Abstract
Purpose:
Active surveillance for patients with low and intermediate risk prostate cancers is becoming a more utilized option in recent years. However, the use of magnetic resonance imaging and imaging-targeted biopsy for monitoring grade progression has been poorly studied in this population. We aim to define the utility of magnetic resonance imaging-targeted biopsy and systematic biopsy in an active surveillance population.
Materials and Methods:
Between July 2007 and January 2020, patients with diagnosed prostate cancer who elected active surveillance were monitored with prostate magnetic resonance imaging, imaging-targeted biopsy and standard systematic biopsy. Patients were eligible for surveillance if diagnosed with any volume Gleason grade 1 disease and select Gleason grade 2 disease. Grade progression (Gleason grade 1 to ≥2 disease and Gleason grade 2 to ≥3 disease) for each biopsy modality was measured at 2 years, 4 years and 6+ years.
Results:
In total, 369 patients had both magnetic resonance imaging-targeted and systematic biopsy and were surveilled for at least 1 year. At 2 years, systematic biopsy, magnetic resonance imaging-targeted biopsy and combined biopsy (systematic+imaging-targeted) detected grade progression in 44 patients (15.9%), 73 patients (26.4%) and 90 patients (32.5%), respectively. Magnetic resonance imaging-targeted biopsy detected more cancer grade progression compared to systematic biopsy in both the low and intermediate risk populations (p <0.001). Of all 90 grade progressions at the 2-year time point 46 (51.1%) were found by magnetic resonance imaging-targeted biopsy alone and missed by systematic biopsy.
Conclusions:
Magnetic resonance imaging-targeted biopsy detected significantly more grade progressions in our active surveillance cohort compared to systematic biopsy at 2 years. Our results provide compelling evidence that prostate magnetic resonance imaging and imaging-targeted biopsy should be included in contemporary active surveillance protocols.
Keywords: multiparametric magnetic resonance imaging, prostatic neoplasms, watchful waiting
Most low grade prostate cancers are increasingly viewed as indolent cancers. Large clinical trials have shown low grade cancers to have a very low cancer specific mortality.1,2 Given the high morbidity risk associated with definitive treatment, active surveillance of lower risk cancers has gained mainstream attention and adoption.3–6 While active surveillance of appropriately selected cancers has been demonstrated to be safe, proper initial staging of patients and accurate detection of progression while on surveillance remains a concern.3,7
Prostate biopsies have traditionally been performed under transrectal ultrasound guidance, allowing the prostate to be sampled systematically according to location (systematic biopsy). Systematic biopsy has been criticized because it overdiagnoses low grade cancers while underdiagnosing high grade cancers.8–11 MRI-targeted biopsies have been shown to improve the detection of clinically significant cancer at initial diagnosis compared to systematic biopsy.9,12–14 However, despite the growing number of patients being enrolled on active surveillance to monitor their disease, the use of mpMRI and MRI-targeted biopsy in today’s diagnostic paradigm is unclear and remains controversial. Therefore, to address the controversy for the use of MRI in this population, we sought to determine whether systematic or MRI-targeted biopsy results in better detection of cancer grade progression among men on active surveillance for GG1 and GG2 prostate cancer.
METHODS
A nationally registered, prospective, institutional review board approved clinical trial was initiated in July 2007 at the National Cancer Institute (Bethesda, Maryland) to evaluate the use of electromagnetic tracking devices for targeted biopsies (clinicaltrails.gov, NCT00102544). The electromagnetic tracking device is now clinically available as the UroNav platform (Philips Healthcare, Andover, Massachusetts).
Men greater than 18 years old with a suspicion of prostate cancer (elevated prostate specific antigen or abnormal digital rectal examination), underwent a prostate MRI. Men found to have a suspicious lesion on prostate MRI met study inclusion criteria, and were offered MRI-targeted and systematic prostate biopsy. All patients included in the study were consented for prostate biopsy prior to enrollment.
MRI Protocol
All patients underwent a multiparametric MRI as previously described.15 Briefly, MRIs were performed using a 3.0 T magnet (Achieva, Philips Healthcare) with an endorectal coil (BPX-30, Medrad®) for all initial evaluation scans. For most subsequent followup scans after the initial evaluation scan, endorectal coil was not used. Prostate MRI studies underwent standardized radiological evaluation by 2 highly experienced genitourinary radiologists. All lesions subsequent to April 2015 were assigned an assessment category using the standardized PI-RADS™ guidelines.16 Lesions identified before the adoption of the PI-RADS system were graded using the National Institutes of Health suspicion score system, previously demonstrated to correlate to the PI-RADS system.17,18 In preparation for biopsy, lesions were identified, labeled and segmented by 1 radiologist using DynaCAD® software.
Biopsy Protocol
All patients underwent both systematic biopsy and MRI-targeted biopsy in the same procedure. MRI-targeted biopsies were performed using the UroNav fusion biopsy system for lesion targeting. MRI images were registered to a real-time prostate ultrasound image, aiding in the identification and sampling of the MRI visible lesions. A minimum of 2 biopsy cores were taken of each lesion with an end-fire transrectal ultrasonographic probe (Philips Healthcare) with the aid of the UroNav system. Following the MRI-targeted biopsy, a standard 12-core systematic biopsy was performed after the targeted biopsy portion of the procedure. The physician was unable to view the MRI target data during this portion of the procedure, and only ultrasound was used for guidance as previously described.19 All pathology specimens were reviewed by a single genitourinary pathologist.
Active Surveillance Protocol
Patients with GG1 and GG2 disease on either systematic biopsy or MRI-targeted biopsy were eligible for our protocol, and risks/benefits of active surveillance were discussed. Patients were eligible if diagnosed with any volume GG1 or GG2 disease if they did not elect definitive therapy and MRI had no aggressive features on evaluation (suggesting ≥T3 disease). No strict exclusion criteria based on PSA or PSA density were applied for eligibility. Patients with current or prior diagnosis of ≥GG3 disease were not eligible. Confirmatory biopsy after initial cancer diagnosis was conducted with MRI-targeted biopsy (if lesions present on mpMRI) and systematic biopsy within 1 year of initial diagnosis. Patients on surveillance were followed with a yearly PSA test and physical examination. Repeat prostate MRI/biopsy was encouraged at 1-year to 2-year intervals based on clinical suspicion, PSA, changes in physical examination or changes on prostate MRI.
Data Analysis
Data were collected between in a prospective manner July 2007 and January 2020 as part of a pretrial designed database. For each patient, the highest Gleason grade detected by each biopsy method was recorded. Only patients on surveillance for ≥1 year at our institution were included in our analysis to assess grade progression.
The main objective of the study was to test the hypothesis that MRI-targeted biopsy has a higher rate of detection of grade progression for clinically significant cancer compared to systematic biopsy at surveillance time points of 2, 4 and 6+ years. Patients were included in the appropriate time point if biopsy was obtained ±12 months from time point analyzed (6-year time point included patients on surveillance >5 years). At each time point, pathology results from both biopsy modalities were compared to the initial biopsy. Grade progression was defined as GG1 to ≥GG2 disease and GG2 to ≥GG3 disease.
Statistical Analysis
Grade progression rates detected by each biopsy method were compared at a specific surveillance time point by McNemar test. Confidence intervals for the grade progression rate were calculated using the Agresti-Coull method.20 Univariate logistic regression was used to identify baseline predictors for overall grade progression of combined biopsy. Variables significant at the univariate analysis were included in the multivariable logistic regression. Clinical variables measured at the followup MRI were correlated with grade progression detected by the addition of a biopsy method via logistic regression analysis. All p values were 2-sided, and p <0.05 was considered statistically significant. All analyses were conducted using R.3.6.1 (R Project for Statistical Computing, Vienna, Austria).
RESULTS
Patients
From June 2007 to January 2020, a total of 2,373 men underwent mpMRI and MRI-ultrasound fusion biopsy at the National Cancer Institute for evaluation of prostate cancer. In total, 1,799 patients were excluded from the analysis for high grade cancer detection, undergoing definitive treatment or electing local provider followup. A further 205 patients who had less than 1-year followup at our institution were excluded from the analysis. The remaining 369 men met the study inclusion criteria and were included in our analysis (fig. 1). Median followup was 30.3 months (IQR 16.5–53.9). On enrollment, all patients in our study had GG1 disease or GG2 favorable disease (per National Comprehensive Cancer Network criteria).21 Table 1 outlines the clinical and demographic characteristics of the study population.
Figure 1.
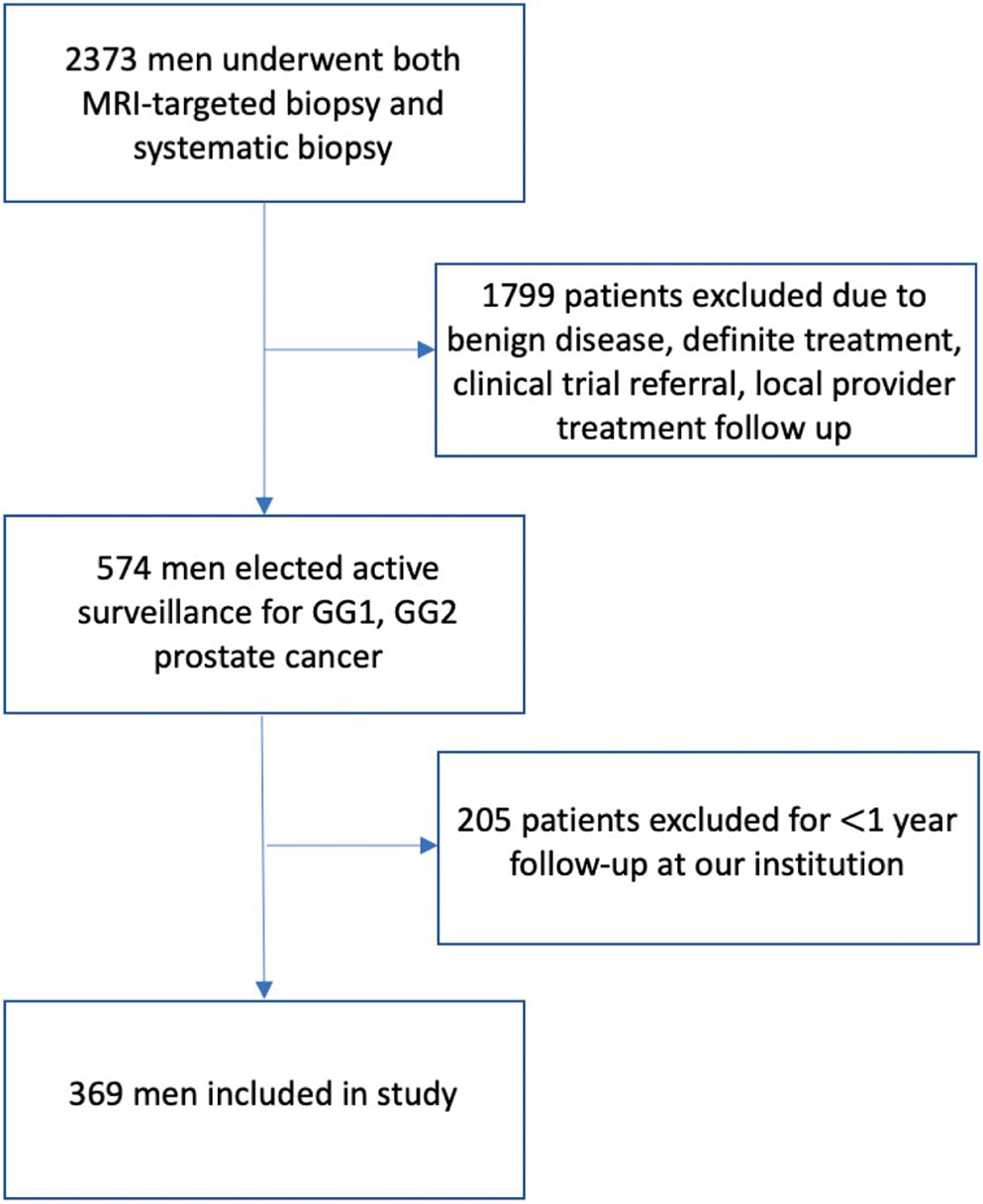
Flow chart of patients included in our study
Table 1.
Patient characteristics at active surveillance enrollment
| All Pts | GG1 at Enrollment | GG2 at Enrollment | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. | 369 | 272 | 97 | |||
| Median mos followup (IQR) | 30.3 | (16.5–53.9) | 34.8 | (19.8–57.7) | 25.9 | (14.5–36.3) |
| Median yrs age (IQR) | 63.3 | (58.4–68) | 62.4 | (57.9–66.6) | 66.8 | (60.3–70.7) |
| Median ng/ml PSA (IQR) | 5.28 | (3.69–7.85) | 5.31 | (3.67–7.64) | 5.27 | (4.01 –8.47) |
| Median cm3 prostate vol (IQR) | 49.75 | (37.25–69) | 50 | (38–68.35) | 48 | (37–74.5) |
| Median ng/cm3 PSA density (IQR) | 0.10 | (0.07–0.14) | 0.10 | (0.07–0.14) | 0.10 | (0.08–0.14) |
| Median No. lesions on initial MRI (range) | 2 | (1–3) | 2 (1 –3) | 2 | (1–3) | |
| Median mm index lesion (IQR) | 10 | (7.38–14) | 10 | (7.12–13) | 10.5 | (8–14) |
Grade Progression on Active Surveillance
At 2 years on surveillance, 277 patients were evaluated with systematic and MRI-targeted biopsy. Systematic biopsy, MRI-targeted biopsy and combined biopsy (systematic + MRI-targeted) detected grade progression in 44 patients (15.9%), 73 patients (26.4%) and 90 patients (32.5%), respectively (fig. 2). MRI-targeted biopsy detected more cancer grade progression compared to systematic biopsy in both the low and intermediate risk populations (p <0.001, table 2). Grade progression was found exclusively by MRI-targeted biopsy in 16.6% of our population (46 of 277 patients, 95% CI 12.7–21.5), compared to 6.1% (17 of 277, 95% CI 3.8–9.7) for systematic biopsy (table 3). In a subgroup analysis, MRI-targeted biopsy found more GG1 to ≥GG2 (p = 0.029) and GG2 to ≥GG3 (p = 0.006) disease grade progression compared to systematic biopsy.
Figure 2.
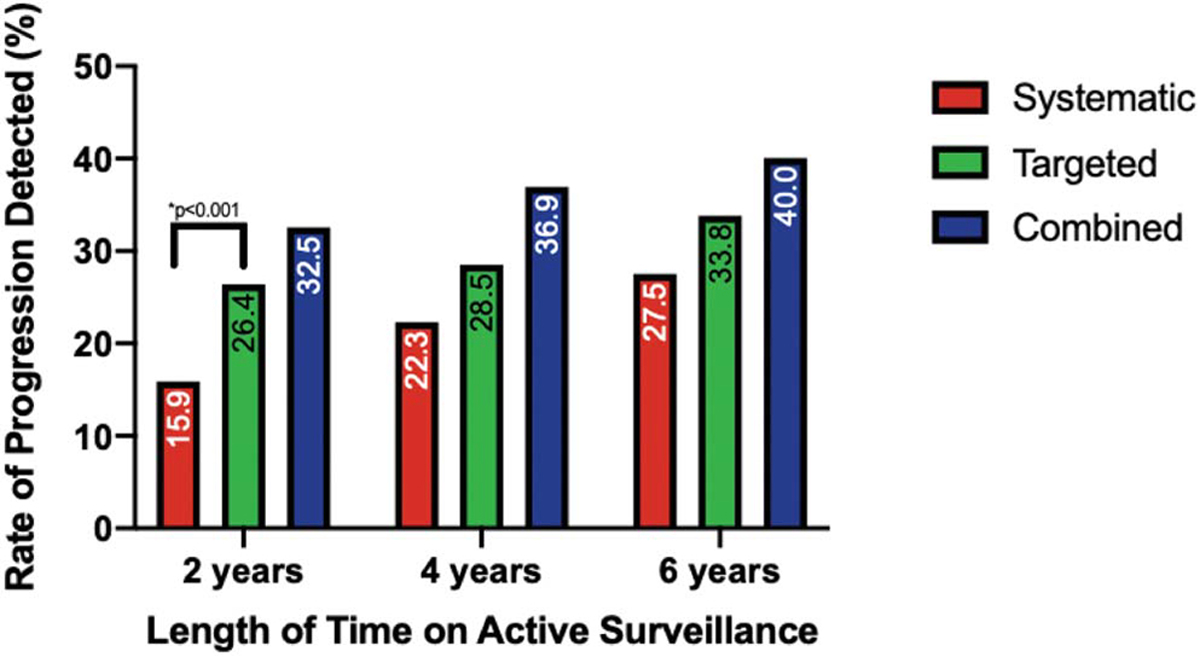
Gleason grade progression detection rates at active surveillance time points stratified by biopsy modality.
Table 2.
Detection of Gleason grade progression for MRI-targeted biopsy compared to systematic biopsy
| 2 Yrs (277 pts) |
4 Yrs (130 pts) |
6+ Yrs (80 pts) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade Progression | MRI-Targeted Biopsy | Systematic Biopsy | p Value | MRI-Targeted Biopsy | Systematic Biopsy | p Value | MRI-Targeted Biopsy | Systematic Biopsy | p Value |
|
| |||||||||
| GG1–≥GG2 (No.) | 23.4% (44) | 15.4% (29) | 0.029 | 31.4% (32) | 22.5% (23) | 0.12 | 34.7% (25) | 26.4% (19) | 0.18 |
| GG2–≥GG3 (No.) | 32.6% (29) | 16.9% (15) | 0.006 | 17.9% (5) | 21.4% (6) | 1 | 25.0% (2) | 37.5% (3) | 1 |
| All progression (No.) | 26.4% (73) | 15.9% (44) | <0.001 | 28.5% (37) | 22.3% (29) | 0.20 | 33.8% (27) | 27.5% (22) | 0.30 |
Grade progression detection difference was significant at 2 years (p <0.001).
Table 3.
Added value and confidence intervals for systematic and targeted biopsy for grade progression by biopsy method
| Systematic Biopsy |
MRI-Targeted Biopsy |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade Progression | Additional No. Pts Detected | Added Value | 95% Lower Control Limit | 95% Upper Control Limit | Additional No. Pts Detected | Added Value | 95% Lower Control Limit | 95% Upper Control Limit |
|
| ||||||||
| 2-Yr time point: | ||||||||
| GG1–GG≥2 | 13 | 6.9% | 4.0% | 11.6% | 28 | 14.9% | 10.5% | 20.7% |
| GG2–GG≥3 | 4 | 4.5% | 1.4% | 11.3% | 18 | 20.2% | 13.1% | 29.8% |
| All progression | 17 | 6.1% | 3.8% | 9.7% | 46 | 16.6% | 12.7% | 21.5% |
| 4-Yr time point: | ||||||||
| GG1–GG≥2 | 9 | 8.8% | 4.5% | 16.1% | 18 | 17.6% | 11.4% | 26.3% |
| GG2–GG≥3 | 2 | 7.1% | 0.9% | 23.7% | 1 | 3.6% | −0.9% | 19.2% |
| All progression | 11 | 8.5% | 4.7% | 14.7% | 19 | 14.6% | 9.5% | 21.8% |
| 6h+-Yr time point: | ||||||||
| GG1–GG≥2 | 4 | 5.6% | 1.8% | 13.8% | 10 | 13.9% | 7.5% | 23.9% |
| GG2–GG≥3 | 1 | 12.5% | 0.1% | 49.2% | 0 | 0 | −4.8% | 37.2% |
| All progression | 5 | 6.1% | 2.4% | 14.1% | 10 | 12.5% | 6.7% | 21.7% |
At 4 and 6+ years, 130 and 80 patients, respectively, were evaluated on active surveillance. Grade progression was found in 48 patients (36.9%) at 4 years and 32 (40.0%) at 6+ years (fig. 2). No significant difference in detection of grade progression was found between biopsy modalities at either time point (table 2). However, MRI-targeted biopsy alone detected more grade progressions compared to systematic biopsy alone, providing a higher added value for detection at all time points (table 3).
On univariate analysis at the 2-year time point, overall grade progression was significantly associated with age (per decade, OR 1.53, 95% CI 1.06–2.22, p=0.024), PSA density (log transformed, OR 2.30, 95% CI 1.41–3.76, p=0.001) and size of index lesion on initial MRI (OR 1.06, 95% CI 1.01–1.12, p=0.035). On multivariate analysis, only age/decade (OR 1.68, 95% CI 1.11–2.53, p=0.013) and PSA density (OR 2.44, 95% CI 1.46–4.09, p=0.001) were associated with grade progression (table 4).
Table 4.
Univariate and multivariable ORs for association of grade progression at 2 years
| Variable | Univariate OR for grade progression (95% CI) | p Value | Multivariable OR for grade progression (95% CI) | p Value |
|---|---|---|---|---|
|
| ||||
| Age (per decade) | 1.53 (1.06–2.22) | 0.024 | 1.68 (1.11 –2.53) | 0.013 |
| log(PSA) | 1.41 (0.94–2.11) | 0.099 | — | |
| log(prostate vol) | 0.61 (0.34–1.09) | 0.174 | — | |
| log(PSA density) | 2.30 (1.41–3.76) | 0.001 | 2.44 (1.46–4.09) | 0.001 |
| No. lesions | 1.07 (0.86–1.32) | 0.563 | — | |
| Size of index lesion | 1.06 (1.01–1.12) | 0.035 | 1.05 (0.99–1.12) | 0.11 |
| PI-RADS 4 vs 1–3 | 0.57 (0.22–1.52) | 0.264 | — | |
| PI-RADS 5 vs 1–3 | 2.29 (0.68–7.74) | 0.184 | — | |
Grade Progression by Systematic and MRI-Targeted Biopsy at 2 Years
At 2 years, grade progression was detected in 90/277 patients (32.5%) in our cohort. Of these patients with grade progression, MRI-targeted biopsy alone found 46/90 patients (51.1%), systematic biopsy alone found 17/90 patients (18.9%) and both biopsy modalities found grade progression in 27/90 patients (30.0%). On analysis with clinical variables, grade progression detected by systematic biopsy alone was found to inversely correlate to prostate volume (OR 0.21, 95% CI 0.072–0.721, p=0.01). Figure 3 illustrates the proportions of grade progressions by the addition of systematic and targeted biopsy according to prostate volume. A threshold analysis was conducted to assess at which prostate volume systematic biopsy would have minimal added value in addition to targeted biopsy, allowing for <2.5% of grade progression to be missed. Using these parameters, systematic biopsy could have been skipped for patients with prostate volume >64 cm3, allowing for 36.8% of all systematic biopsies to be skipped for patients on surveillance, while only missing 2.2% (2/90) of grade progressions in our cohort.
Figure 3.
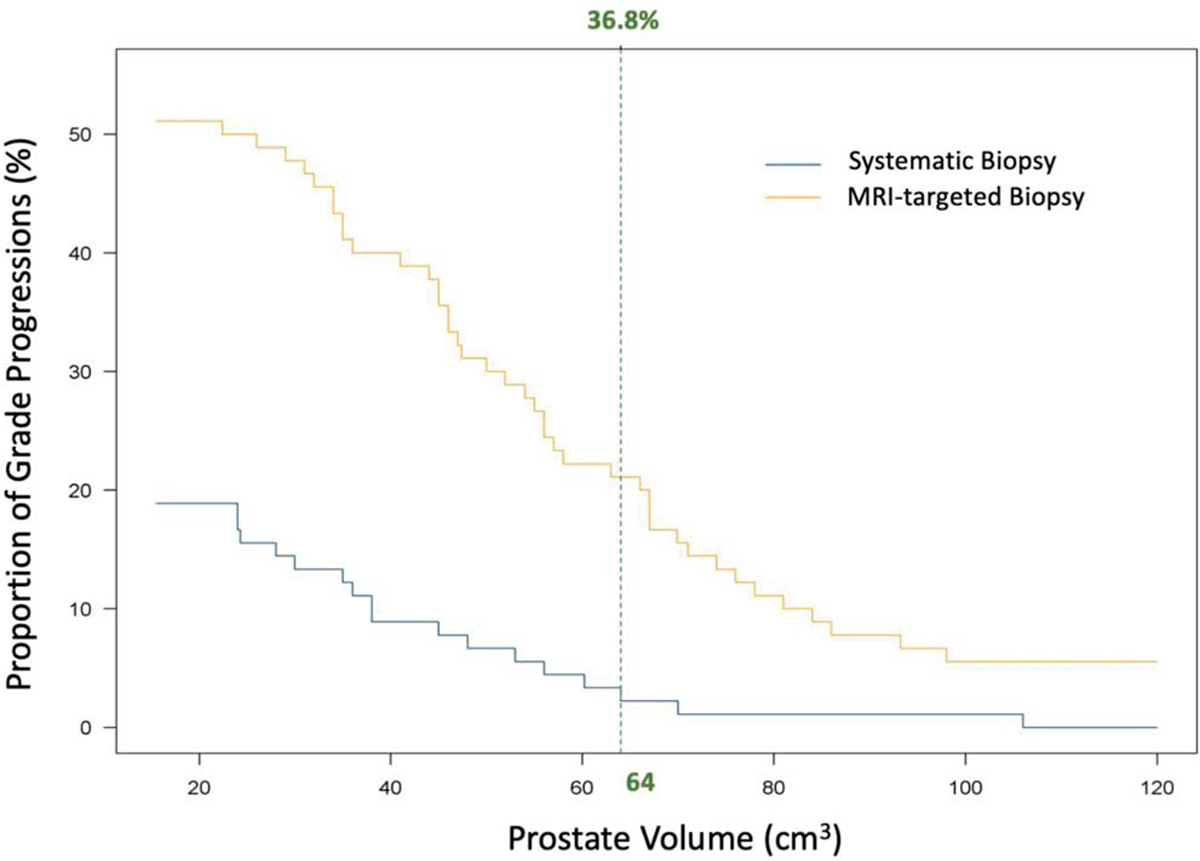
Threshold analysis of grade progression detection rates and prostate volume. Vertical green line indicates volume threshold at which systematic biopsy could be skipped if allowing for 2.2% grade progression to be missed. If only MRI-targeted biopsy was utilized for surveillance in patients with prostate volumes >64 cm3, 97.8% (88/90) of all grade progressions would still be detected and 36.8% of systematic biopsies would have been avoided.
DISCUSSION
The use of prostate MRI has expanded significantly in the diagnosis and treatment of prostate cancer in recent years. Prostate MRI and MRI-targeted biopsy’s utility in the diagnosis of prostate cancer has been proven in many studies, including the recent PRECISION study.14 Our group also recently published on the improved utility of MRI-targeted biopsy over systematic biopsy in accurately profiling patients.19 Given MRI’s growing utilization in the diagnosis of cancer, clinicians are expanding the use of prostate MRI and MRI-targeted biopsy in patients on active surveillance, despite limited comparative evidence.
In our study, we found that MRI-targeted biopsy detected grade progression at higher rates than systematic biopsy in both the low and intermediate risk patients. We aimed to show the utility of MRI-targeted biopsy at typical surveillance time points, given that a common treatment intervention time point for patients on surveillance is around 2 years.22 At the 2-year surveillance time point, MRI-targeted biopsy detected more GG1 to ≥GG2 and GG2 to ≥GG3 disease grade progression compared to systematic biopsy. This result supports the use of MRI-targeted biopsy by urologists to better surveil their patients. Furthermore, in patients with GG2 disease at enrollment, MRI-targeted biopsy exclusively found progression to ≥GG3 in 20.2% of patients, while systematic biopsy found only 4.5% of patients. If patients with GG2 prostate cancer are enrolled in active surveillance protocols, we recommend surveillance with the addition of MRI-targeted biopsy as it proved its superiority in detecting grade group progression compared to standard systematic biopsy. There was no statistical difference noted at 4 and 6+ years, likely due to the fewer patients included in the analysis at those time points given that some men with progression were removed from surveillance and that patients tended to transfer care to their local urologist over time.
Our study also provided evidence that MRI-targeted biopsy found a majority of patients with grade progression at all time points. In our 2-year analysis, MRI-targeted biopsy alone found over 51% of the patients who had grade progression. If only systematic biopsy was used in our protocol, more than half of all patients with progression would have been missed and incorrectly risk stratified on our surveillance regimen. This is further evidence of the added value of MRI-targeted biopsy in this population. However, we do not advocate elimination of systematic biopsy from our surveillance protocol, given that it exclusively detected almost 18% of grade progressions. Instead, we demonstrate that the utility of systematic biopsy to detect grade progression was nominal for patients with prostate volumes greater than 64 cm3. By only utilizing MRI-targeted biopsy in these patients, 97.8% of the grade progressions would have been detected and more than a third of patients in our cohort would have been spared a 12-core systematic biopsy. Given the associated of morbidity with additional biopsy cores,23 this strategy could prove to be a safe, effective and cost-conscious way to surveil patients.
Several earlier studies revealed mixed results in the use of MRI in patients on active surveillance. A recent study by Chesnut et al found that if a change on MRI at 3 years on surveillance is used as a reason to biopsy or not, clinically significant cancer would be missed in 53% of patients.24 Similarly, the utility of MRI-targeted biopsy in patients on surveillance remains in question. In the recent ASIST trial, the authors showed no difference in the grade progression to ≥GG2 on confirmatory biopsy between the MRI-targeted arm and standard biopsy arm.25 However, in a 2-year followup study published by the same group, the authors reported a significant difference in active surveillance failures in the 2 groups.26 The authors suggest that MRI helped select for a lower risk cohort at the time of confirmatory biopsy, thus resulting in a group of patients more likely to be followed on active surveillance. Our data show a clear benefit to the addition of prostate MRI and MRI-targeted biopsies in a surveillance protocol, which should be utilized by clinicians when available.
Our study has a number of strengths and limitations. One strength of the study is our inclusion of GG2 disease at enrollment in surveillance, creating a more heterogeneous cohort for analysis, reflecting real-world practice patterns. Given the emerging evidence that patients with GG2 disease may be safe to surveil, we believe these patients should be monitored closely with MRI-targeted biopsy to better identify if and when progression occurs.27–29 Conversely, a limitation of our study is that patients with a negative prostate MRI were excluded, limiting the generalizability to the overall population seen in the community. In addition, the use of GG alone as an indicator of progression and not volume of cancer or cores is a limitation. MRI-targeted biopsy has been shown to be associated with downgrading at final pathology (likely oversampling a lesion), and thus may overestimate the true GG of the lesion.30 Lastly, our institution serves as a quaternary referral center, with a specific population of patients referred to us for evaluation, creating the possibility of a selection bias in our cohort. Our institution has proficiency with MRI acquisition and interpretation, and performing MRI-targeted biopsies. Thus, these results may not be replicable in an institution without expertise in these practices, limiting the applicability of our findings to the general population.
CONCLUSIONS
For patients on active surveillance for prostate cancer, MRI-targeted biopsy detected higher rates of grade progression compared to standard transrectal ultrasound biopsy. At 2 years, MRI-targeted biopsy exclusively detected 51.1% of all grade progressions in our cohort that systematic biopsy missed. Our study provides strong evidence that prostate MRI and MRI-targeted biopsy should be included in contemporary active surveillance protocols for prostate cancer.
Abbreviations and Acronyms
- GG
Gleason grade
- mpMRI
multiparametric magnetic resonance imaging
- MRI
magnetic resonance imaging
- PI-RADS
Prostate Imaging Reporting and Data System
- PSA
prostate specific antigen
Footnotes
Financial interest and/or other relationship with NVIDIA and NIH (which has cooperative research and development agreements with Philips, Celsion Corp., Boston Scientific, Siemens and Exact Robotics, and MTA support with Exact Imaging, Canon Medical, QT Imaging and Angiodynamics).
NIH and Philips have a Cooperative Research and Development Agreement. NIH has intellectual property in the field, including among other patents and patent applications, Patent: “System, methods, and instrumentation for image guided prostate treatment,” U.S. Patent No. 8948845, with inventors/authors including PLC, BW and PP. NIH and Philips (InVivo Inc) have a licensing agreement. NIH and authors PLC, BW and PP receive royalties for a licensing agreement with Philips/InVivo Inc. NIH does not endorse or recommend any commercial products, processes or services. The views and personal opinions of the authors expressed herein do not necessarily reflect those of the U.S. Government, nor reflect any official recommendation nor opinion of the NIH nor NCI.
Contributor Information
Nitin K. Yerram, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Lori Long, Center for Interventional Oncology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Luke P. O’Connor, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Alex Z. Wang, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Michael Ahdoot, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Amir H. Lebastchi, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Sandeep Gurram, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Johnathan Zeng, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Heather Chalfin, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Stephanie A. Harmon, Clinical Research Directorate, Frederick National Laboratory for Cancer Research sponsored by the National Cancer Institute, Bethesda, Maryland
Sherif Mehralivand, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Maria J. Merino, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Howard L. Parnes, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Peter L. Choyke, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Joanna Shih, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Bradford J. Wood, Center for Interventional Oncology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Baris Turkbey, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Peter A. Pinto, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
REFERENCES
- 1.Hamdy FC, Donovan JL, Lane JA et al. : 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Garmo H et al. : Radical prostatectomy or watchful waiting in prostate cancer—29-year follow-up. N Engl J Med 2018; 379: 2319. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015; 33: 272. [DOI] [PubMed] [Google Scholar]
- 4.Morash C, Tey R, Agbassi C et al. : Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J 2015; 9: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz L: Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol 2004; 172: S48. [DOI] [PubMed] [Google Scholar]
- 6.Loeb S, Gonzalez CM, Roehl KA et al. : Pathological characteristics of prostate cancer detected through prostate specific antigen based screening. J Urol 2006; 175: 902. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Musunuru HB, Vesprini D et al. : Metastatic prostate cancer in men initially treated with active surveillance. J Urol 2016; 195: 1409. [DOI] [PubMed] [Google Scholar]
- 8.Kasivisvanathan V, Stabile A, Neves JB et al. : Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol 2019; 76: 284. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed HU, El-Shater Bosaily A, Brown LC et al. : Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815. [DOI] [PubMed] [Google Scholar]
- 10.Filson CP, Natarajan S, Margolis DJ et al. : Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016; 122: 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouviere O, Puech P, Renard-Penna R et al. : Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019; 20: 100. [DOI] [PubMed] [Google Scholar]
- 12.De Visschere PJ, Briganti A, Futterer JJ et al. : Role of multiparametric magnetic resonance imaging in early detection of prostate cancer. Insights Imaging 2016; 7: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futterer JJ, Briganti A, De Visschere P et al. : Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015; 68: 1045. [DOI] [PubMed] [Google Scholar]
- 14.Kasivisvanathan V, Rannikko AS, Borghi M et al. : MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378: 1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkbey B, Mani H, Aras O et al. : Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 2013; 268: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinreb JC, Barentsz JO, Choyke PL et al. : PI-RADS Prostate Imaging—Reporting and Data System: 2015, version 2. Eur Urol 2016; 69: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaur S, Harmon S, Mehralivand S et al. : Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 2018; 48: 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rais-Bahrami S, Siddiqui MM, Turkbey B et al. : Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013; 190: 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahdoot M, Wilbur AR, Reese SE et al. : MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 2020; 382: 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agresti A and Caffo B: Simple and effective confidence intervals for proportions and differences of proportions result from adding two successes and two failures. Am Statistician 2000; 54: 280. [Google Scholar]
- 21.National Comprehensive Cancer Network: Prostate Cancer (version 1.2020). 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 22.Tosoian JJ, Trock BJ, Landis P et al. : Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol 2011; 29: 2185. [DOI] [PubMed] [Google Scholar]
- 23.Papagiannopoulos D, Abern M, Wilson N et al. : Predictors of infectious complications after targeted prophylaxis for prostate needle biopsy. J Urol 2018; 199: 155. [DOI] [PubMed] [Google Scholar]
- 24.Chesnut GT, Vertosick EA, Benfante N et al. : Role of changes in magnetic resonance imaging or clinical stage in evaluation of disease progression for men with prostate cancer on active surveillance. Eur Urol 2020; 77: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klotz L, Loblaw A, Sugar L et al. : Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol 2019; 75: 300. [DOI] [PubMed] [Google Scholar]
- 26.Klotz L, Pond G, Loblaw A et al. : Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year postbiopsy follow-up. Eur Urol 2020; 77: 311. [DOI] [PubMed] [Google Scholar]
- 27.Luzzago S, de Cobelli O, Cozzi G et al. : A novel nomogram to identify candidates for active surveillance among patients with ISUP grade group 1 or ISUP grade group 2 prostate cancer, according to multiparametric magnetic resonance imaging findings. BJU Int 2020; 126: 104. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson S, Benfante N, Alvim R et al. : Risk of metastasis in men with grade group 2 prostate cancer managed with active surveillance at a tertiary cancer center. J Urol 2020; 203: 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stavrinides V, Giganti F, Trock B et al. : Five-year outcomes of magnetic resonance imaging-based active surveillance for prostate cancer: a large cohort study. Eur Urol 2020; 78: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoag JE, Cai PY, Gross MD et al. : Impact of prebiopsy magnetic resonance imaging on biopsy and radical prostatectomy grade concordance. Cancer 2020; 126: 2986. [DOI] [PubMed] [Google Scholar]


