Introduction
Meningiomas account for about one third of adult primary brain tumors. Although most meningiomas are benign, their intracranial location and corresponding risk of complications from therapy may cause life-threatening morbidity.1 Radiation therapy (RT) after surgical resection is often recommended to reduce the risk of local failure in patients with recurrent or higher-grade disease.2
Radiation necrosis (RN) is one of the most severe late effects of RT to the brain, causing significant morbidity in symptomatic patients.3 Reports on the incidence of RN among patients with meningioma vary considerably (0.1%-23%),4,5 and onset can be delayed beyond the typical window of 6 to 18 months.6, 7, 8 Identification of RN after treatment remains a diagnostic challenge for clinicians given the similarities in radiographic appearance of necrotic lesions and progressing tumor on conventional multisequence magnetic resonance imaging (MRI).3
Proton RT (PRT) is under prospective investigation for the treatment of meningioma, because PRT decreases the radiation dose to normal tissue, potentially resulting in the preservation of functional capabilities and decreased risk of radiation-related toxicities.9, 10, 11 These considerations are essential for patients with meningioma who can anticipate longer survival times than patients with other primary brain tumors.12 However, in PRT, relative biologic effectiveness (RBE) varies along the treatment beam, resulting in an increase in biologically effective dose and linear energy transfer (LET) deposition at the distal segments of the spread-out Bragg peak, which is hypothesized to contribute to RN development.13,14
We present an unusual case of RN after PRT that presented as new contrast-enhancing lesions, many of which were outside of the high-dose treatment volume. Given the significant concern over whether these lesions were new metastatic disease or recurrent meningioma, we used multiparametric MRI and proton biologic effectiveness analyses to ascertain intrinsic physical factors contributing to RN development.
Case Report
A 48-year-old White man presented with 4 weeks of progressive memory difficulty, visual decline, and headaches. His previous medical history was significant for benign meningioma of the left occipital region, for which he had undergone a craniotomy and resection 13 years prior. On this encounter, MRI revealed a complex, solid-cystic, intracranial mass within the previous resection cavity involving the torcula, with extension anteriorly along the falcotentorial junction (Fig. 1).
Figure 1.
Narrative of clinical course and correlates of advanced brain tumor imaging. Multiparametric magnetic resonance imaging was used before treatment, at the time of diagnosis of radiation necrosis (RN), and at 3 months and 2 years of follow up. Preoperative imaging showed complex solid and cystic intracranial mass, with cystic component measuring 6.5 cm × 2.7 cm × 2.7 cm in anteroposterior, transverse, and craniocaudal planes, and solid nodular enhancing component 2.9 cm × 2.3 cm × 3.2 cm. When RN was diagnosed, T1 + contrast and T2-weighted fluid-attenuated inversion recovery images revealed contrast-enhancing lesions in cerebellum with mild edema indistinguishable from active disease. Permeability and relative cerebral blood flow mapping at level of contrast-enhancing lesions were normal, supporting the diagnosis of RN. On follow-up surveillance imaging, patient had waxing and waning pattern of neurologic symptoms requiring medical intervention with steroids and bevacizumab therapy.
Abbreviations: RN = radiation necrosis.
The patient underwent a craniotomy and resection of the mass. A histopathologic examination of the resected lesion revealed a solitary fibrous tumor with 4 mitoses/10 hpf. Immunostaining showed a mildly elevated Ki-67 labeling index at 10%. Tumor cells were positive for STAT6, CD34, and BCL2, and negative for epithelial membrane antigen. These features classified the tumor as a World Health Organization grade II, meningioma. There was no evidence of brain invasion.
Postoperative MRI revealed residual nodular tumor along the posterior falx and extending into the right tentorium. Given the findings of recurrent World Health Organization grade II meningioma with residual disease after resection, the patient underwent postoperative RT with passive-scatter PRT to a dose of 60 Gy RBE in 30 fractions. The surgical bed and residual tumor consisted the gross tumor volume, and a 0.5-cm anatomically constrained margin was added for the clinical treatment volume (CTV), which included the cerebellar tentorium. A 0.3-cm planning target volume margin was added with daily kV imaging for image guidance. The patient tolerated treatment well, with only fatigue and grade 1 dermatitis.
Follow-up brain MRI obtained at 14 months after completion of RT revealed stable treated disease. However, numerous new ring-enhancing lesions were present in the bilateral superior cerebellar hemispheres, as well as enhancing nodules along the superior vermis (Fig. 1). These areas raised concern for recurrent meningioma versus new intracranial primary or metastatic disease versus postradiation imaging changes. Review by a multidisciplinary health care team led to a recommendation for short-interval follow up to include brain and spine MRI. Four weeks later, conventional MRI redemonstrated well-defined regions of T2 hyperintensity and superficial enhancement along the superior cerebellar vermis. Multiparametric MRI findings favored RN rather than tumor progression (Fig. 1). Spine MRI showed no evidence of metastatic disease to the bone or leptomeninges.
Due to progressive symptoms of double vision, dizziness, and headaches, a 3-month treatment period with dexamethasone was begun, and improvement was noted. Two years after diagnosis, a course of bevacizumab (7.5 mg/kg every 2 weeks) was initiated for progressive symptoms corresponding with increased lesion-associated edema noted on T2-weighted fluid-attenuated inversion recovery (T2-FLAIR). The symptoms and FLAIR signal improved; however, bevacizumab was discontinued after 3 cycles due to imaging findings suggestive of acute ischemia. Ten months after bevacizumab discontinuation, the patient had worsening symptoms of headache, imbalance, and blurry vision. Treatment options considered included surgical resection, steroids, and reinitiation of bevacizumab. Given the patient's medical comorbidities and large area of RN, surgical resection was not recommended. Due to the patient's history of insulin-dependent diabetes and poor tolerance of steroids in the past, he was restarted on a lower dose of bevacizumab (5 mg/kg every 3 weeks) and continued for 6 cycles. The patient has remained off therapy for 8 months with no new areas of necrosis or imaging findings suggestive of progressive disease.
Discussion
RN is thought to develop from collateral radiation exposure to normal brain tissue leading to glial cell and vascular injury and ultimately resulting in local ischemia, chronic inflammation, and death of surrounding parenchyma.15,16 Malignant brain tumors are supported by angiogenic vasculature, a poorly functioning, chaotically organized, leaky vessel network that may be more susceptible to vascular injury caused by radiation exposures.17 The permeability of the blood–brain barrier and chronic tumor-associated inflammation may also prime surrounding brain tissue to radiation injury. Thus, the diagnosis of RN is often supported by the anatomic location of the lesion, with lesions near the tumor site and within the radiation treatment volume more likely to be necrosis than tumor recurrence. Likewise, the primary risk factors associated with the development of RN center around RT variables include volume, fraction size, and dose,18,19 with secondary risk factors of prior radiation exposure, disease histology, and use of systemic therapy.6,20,21 Medical management with corticosteroids or antiangiogenic therapies, such as bevacizumab used herein, is preferred initially for patients experiencing symptoms.7 Cases refractory to medical management may require surgical intervention.
For this patient, the anatomic location of new contrast-enhancing lesions on MRI did not fit the profile of RN. Although the treated cavity was in the supratentorium and had no evidence of treatment-related changes, the new lesions were in the cerebellum. RN risk in the cerebellum was unlikely to have been influenced by tumor-associated factors, such as angiogenic vasculature or inflammation, but more likely reflected RT variables because part of the cerebellum was included in the treatment field. However, several cerebellar RN lesions were outside of the high-dose treatment volume, further complicating a diagnosis based on conventional radiologic assessment and treatment history alone.
Proton biologic effectiveness: A player in radiation necrosis induction?
During radiation treatment planning, the proton radiation dose is converted to a photon-dose equivalent based on a uniform RBE factor of 1.1. However, preclinical studies have demonstrated that the RBE of PRT increases with LET.13,14,22 For protons, high-LET particles with increased RBE are present within the falloff region of the Bragg curve. Clinically, the distal edge of the proton beam typically lies beyond the tumor target and within normal tissue. In one retrospective study, the incidence and location of normal brain tissue damage after PRT were associated with regions of increased LET.23 Specifically, hyperintensity on posttreatment T2-FLAIR MRI scans from pediatric patients who underwent postoperative PRT for ependymoma were dependent on LET and dose.23 Investigators have hypothesized that RN risk may also be greater in high-LET regions. However, another retrospective review of PRT plans found no evidence of increased LET within necrotic regions.24 That study was limited by small patient groups, and the investigators concluded that the contribution of LET to RN is probably confounded by variations in absolute radiation dose and patients’ inherent sensitivity to radiation.
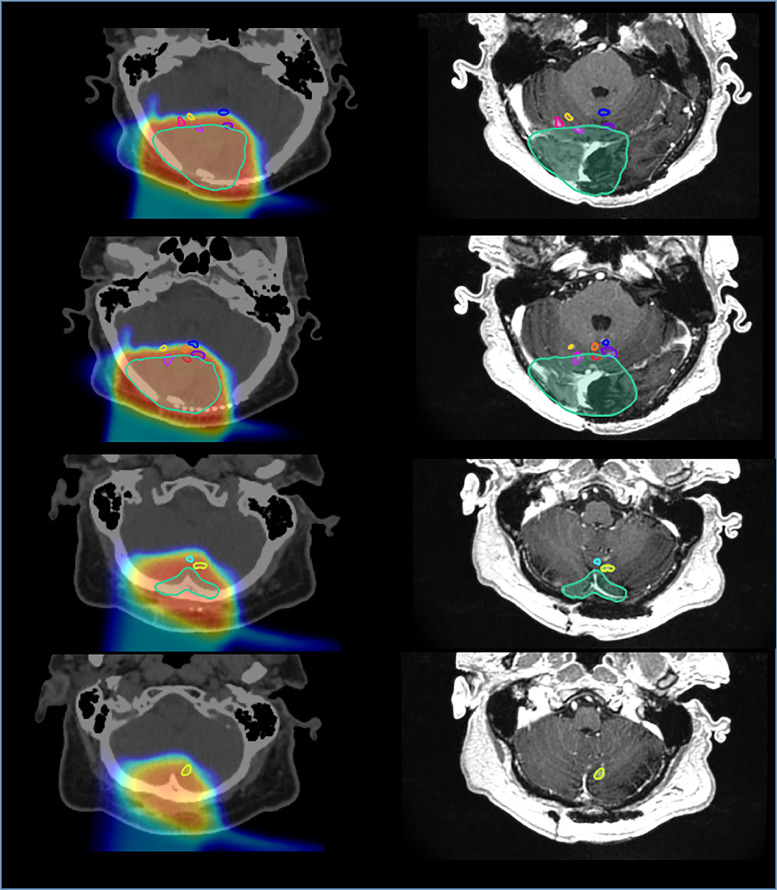
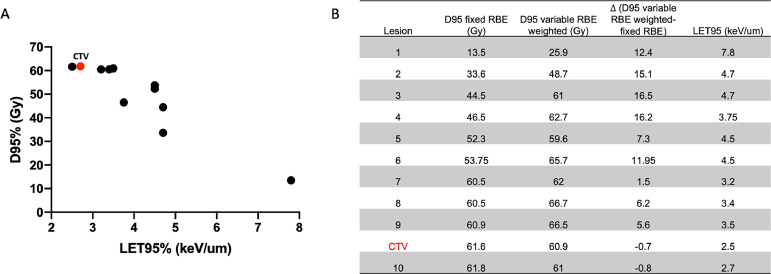
In this case, when RN lesions were contoured on MRI and co-registered to the treatment planning computed tomography, 9 of 10 lesions located in the anterior cerebellum had no overlap with the CTV (Fig. 2). Our analysis of the dose delivered to the area of the brain in which RN developed showed that 6 of 10 lesions received a dose of <60 Gy RBE to 95% of the volume (D95). As for the potential contribution of LET, our Monte Carlo predictions25 revealed that dose and LET distribution in RN lesions followed an inverse linear trend (Fig. 3). For example, a lesion that received the lowest D95 (13.5 Gy RBE) received the highest LET (LET95 of 7.8 keV/μm). In comparison, the CTV received a D95 of 62.5 Gy RBE with an LET95 of 2.5 keV/um.
Figure 2.
Necrosis lesions contoured on diagnostic magnetic resonance imaging (right) and fused with original planning computed tomography scan (left). Location of lesions with respect to clinical treatment volume (cyan line) and dose gradient delivered shown at several levels.
Figure 3.
A, Radiation dose (Gy) versus linear energy transfer (keV/μm) for 10 identified radiation necrosis lesions (black dots) and clinical treatment volume (red); and B, D95 for fixed-relative biologic effective (RBE) dose, variable RBE-weighted dose, (D95 variable RBE weighted–fixed RBE), and LET95 shown in tabular format at right.
Abbreviations: RBE = relative biologic effective.
Clinical correlations between brain RN and dosimetric variables in RT are still being defined, and the contributions of LET to charged particle RT also remain unclear. This single-patient analysis highlights the significance of accounting for high-LET regions of the beam during PRT planning to reduce the risk of necrosis in normal tissue. In clinical practice, beam arrangements that result in an overlap of multiple high-LET beam regions in critical structures, including the brain stem, optic nerves and chiasm should be avoided. Furthermore, we support the use of biologic response modeling to comprehensively describe variable RBE at the treatment planning stage.25, 26, 27
Digging deeper for a diagnosis: Role of advanced imaging in distinguishing tumor progression from radiation necrosis
Both the radiographic features and clinical course of RN overlap considerably with tumor progression, which poses diagnostic challenges to determine the most appropriate intervention.28 In many cases, progression of previously stable neurologic symptoms often prompts urgent intervention. Alternative imaging studies, such as dual-phase fluorodeoxyglucose and amino-acid positron emission tomography imaging have shown promise in detecting tumor progression versus radiation treatment effects, but have not been routinely implemented in clinical practice.29 Obtaining a tissue biopsy sample remains the gold standard for a definitive diagnosis of RN; however, in most practical settings, the diagnosis is made using imaging features alone. A significant limitation relying on imaging features is that tumor and necrosis can present simultaneously, as found in >70% of cases in pathologic confirmation studies.30
Multiparametric MRI is used as a noninvasive alternative to tissue biopsy in diagnosing RN, and provides insight into tissue biology and metabolism. Information on the biophysical processes occurring in brain tissue aids in distinguishing between necrotic and viable tumor tissue.17,31,32 A panel of perfusion parameters, including relative cerebral blood volume (rCBV), permeability (Ktrans), and cerebral blood flow (CBF), are acquired through the measurements of dynamic susceptibility contrast (DSC), dynamic contrast-enhancement (DCE), and arterial spin labeling (ASL), respectively.32 Although high-grade proliferative tumors generally have increased rCBV values due to their higher microvascular density and the presence of collateral vasculature, RN usually has lower rCBV values. Likewise, DCE, which assesses contrast leakage across the blood–brain barrier, is used as a surrogate marker of permeability and quantified by the volume transfer constant (Ktrans). Areas of RN typically have lower Ktrans values than those of tumor progression.32 Unlike DSC and DCE, ASL does not depend on the intravenous administration of gadolinium contrast, but rather quantifies magnetically labeled blood, which acts as an endogenous tracer in the measurement of CBF. Although another measure of perfusion, CBF is not affected by leakage effects in the blood–brain barrier, making CBF more quantitatively accurate than DSC.32 Expected differences in perfusion parameter findings between tumor and necrotic brain tissue are summarized in Table 1.
Table 1.
Distinction between perfusion parameters for tumor versus necrosis
| Perfusion parameter | Measurement | Metabolically active tumor | Necrosis |
|---|---|---|---|
| Cerebral blood volume | Dynamic susceptibility contrast | High | Low |
| Permeability | Dynamic contrast-enhancement/Ktrans | High | Low |
| Cerebral blood flow | Arterial spin labeling | High | Low |
The contrast-enhancing lesions identified on this patient's follow-up MRI were indistinguishable from those of tumor progression, and a perfusion analysis provided a comprehensive characterization of tissue physiology (Fig. 1). On ASL, no elevation in CBF was seen near the areas of enhancement. Although mild increased permeability (DCE) was associated with enhancing areas, there was no elevation in CBV. On follow-up imaging at 3 months and 2 years after the initial diagnosis of RN, both the size and number of lesions were stable. An overall decrease in contrast enhancement at that time supported the diagnosis of RN. Moreover, perfusion parameters and CBF remained low in areas with associated contrast enhancement. At 3 months after diagnosis, the mean DSC was 203 over 115 (standard deviation [SD]: 42/47), Ktrans was not elevated, mean ASL was 34 (SD: 7.5), and mean ASL in the cerebellum was 31 (SD: 5.5).
Conclusions
Herein, we present a case in which the diagnosis of RN after PRT was challenging, because several lesions were located outside of the target treatment volume. Considerable resources were dedicated to confirming the diagnosis, and the patient's clinical course of waxing and waning symptoms led to a significant decrease in quality of life and required multiple lines of medical therapy. Although PRT has the dosimetric advantage of a reduced integral dose to organs at risk, the location of the high-LET region of the beam should be considered during treatment design. Indeed, LET-optimized planning should be explored in this context to maximize the therapeutic window when choosing PRT over other radiation modalities. Multiparametric MRI, particularly perfusion parameters, and the use of multimodal imaging, such as positron emission tomography, allow for the noninvasive detection of radiation-induced damage with more accuracy than conventional MRI. This case highlights the need to better understand the induction, incidence, and severity of RN to facilitate strategies for improved prevention and diagnosis.
Acknowledgments
The authors thank Christine Wogan from the Department of Radiation Oncology Research for reviewing the manuscript.
Footnotes
Sources of support: Supported, in part, by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center (P.W. Pisters).
Disclosures: None.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author
References
- 1.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers CL, Won M, Vogelbaum MA, et al. High-risk meningioma: Initial outcomes from NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106:790–799. doi: 10.1016/j.ijrobp.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: Imaging features and differentiation from tumor recurrence. Radiographics. 2012;32:1343–1359. doi: 10.1148/rg.325125002. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg LA, Prayson RA, Lee J, et al. Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys. 2009;74:427–432. doi: 10.1016/j.ijrobp.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Pasquier D, Bijmolt S, Veninga T, et al. Atypical and malignant meningioma: Outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388–1393. doi: 10.1016/j.ijrobp.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Chung C, Bryant A, Brown PD. Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery. Cochrane Database Syst Rev. 2018;7 doi: 10.1002/14651858.CD011492.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawley T, Rana Z, Abou-Al-Shaar H, Goenka A, Schulder M. Major complications from radiotherapy following treatment for atypical meningiomas. Neurosurg Focus. 2019;46:E5. doi: 10.3171/2019.3.FOCUS1930. [DOI] [PubMed] [Google Scholar]
- 9.U.S. National Library of Medicine. Proton dose escalation for patients with atypical or anaplastic meningiomas. Available at: https://ClinicalTrials.gov/show/NCT02978677. Accessed June 27, 2022.
- 10.U.S. National Library of Medicine. A trial of increased dose intensity modulated proton therapy (IMPT) for high-grade meningiomas. Available at: https://ClinicalTrials.gov/show/NCT02693990. Accessed June 27, 2022.
- 11.U.S. National Library of Medicine. Proton radiation for meningiomas and hemangiopericytomas. Available at: https://ClinicalTrials.gov/show/NCT01117844. Accessed June 27, 2022.
- 12.Weber DC, Lim PS, Tran S, et al. Proton therapy for brain tumours in the area of evidence-based medicine. Br J Radiol. 2020;93 doi: 10.1259/bjr.20190237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 14.Guan F, Bronk L, Titt U, et al. Spatial mapping of the biologic effectiveness of scanned particle beams: Towards biologically optimized particle therapy. Sci Rep. 2015;5:9850. doi: 10.1038/srep09850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belka C, Budach W, Kortmann RD, Bamberg M. Radiation-induced CNS toxicity—Molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008;25:51–58. doi: 10.1007/s10014-008-0233-9. [DOI] [PubMed] [Google Scholar]
- 17.Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134:495–504. doi: 10.1007/s11060-017-2375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitpanit S, Lee A, Pitter KL, et al. Temporal lobe necrosis in head and neck cancer patients after proton therapy to the skull base. Int J Part Ther. 2020;6:17–28. doi: 10.14338/IJPT-20-00014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Smith MC, Ryken TC, Buatti JM. Radiotoxicity after conformal radiation therapy for benign intracranial tumors. Neurosurg Clin N Am. 2006;17:169–180. doi: 10.1016/j.nec.2006.04.002. vii. [DOI] [PubMed] [Google Scholar]
- 21.Lee AW, Kwong DL, Leung SF, et al. Factors affecting risk of symptomatic temporal lobe necrosis: Significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys. 2002;53:75–85. doi: 10.1016/s0360-3016(02)02711-6. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhary P, Marshall TI, Perozziello FM, et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int J Radiat Oncol Biol Phys. 2014;90:27–35. doi: 10.1016/j.ijrobp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Peeler CR, Mirkovic D, Titt U, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. 2016;121:395–401. doi: 10.1016/j.radonc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemierko A, Schuemann J, Niyazi M, et al. Brain necrosis in adult patients after proton therapy: Is there evidence for dependency on linear energy transfer? Int J Radiat Oncol Biol Phys. 2021;109:109–119. doi: 10.1016/j.ijrobp.2020.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara AL, Schuemann J, Paganetti H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys Med Biol. 2015;60:8399–8416. doi: 10.1088/0031-9155/60/21/8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flint DB, Ruff CE, Bright SJ, et al. An empirical model of proton RBE based on the linear correlation between x-ray and proton radiosensitivity. Med Phys. 2022 doi: 10.1002/mp.15850. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wedenberg M, Lind BK, Hardemark B. A model for the relative biological effectiveness of protons: The tissue specific parameter alpha/beta of photons is a predictor for the sensitivity to LET changes. Acta Oncol. 2013;52:580–588. doi: 10.3109/0284186X.2012.705892. [DOI] [PubMed] [Google Scholar]
- 28.Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9:180–188. doi: 10.1097/01.nrl.0000080951.78533.c4. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Deng L, Bai HX, et al. Diagnostic accuracy of amino acid and FDG-PET in differentiating brain metastasis recurrence from radionecrosis after radiotherapy: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2018;39:280–288. doi: 10.3174/ajnr.A5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detsky JS, Keith J, Conklin J, et al. Differentiating radiation necrosis from tumor progression in brain metastases treated with stereotactic radiotherapy: Utility of intravoxel incoherent motion perfusion MRI and correlation with histopathology. J Neurooncol. 2017;134:433–441. doi: 10.1007/s11060-017-2545-2. [DOI] [PubMed] [Google Scholar]
- 31.Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: A meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thust SC, van den Bent MJ, Smits M. Pseudoprogression of brain tumors. J Magn Reson Imaging. 2018;48:571–589. doi: 10.1002/jmri.26171. [DOI] [PMC free article] [PubMed] [Google Scholar]