and 0·99 (0·97, 1·02) for all-cause mortality.
Figure 1.
Hazard ratios of CVD, ESKD, all-site cancer, all-site infection, and all-cause mortality in people with prediabetes stratified by age groups. All p-values for interaction test are statistically significant. Model 1: adjusting for baseline age, sex, and calendar year. Model 2: model 1 + excluding subsequent development of diabetes. Model 3: model 2 + further adjustment of metabolic factors including baseline LDL-C, triglyceride/HDL-C, haemoglobin, albumin, use of lipid-regulating drugs and blood pressure medications. CVD: cardiovascular disease; ESKD: end-stage kidney disease; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
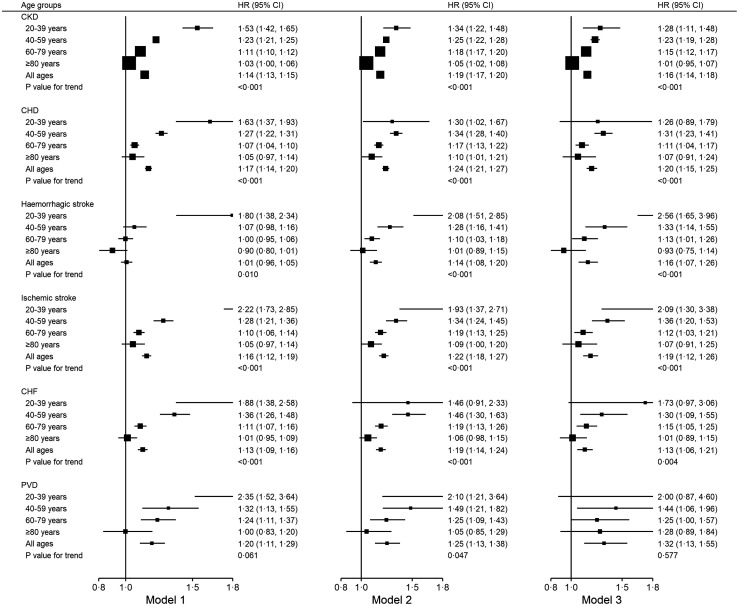
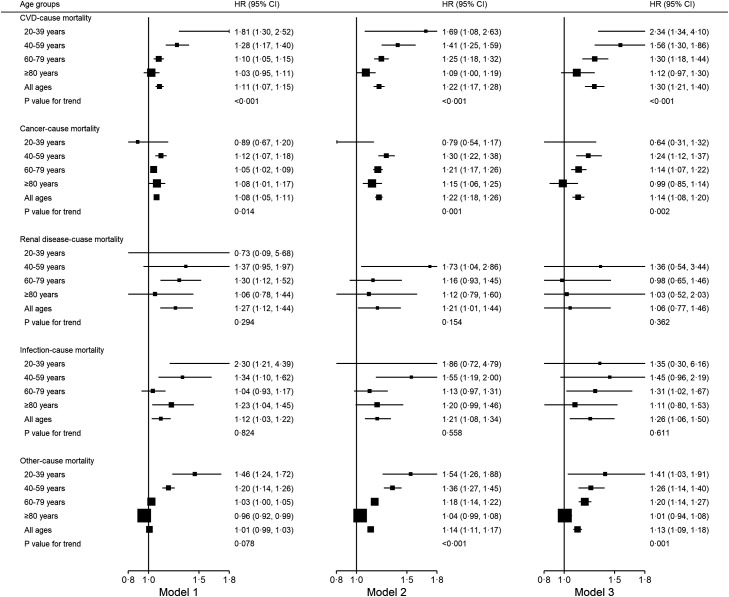
Similar age-related trends were detected in associations of prediabetes with incident CKD, CHD, haemorrhagic stroke, ischemic stroke, CHF, and mortality due to CVD (Figures 2,3). For incident cancer, PVD, mortality due to cancer, mortality due to kidney disease, mortality due to infection, and other-cause mortality, no clear or significant trend with age was observed for the association with prediabetes.
Figure 2.
Hazard ratios of CKD and subtypes of CVD in people with prediabetes stratified by age groups. All p-values for interaction test are statistically significant. Model 1: adjusting for baseline age, sex, and calendar year. Model 2: model 1 + excluding subsequent development of diabetes. Model 3: model 2 + further adjustment of metabolic factors including baseline LDL-C, triglyceride/HDL-C, haemoglobin, albumin, use of lipid-regulating drugs and blood pressure medications. CHD: coronary heart disease; CHF: congestive heart failure; CKD: chronic kidney disease; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; PVD: Peripheral vascular disease.
Figure 3.
Hazard ratios of cause-specific mortality in people with prediabetes stratified by age groups. All p-values for interaction test are statistically significant. Model 1: adjusting for baseline age, sex, and calendar year. Model 2: model 1 + excluding subsequent development of diabetes. Model 3: model 2 + further adjustment of metabolic factors including baseline LDL-C, triglyceride/HDL-C, haemoglobin, albumin, use of lipid-regulating drugs and blood pressure medications. HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol.
The strength of the association between prediabetes and incident events were attenuated but remained significant after excluding people who subsequently developed diabetes. Further adjustment for other metabolic factors and drug use had little influence on the associations. In the meta-regression models, HRs of prediabetes for outcome events of interest decreased with increasing age (Figure S1). The ARDs of events associated with prediabetes are shown in Table S3. The lowest ARDs were observed in the youngest age groups, and the magnitude of ARDs reflected differences in incidence rates of events across age groups.
Risk of outcome events in prediabetes defined using FPG or HbA1c
Among 528,948 people with prediabetes, 339,747 (64·2%) had IFG only, 152,747 (28·9%) had elevated HbA1c (HbA1c 5·7-6·4%) only, and 34,884 (6·6%) had prediabetes defined using both FPG and HbA1c. Compared to people with elevated HbA1c, those with IFG had higher levels of total cholesterol, LDL-C, triglyceride/HDL-C, haemoglobin, and albumin, but lower frequencies of pre-existing CVD, CKD, CHF and cancer, and use of lipid-regulating drugs (Table S4). People with IFG had increased hazards of all incident events compared with people without IFG, and the trend in effect sizes across age subgroups was also significant (Table 2). People with prediabetes defined using HbA1c had increased hazards of incident CVD but not ESKD, all-site cancer, all-site infection or all-cause mortality. A decreasing trend in HRs with increase age was detected for incident CVD, all-site cancer, and all-site infection but not for other outcomes. Similar age-related trends were found for the associations of continuous levels of FPG and HbA1c with outcomes.
Table 2.
Hazards ratios of CVD, ESKD, all-site cancer, all-site infection, and all-cause mortality associated with prediabetes defined by IFG (FPG 5·6-6·9 mmol/L), prediabetes defined by elevated HbA1c (HbA1c 5·7-6·4%), per 1-unit increase in FPG, and per 1- unit increase in HbA1c across age groups.
| Prediabetes defined by IFG | Prediabetes defined by elevated HbA1c | Per 1-unit (mmol/L) increase in FPG | Per 1-unit (%) increase in HbA1c | |
|---|---|---|---|---|
| CVD | ||||
| 20–39 years | 1·85 (1·62, 2·10) | 1·91 (1·44, 2·53) | 1·55 (1·41, 1·70) | 2·42 (1·63, 3·60) |
| 40–59 years | 1·22 (1·18, 1·25) | 1·19 (1·10, 1·29) | 1·17 (1·14, 1·20) | 1·25 (1·12, 1·40) |
| 60–79 years | 1·04 (1·02, 1·07) | 1·03 (0·96, 1·10) | 1·04 (1·02, 1·06) | 1·13 (1·03, 1·24) |
| ≥80 years | 1·00 (0·95, 1·06) | 0·95 (0·83, 1·09) | 0·98 (0·94, 1·02) | 0·97 (0·81, 1·16) |
| All ages | 1·11 (1·09, 1·13) | 1·12 (1·06, 1·17) | 1·10 (1·09, 1·12) | 1·21 (1·13, 1·29) |
| P value for trend | <0·001 | <0·001 | <0·001 | <0·001 |
| ESKD | ||||
| 20–39 years | 1·84 (1·48, 2·28) | 1·61 (1·02, 2·56) | 1·44 (1·24, 1·68) | 2·14 (1·12, 4·09) |
| 40–59 years | 1·46 (1·36, 1·57) | 1·15 (0·95, 1·39) | 1·27 (1·19, 1·34) | 1·25 (0·97, 1·61) |
| 60–79 years | 1·20 (1·15, 1·25) | 1·01 (0·89, 1·16) | 1·17 (1·13, 1·22) | 1·11 (0·94, 1·32) |
| ≥80 years | 1·11 (1·02, 1·21) | 0·83 (0·68, 1·02) | 1·09 (1·02, 1·17) | 0·80 (0·61, 1·05) |
| All ages | 1·22 (1·18, 1·26) | 0·99 (0·90, 1·08) | 1·15 (1·12, 1·18) | 1·03 (0·91, 1·17) |
| P value for trend | 0·062 | 0·154 | 0·020 | 0·205 |
| All-site cancer | ||||
| 20–39 years | 1·06 (0·91, 1·24) | 1·03 (0·78, 1·37) | 1·06 (0·96, 1·16) | 1·07 (0·72, 1·58) |
| 40–59 years | 1·07 (1·04, 1·10) | 0·99 (0·91, 1·07) | 1·04 (1·02, 1·07) | 0·93 (0·83, 1·04) |
| 60–79 years | 1·03 (1·00, 1·05) | 0·98 (0·92, 1·06) | 1·03 (1·01, 1·05) | 0·96 (0·88, 1·06) |
| ≥80 years | 1·03 (0·96, 1·10) | 0·89 (0·76, 1·05) | 1·05 (0·99, 1·11) | 0·96 (0·76, 1·19) |
| All ages | 1·05 (1·03, 1·07) | 1·02 (0·97, 1·07) | 1·05 (1·04, 1·07) | 1·01 (0·95, 1·08) |
| P value for trend | <0·001 | <0·001 | <0·001 | 0·004 |
| All-site infection | ||||
| 20–39 years | 1·36 (1·25, 1·48) | 1·38 (1·19, 1·60) | 1·21 (1·15, 1·28) | 1·82 (1·48, 2·25) |
| 40–59 years | 1·08 (1·05, 1·11) | 0·90 (0·84, 0·97) | 1·02 (1·00, 1·05) | 0·89 (0·81, 0·99) |
| 60–79 years | 1·00 (0·98, 1·02) | 0·92 (0·87, 0·98) | 0·98 (0·96, 1·00) | 0·92 (0·85, 0·99) |
| ≥80 years | 0·94 (0·90, 0·97) | 0·83 (0·76, 0·92) | 0·91 (0·88, 0·94) | 0·76 (0·67, 0·86) |
| All ages | 0·99 (0·97, 1·00) | 0·86 (0·83, 0·90) | 0·94 (0·93, 0·95) | 0·82 (0·78, 0·87) |
| P value for trend | <0·001 | <0·001 | <0·001 | 0·002 |
| All-cause mortality | ||||
| 20–39 years | 1·37 (1·18, 1·59) | 1·17 (0·89, 1·52) | 1·17 (1·06, 1·30) | 1·06 (0·73, 1·55) |
| 40–59 years | 1·18 (1·14, 1·22) | 0·81 (0·75, 0·89) | 1·08 (1·05, 1·11) | 0·74 (0·66, 0·84) |
| 60–79 years | 1·02 (1·00, 1·04) | 0·87 (0·82, 0·91) | 1·00 (0·98, 1·01) | 0·84 (0·79, 0·90) |
| ≥80 years | 0·97 (0·94, 1·01) | 0·77 (0·72, 0·83) | 0·93 (0·90, 0·95) | 0·73 (0·66, 0·81) |
| All ages | 1·02 (1·01, 1·04) | 0·81 (0·78, 0·84) | 0·98 (0·97, 0·99) | 0·76 (0·73, 0·80) |
| P value for trend | <0·001 | 0·753 | 0·196 | 0·109 |
Adjusting for baseline age, sex, and calendar year. All p-values for interaction test are statistically significant. ADA: American Diabetes Association; CVD: cardiovascular disease; ESKD: end-stage kidney disease; IFG: impaired fasting glucose.
Sensitivity analysis
Sensitivity analysis adjusting for the competing risk of death using the Fine-Gary model showed similar results to the primary analysis (Table S5). The E-values for the point estimates (ranging from 1·1 to 2·7) and confidence intervals (ranging from 1·0 to 2·0) of associations between prediabetes and major clinical events are shown in Table S6. Comparisons between the full sample and the sample with complete data on metabolic factors showed generally similar effect sizes, indicating limited heterogeneity existed between these two samples and therefore limited bias caused by missing data (Table S7).
Discussion
Role of age in health risk of prediabetes
In this population-based study on the long-term health outcomes of prediabetes, we found that prediabetes was associated with a higher risk of adverse clinical outcomes and mortality in younger than older people. Many studies have reported the role of age at diagnosis on the risk of developing complications in people with diabetes18 but few studies have examined whether age modifies the risk association between abnormal blood glucose and major morbidities in people who have not yet developed diabetes. Based on a nationwide health insurance dataset including 12 million people with a mean follow-up of 10·8 years, a Korean study reported a 30% increased risk of all-cause mortality per 1 mmol/L rise in FPG in people aged 18-34 years, but the corresponding increase in risk was only 10% in those aged 75-99 years.9 In the nationwide German National Health Interview and Examination Survey with 6299 individuals followed for an average of 11·6 years,8 a steeper increase was observed in mortality risk in both low and high range of HbA1c among participants aged below 55 years compared with those aged above 55 years.
The mechanisms for the modifying role of age on health hazards associated with prediabetes are not known although there are several possible reasons. Firstly, young people with prediabetes have greater insulin resistance and exhibit different trajectories of beta-cell dysfunction over time compared with their older counterparts.19 The faster progression of dysglycaemia in young people may contribute to more severe changes to the vasculatures and other organs leading to higher risks of clinical events. Secondly, young people are more likely to adopt unhealthy habits due to poor health awareness and competing life priorities. In the present study, we have adjusted for some metabolic factors. However, information on other important risk factors such as smoking, obesity, diet and exercise levels were not available, which could have confounded the association between prediabetes and outcome. Thirdly, prediabetes is asymptomatic and is usually diagnosed during planned or opportunistic screening. Due to differences in health seeking behaviour with younger people being less inclined to undergo screening, detection of prediabetes could be delayed which adds to the adverse effects of abnormal glucose metabolism in this age group. Fourthly, low blood glucose may indicate poor nutrition and multiple morbidities especially among the elderly. However, we have excluded events occurring within one year from baseline to minimise potential reverse causality. Lastly, the association of prediabetes with health outcomes may be attenuated in older people with age-related conditions that have greater contribution to outcome events and mortality. In this connection, the strength of evidence for the association between prediabetes and incident CVD and mortality was weaker in people with pre-existing diseases than in the general population.5
Diabetes and metabolic factors in mediating long term health risks of prediabetes
The relationships between prediabetes and incident events might be explained by concurrent metabolic risk factors and subsequent progression to diabetes.20 In the present study, we found that exclusion of people who later developed diabetes and adjustment of metabolic factors attenuated, but not removed, the association between prediabetes and incident events.21,22 Our results corroborate findings from earlier studies showing that prediabetes is a risk determinant for major clinical events and premature mortality, independent of progression to diabetes. In a sample of 2,620 Finnish observed for 10 years, people who had prediabetes at baseline and did not progress to diabetes during the follow-up had higher hazards of incident CHD and mortality, compared to their counterparts with normoglycaemia at baseline.22 Similarly, the health hazards of prediabetes persisted after adjustment for development of diabetes or excluding subsequent diabetes cases, as reported among people in the UK23 and Japan.21
Glycaemic measurements and health consequences of prediabetes
We observed a risk differential between prediabetes and incident events according to the glycaemic index used to define prediabetes. IFG was linked to greater risks of all major events, whereas prediabetes defined using HbA1c was associated with increased hazard of incident CVD, but not mortality. Previous studies comparing the mortality risk in prediabetes defined by IFG and HbA1c showed mixed results. In the Whitehall II cohort including 5427 British civilians, HbA1c-defined prediabetes was associated with higher mortality risk, whereas no association with mortality was observed for IFG or IGT.24 Similarly, the US National Health and Nutrition Examination Survey reported that prediabetes defined by HbA1c but not IFG or IGT was predictive of mortality.25,26 On the other hand, the Atherosclerosis Risk in Communities study including four communities in the US found that all subtypes of prediabetes, namely IFG, IGT, and elevated HbA1c, were associated with higher mortality risk.27 A recent umbrella review of studies on prediabetes and clinical outcomes synthesised the existing evidence and suggested that the associations with mortality were only observed in prediabetes defined by IFG or IGT, but not prediabetes defined using HbA1c.5
Observations in our study, compatible to those of the umbrella review, may in part be explained by differences in pathophysiological characteristics of prediabetes defined by the two indices. FPG has been shown to be more strongly correlated with insulin sensitivity and insulin secretion compared with HbA1c.28 Furthermore, levels of HbA1c are determined by factors other than glycaemia, such as age and haemoglobin level.29, 30, 31 As fewer than 2% of people in this territory-wide cohort underwent an OGTT, we were unable to compare IGT against other glycaemic indices for the association with clinical outcomes.
Implications
A meta-analysis previously showed a risk differential in associations of prediabetes with all-cause mortality and CVD risk among individuals <60 years versus those 60 years or above,13 although these findings might be confounded by the heterogeneity in background comorbidities, duration of follow-up and definitions of glycaemic measurements across the included studies. By dividing individuals into 20-year age groups, our study provides new evidence on age-specific associations between prediabetes and a wide range of health outcomes including all-cause mortality, CVD, and other important clinical events, in a population-based cohort. These findings extend our understanding of prediabetes in the following ways. Firstly, our study emphasizes the importance of age in identifying and managing high-risk population with prediabetes. The Da Qing Diabetes Prevention Outcome Study (Da Qing study) 6 but not the Diabetes Prevention Program Outcomes Study (DPPOS) 7 showed mortality benefits in those assigned active lifestyle intervention. The discrepant results might be explained by differences in clinical profile of the study participants and the intensity of the lifestyle intervention with the Da Qing study intervening people with a healthier metabolic profile for a longer period. Importantly, the lower age of participants at study entrance to the Da Qing study (45·2 years versus 50·6 years in DPPOS) might also contribute to the observed difference in study outcome, as supported by findings from the current study. It is noteworthy that a post-hoc analysis of the diabetes prevention study PREVIEW also suggested that younger adults achieved greater improvement in body composition and cardiometabolic markers from lifestyle intervention than older adults.32 Our results called for age-stratified preventive strategies, in particular early screening for prediabetes and intervention in young people who have the potential to benefit more from the prevention efforts. Secondly, prediabetes itself is associated with major clinical events even excluding people who subsequently developed diabetes and adjusting for metabolic factors. Improved public education and awareness of this condition is needed to stimulate active screening and healthy behaviour. Thirdly, the health hazards of prediabetes were observed in IFG, but not in elevated HbA1c, among Chinese, although further studies are needed to confirm this.
Strengths and limitations
The main strength of our study is the use of a territory-wide database representative of the local population with up to 20 years of observation time. The large sample size should provide sufficient statistical power to generate reliable epidemiological evidence. There are also some limitations to note. Firstly, information on weight, blood pressure, smoking, drinking and lifestyle were not available for the majority of people without diabetes in the HKDSD. As such, we were not able to fully examine the extent to which the risk association between prediabetes and clinical outcomes are confounded by accompanying metabolic risk factors, although we adjusted for triglyceride/HDL-C as surrogate for central obesity 33 and baseline use of blood pressure-lowering drugs as surrogate for hypertension. The E-values for primary outcomes were relatively small, indicating an unmeasured confounding with small effect size would nullify the associations in primary analysis, although the large sample size in the present study would also lead to smaller E-values. We reviewed all of the studies included in the recent meta-analysis on the association between prediabetes and CVD and/or mortality (Table S8).13 Among 19 studies reporting risk association between prediabetes and clinical outcomes before and after statistical adjustment for lifestyle factors, the excess risks of CVD and/or mortality persisted after adjusting for lifestyle factors in 14 studies. Secondly, only people who used public health services were included in the HKDSD who may have different demographic, socioeconomic status and prognosis to those who only use private health services. However, the proportion of health services provided by private sectors to the whole health services in Hong Kong is only about 10%.14 Thirdly, the HKDSD was curated from electronic medical records of the Hong Kong HA, comprising individuals who have ever had glycaemic measurements on one occasion or more during the surveillance period. Therefore, some people were included from routine screening and others from opportunistic testing. The overall sample of the HKDSD may still be different from the Hong Kong general population resulting in possible selection bias towards the inclusion of a more high risk group. This may potentially inflate the risk associations between prediabetes and clinical outcomes but the trends across age groups are unlikely to be affected. Lastly, people might also be lost to follow due to external migration although the net movement rate in Hong Kong from 2001 to 2019 was less than 1%.34 Incomplete capture of incident events or death due to migration should therefore be minimal.
Conclusion
Prediabetes is associated with higher risks of major clinical events and mortality, even excluding the development of diabetes and adjusting for metabolic factors, which is more pronounced in younger than older people. Age-specific strategies should be considered in screening, intervention, and monitoring for prediabetes.
Contributors
XZ contributed to conception of the article, statistical analysis, results interpretation, drafted the manuscript, revised the manuscript critically and approved the final version. AOYL and RCWM contributed to conception of the article, results interpretation, revised the manuscript critically and approved the final version. HW, BF, MS, ESHL, and AY contributed to conception of the article, results interpretation, revised the manuscript critically and approved the final version. EC, APSK, and JCNC contributed to conception of the article, revised the manuscript critically and approved the final version. AOYL is the guarantor of this work, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interests
AOYL has served as a member of advisory panel for Amgen, AstraZeneca, Boehringer Ingelheim and Sanofi and received research support from Amgen, Asia Diabetes Foundation, Bayer, Boehringer Ingelheim, Lee's Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Sugardown Ltd, Takeda, outside the submitted work. APSK has received research grants and/or speaker honoraria from Abbott, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli-Lilly, Kyowa Kirin, Merck Serono, Novo Nordisk, Pfizer and Sanofi, outside the submitted work. JCNC has received grants from Astra Zeneca, Lilly, Hua Medicine, Lee Powder; grants and personal fees from Bayer, MSD, Merck, and Sanofi, acts as CEO in Asia Diabetes Foundation and founding director in GemVCare, outside the submitted work. In addition, JCNC has a patent Genetic marker for DKD issued. RCWM has received grants from AstraZeneca, Bayer, Pfizer, Novo Nordisk, Sanofi, Tricida Inc., speaker honorarium from AstraZeneca, Bayer and Boehringer Ingelheim, and has received support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU), as co-founder of a technology start-up GemVCare which provides genetic testing for diabetes and diabetes complications, outside the submitted work.
Acknowledgements/Funding
We acknowledge the Hong Kong Hospital Authority for provision of anonymised clinical data for research. RCWM acknowledges partial support from a Research Grants Council Research Impact Fund (R4012-18) and a Croucher Foundation Senior Medical Research Fellowship.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100599.
Contributor Information
Ronald C.W Ma, Email: rcwma@cuhk.edu.hk.
Andrea O.Y Luk, Email: andrealuk@cuhk.edu.hk.
Appendix. Supplementary materials
References
- 1.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . 10th Ed. 2021. IDF Diabetes Atlas.https://www.diabetesatlas.org [Google Scholar]
- 3.Ligthart S, van Herpt TT, Leening MJ, et al. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):44–51. doi: 10.1016/S2213-8587(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Wu H, Fan B, et al. Lifetime risk of developing diabetes in Chinese people with normoglycemia or prediabetes: a modeling study. PLoS Med. 2022;19(7) doi: 10.1371/journal.pmed.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlesinger S, Neuenschwander M, Barbaresko J, et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65(2):275–285. doi: 10.1007/s00125-021-05592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol. 2019;7(6):452–461. doi: 10.1016/S2213-8587(19)30093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CG, Heckman-Stoddard B, Dabelea D, et al. Effect of metformin and lifestyle interventions on mortality in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2021;44(12):2775–2782. doi: 10.2337/dc21-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paprott R, Schaffrath Rosario A, Busch MA, et al. Association between hemoglobin A1c and all-cause mortality: results of the mortality follow-up of the German national health interview and examination survey 1998. Diabetes Care. 2015;38(2):249–256. doi: 10.2337/dc14-1787. [DOI] [PubMed] [Google Scholar]
- 9.Yi S-W, Park S, Lee Y-h, Park H-J, Balkau B, Yi J-J. Association between fasting glucose and all-cause mortality according to sex and age: a prospective cohort study. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-08498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinning C, Makarova N, Völzke H, et al. Association of glycated hemoglobin A1c levels with cardiovascular outcomes in the general population: results from the BiomarCaRE (biomarker for cardiovascular risk assessment in Europe) consortium. Cardiovasc Diabetol. 2021;20(1):223. doi: 10.1186/s12933-021-01413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YS, Park YM, Han KD, Yun JS, Ahn YB, Ko SH. Fasting glucose level and all-cause or cause-specific mortality in Korean adults: a nationwide cohort study. Korean J Intern Med. 2021;36(3):647–658. doi: 10.3904/kjim.2019.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue K, Nianogo R, Telesca D, et al. Low HbA1c levels and all-cause or cardiovascular mortality among people without diabetes: the US national health and nutrition examination survey 1999–2015. Int J Epidemiol. 2021;50(4):1373–1383. doi: 10.1093/ije/dyaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2297. m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong Kong SAR, Census and Statistics Department. Hong Kong Special Administrative Region . Thematic Household Survey Report No. 50; 2013. [Google Scholar]
- 15.Wu H, Lau ESH, Yang A, et al. Data resource profile: The Hong Kong diabetes surveillance database (HKDSD) Int J Epidemiol. 2022;51(2):e9–e17. doi: 10.1093/ije/dyab252. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 17.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Lau ES, Yang A, et al. Young age at diabetes diagnosis amplifies the effect of diabetes duration on risk of chronic kidney disease: a prospective cohort study. Diabetologia. 2021;64(9):1990–2000. doi: 10.1007/s00125-021-05494-4. [DOI] [PubMed] [Google Scholar]
- 19.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. observations using the hyperglycemic clamp. Diabetes Care. 2018;41(8):1696–1706. doi: 10.2337/dc18-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet North Am Ed. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Mizoue T, Sasaki N, et al. Prediabetes and cardiovascular disease risk: a nested case-control study. Atherosclerosis. 2018;278:1–6. doi: 10.1016/j.atherosclerosis.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Qiao Q, Jousilahti P, Eriksson J, Tuomilehto J. Predictive properties of impaired glucose tolerance for cardiovascular risk are not explained by the development of overt diabetes during follow-up. Diabetes Care. 2003;26(10):2910–2914. doi: 10.2337/diacare.26.10.2910. [DOI] [PubMed] [Google Scholar]
- 23.Eastwood SV, Tillin T, Sattar N, Forouhi NG, Hughes AD, Chaturvedi N. Associations between prediabetes, by three different diagnostic criteria, and incident CVD differ in South Asians and Europeans. Diabetes Care. 2015;38(12):2325–2332. doi: 10.2337/dc15-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the Whitehall II study. Diabetes Care. 2018;41(4):899–906. doi: 10.2337/dc17-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 2001;24(8):1397–1402. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- 26.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3(6):661–667. doi: 10.1161/CIRCOUTCOMES.110.957936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren B, Pankow JS, Matsushita K, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the atherosclerosis risk in communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34–42. doi: 10.1016/S2213-8587(16)30321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the insulin resistance atherosclerosis study (IRAS) Diabetes Care. 2010;33(9):2104–2109. doi: 10.2337/dc10-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soranzo N, Sanna S, Wheeler E, et al. Common variants at 10 genomic loci influence hemoglobin A₁(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the framingham offspring study and the national health and nutrition examination survey 2001-2004. Diabetes Care. 2008;31(10):1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fizelova M, Stančáková A, Lorenzo C, et al. Glycated hemoglobin levels are mostly dependent on nonglycemic parameters in 9398 finnish men without diabetes. J Clin Endocrinol Metabol. 2015;100(5):1989–1996. doi: 10.1210/jc.2014-4121. [DOI] [PubMed] [Google Scholar]
- 32.Zhu R, Craciun I, Bernhards-Werge J, et al. Age- and sex-specific effects of a long-term lifestyle intervention on body weight and cardiometabolic health markers in adults with prediabetes: results from the diabetes prevention study PREVIEW. Diabetologia. 2022;68(8):1262–1277. doi: 10.1007/s00125-022-05716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 34.The Census and Statistics Department of Hong Kong. Population estimates. 22 April, 2021. https://www.censtatd.gov.hk/tc/scode150.html. Accessed 18 November 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.