Abstract
Purpose
Patients with head and neck (H&N) and esophageal cancer are at high risk for treatment-related symptomatic dehydration, often leading to interventions and hospital admissions. We tested the hypothesis that preemptive daily oral hydration during curative-intent radiation therapy would decrease dehydration as measured by intravenous fluid (IVF) delivery, acute care clinic (ACC) visits, and emergency department (ED) presentations.
Methods and Materials
Patients with H&N or esophageal cancer undergoing definitive radiation therapy were enrolled in this prospective pilot study. Beyond standard nutritional counseling, patients were given one 20-oz bottle of an electrolyte-infused solution (EIS) daily throughout treatment. Compliance, presentations to the hospital ACC and/or ED for dehydration-related indications, and IVF infusions were documented and compared with a matched contemporary control cohort. The incidence and frequency of outcomes were compared with the Fisher exact test and Wilcoxon rank-sum test, respectively.
Results
Thirty-one patients were compared during a 6-month period. Mean and median compliance rates were 87.4% and 100%, respectively. There were 0 unplanned dehydration-related ED presentations in the study group versus 3 (9.7%) among controls (P = .08). Of patients in the intervention cohort, 32.3% required presentation to the ACC, versus 64.5% in the control cohort (P = .02), with a total of 26 versus 117 visits, respectively (P = .002). On multivariable analysis, receipt of the EIS in the intervention cohort was the only significantly associated factor (P = .02). Among patients in the intervention cohort, 35.5% required IVF during treatment, versus 64.5% among controls (P = .004). The difference in ACC visits (P = .003) and IVF received (P = .008) was especially notable among patients with esophageal cancer. Patients with ≥60% EIS compliance had slightly fewer ACC visits versus those with <60% compliance (P = .067).
Conclusions
Regimented oral hydration during radiation for H&N and esophageal cancer was associated with a significant decrease in ACC visits and IVF delivery during definitive radiation therapy. This noninvasive and inexpensive preventative program in a high-risk cohort warrants further study.
Introduction
As improvements in treatment for patients with cancer continue to yield better disease control and survival, there is a heightened awareness of maximizing the safety and minimizing the toxicity of cancer therapy. A focus on optimizing quality care delivery is particularly relevant in the setting of a rising median age of cancer patients nationally and an increasing average burden of comorbid conditions.1, 2, 3, 4
Serious adverse effects of standard treatment modalities including surgery, radiation, and systemic therapies can lead to complications requiring additional interventions and inpatient management. Recent data from the largest nationally representative emergency department (ED) database identified 29.5 million ED visits by adults with a cancer diagnosis, more of which result in hospitalization compared with visits associated with other diagnoses.5,6 Among patients receiving chemotherapy and/or radiation, one of the most common reasons for cancer-related ED visits was dehydration and/or electrolyte imbalances.5 Toxic effects of this degree represent significant health risks and increased financial burden to patients, hospitals, and the health care system.7, 8, 9, 10
Patients with primary malignancies of the head and neck (H&N) and esophagus are at particularly high risk of radiation-induced symptomatic dehydration resulting in interventions and referral to higher-acuity care. As part of quality data collected at our institution, during a selected 6-month period, 22% of these patients presented to the ED with dehydration-related diagnoses, 48.1% of whom were referred to our acute care clinic (ACC) for intravenous fluids (IVF); of those patients, 61.5% required >1 ACC visit. Severe dehydration can further result in missed days of treatment and prolongation of the overall treatment course, known to negatively affect oncologic outcomes.11,12
Based on this, we carried out a prospective pilot study of prescribed preemptive oral hydration to decrease rates of symptomatic dehydration in this high-risk cohort. Using an inexpensive and generalizable intervention, we hypothesized that regimented daily fluid and electrolyte consumption throughout radiation would decrease symptomatic dehydration necessitating intervention compared with matched contemporary controls, as measured by the need for ACC referral, ED presentation, and IVF infusion.
Methods and Materials
Institutional review board approval was obtained for a prospective pilot trial of a prescribed electrolyte-infused solution (EIS) (ie, Gatorade) in patients aged ≥18 years undergoing curative-intent radiation therapy for primary H&N or esophageal cancer with or without chemotherapy. Beginning on day 1 of radiation, patients received a 20-oz bottle (flavor and sugar content per patient preference and/or relevant comorbidities) to be consumed in the department of radiation oncology, either by mouth or feeding tube, with additional bottles provided for off-treatment days.
To prospectively document compliance, all patients completed a daily fluids log (Appendix E1) to document EIS consumption (majority of the bottle) in the intervention group, as well as that of any other fluids during the day for both groups. Logs were collected and reviewed weekly. All patients received standard-of-care nutritional support and counseling from a dietician specializing in the care of this patient cohort.
As per standard institutional practice, all patients in both groups were formally assessed twice weekly for signs and symptoms of dehydration requiring referral to the ACC for IVF and/or possible referral to the ED. This comprised a weekly on-treatment visit with the nurse and treating physician, performed at minimum within every group of 5 fractions, as well as a weekly evaluation by the specialized department dietician. The number and frequency of presentations to the ACC and ED as well as the number and frequency of IVF liters delivered were recorded as surrogates for symptomatic dehydration. Patients were not required to consume the beverage if they were inpatients.
A cohort of matched controls was identified from our institution, each treated within 1 year of the paired trial participant. Patients were matched for treating physician, site of disease, age, clinical T and N category, receipt of systemic therapy, number of systemic therapy agents, histology, and radiation therapy dose.
Funding was obtained for a limited sample size. We hypothesized that our intervention could decrease the rate of ACC or ED presentation risk by 50%. With an assumption of dichotomous endpoints, a minimum of 31 patients was required to achieve an α of .05 and β of 80%. As a prospective single-arm clinical trial with matched contemporary controls, descriptive statistics (numbers and proportions for categorical variables and means, medians, and ranges for continuous variables) of covariates and outcomes were assessed and compared with the contemporary control cohort as described. The associations between relevant covariates and ACC or ED visits were estimated using logistic regression, stratified by administration of the hydration supplementation. Frequencies of ED and ACC visits and IVF administration were compared using the Wilcoxon rank-sum test, and incidences of any ED or ACC visits were compared with the Fisher exact test. The percentage of adherence with the EIS supplement per patient was compared between patients with and without ACC or ED visits with a χ2 test.
Results
A total of 31 patients were enrolled (18 with H&N cancer, 13 with esophageal cancer) and compared with 31 matched controls based on the criteria described previously; all were treated within the same period (maximum difference of 1 year). Tumor and treatment characteristics were balanced between groups (Table 1). In addition, 94% of patients were paired for early- versus late-stage (stage 1-2 vs stage 3-4) disease. Mean and median compliance with successful daily consumption of the EIS through treatment were 87.4% and 100%, respectively. Few patients underwent placement of a feeding tube, 2 versus 3 prophylactically and 2 in each group during radiation treatment in the intervention and control groups, respectively.
Table 1.
Baseline characteristics of the interventional cohort and matched contemporary controls
| Characteristic | Intervention arm (n = 31) | Matched controls (n = 31) |
|---|---|---|
| Age, y | 63 (44-87) | 59 (28-88) |
| Disease site, n (%) | ||
| Esophagus | 13 (41.9) | 13 (41.9) |
| Head and neck | 18 (58.1) | 18 (58.1) |
| Oropharynx | 9 | 9 |
| Oral cavity | 4 | 4 |
| Larynx | 3 | 3 |
| Hypopharynx | 1 | 2 |
| Salivary gland | 1 | 0 |
| T stage, n (%) | ||
| 1 | 2 (6.5) | 2 (6.5) |
| 2 | 8 (25.8) | 6 (19.4) |
| 3 | 16 (51.6) | 17 (54.8) |
| 4 | 4 (12.9) | 5 (16.2) |
| N stage, n (%) | ||
| 0 | 9 (29.0) | 8 (25.8) |
| 1 | 10 (32.3) | 10 (32.3) |
| 2 | 10 (32.3) | 9 (29.0) |
| 3 | 2 (6.5) | 3 (9.7) |
| Histology, n (%) | ||
| Adenocarcinoma | 12 (38.7) | 13 (41.9) |
| Squamous cell carcinoma | 18 (58.1) | 17 (54.8) |
| Other | 1 (3.2) | 1 (3.2) |
| Systemic therapy, n (%) | ||
| None | 2 (6.5) | 2 (6.5) |
| Chemotherapy | 29 (93.5) | 26 (83.9) |
| Cetuximab | 0 (0) | 3 (9.7) |
| Chemotherapy agents, n (%) | ||
| 0 | 2 (6.5) | 2 (6.5) |
| 1 | 16 (51.6) | 17 (54.8) |
| 2 | 13 (41.9) | 12 (38.7) |
| Total (range) radiation therapy dose, Gy | 60 (45-70) | 60 (45-70) |
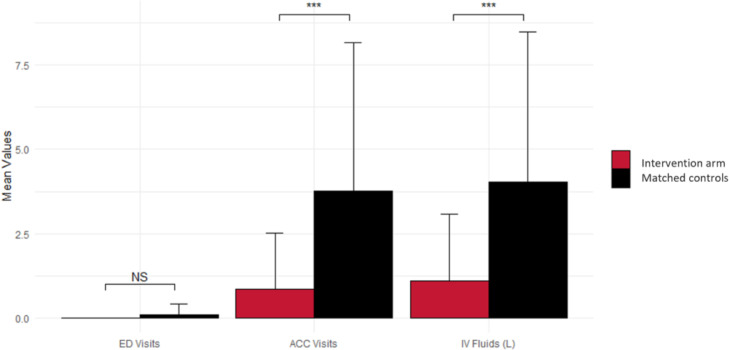
Patients in the intervention cohort experienced a decreased rate of all 3 primary outcome measures (ED and ACC admissions and IVF) (Fig. 1). Of the patients in the intervention cohort, 32.3% presented to the ACC, compared with 64.5% among the matched controls (P = .02), with a total number of 26 versus 117 visits between the groups, respectively (P = .002). On multivariable analysis including age, total radiation dose, site of disease (including H&N subsites), T-stage, N-stage, receipt of platinum chemotherapy, and receipt of EIS, receipt of the EIS was the only significantly associated factor (P = .02). Among patients with ≥60% EIS compliance, only 23.1% required a visit to the ACC, compared with 75% of those with <60% compliance (P = .067). Significantly fewer patients in the study cohort required IVF infusion during the course of treatment: 35.5% versus 64.5%, respectively (P = .004). There were few instances of unplanned ED presentations for dehydration-related complications overall: none in the intervention group versus 3 (9.7%) among controls (P = .08).
Figure 1.
Comparison of primary outcome measures between the intervention cohort and matched controls. Abbreviations: ACC = acute care clinic; ED = emergency department; IVF = intravenous fluids.
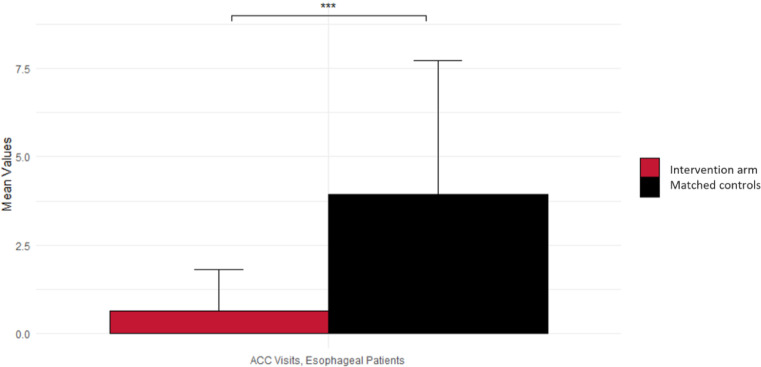
Although overall trial numbers were small, a subgroup analysis was performed comparing the cohorts with H&N and esophageal cancer separately. Among patients with esophageal primary cancer, the difference between controls and the intervention group in ACC visits (51 vs 6; P = .003) and liters of IVF received (58 vs 10; P = .008) was especially notable (Fig. 2).
Figure 2.
Difference between the intervention cohort and matched controls in acute care clinic (ACC) presentations among patients with esophageal cancer.
Discussion
Symptomatic dehydration during radiation therapy in the treatment of H&N and esophageal cancer can result in patients requiring additional invasive interventions, demand time from patients and staff, and increase resource use. In more severe instances, it can necessitate referral to higher-acuity management. Unplanned hospital admissions often result in missed days of treatment, which can negatively affect survival outcomes. An ideal preventive measure would substantially decrease rates of referrals for urgent care services such as an ACC, limit the need for medical intervention, and potentially even affect ED admissions, while simultaneously being inexpensive, noninvasive, and widely available among practice settings. Our prospective pilot study of prescribed daily hydration demonstrated a significant decrease (by approximately half) in the rate of ACC presentations and IVF requirements. Although the number of patients requiring ED presentation was low overall, there were no instances among the intervention cohort.
Compliance with the intervention was excellent, with a mean compliance rate of 87.4% and median compliance rate of 100% of collective days with patients successfully consuming a majority of the EIS. Despite a concern of esophagitis preventing patients’ ability to swallow an acidic beverage, patients were able to tolerate the EIS on the vast majority of days.
In addition to the EIS itself replenishing fluids, patients subjectively reported improved motivation and recollection to consume daily fluids in the weekly submitted fluid diary/survey, with responses confirmed directly during weekly on-treatment visits. The responsibility of logging fluid intake in real time, which was collected by the nutritionist weekly, served as a reminder for both the patient and caretakers and entailed no additional cost or access-limiting technologies. Prescribing a daily routine of scheduled hydration with required and collected documentation from the patient may therefore influence behavioral changes and lead to better maintenance of hydration in general.
Improving the quality of cancer care delivery and minimizing adverse effects is an important goal in the treatment of oncology patients. Morbidity resulting from radiation effects is distressing to the patient, can lead to further complications with associated risks, and substantially increases cost. This “financial toxicity” to patients is now better appreciated as an important risk factor affecting mental and physical health, far beyond the often-strained ability to pay for medical and health care–related services.13
Initiatives have been developed to try to address unplanned hospital admissions. At the University of North Carolina, a weekly clinic run by registered nurses and nurse practitioners was established for patients with H&N cancer to decrease rates of ED visits and admissions. Although ED visits declined and there were associated cost savings, additional strain on providers was reported.14 An ACC acts as a preventive service to obviate ED presentations, but it too demands time, expense, and the risks associated with interventions. We found that our daily hydration regimen of an EIS achieved similar goals but with negligible cost and minimal increased provider effort.
Limitations of our study include its single-arm design without a prospectively collected control group and its relatively small sample size. Nonetheless, in this well-matched cohort, a significant decrease in ACC presentations and required IVF infusions emerged. These outcomes, combined with a potential signal of benefit extending to ED referrals, are promising for the potential of further statistically significant findings with a larger randomized study.
Conclusions
Regimented daily oral electrolyte hydration during radiation for H&N and esophageal cancer was associated with a significant decrease in the rate of referral to an ACC and need for therapeutic IVF infusion for symptomatic dehydration during the course of definitive radiation treatment. These pilot data supporting a noninvasive, simple, and inexpensive program of preemptive daily fluid consumption to decrease symptomatic dehydration warrant further investigation in a larger randomized trial.
Acknowledgments
We are grateful for the contributions of our nutrition staff in delivering the intervention, including Amy LeJeune, MS, RDN, CSO, LD; Kelly Andrus, MS, RDN, LD; and April Moss, MS, RDN, LD.
Footnotes
Sources of support: Funding for this study was provided by the Case Comprehensive Cancer Center and Seidman Cancer Center of University Hospitals Cleveland Medical Center.
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.adro.2022.101026.
Appendix. Supplementary materials
References
- 1.Parry C, Kent EE, Mariotto AB, et al. Cancer survivors: A booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey WH. Baby boomers and the new demographics of America's seniors. Generations. 2010;34:28–37. [Google Scholar]
- 3.de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowland JH, Bellizzi KM. Cancer survivorship issues: Life after treatment and implications for an aging population. J Clin Oncol. 2014;32:2662–2668. doi: 10.1200/JCO.2014.55.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera DR, Gallicchio L, Brown J, et al. Trends in adult cancer-related emergency department utilization: An analysis of data from the Nationwide Emergency Department Sample. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer DK, Travers D, Wyss A, et al. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol. 2011;29:2683–2688. doi: 10.1200/JCO.2010.34.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basoor A, Doshi NC, Cotant JF, et al. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013;19:200–206. doi: 10.1111/chf.12031. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services. National health expenditure projections 2012 to 2022. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData. Accessed July 15, 2021.
- 9.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 10.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: Paying for coordinated quality care. JAMA. 2011;306:1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal DI, Mohamed ASR, Garden AS, et al. Final report of a prospective randomized trial to evaluate the dose-response relationship for postoperative radiation therapy and pathologic risk groups in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2017;98:1002–1011. doi: 10.1016/j.ijrobp.2017.02.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graboyes EM, Kompelli AR, Neskey DM, et al. Association of treatment delays with survival for patients with head and neck cancer: A systematic review. JAMA Otolaryngol Head Neck Surg. 2019;145:166–177. doi: 10.1001/jamaoto.2018.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68:153–165. doi: 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terzo L, Fleming M, Yechoor A, et al. Reducing unplanned admissions: Focusing on hospital admissions and emergency department visits for patients with head and neck cancer during radiation therapy. Clin J Oncol Nurs. 2017;21:363–369. doi: 10.1188/17.CJON.363-369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.