Abstract
Purpose
Proton radiation therapy (PR) is well established in the treatment of pediatric malignancies in the central nervous system (CNS) with dosimetric advantages that reduce late radiation therapy (RT) effects. In this analysis, we sought to evaluate the utilization of PR in children with primary CNS malignancies and characterize the clinical and sociodemographic factors predictive of receipt of PR.
Methods and Materials
The National Cancer Database was queried to identify all pediatric patients with primary CNS malignancies treated with curative intent RT from 2004 to 2017. Clinical characteristics and demographics were analyzed using standard t and χ2 testing. Predictors of PR receipt were identified with univariable and multivariable logistic regression.
Results
We identified 9126 patients ≤18 years of age treated with RT between 2004 and 2017, of which 1045 (11.5%) received PR. PR usage continued to increase significantly, from <1% in 2004 to 28% in 2017. The proportion of white and Asian patients receiving PR for nonhigh-grade glioma and nonmeningioma CNS malignancies during the study period rose from <1% for both to 35% and 44%, respectively, and in black patients the proportion rose from <1% to 26%. Multivariable predictors of receipt of PR include year of diagnosis, age <6 years, income level, distance from PR facility, and histology; multivariable predictors of receipt of photon RT include black race, rural residence, and Medicaid insurance. These factors remained significant when isolating the most recent 5 years of data.
Conclusions
Proton radiation therapy usage for CNS malignancies increased significantly during the study period. Despite the potential clinical advantages of PR for pediatric primary CNS malignancies, there are notable socioeconomic, geographic, and racial disparities in the receipt of PR that persisted despite the increased availability and accessibility. Further study is warranted to identify how to address the disparities and better support these patients.
Introduction
Proton radiation therapy (PR) is well established in the treatment of pediatric primary central nervous system (CNS) malignancies, with known dosimetric advantages compared with traditional photon radiation and increasing retrospective evidence showing reduced risk of late effects including cognitive decline and secondary malignancy.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Prior publications have reported on national PR practice patterns, which were notable for racial and sociodemographic disparities in the application of proton therapy.19 In the years since the most recent national level database analysis was published, the number of available proton therapy centers increased by about 200%.20,21 In this analysis, we sought to evaluate the effect of increased availability and access to PR on patterns of care reported in a national level database.
Methods and Materials
The National Cancer Database is a national level registry of deidentified patient clinical and sociodemographic data representing about 70% of cancer diagnoses and collected from about 1500 medical centers in the United States. We queried the database to identify all pediatric patients ≤18 years of age who received a primary diagnosis of CNS malignancy between 2004 and 2017. Patients were included for analysis if they received nonpalliative intent radiation therapy and were nonmetastatic at presentation. Additional clinical and sociodemographic data included tumor histology, radiation technique, year of diagnosis, age at diagnosis, race, ethnicity, insurance type, Charlson comorbidity index, distance from treatment facility, community type, and family income. Geographic region and type of treatment center (academic vs community) were unavailable as they are not coded for patients ≤18 years of age. Tumor histology was divided into low-grade glioma (LGG), high-grade glioma, ependymoma (EP), medulloblastoma (MB), primitive neuroectodermal tumor (PNET), craniopharyngioma (CPG), germ cell tumors (GCT), meningioma, atypical teratoid or rhabdoid tumor (ATRT), and other.
Standard t and χ2 testing were used to characterize clinical and sociodemographic differences between proton and photon groups. Univariable and multivariable logistic regression models were used to identify predictors of receipt of PR. A significance threshold was set at P < .05 for all analyses. Statistical analysis was performed in STATA/IC-14.
Results
We identified 9126 patients between the age of 0 and 18 years with primary CNS malignancies, of which 1045 (11.3%) received PR and 8081 (87.7%) received photons. The mean ages of patients receiving PR and non-PR were 8.1 and 9.2 years, respectively (P < .01). Patients receiving PR were more likely to be ≤5 years of age (P < .01), be treated after 2009 (P < .01), be white (P < .01), be privately insured (P < .01), belong to a higher income bracket (P < .01), live >50 miles from the treatment center (P = .02), reside in a metropolitan area (P < .01), and have EP, MB, PNET, GCT, or ATRT tumor histology (P < .01). Baseline characteristics between treatment groups are shown in Table 1.
Table 1.
Baseline patient characteristics
| Characteristic | Photons, n (%) | Protons, n (%) | P value | ||
|---|---|---|---|---|---|
| Age, y | <.01 | ||||
| 0-5 | 2279 | 28 | 373 | 29 | |
| 6-10 | 2519 | 31 | 339 | 31 | |
| 11-18 | 3283 | 41 | 333 | 32 | |
| Year of diagnosis | <.01 | ||||
| 2004-2008 | 3022 | 37 | 84 | 8 | |
| 2009-2012 | 2411 | 30 | 231 | 22 | |
| 2013-2017 | 2648 | 33 | 730 | 70 | |
| Race | <.01 | ||||
| White | 6189 | 77 | 794 | 76 | |
| Black | 1079 | 13 | 68 | 7 | |
| Asian | 329 | 4 | 50 | 5 | |
| Native American/Eskimo | 55 | 1 | 10 | 1 | |
| Native HI/Pacific Islander | 20 | <1 | 4 | <1 | |
| Other/unknown | 409 | 5 | 119 | 11 | |
| Ethnicity | .81 | ||||
| Non-Hispanic/unreported | 6820 | 85 | 878 | 86 | |
| Hispanic white | 1136 | 14 | 141 | 14 | |
| Hispanic black | 32 | <1 | 3 | <1 | |
| Insurance status | <.01 | ||||
| Privately insured | 4734 | 63 | 702 | 72 | |
| Medicaid | 2768 | 37 | 276 | 28 | |
| Charlson comorbidity index | .18 | ||||
| 0 | 7407 | 92 | 977 | 93 | |
| 1 | 359 | 4 | 39 | 4 | |
| 2 | 245 | 3 | 22 | 2 | |
| 3 + | 70 | 1 | 7 | 1 | |
| Income ($) | <.01 | ||||
| <30,000 | 1049 | 15 | 76 | 8 | |
| 30,000-34,999 | 1256 | 17 | 116 | 12 | |
| 35,000-45,999 | 2025 | 28 | 284 | 31 | |
| 46,000 + | 2892 | 40 | 450 | 49 | |
| Distance from treatment, miles | 0.02 | ||||
| <50 | 5349 | 72 | 643 | 67 | |
| 51-200 | 1733 | 23 | 255 | 27 | |
| >200 | 391 | 5 | 59 | 6 | |
| Community type | <.01 | ||||
| Metro | 6413 | 83 | 892 | 90 | |
| Urban | 853 | 11 | 72 | 7 | |
| Rural | 485 | 6 | 27 | 3 | |
| Histology | <.01 | ||||
| Low-grade glioma | 1862 | 24 | 114 | 11 | |
| High-grade glioma | 1803 | 23 | 99 | 10 | |
| Ependymoma | 1034 | 13 | 239 | 23 | |
| Medulloblastoma | 1967 | 25 | 379 | 37 | |
| PNET | 286 | 4 | 40 | 4 | |
| Craniopharyngioma | 59 | 1 | 9 | 1 | |
| Germ cell tumors | 350 | 4 | 59 | 6 | |
| Meningioma | 119 | 2 | 11 | 1 | |
| ATRT | 203 | 3 | 51 | 5 | |
| Other | 153 | 2 | 28 | 3 | |
Abbreviations: ATRT = atypical teratoid/rhabdoid tumor; HI = Hawaiian Islander; PNET = primitive neuroectodermal tumor.
The results of univariable and multivariable analysis of the entire patient cohort for the receipt of PR is shown in Table 2. Factors predictive of receipt of PR include age ≤5 years of age (P < .01), year of diagnosis 2009 and later (P < .01), income >$35,000, >50-mile distance from the treatment facility, and EP, MB, PNET, GCT, ATRT, and other histology (all P < .01). CPG was not predictive of PR. Patients were less likely to receive PR if they were of the black race (P < .01), had Medicaid insurance (P < .01), and had an urban (P = .05) or rural (P <.01) residence. The same factors remained significantly predictive if we restricted analysis to the most recent 5 years of data.
Table 2.
Predictors of receipt of proton radiation therapy
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Predictor | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value |
| Age, y | ||||||
| 0-5 | 1.6 | 1.4-1.9 | <.01 | 1.3 | 1.1-1.6 | .01 |
| 6-10 | 1.3 | 1.1-1.6 | <.01 | 1.1 | 0.9-1.4 | .31 |
| 11-18 | - | - | - | - | - | - |
| Year of diagnosis | ||||||
| 2004-2008 | - | - | - | - | - | - |
| 2009-2012 | 3.5 | 2.7-4.5 | <.01 | 4.2 | 3.2-5.7 | <.01 |
| 2013-2017 | 10 | 7.9-13 | <.01 | 11 | 8.5-15 | <.01 |
| Race | ||||||
| White | - | - | - | - | - | - |
| Black | 0.48 | 0.38-0.63 | <.01 | 0.62 | 0.45-0.85 | <.01 |
| Asian | 1.2 | 0.87-1.6 | .28 | 1.1 | 0.77-1.6 | .56 |
| Native American/Eskimo | 1.4 | 0.72-2.8 | .31 | 1.4 | 0.54-3.4 | .52 |
| Native HI/Pacific Islander | 1.6 | 0.53-4.6 | .42 | 1.8 | 0.55-6.1 | .33 |
| Other/unknown | 2.3 | 1.8-2.8 | <.01 | 2.3 | 1.7-3.1 | <.01 |
| Ethnicity | ||||||
| Non-Hispanic/unreported | - | - | - | - | - | - |
| Hispanic white | 0.96 | 0.80-1.2 | .70 | 1.0 | 0.80-1.3 | .83 |
| Hispanic black | 0.73 | 0.22-2.4 | .60 | 0.63 | 0.08-5.0 | .66 |
| Insurance status | ||||||
| Privately insured | - | - | - | - | - | - |
| Medicaid | 0.67 | 0.58-0.78 | <.01 | 0.62 | 0.51-0.75 | <.01 |
| Charlson comorbidity index | ||||||
| 0 | - | - | - | - | - | - |
| 1 | 0.82 | 0.59-1.2 | .26 | 0.70 | 0.45-1.1 | .10 |
| 2 | 0.68 | 0.44-1.1 | .09 | 0.90 | 0.52-1.5 | .68 |
| 3 + | 0.76 | 0.35-1.7 | .49 | 1.0 | 0.38-2.6 | .98 |
| Income ($) | ||||||
| <30,000 | - | - | - | - | - | - |
| 30,000-34,999 | 1.3 | 0.94-1.7 | .11 | 1.1 | 0.75-1.5 | .74 |
| 35,000-45,999 | 1.9 | 1.5-2.5 | <.01 | 1.5 | 1.1-2.0 | .02 |
| 46,000 + | 2.2 | 1.7-2.8 | <.01 | 1.6 | 1.2-2.3 | .01 |
| Distance from treatment, miles | ||||||
| <50 | - | - | - | - | - | - |
| 51-200 | 1.2 | 1.1-1.4 | <.01 | 1.4 | 1.2-1.8 | <.01 |
| >200 | 1.3 | 0.94-1.7 | .12 | 1.6 | 1.1-2.5 | .02 |
| Community type | ||||||
| Metro | - | - | - | - | - | - |
| Urban | 0.61 | 0.47-0.78 | <.01 | 0.72 | 0.53-0.99 | .05 |
| Rural | 0.4 | 0.27-0.59 | <.01 | 0.47 | 0.29-0.75 | <.01 |
| Histology | ||||||
| Low-grade glioma | - | - | - | - | - | - |
| High-grade glioma | 0.9 | 0.68-1.2 | .44 | 0.82 | 0.59-1.2 | .25 |
| Ependymoma | 3.8 | 3.0-4.8 | <.01 | 3.9 | 2.9-5.2 | <.01 |
| Medulloblastoma | 3.2 | 2.5-3.9 | <.01 | 3.6 | 2.8-4.7 | <.01 |
| PNET | 2.3 | 1.6-3.3 | <.01 | 3.3 | 2.1-5.2 | <.01 |
| Craniopharyngioma | 2.5 | 1.2-5.2 | .01 | 1.9 | 0.72-5.3 | .19 |
| Germ cell tumors | 2.8 | 2.0-3.9 | <.01 | 3.4 | 2.2-5.0 | <.01 |
| Meningioma | 1.5 | 0.79-2.9 | .21 | 2.0 | 0.95-4.0 | .07 |
| ATRT | 4.1 | 2.9-5.9 | <.01 | 3.7 | 2.3-5.7 | <.01 |
| Other | 3.0 | 1.9-4.7 | <.01 | 3.6 | 2.1-6.2 | <.01 |
Abbreviations: ATRT = atypical teratoid/rhabdoid tumor; CI = confidence interval; HI = Hawaiian Islander; PNET = primitive neuroectodermal tumor.
We performed a subset multivariable analysis for receipt of PR isolating histologies for which data support PR, including LGG, EP, MB, PNET, CPG, GCT, and ATRT (n = 6652), as shown in Table 3. The factors predictive of receipt or nonreceipt of PR were identical to those of the entire cohort, with the exception of urban residence no longer being significant.
Table 3.
Predictors of receipt of proton therapy limited to LGG, EP, MB, PNET, CPG, GCT, and ATRT
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Predictor | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value |
| Age, y | ||||||
| 0-5 | 1.3 | 1.1-1.6 | <.01 | 1.3 | 1.1-1.7 | <.01 |
| 6-10 | 1.2 | 1.01-1.4 | .04 | 1.2 | 0.9-1.5 | .21 |
| 11-18 | - | - | - | - | - | - |
| Year of diagnosis | ||||||
| 2004-2008 | - | - | - | - | - | - |
| 2009-2012 | 4.3 | 3.2-5.8 | <.01 | 5.0 | 3.6-6.8 | <.01 |
| 2013-2017 | 13 | 10-17 | <.01 | 13 | 9.7-17.8 | <.01 |
| Race | ||||||
| White | - | - | - | - | - | - |
| Black | 0.48 | 0.36-.63 | <.01 | 0.63 | 0.45-0.90 | .01 |
| Asian | 1.3 | 0.95-1.8 | .10 | 1.2 | 0.84-1.8 | .29 |
| Native American/Eskimo | 1.6 | 0.80-3.2 | .19 | 1.5 | 0.58-3.9 | .41 |
| Native HI/Pacific Islander | 1.6 | 0.46-5.8 | .45 | 1.6 | 0.40-6.6 | .50 |
| Other/unknown | 2.2 | 1.7-2.8 | <.01 | 2.1 | 1.9-4.0 | <.01 |
| Ethnicity | ||||||
| Non-Hispanic/unreported | - | - | - | - | - | - |
| Hispanic white | 0.87 | 0.71-1.1 | .20 | 0.94 | 0.71-1.2 | .66 |
| Hispanic black | 0.56 | 0.13-2.4 | .43 | 0.70 | 0.08-5.8 | .74 |
| Insurance status | ||||||
| Privately insured | - | - | - | - | - | - |
| Medicaid | 0.66 | 0.56-0.78 | <.01 | 0.62 | 0.50-0.76 | <.01 |
| Charlson comorbidity index | ||||||
| 0 | - | - | - | - | - | - |
| 1 | 0.84 | 0.58-1.2 | .34 | 0.72 | 0.45-1.2 | .18 |
| 2 | 0.85 | 0.51-1.4 | .53 | 0.90 | 0.46-1.7 | .74 |
| 3 + | 1.2 | 0.49-2.8 | .73 | 1.5 | 0.54-4.3 | .42 |
| Income ($) | ||||||
| <30,000 | - | - | - | - | - | - |
| 30,000-34,999 | 1.3 | 0.94-1.9 | .10 | 1.2 | 0.80-1.8 | .40 |
| 35,000-45,999 | 2.1 | 1.6-2.8 | <.01 | 1.7 | 1.2-2.4 | .01 |
| 46,000 + | 2.4 | 1.8-3.2 | <.01 | 1.9 | 1.3-2.7 | <.01 |
| Distance from treatment, miles | ||||||
| <50 | - | - | - | - | - | - |
| 51-200 | 1.26 | 1.06-1.49 | .01 | 1.6 | 1.3-2.0 | <.01 |
| >200 | 1.35 | 0.98-1.87 | .07 | 1.7 | 1.1-2.7 | .02 |
| Community type | ||||||
| Metro | - | - | - | - | - | - |
| Urban | 0.65 | 0.50-0.85 | <.01 | 0.76 | 0.54-1.1 | .11 |
| Rural | 0.40 | 0.26-0.61 | <.01 | 0.46 | 0.27-0.78 | <.01 |
| Histology | ||||||
| Low-grade glioma | - | - | - | - | - | - |
| Ependymoma | 3.8 | 3.0-4.8 | <.01 | 3.9 | 2.9-5.2 | <.01 |
| Medulloblastoma | 3.2 | 2.5-3.9 | <.01 | 3.6 | 2.8-4.7 | <.01 |
| PNET | 2.3 | 1.6-3.3 | <.01 | 3.3 | 2.1-5.3 | <.01 |
| Craniopharyngioma | 2.5 | 1.2-5.2 | .01 | 1.9 | 0.70-5.2 | .21 |
| Germ cell tumors | 2.8 | 2.0-3.9 | <.01 | 3.4 | 2.2-5.1 | <.01 |
| ATRT | 4.1 | 2.9-5.9 | <.01 | 3.7 | 2.4-5.8 | <.01 |
Abbreviations: ATRT = atypical teratoid/rhabdoid tumor; CI = confidence interval; EP = ependymoma; CPG = craniopharyngioma; GCT = germ cell tumors; HI = Hawaiian Islander; LGG = low-grade glioma; MB = medulloblastoma; PNET = primitive neuroectodermal tumor.
Isolating patients with EP only (n = 1273), multivariable logistic regression demonstrated that Asian race was predictive of receipt of PR (hazard ratio [HR], 2.79; P = .01), and predictors of nonreceipt included black race (HR, 0.44; P = .04) and Medicaid insurance (HR, 0.50; P <.01). For patients with MB (n = 2346), black race was no longer a significant predictor of PR use, although higher income levels (HR, 1.88; P = .04) and Medicaid insurance (HR, 0.60; P <.01) remained significant.
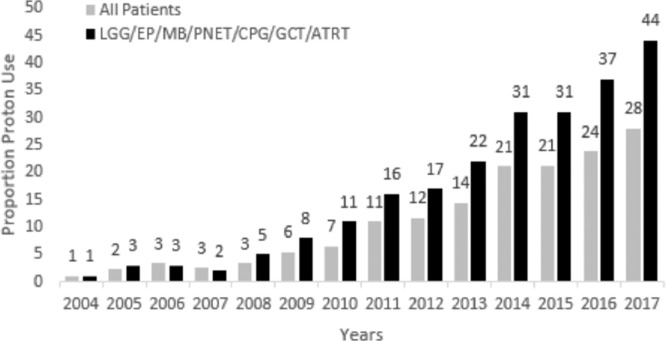
During the study period, the proportion of pediatric patients receiving PR increased from <1% in 2004 to 28% in 2017. Among the group of patients with LGG, EP, MB, PNET, GCT, and ATRT, that proportion increased from 1% to 44% during the same period, with 45% of EP and 47% of patients with MB receiving PR in 2017 (Fig. 1). The proportion of white and Asian patients receiving PR for LGG, EP, MB, PNET, GCT, and ATRT during the study period rose from <1% for both to 35% and 44%, respectively, and in black patients the proportion rose from <1% to 26%. Mean proportion of white, Asian, and black patients receiving PR across the study period was 14%, 15%, and 7%, respectively.
Figure 1.
Proportion of pediatric patients receiving proton therapy by year. Gray bars show entire patient cohort and black bars show cohort limited to patients with LGG, EP, MB, PNET, CPG, GCT, and ATRT. Abbreviations: ATRT = atypical teratoid/rhabdoid tumor; EP = ependymoma; CPG = craniopharyngioma; GCT = germ cell tumors; LGG = low-grade glioma; MB = medulloblastoma; PNET = primitive neuroectodermal tumor.
Discussion
In this report, we have detailed the national level practice patterns of PR in the treatment of pediatric primary CNS malignancies. Higher income levels, private insurance, greater distance from the treatment facility, nonrural residence, and nonblack race were independent predictors of receipt of PR on multivariable analysis.
Expectedly, PR usage has expanded significantly in recent years, with around a quarter of all reported pediatric patients with CNS receiving PR in 2016 and 2017, including nearly half of all patients with EP and MB. This finding is in concert with the increased number and improved geographic distribution of PR centers across the United States and may have been influenced by reports on reduced rates of late cognitive decline, slowing of processing speed, and secondary malignancies that were published during the study period.13, 14, 15,20
Unfortunately, there continues to be a statistically significant deficit in PR usage for patients who are black, live in rural areas, and have Medicaid insurance. Although the cost-effectiveness for the health care system of PR for pediatric CNS malignancies has been studied, it may still be that insurance coverage and travel and housing costs for the patient may be a deterrent, as the majority of proton patients in this cohort traveled >50 miles for treatment.22, 23, 24 When isolating the most recent 5 years of data, the same predictors were significant, suggesting that there has not been an improvement in the racial and socioeconomic disparities in PR use. This is especially concerning when a recent SEER analysis demonstrated that black children with CNS malignancies have worse survival compared with matched white, non-Hispanic children.25 More study is needed to determine why these disparities in utilization have persisted and how we can better support patients who are black, from lower socioeconomic groups, or geographically isolated to ensure they have equal access to best care practices.
Although the proportion of pediatric patients receiving PR for CNS malignancies has greatly increased over time owing to the increased number and wider distribution of PR centers, there continue to be disparities in access, with patients who are nonblack, privately insured, or from higher-income households being more likely to receive PR. Given the increasing data showing important late toxicity benefits to PR compared with photon radiation therapy, our field needs to address how to improve PR accessibility for all families.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: All authors have no financial disclosures and no conflicts of interest.
Data sharing statement: All data from the National Cancer Database are available through the American College of Surgeons. Research STATA code available on request.
References
- 1.Cotter SE, McBride SM, Yock TI. Proton radiotherapy for solid tumors of childhood. Technol Cancer Res Treat. 2012;11:267–278. doi: 10.7785/tcrt.2012.500295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foote RL, Stafford SL, Peterson IA, et al. The clinical case for proton beam therapy. Radiat Oncol. 2012;7:174. doi: 10.1186/1748-717X-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon M, Shin DH, Kim J, et al. Craniospinal irradiation techniques: A dosimetric comparison of proton beams with standard and advanced photon radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:637–646. doi: 10.1016/j.ijrobp.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald SM, Sethi R., Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: Clinical outcomes for 70 patients. Neuro Oncol. 2013;15:1552–1559. doi: 10.1093/neuonc/not121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez RB, Sethi R, Depauw N, et al. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: Outcomes for very young children treated with upfront chemotherapy. Int J Radiat Oncol Biol Phys. 2013;87:120–126. doi: 10.1016/j.ijrobp.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Indelicato DJ, Rotondo RL, Uezono H, et al. Outcomes following proton therapy for pediatric low-grade glioma. Int J Radiat Oncol Biol Phys. 2019;104:149–156. doi: 10.1016/j.ijrobp.2019.01.078. [DOI] [PubMed] [Google Scholar]
- 7.Indelicato DJ, Ioakeim-Ioannidou M, Bradley JA, et al. Proton therapy for pediatric ependymoma: Mature results from a bicentric study. Int J Radiat Oncol Biol Phys. 2021;110:815–820. doi: 10.1016/j.ijrobp.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Patteson BE, Baliga S, Bajaj BVM, et al. Clinical outcomes in a large pediatric cohort of patients with ependymoma treated with proton radiotherapy. Neuro Oncol. 2021;23:156–166. doi: 10.1093/neuonc/noaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahalley LS, Peterson R, Ris MD, et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol. 2020;38:454–461. doi: 10.1200/JCO.19.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald SM, Trofimov A, Safai S, et al. Proton radiotherapy for pediatric central nervous system germ cell tumors: Early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79:121–129. doi: 10.1016/j.ijrobp.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 11.De Amorim Bernstein K, Sethi R, Trofimov A, et al. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. Int J Radiat Oncol Biol Phys. 2013;86:114–120. doi: 10.1016/j.ijrobp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 12.McGovern SL, Okcu MF, Munsell MF, et al. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys. 2014;90:1143–1152. doi: 10.1016/j.ijrobp.2014.08.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93:400–407. doi: 10.1016/j.ijrobp.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016;17:287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 15.Fortin D, Tsang D, Ny A, et al. Monte Carlo-driven predictions of neurocognitive and hearing impairments following proton and photon radiotherapy for pediatric brain-tumor patients. J Neurooncol. 2017;135:521–528. doi: 10.1007/s11060-017-2597-3. [DOI] [PubMed] [Google Scholar]
- 16.Hidaka K, Fukushia H, Hosaka S, et al. Cognitive functions of pediatric brain tumor survivors treated with proton beam therapy: A case series. J Pediatr Hematol Oncol. 2021;43:e1205–e1209. doi: 10.1097/MPH.0000000000002011. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Kato T, Murakami M. Impact of lifetime attributable risk of radiation-induced secondary cancer in proton craniospinal irradiation with vertebral-body-sparing for young pediatric patients with medulloblastoma. J Radiat Res. 2021;62:186–197. doi: 10.1093/jrr/rraa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indelicato DJ, Bates JE, Mailhot Vega RB, et al. Second tumor risk in children treated with proton therapy. Pediatr Blood Cancer. 2021:e28941. doi: 10.1002/pbc.28941. [DOI] [PubMed] [Google Scholar]
- 19.Odei B, Frandsen JE, Boothe D, Ermoian RP, Poppe MM. Patterns of care in proton radiation therapy for pediatric central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2017;97:60–63. doi: 10.1016/j.ijrobp.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 20.The National Association for Proton Therapy. Proton therapy centers in the U.S. Available at: https://www.proton-therapy.org/map/. Accessed March 21, 2020.
- 21.Bitterman DS, Bona K, Laurie F, et al. Race disparities in proton radiotherapy use for cancer treatment in patients enrolled in children's oncology group trials. JAMA Oncol. 2020;6:1465–1468. doi: 10.1001/jamaoncol.2020.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundkvist J, Ekman M, Ericsson SR, Jönsson B, Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 2005;44:850–861. doi: 10.1080/02841860500341157. [DOI] [PubMed] [Google Scholar]
- 23.Lundkvist J, Vianna CMM, Guerra RL, et al. Cost-effectiveness of proton radiation in the treatment of childhood medulloblastoma. Cancer. 2005;103:793–801. doi: 10.1002/cncr.20844. [DOI] [PubMed] [Google Scholar]
- 24.Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122:1483–1501. doi: 10.1002/cncr.29882. [DOI] [PubMed] [Google Scholar]
- 25.Fineberg R, Zahedi S, Eguchi M, et al. Population-based analysis of demographic and socioeconomic disparities in pediatric CNS cancer survival in the United States. Sci Rep. 2020;10:4588. doi: 10.1038/s41598-020-61237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]