Abstract
Transactive response DNA-binding protein 43 kDa (TDP-43) proteinopathy is the hallmark of limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC). LATE-NC is a common copathology with Alzheimer disease neuropathologic change (ADNC). Data from the National Alzheimer’s Coordinating Center were analyzed to compare clinical features and copathologies of autopsy-confirmed ADNC with versus without comorbid LATE-NC. A total of 735 participants with ADNC alone and 365 with ADNC with LATE-NC were included. Consistent with prior work, brains with LATE-NC had more severe ADNC, more hippocampal sclerosis, and more brain arteriolosclerosis copathologies. Behavioral symptoms and cognitive performance on neuropsychological tests were compared, stratified by ADNC severity (low/intermediate vs high). Participants with ADNC and LATE-NC were older, had higher ADNC burden, and had worse cognitive performance than participants with ADNC alone. In the low/intermediate ADNC strata, participants with comorbid LATE-NC had higher prevalence of behavioral symptoms (apathy, disinhibition, agitation, personality change). They also had worsened performance in episodic memory and language/semantic memory. Differences narrowed in the high ADNC strata, with worsened performance in only episodic memory in the comorbid LATE-NC group. The co-occurrence of LATE-NC with ADNC is associated with a different pattern of behavioral and cognitive performance than ADNC alone, particularly in people with low/intermediate ADNC burden.
Keywords: Alzheimer disease, Depression, Lewy body, Limbic predominant age-related TDP-43 encephalopathy, Neuropsychiatric, TDP-43
INTRODUCTION
Limbic-predominant age-related transactive response DNA-binding protein 43 kDa (TDP-43) encephalopathy neuropathologic change (LATE-NC) is a prevalent neuropathology in older age (1, 2). This condition has been detected in over 20% of brains in community autopsy series and is especially common (30%–50%) in people over 80 years (1, 3–5). LATE-NC often coexists with other neuropathologies, such as Alzheimer disease neuropathologic change (ADNC) (1, 6). In isolation, the cognitive decline associated with LATE-NC tends to be milder and slower in course than in persons with “pure” ADNC. The cognitive decline associated with LATE-NC is more severe when it coexists with other neuropathologies, such as ADNC or Lewy body disease (2, 3, 7–9).
Given the high prevalence of both ADNC and LATE-NC, and their common coexistence in the same brains, it is important to understand their potential interactions (10). Kapasi et al (8) found that cognitive decline was most severe in the presence of ADNC + LATE-NC, followed by ADNC alone, followed by LATE-NC alone, followed by neither pathology. The presence of LATE-NC has been shown to be associated with worsened cognitive decline across the spectrum of Braak neurofibrillary tangle (NFT) stages (11). One study also looked at neuropsychiatric symptoms and found no difference between ADNC alone and ADNC with LATE-NC (12).
Cognitive decline in ADNC with and without LATE-NC has mostly been evaluated using global measures, such as the Mini-Mental State Exam (MMSE) (1, 9–11, 13, 14). A few studies have looked at different neuropsychological domains. Wilson et al investigated the independent effects of several neuropathologies (ADNC, LATE-NC, Lewy bodies, and hippocampal sclerosis [HS]) on these domains. Each neuropathology independently decreased episodic memory 10 to 16 years before death. As time to death got closer, ADNC, Lewy bodies, and HS all were associated with decreased function in all other domains. However, the detected associations with LATE-NC were confined to decreased episodic memory (15).
In one of the few direct comparisons of ADNC alone versus with LATE-NC that evaluated neuropsychological domains, Kapasi et al (8) used 16 neuropsychological tests in 5 domains and showed that LATE-NC with ADNC was associated with worsened global performance and worsened performance in all 5 domains in comparison to ADNC alone. Given the paucity of literature on this topic, particularly with respect to neuropsychological domains, we sought to assess the associations of ADNC, with and without comorbid LATE-NC, with a panel of outcome measures, including clinical symptoms (cognitive, behavioral, and motor) and performance in 5 neuropsychological domains.
MATERIALS AND METHODS
Participants and Data Source
Data were obtained from the National Alzheimer’s Coordinating Center (NACC), which is the data repository for past and present Alzheimer’s Disease Research Centers (ADRC) funded by the National Institute on Aging (NIA). Participants are assessed using the standardized Uniform Data Set (UDS) at their local ADRC approximately annually. The UDS collects a robust set of data including participant demographics, health history, physical and neurological exams, symptomatology of AD and related dementias, the Clinical Dementia Rating (CDR) Dementia Staging Instrument plus NACC frontotemporal lobar degeneration (FTLD) Behavior and Language Domains, and a neuropsychological test battery. Participants who met the study eligibility criteria were selected from the December 2021 data freeze, which included cross-sectional data from the participant’s most recent UDS visit prior to death, collected from January 2011 to December 2021. Additional details about the UDS are described elsewhere (16–20). ADRCs obtained written informed consent from their participants and maintain their own separate IRB review and approval from their institution prior to submitting data to NACC.
Neuropathologic Features
Standardized data collected on neuropathological features present at the time of death are available for participants who were assessed with the UDS and who consented to autopsy (16, 20). The NACC Neuropathology (NP) form is used by the ADRCs, and provides guidance based on established criteria for evaluation of the presence of amyloid β, tau, TDP-43, α-synuclein, cerebrovascular injuries, as well as less common pathologies. Version 10 of the NACC NP form, implemented in January 2014, introduced the assessment of FTLD-TDP and more generally, the presence of TDP-43-immunoreactive inclusions in the spinal cord, amygdala, hippocampus, entorhinal/inferior temporal cortex, and neocortex. In this study, we defined Alzheimer pathology using the NIA-AA ADNC score. LATE-NC was defined as the presence of TDP-43 inclusions in amygdala, hippocampus, and/or neocortex. The ADNC group included participants with low, intermediate, or high ADNC and no LATE-NC pathology, while the ADNC plus LATE-NC group included participants with low, intermediate, or high ADNC and LATE-NC pathology.
Inclusion Criteria
Our sample includes participants who were 65 years or older at death, died within 3 years of their last UDS visit, and have neuropathology data from the NPv10 or NPv11 form. We excluded participants with rare pathologies present (such as Down syndrome, pigment-spheroid degeneration/neurodegeneration with brain iron accumulation, multiple system atrophy, trinucleotide disease, Huntington disease, spinocerebellar ataxia, or other), malformation of cortical development, metabolic/storage disorder of any type, white matter disease (leukodystrophy, multiple sclerosis, or other demyelinating disease), contusion/traumatic brain injury of any type (acute or chronic), neoplasm (primary or metastatic), infectious process of any type (encephalitis, abscess, etc.), herniation (any site), prion disease, FTLD with tau pathology (FTLD-tau) or other tauopathy, ALS/motor neuron disease, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, or other FTLD. Participants were also excluded if they did not have ADNC pathology, were missing data on TDP-43 inclusions in the amygdala, hippocampus, and neocortex, or if they had FTLD-TDP-43 pathology present. Participants in either the ADNC or the ADNC + LATE-NC groups were not excluded if their primary clinical diagnosis while living had been frontotemporal disorder.
Cognitive Measurements
Participants were assessed using the CDR and the UDS neuropsychological test batteries (C1 prior to March 2015, C2 after March 2015) (21, 22). These batteries included the Logical Memory Immediate and Delayed Recall tests for C1 (Craft Story 21 Immediate and Delayed Recall tests for C2), Digit Span Forward and Backward tests (for both C1 and C2), the Boston Naming test for C1 (MINT for C2), animal and vegetable naming tests (for both C1 and C2), the Wechsler Adult Intelligence Scale-Revised Digit Symbol test (WAIS-R Digit Symbol, for both C1 and C2), and Trail Making tests A and B (for both C1 and C2). Z-scores for each test were calculated by subtracting the score from the mean test score and dividing it by the standard deviation (SD) of all UDS initial visit scores among cognitively normal participants (i.e. CDR = 0). Tests were grouped by cognitive domains (i.e. episodic memory, attention/working memory, language/semantic memory, executive function), which were established by Hayden et al (23) using factor analysis, and z-scores for the tests within a domain were then averaged to calculate a domain z-score. A global composite score was created by averaging the domain z-scores.
Statistical Analyses
To compare the demographic characteristics, clinical measures, and neuropathologic features between ADNC and ADNC plus LATE-NC groups, Pearson chi-square or Fisher exact tests for the categorical variables, and 2-sample t-tests for the continuous variables were applied. Clinical characteristics examined included age at death, presence of the APOE ε4 allele, cognitive status at most recent UDS visit, presumptive etiologic diagnosis at most recent visit, CDR sum of boxes, and global CDR scores at most recent UDS visit. Neuropathologic features investigated include ADNC score, Thal phase, Braak NFT stage, Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaque density, Lewy bodies, LATE-NC stage, HS, brain arteriolosclerosis, infarcts or lacunes, microinfarcts, and hemorrhages and microbleeds.
We assessed the association of ADNC versus ADNC + LATE-NC with 2 sets of outcome measures: clinical symptoms based on clinician judgment (cognitive, behavioral, and motor symptoms) and performance in 5 neuropsychological domains. We first conducted bivariate analyses, followed by multivariable models.
Clinical symptoms were compared between the 2 groups (ADNC vs ADNC + LATE-NC). Differences in the presence of these symptoms were investigated using Pearson chi-square or Fisher exact tests. These comparisons were stratified by ADNC severity score (low/intermediate vs high) to examine whether differences were present within groups of participants with similar severity of ADNC. Separate logistic regression models with generalized estimating equations were run to test for significance of odds ratios for presence of the above noted cognitive, behavioral, and motor symptoms. Models were adjusted for age at death, time between last visit and death, sex, years of education, the presence of an APOE ε4 allele, the presence of vascular pathology (i.e. moderate to severe arteriolosclerosis), and the presence of Lewy bodies. The covariates included known confounders in dementia research (i.e. age at death, sex, years of education), or potential confounders in the relationship between ADNC or ADNC plus LATE-NC with symptoms that were found to have significantly different (at p < 0.05) distributions between our LATE-NC and ADNC plus LATE-NC samples (time between last visit and death, presence of Lewy bodies, or brain arteriosclerosis). Models were stratified by ADNC severity score (low/intermediate vs high) to examine whether differences are present within groups of participants with similar severity of ADNC.
Separate linear regression models with generalized estimating equations were run to test for marginal mean differences between the 2 groups on 5 cognitive domain z-scores calculated from neuropsychological test scores at last UDS visit. Unadjusted and adjusted models were included; covariates in adjusted models were age at death, time between last visit and death, sex, years of education, neuropsychological test battery (i.e. C1 vs C2), the presence of an APOE ε4 allele, the presence of vascular pathology (i.e. moderate to severe arteriolosclerosis), and the presence of Lewy bodies. Criteria for selecting potentially confounding variables to include in the model were as discussed above. Models were stratified by ADNC severity score (low/intermediate vs high). All analyses were run using SAS version 9.4. We used p < 0.05 as the level of statistical significance. No adjustments were made for multiple comparisons.
We excluded cases that were diagnosed with neuropathologic FTLD-TDP. Given that LATE-NC and FTLD-TDP both had TDP-43 inclusions and that the neuropathologic distinction between the 2 groups is evolving, we conducted a sensitivity analysis, in which we compared the clinical symptoms and neuropsychological test scores of the group with ADNC + LATE-NC with the group who had FTLD-TDP. The purpose of this sensitivity analysis was to demonstrate that these 2 groups behave differently and hence excluding the FTLD-TDP participants for the main analysis is logical. The same inclusion and exclusion criteria were applied to the FTLD-TDP group as were applied in the main analysis. Participants with FTLD-tau and ALS/MND were excluded, as they had been in the main analysis. No exclusions were applied to the FTLD-TDP group based on their clinical diagnosis.
RESULTS
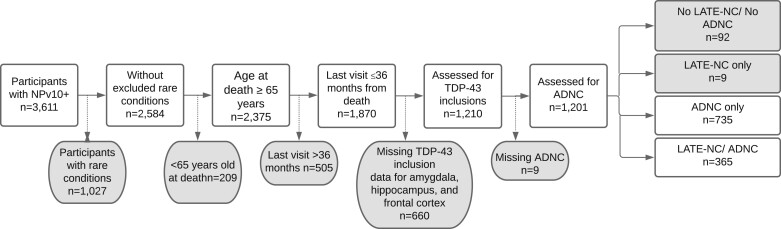
The final analytic sample included 1100 participants: 735 with ADNC and 365 with ADNC plus LATE-NC (Fig. 1). Participants with ADNC plus LATE-NC were significantly older at death on average than those with ADNC (mean 84.5 years, SD 8.5 years vs mean 82.5 years, SD 8.9 years, p < 0.001; Table 1). Participants with ADNC plus LATE-NC were more likely than the ADNC group to be APOE ε4 carriers (55.3% vs 44.6%, p = 0.003) and were more likely to have more severe cognitive impairment, having higher CDR sum of boxes scores and more severe global CDR scores, as well as being more likely to have received a diagnosis of dementia at their most recent UDS visit. Times between last UDS visit and death were slightly longer for those with ADNC + LATE-NC, but only for the high ADNC strata. Those with ADNC plus LATE-NC were primarily diagnosed with AD clinically at their most recent visit prior to autopsy (82.5%) and were additionally diagnosed with Lewy body disease (4.4%) and frontotemporal disorders (3.3%). Participants with ADNC were also primarily diagnosed with AD clinically at their most recent UDS visit (63.3%), while 7.6% were diagnosed with Lewy body disease and 5.0% were diagnosed with frontotemporal disorders. The percentages of participants diagnosed with frontotemporal disorders were similar in the 2 groups (ADNC 5.0% vs ADNC + LATE-NC 3.3%, p = 0.19). The distribution of subtypes of frontotemporal disorders was similar with each group: ADNC: other frontotemporal disorder (3.8%), corticobasal degeneration (1%), progressive supranuclear palsy (0.3%); ADNC + LATE-NC: other frontotemporal disorder (2.5%), corticobasal degeneration (0.6%), frontotemporal disorder with motor neuron disease (0.3%).
FIGURE 1.
Sample inclusion and exclusion criteria.
TABLE 1.
Demographic and Clinical Characteristics Among Participants With ADNC Only Versus ADNC + LATE-NC
| Characteristic | All n = 1100 | ADNC n = 735 | ADNC and LATE-NC n = 365 | p value |
|---|---|---|---|---|
| Age at death, mean (SD) | 83.2 (8.8) | 82.5 (8.9) | 84.5 (8.5) | <0.001 |
| Months between last UDS visit and death, mean (SD) | 12.5 (9.3) | 12.3 (9.1) | 13.1 (9.8) | 0.195 |
| Years of education, mean (SD) | 15.9 (3.1) | 15.8 (3.2) | 16 (3.0) | 0.206 |
| Female, n (%) | 537 (48.8) | 346 (47.1) | 191 (52.3) | 0.101 |
| Nonwhite race, n (%) | 77 (7.0) | 48 (6.5) | 29 (8.0) | 0.725 |
| APOE ε4 carrier, n (%) | 530 (48.2) | 328 (44.6) | 202 (55.3) | 0.003 |
| Cognitive status at last UDS visit, n (%) | <0.001 | |||
| Normal cognition | 126 (11.5) | 112 (15.2) | 14 (3.8) | |
| Impaired, not MCI | 20 (1.8) | 16 (2.2) | 4 (1.1) | |
| MCI | 111 (10.1) | 94 (12.8) | 17 (4.7) | |
| Dementia | 843 (76.6) | 513 (69.8) | 330 (90.4) | |
| Primary clinical diagnosis, n (%) | <0.001 | |||
| Normal cognition | 126 (11.5) | 112 (15.2) | 14 (3.8) | |
| Alzheimer disease | 766 (69.6) | 465 (63.3) | 301 (82.5) | |
| Lewy body disease | 72 (6.6) | 56 (7.6) | 16 (4.4) | |
| Frontotemporal disorders* | 49 (4.5) | 37 (5.0) | 12 (3.3) | |
| Other | 84 (7.6) | 63 (8.6) | 21 (5.8) | |
| CDR sum of boxes, mean (SD) | 10.3 (6.8) | 9.3 (7.0) | 12.4 (5.7) | <0.001 |
| CDR global score, n (%) | <0.001 | |||
| None | 124 (11.3) | 110 (15) | 14 (4.2) | |
| Questionable | 166 (15.1) | 133 (18.1) | 31 (9.3) | |
| Mild | 156 (14.2) | 107 (14.6) | 47 (14.1) | |
| Moderate | 233 (21.2) | 136 (18.5) | 88 (26.4) | |
| Severe | 421 (38.3) | 249 (33.9) | 154 (46.1) |
Includes MSA, PSP, CBD, FTLD with motor neuron disease (e.g. ALS), and other FTLD.
Missing data: ADNC: Education (n=7), Race (n=2), APOE ε4 (n=72), Primary clinical diagnosis (n=2); ADNC + LATE: Education (n=3), Race (n=1), APOE ε4 (n=31), Primary clinical diagnosis (n=1).
Bold values indicate statistical significance at the p<0.05 level.
Participants with ADNC plus LATE-NC were more likely to have higher ADNC scores as well as more severe Thal Aβ phases, Braak NFT stages, and CERAD neuritic plaque densities compared to those with ADNC alone (Tables 2 and 3). Participants with ADNC plus LATE-NC were also more likely than ADNC participants to have Lewy body pathology (p < 0.001), HS (34.3% vs 4.6%, p < 0.001), and moderate to severe brain arteriolosclerosis (56.2% vs 46.0%, p < 0.001). No differences were observed between the 2 groups for infarcts/lacunes, microinfarcts, hemorrhages, or microbleeds.
TABLE 2.
Co-neuropathologic Features Present at Autopsy Among Participants With ADNC Only Versus ADNC + LATE-NC
| All n = 1100 | ADNC n = 735 | ADNC and LATE-NC n = 365 | p value | |
|---|---|---|---|---|
| AD neuropathologic change, n (%) | <0.001 | |||
| Not AD | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Low | 185 (16.8) | 149 (20.3) | 36 (9.9) | |
| Intermediate | 264 (24.0) | 202 (27.5) | 62 (17.0) | |
| High | 651 (59.2) | 384 (52.2) | 267 (73.2) | |
| Thal phase, n (%) | <0.001 | |||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 1–2 | 116 (10.6) | 90 (12.2) | 26 (7.1) | |
| 3–4 | 400 (36.4) | 309 (42.0) | 91 (24.9) | |
| 5 | 584 (53.1) | 336 (45.7) | 248 (68.0) | |
| Braak stage, n (%) | <0.001 | |||
| 0 | 7 (0.6) | 5 (0.7) | 2 (0.6) | |
| I–II | 148 (13.5) | 124 (16.9) | 24 (6.6) | |
| III–IV | 229 (20.8) | 174 (23.7) | 55 (15.1) | |
| V–VI | 716 (65.1) | 432 (58.8) | 284 (77.8) | |
| Neuritic plaque density, n (%) | <0.001 | |||
| None | 117 (10.6) | 96 (13.1) | 21 (5.8) | |
| Sparse | 135 (12.3) | 104 (14.2) | 31 (8.5) | |
| Moderate | 233 (21.2) | 162 (22.0) | 71 (19.5) | |
| Frequent | 615 (55.9) | 373 (50.8) | 242 (66.3) | |
| LATE-NC stage, n (%) | <0.001 | |||
| 0 | 735 (66.8) | 735 (100.0) | 0 (0.0) | |
| 1 | 69 (6.3) | 0 (0.0) | 69 (18.9) | |
| 2 | 259 (23.6) | 0 (0.0) | 259 (71.0) | |
| 3 | 37 (3.4) | 0 (0.0) | 37 (10.1) | |
| Lewy Bodies, n (%) | <0.001 | |||
| No Lewy body pathology | 612 (55.6) | 445 (60.5) | 167 (45.8) | |
| Brainstem predominant | 45 (4.1) | 32 (4.4) | 13 (3.6) | |
| Limbic or amygdala predominant | 237 (21.6) | 133 (18.1) | 104 (28.5) | |
| Neocortical | 175 (15.9) | 109 (14.8) | 66 (18.1) | |
| Region unspecified | 29 (2.6) | 15 (2.0) | 14 (3.8) | |
| Hippocampal sclerosis, n (%) | 159 (14.5) | 34 (4.6) | 125 (34.3) | <0.001 |
| Vascular brain injury, n (%) | ||||
| Brain arteriolosclerosis (moderate/severe) | 543 (49.4) | 338 (46.0) | 205 (56.2) | <0.001 |
| Infarcts or lacunes | 169 (15.4) | 116 (15.8) | 53 (14.5) | 0.568 |
| Microinfarcts | 268 (24.4) | 170 (23.1) | 98 (26.9) | 0.339 |
| Hemorrhages and microbleeds | 66 (6.0) | 49 (6.7) | 17 (4.7) | 0.185 |
Missing data: ADNC: Lewy bodies (n = 1), Hippocampal sclerosis (n = 2), Brain arteriolosclerosis (n = 3), Infarcts or lacunes (n = 8), Microinfarcts (n = 4), Hemorrhages and microbleeds (n = 7); TDP-43 inclusions in amygdala (n = 138), in hippocampus (n = 0), in neocortex (n = 97); ADNC + LATE: Lewy bodies (n = 1), Hippocampal sclerosis (n = 2), Brain arteriolosclerosis (n = 7), Infarcts or lacunes (n = 2), Microinfarcts (n = 1), Hemorrhages and microbleeds (n = 1); TDP-43 inclusions in amygdala (n = 54), in hippocampus (n = 4), in neocortex (n = 31).
Bold values indicate statistical significance at the p<0.05 level.
TABLE 3.
Co-neuropathologic Features Present at Autopsy Among Participants With ADNC Only Versus ADNC + LATE-NC, Stratified by ADNC Score
| All n = 1100 | Low/intermediate ADNC |
p value | High ADNC |
p value | |||
|---|---|---|---|---|---|---|---|
| ADNC n = 351 | ADNC and LATE-NC n = 98 | ADNC n = 384 | ADNC and LATE-NC n = 267 | ||||
| AD neuropathologic change, n (%) | 0.310 | — | |||||
| Not AD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Low | 185 (16.8) | 149 (42.5) | 36 (36.7) | 0 (0.0) | 0 (0.0) | ||
| Intermediate | 264 (24.0) | 202 (57.6) | 62 (63.3) | 0 (0.0) | 0 (0.0) | ||
| High | 651 (59.2) | 0 (0.0) | 0 (0.0) | 384 (100.0) | 267 (100.0) | ||
| Thal phase, n (%) | 0.029 | ||||||
| 0 | 0 (0.0) | 0.005 | 0 (0.0) | 0 (0.0) | |||
| 1–2 | 116 (10.6) | 90 (25.6) | 26 (26.5) | 0 (0.0) | 0 (0.0) | ||
| 3–4 | 400 (36.4) | 222 (63.3) | 49 (50.0) | 87 (22.7) | 42 (15.7) | ||
| 5 | 584 (53.1) | 39 (11.1) | 23 (23.5) | 297 (77.3) | 225 (84.3) | ||
| Braak stage, n (%) | 0.233 | — | |||||
| 0 | 7 (0.6) | 5 (1.4) | 2 (2.0) | 0 (0.0) | 0 (0.0) | ||
| I–II | 148 (13.5) | 124 (35.3) | 24 (24.5) | 0 (0.0) | 0 (0.0) | ||
| III–IV | 229 (20.8) | 174 (49.6) | 55 (56.1) | 0 (0.0) | 0 (0.0) | ||
| V–VI | 716 (65.1) | 48 (13.7) | 17 (17.4) | 384 (100.0) | 267 (100.0) | ||
| Neuritic plaque density, n (%) | 0.483 | 0.620 | |||||
| None | 117 (10.6) | 96 (27.4) | 21 (21.4) | 0 (0.0) | 0 (0.0) | ||
| Sparse | 135 (12.3) | 104 (29.6) | 31 (31.6) | 0 (0.0) | 0 (0.0) | ||
| Moderate | 233 (21.2) | 107 (30.5) | 29 (29.6) | 55 (14.3) | 42 (15.7) | ||
| Frequent | 615 (55.9) | 44 (12.5) | 17 (17.4) | 329 (85.7) | 225 (84.3) | ||
| LATE-NC stage, n (%) | <0.001 | <0.001 | |||||
| 0 | 735 (66.8) | 351 (100.0) | 0 (0.0) | 384 (100.0) | 0 (0.0) | ||
| 1 | 69 (6.3) | 0 (0.0) | 19 (19.4) | 0 (0.0) | 50 (18.7) | ||
| 2 | 259 (23.6) | 0 (0.0) | 63 (64.3) | 0 (0.0) | 196 (73.4) | ||
| 3 | 37 (3.4) | 0 (0.0) | 16 (16.3) | 0 (0.0) | 21 (7.9) | ||
| Lewy Bodies, n (%) | 0.141 | 0.008 | |||||
| No Lewy body pathology | 612 (55.6) | 234 (66.7) | 58 (59.2) | 211 (55) | 109 (40.8) | ||
| Brainstem predominant | 45 (4.1) | 24 (6.8) | 10 (10.2) | 8 (2.1) | 3 (1.1) | ||
| Limbic or amygdala predominant | 237 (21.6) | 38 (10.8) | 12 (12.2) | 95 (24.7) | 92 (34.5) | ||
| Neocortical | 175 (15.9) | 52 (14.8) | 14 (14.3) | 57 (14.8) | 52 (19.5) | ||
| Region unspecified | 29 (2.6) | 3 (0.9) | 3 (3.1) | 12 (3.1) | 11 (4.1) | ||
| Hippocampal sclerosis, n (%) | 159 (14.5) | 16 (4.6) | 39 (39.8) | <0.001 | 18 (4.7) | 86 (32.2) | <0.001 |
| Vascular brain injury, n (%) | |||||||
| Brain arteriolosclerosis (moderate/severe) | 543 (49.4) | 145 (41.3) | 57 (58.2) | 0.002 | 193 (50.3) | 148 (55.4) | 0.032 |
| Infarcts or lacunes | 169 (15.4) | 66 (18.8) | 17 (17.4) | 0.912 | 50 (13) | 36 (13.5) | 0.871 |
| Microinfarcts | 268 (24.4) | 90 (25.6) | 32 (32.7) | 0.320 | 80 (20.8) | 66 (24.7) | 0.514 |
| Hemorrhages and microbleeds | 66 (6.0) | 21 (6.0) | 7 (7.1) | 0.583 | 28 (7.3) | 10 (3.8) | 0.157 |
Missing data, stratified: LOW/INTERMEDIATE ADNC, ADNC: Brain arteriolosclerosis (n = 2), Infarcts or lacunes (n = 5), Microinfarcts (n = 2), Hemorrhages and microbleeds (n = 5); TDP-43 inclusions in amygdala (n = 60), in hippocampus (n = 0), in neocortex (n = 58). ADNC + LATE: Lewy bodies (n = 1), Brain arteriolosclerosis (n = 2), Infarcts or lacunes (n = 1); TDP-43 inclusions in amygdala (n = 19), in hippocampus (n = 1), in neocortex (n = 7). HIGH ADNC, ADNC: Lewy bodies (n = 1), Hippocampal sclerosis (n = 2), Brain arteriolosclerosis (n = 1), Infarcts or lacunes (n = 3), Microinfarcts (n = 2), Hemorrhages and microbleeds (n = 2); TDP-43 inclusions in amygdala (n = 78), in hippocampus (n = 0), in neocortex (n = 39). ADNC + LATE: Hippocampal sclerosis (n = 2), Brain arteriolosclerosis (n = 5), Infarcts or lacunes (n = 1), Microinfarcts (n = 1), Hemorrhages and microbleeds (n = 1); TDP-43 inclusions in amygdala (n = 35), in hippocampus (n = 3), in neocortex (n = 24).
Bold values indicate statistical significance at the p<0.05 level.
A small number (n = 36) of participants had low ADNC + LATE-NC. Their clinical diagnoses were: Alzheimer disease (n = 20, 55.6%), normal cognition (n = 7, 19.4%), Lewy body disease (n = 3, 8.3%), and other (n = 6, 16.7%). Their classification by LATE-NC stage was: Stage 1 (n = 6, 16.7%), Stage 2 (n = 26, 72.2%), and Stage 3 (n = 4, 11.1%).
When stratified by ADNC Score, cognitive status at last visit, CDR sum of boxes, and CDR global score were all significantly worse for participants with comorbid LATE-NC than the participants with ADNC alone in the low/intermediate ADNC strata (Table 4). However, these measures of cognitive status were either not significantly different or were (in the case of CDR sum of boxes) minimally different between participants with ADNC alone versus ADNC with LATE-NC in the high ADNC strata.
TABLE 4.
Demographic and Clinical Characteristics Among Participants With ADNC Only Versus ADNC + LATE-NC, Stratified by ADNC Score
| Characteristic | All n = 1100 | Low/intermediate ADNC |
p value | High ADNC |
p value | ||
|---|---|---|---|---|---|---|---|
| ADNC n = 351 | ADNC and LATE-NC n = 98 | ADNC n = 384 | ADNC and LATE-NC n = 267 | ||||
| Age at death, mean (SD) | 83.2 (8.8) | 86.2 (7.8) | 89.3 (7.6) | <0.001 | 79.2 (8.5) | 82.7 (8.2) | <0.001 |
| Years between last UDS visit and death, mean (SD) | 12.5 (9.3) | 12.9 (8.8) | 12.6 (9.2) | 0.733 | 11.7 (9.3) | 13.3 (10.1) | 0.044 |
| Years of education, mean (SD) | 15.9 (3.1) | 15.9 (3.2) | 16.2 (3.2) | 0.414 | 15.7 (3.1) | 16 (2.9) | 0.248 |
| Female, n (%) | 537 (48.8) | 163 (46.4) | 52 (53.1) | 0.246 | 183 (47.7) | 139 (52.1) | 0.269 |
| Nonwhite race, n (%) | 77 (7.0) | 24 (6.8) | 11 (11.2) | 0.152 | 24 (6.3) | 18 (6.7) | 0.935 |
| APOE ε4 carrier, n (%) | 530 (48.2) | 110 (31.3) | 37 (37.8) | 0.285 | 218 (56.8) | 165 (61.8) | 0.437 |
| Cognitive status at last UDS visit, n (%) | <0.001 | 0.052 | |||||
| Normal cognition | 126 (11.5) | 108 (30.8) | 13 (13.3) | 4 (1.0) | 1 (0.4) | ||
| Impaired, not MCI | 20 (1.8) | 13 (3.7) | 3 (3.1) | 3 (0.8) | 1 (0.4) | ||
| MCI | 111 (10.1) | 75 (21.4) | 13 (13.3) | 19 (5.0) | 4 (1.5) | ||
| Dementia | 843 (76.6) | 155 (44.2) | 69 (70.4) | 358 (93.2) | 261 (97.8) | ||
| Primary clinical diagnosis, n (%) | <0.001 | 0.318 | |||||
| Normal cognition | 126 (11.5) | 108 (30.8) | 13 (13.3) | 4 (1.0) | 1 (0.4) | ||
| Alzheimer disease | 766 (69.6) | 138 (39.3) | 63 (64.3) | 327 (85.2) | 238 (89.1) | ||
| Lewy body disease | 72 (6.6) | 46 (13.1) | 6 (6.1) | 10 (2.6) | 10 (3.8) | ||
| Frontotemporal disorders* | 49 (4.5) | 6 (1.7) | 0 (0.0) | 31 (8.1) | 12 (4.5) | ||
| Other | 84 (7.6) | 52 (14.8) | 15 (15.3) | 11 (2.9) | 6 (2.3) | ||
| CDR sum of boxes, mean (SD) | 10.3 (6.8) | 5.1 (6.1) | 8.4 (6.5) | <0.001 | 13.1 (5.3) | 13.9 (4.5) | 0.047 |
| CDR global score, n (%) | 0.001 | 0.078 | |||||
| None | 124 (11.3) | 107 (30.5) | 13 (13.3) | 3 (0.8) | 1 (0.4) | ||
| Questionable | 166 (15.1) | 105 (29.9) | 24 (24.5) | 28 (7.3) | 9 (3.4) | ||
| Mild | 156 (14.2) | 46 (13.1) | 17 (17.4) | 61 (15.9) | 32 (12.0) | ||
| Moderate | 233 (21.2) | 41 (11.7) | 16 (16.3) | 95 (24.7) | 81 (30.3) | ||
| Severe | 421 (38.3) | 52 (14.8) | 28 (28.6) | 197 (51.3) | 144 (53.9) | ||
Includes MSA, PSP, CBD, FTLD with motor neuron disease (e.g. ALS), and other FTLD.
Missing Data, stratified: LOW/INTERMEDIATE ADNC, ADNC: Education (n = 4), APOE ε4 (n = 27), Primary clinical diagnosis (n = 1); ADNC + LATE: Education (n = 1), Race (n = 1), APOE ε4 (n = 4), Primary clinical diagnosis (n = 1); HIGH ADNC, ADNC: Education (n = 3), Race (n = 2), APOE ε4 (n = 45), Primary clinical diagnosis (n = 1); ADNC + LATE: Education (n = 2), Race (n = 1), APOE ε4 (n = 27).
Bold values indicate statistical significance at the p<0.05 level.
While there were minimal differences in cognitive and behavioral symptoms present at the most recent UDS visit prior to death between ADNC and ADNC plus LATE-NC participants with high ADNC scores, multiple differences were observed between the groups when comparing those with low/intermediate ADNC scores (Tables 5 and 6). Most of the differences found on bivariate analysis persisted on multivariable analysis. Among participants with low/intermediate ADNC scores, the ADNC plus LATE-NC group was more likely to exhibit impairment in the following cognitive symptoms than the ADNC group: memory (adjusted odds ratio [AOR] 4.15, p < 0.001), executive function (AOR 3.47, p < 0.001), language (AOR 2.84, p < 0.001), visuospatial (AOR 1.93, p = 0.001), and attention (AOR 2.06, p = 0.002). Participants with ADNC plus LATE-NC in the low/intermediate ADNC strata also had relatively more behavioral symptoms, some of which are associated with behavioral-variant frontotemporal dementia (bvFTD), including apathy (AOR 2.62, p = 0.010), disinhibition (AOR 2.79, p < 0.001), and agitation (AOR 4.10, p = 0.003). Note that these behavioral symptoms were lacking in most of the participants, and very few of the participants had been diagnoses with FTD clinical syndrome. Further, no differences between the groups were observed in motor symptoms.
TABLE 5.
Clinical Symptoms at Most Recent Visit Prior to Death Present Among Participants With ADNC Only Versus ADNC + LATE-NC, Stratified by ADNC Score
| All n = 1100 | Low/intermediate ADNC |
p value | High ADNC |
p value | |||
|---|---|---|---|---|---|---|---|
| ADNC n = 351 | ADNC and LATE-NC n = 98 | ADNC n = 384 | ADNC and LATE-NC n = 267 | ||||
| Cognitive symptoms, n (%) | |||||||
| Memory | 953 (86.6) | 227 (64.7) | 85 (86.7) | <0.001 | 376 (97.9) | 265 (99.3) | 0.472 |
| Executive function | 898 (81.6) | 187 (53.3) | 74 (75.5) | <0.001 | 374 (97.4) | 263 (98.5) | 0.415 |
| Language | 747 (67.9) | 145 (41.3) | 56 (57.1) | 0.001 | 320 (83.3) | 226 (84.6) | 0.554 |
| Visuospatial | 620 (56.4) | 106 (30.2) | 40 (40.8) | 0.006 | 276 (71.9) | 198 (74.2) | 0.402 |
| Attention | 621 (56.5) | 119 (33.9) | 45 (45.9) | 0.001 | 257 (66.9) | 200 (74.9) | 0.091 |
| Fluctuating cognition | 176 (16.0) | 47 (13.4) | 10 (10.2) | 0.518 | 74 (19.3) | 45 (16.9) | 0.576 |
| Behavioral symptoms, n (%) | |||||||
| Apathy | 508 (46.2) | 95 (27.1) | 41 (41.8) | 0.012 | 223 (58.1) | 149 (55.8) | 0.838 |
| Depressed mood | 328 (29.8) | 91 (25.9) | 29 (29.6) | 0.319 | 123 (32.0) | 85 (31.8) | 0.818 |
| Visual hallucinations | 157 (14.3) | 39 (11.1) | 9 (9.2) | 0.791 | 71 (18.5) | 38 (14.2) | 0.162 |
| Auditory hallucinations | 45 (4.1) | 12 (3.4) | 3 (3.1) | 0.113 | 22 (5.7) | 8 (3.0) | 0.203 |
| Delusions | 180 (16.4) | 26 (7.4) | 11 (11.2) | 0.453 | 84 (21.9) | 59 (22.1) | 0.881 |
| Disinhibition | 201 (18.3) | 29 (8.3) | 15 (15.3) | 0.006 | 92 (24.0) | 65 (24.3) | 0.749 |
| Irritability | 352 (32.0) | 70 (19.9) | 29 (29.6) | 0.068 | 157 (40.9) | 96 (36.0) | 0.404 |
| Agitation | 286 (26.0) | 32 (9.1) | 22 (22.5) | 0.001 | 138 (35.9) | 94 (35.2) | 0.539 |
| Personality change | 141 (12.8) | 23 (6.6) | 7 (7.1) | 0.006 | 70 (18.2) | 41 (15.4) | 0.525 |
| Motor symptoms, n (%) | |||||||
| Gait disorder | 478 (43.5) | 123 (35) | 35 (35.7) | 1.000 | 189 (49.2) | 131 (49.1) | 0.848 |
| Falls | 254 (23.1) | 77 (21.9) | 17 (17.4) | 0.104 | 101 (26.3) | 59 (22.1) | 0.326 |
| Tremors | 236 (21.5) | 68 (19.4) | 20 (20.4) | 0.069 | 89 (23.2) | 59 (22.1) | 0.540 |
| Slowness | 482 (43.8) | 127 (36.2) | 35 (35.7) | 0.772 | 187 (48.7) | 133 (49.8) | 0.498 |
Missing data, stratified: LOW/INTERMEDIATE ADNC, ADNC: Executive function (n = 4), Language (n = 2), Visuospatial (n = 16), Attention (n = 7), Fluctuating cognition (n = 52), Apathy (n = 3), Depressed mood (n = 1), Visual hallucinations (n = 5), Auditory hallucinations (n = 7), Delusions (n = 9), Disinhibition (n = 1), Irritability (n = 4), Agitation (n = 2), Personality change (n = 2), Gait disorder (n = 5), Falls (n = 4), Tremors (n = 3), Slowness (n = 4); ADNC + LATE: Executive function (n = 2), Language (n = 3), Visuospatial (n = 10), Attention (n = 7), Fluctuating cognition (n = 64), Apathy (n = 1), Depressed mood (n = 2), Visual hallucinations (n = 2), Auditory hallucinations (n = 6), Delusions (n = 3), Disinhibition (n = 3), Irritability (n = 2), Agitation (n = 2), Personality change (n = 5), Gait disorder (n = 1), Falls (n = 4), Tremors (n = 4), Slowness (n = 2); HIGH ADNC, ADNC: Memory (n = 2), Executive function (n = 2), Language (n = 3), Visuospatial (n = 20), Attention (n = 21), Fluctuating cognition (n = 26), Apathy (n = 6), Depressed mood (n = 15), Visual hallucinations (n = 22), Auditory hallucinations (n = 29), Delusions (n = 25), Disinhibition (n = 9), Irritability (n = 7), Agitation (n = 5), Personality change (n = 8), Gait disorder (n = 10), Falls (n = 14), Tremors (n = 21), Slowness (n = 15); ADNC + LATE: Memory (n = 0), Executive function (n = 2), Language (n = 4), Visuospatial (n = 18), Attention (n = 11), Fluctuating cognition (n = 15), Apathy (n = 4), Depressed mood (n = 8), Visual hallucinations (n = 23), Auditory hallucinations (n = 25), Delusions (n = 20), Disinhibition (n = 4), Irritability (n = 4), Agitation (n = 1), Personality change (n = 4), Gait disorder (n = 9), Falls (n = 7), Tremors (n = 10), Slowness (n = 6).
Bold values indicate statistical significance at the p<0.05 level.
TABLE 6.
Multivariable Analysis of Clinical Symptoms at Most Recent Visit Prior to Death Present Among Participants With ADNC Only Versus ADNC + LATE-NC, Stratified by ADNC Score
| Clinical symptoms | Unadjusted OR (95%) | p value | ADNC n | ADNC + LATE n | Adjusted OR* (95%) | p value |
|---|---|---|---|---|---|---|
| Low/intermediate ADNC | ||||||
| Cognitive symptoms, n (%) | ||||||
| Memory | 3.82 (2.42, 6.03) | <0.001 | 320 | 91 | 4.15 (2.25, 7.65) | <0.001 |
| Executive function | 3.36 (2.19, 5.14) | <0.001 | 317 | 90 | 3.47 (2.09, 5.77) | <0.001 |
| Language | 2.45 (1.74, 3.45) | <0.001 | 319 | 89 | 2.84 (1.81, 4.46) | <0.001 |
| Visuospatial | 1.96 (1.44, 2.66) | <0.001 | 307 | 82 | 1.93 (1.31, 2.85) | 0.001 |
| Attention | 1.95 (1.39, 2.75) | <0.001 | 314 | 86 | 2.06 (1.29, 3.27) | 0.002 |
| Behavioral symptoms, n (%) | ||||||
| Apathy | 1.95 (1.01, 3.76) | 0.047 | 317 | 90 | 2.62 (1.26, 5.44) | 0.010 |
| Disinhibition | 2.13 (1.31, 3.48) | 0.003 | 319 | 88 | 2.79 (1.62, 4.81) | <0.001 |
| Agitation | 2.88 (1.2, 6.91) | 0.018 | 318 | 89 | 4.10 (1.61, 10.45) | 0.003 |
| Personality change | 1.27 (0.50, 3.26) | 0.614 | 318 | 86 | 1.95 (0.73, 5.23) | 0.183 |
| High ADNC | ||||||
| Cognitive symptoms, n (%) | ||||||
| Memory | 2.09 (0.50, 8.71) | 0.311 | 335 | 235 | 2.46 (0.44, 13.75) | 0.306 |
| Executive function | 2.72 (0.29, 0.00) | 0.378 | 334 | 233 | 3.34 (0.45, 24.57) | 0.237 |
| Language | 1.16 (0.83, 1.63) | 0.372 | 333 | 231 | 1.46 (0.82, 2.58) | 0.195 |
| Visuospatial | 1.30 (0.82, 2.06) | 0.260 | 318 | 220 | 1.45 (0.87, 2.41) | 0.153 |
| Attention | 1.43 (1.00, 2.05) | 0.053 | 319 | 224 | 1.51 (1.05, 2.18) | 0.025 |
| Behavioral symptoms, n (%) | ||||||
| Apathy | 0.89 (0.66, 1.20) | 0.443 | 332 | 231 | 0.99 (0.77, 1.27) | 0.950 |
| Disinhibition | 1.00 (0.65, 1.53) | 0.996 | 329 | 231 | 1.17 (0.79, 1.73) | 0.423 |
| Agitation | 1.00 (0.72, 1.38) | 0.983 | 332 | 234 | 1.18 (0.81, 1.74) | 0.387 |
| Personality change | 0.82 (0.56, 1.22) | 0.335 | 330 | 231 | 0.93 (0.67, 1.29) | 0.654 |
Adjusted for age at death, time between last UDS visit and death, sex, years of education, APOEe4 carrier status, presence of Lewy bodies, presence of vascular pathology (i.e. moderate to severe arteriolosclerosis). OR >1 implies higher percentage of symptoms in ADNC + LATE-NC group compared with ADNC group.
Bold values indicate statistical significance at the p<0.05 level.
Cognitive domain scores on neuropsychological tests were examined by bivariate analysis and then by multivariable linear regression, with similar results (Table 7). On multivariable analysis, when examining the cognitive domain scores, participants with low/intermediate ADNC and with comorbid LATE-NC performed significantly worse in episodic memory (p < 0.001), language/semantic memory (p = 0.009), and global cognition (p = 0.018) compared to those with low/intermediate ADNC lacking LATE-NC. Among those with high ADNC, the ADNC plus LATE-NC participants performed significantly worse in only episodic memory (p = 0.002) compared to those with high ADNC and no comorbid LATE-NC.
TABLE 7.
Cognitive Domain Z-Scores at Most Recent Visit Prior to Death Present Among Participants With ADNC Only Versus ADNC + LATE-NC, Stratified by ADNC Score
| Low/intermediate ADNC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive domains, mean (SD) | ADNC n = 258 mean z-score, SD | ADNC and LATE-NC n = 66 mean z-score, SD | ADNC n | ADNC + LATE n | Unadjusted mean difference β est. (95%) | p value | ADNC n | ADNC + LATE n | Adjusted mean difference*β est. (95%) | p value | |||
| Episodic memory | −0.72 | 1.41 | −1.50 | 1.50 | 223 | 54 | −0.82 (−1.15, −0.48) | <0.001 | 206 | 52 | −0.73 (−1.05, −0.4) | <0.001 | |
| Attention/working memory | −0.51 | 1.01 | −0.59 | 0.83 | 225 | 55 | −0.12 (−0.38, 0.15) | 0.391 | 209 | 53 | −0.04 (−0.29, 0.21) | 0.736 | |
| Executive function | −1.24 | 1.63 | −1.56 | 1.61 | 169 | 41 | −0.28 (−1.00, 0.44) | 0.445 | 154 | 39 | −0.07 (−0.88, 0.73) | 0.855 | |
| Language/semantic memory | −0.88 | 1.15 | −1.33 | 1.10 | 222 | 52 | −0.54 (−0.83, −0.25) | <0.001 | 205 | 50 | −0.42 (−0.73, −0.11) | 0.009 | |
| Global composite | −0.61 | 0.89 | −1.11 | 0.95 | 166 | 40 | −0.49 (−0.81, −0.18) | 0.002 | 152 | 38 | −0.42 (−0.76, −0.08) | 0.016 | |
| High ADNC |
|||||||||||||
| Cognitive domains, mean (SD) | ADNC n = 209 mean z-score, SD | ADNC and LATE-NC n = 134 mean z-score, SD | ADNC n | ADNC + LATE n | Unadjusted mean difference β est. (95%) | p value | ADNC n | ADNC + LATE n | Adjusted mean difference*β est. (95%) | p value | |||
| Episodic memory | −2.49 | 1.10 | −2.86 | 0.73 | 126 | 85 | −0.35 (−0.57, −0.14) | 0.002 | 115 | 79 | −0.4 (−0.66, −0.15) | 0.002 | |
| Attention/working memory | −1.54 | 1.22 | −1.47 | 1.11 | 136 | 92 | 0.07 (−0.25, 0.40) | 0.653 | 124 | 86 | −0.04 (−0.38, 0.31) | 0.828 | |
| Executive function | −2.57 | 1.86 | −3.06 | 1.80 | 59 | 30 | −0.55 (−1.03, −0.07) | 0.025 | 52 | 27 | −0.05 (−0.73, 0.63) | 0.886 | |
| Language/semantic memory | −2.68 | 1.54 | −2.99 | 1.39 | 124 | 82 | −0.09 (−0.70, 0.51) | 0.758 | 114 | 77 | −0.30 (−0.91, 0.30) | 0.329 | |
| Global composite | −1.81 | 1.21 | −1.98 | 0.71 | 53 | 29 | −0.17 (−0.39, 0.05) | 0.123 | 47 | 26 | 0.05 (−0.34, 0.43) | 0.815 | |
Adjusted for age at death, time between last UDS visit and death, sex, years of education, APOEe4 carrier status, neuropsychological test battery (i.e. C1 vs C2), presence of Lewy bodies, presence of vascular pathology (i.e. moderate to severe arteriolosclerosis). Negative value implies worsened performance for ADNC + LATE-NC group compared with ADNC group.
Bold values indicate statistical significance at the p<0.05 level.
Around one-third of cases (n = 660) that might have met inclusion criteria for the study (n = 1870, Fig. 1) were excluded due to missing TDP-43 pathology data for all of amygdala, hippocampus, and frontal cortex, thus prohibiting any determination of whether LATE-NC was present or not. There was considerable variability in the number of LATE-NC cases contributed by center, with a range of 0 cases (2 centers) to 178 cases (1 center, accounting for 14.7% of TDP cases), and a median of 36 cases. Likewise, the number of cases that were excluded due to missing TDP-43 pathology data ranged from 0 cases (9 centers) to 135 cases (1 center), with a median of 5 cases. Finally, the percent of all cases at a given center for which there was missing data ranged from 0% missing data (9 centers) to 100% missing data (2 centers), with a median of 14.1%.
We ran a sensitivity analysis to evaluate the differences between ADNC + LATE-NC participants in the analytic sample and FTLD-TDP participants who had been excluded from the main analysis. There were 81 such FTLD-TDP participants, of whom 52 had some degree of comorbid ADNC. As shown in Supplementary DataTables S1 and S2, the ADNC + LATE-NC and ADNC + FTLD-TDP participants differed considerably in their clinical symptoms and neuropsychological test scores, commensurate with prior work showing the differences between LATE-NC and FTLD-TDP. This reinforces the validity of excluding FTLD-TDP cases from the main analysis (1, 24, 25). By way of further detail on the differences between these 2 groups, we also examined their neuropathologic differences. For the 52 participants for whom test score data were available, ADNC was more advanced in the ADNC + LATE-NC group, but only in the low/intermediate ADNC strata (Supplementary DataTable S3). FTLD-TDP participants were more likely to have neocortical TDP, in both low/intermediate and high ADNC strata. ADNC + LATE-NC participants were more likely to have brain arteriosclerosis, but only in the high ADNC strata. Finally, for the 52 FTLD-TDP participants for whom test score data were available, the breakdown of clinical diagnoses was: frontotemporal disorder (not further specified) (n = 30, 57.7%), frontotemporal disorder (corticobasal degeneration) (n = 4, 7.7%), Alzheimer disease (n = 17, 32.7%), Lewy body disease (n = 1, 1.9%).
A notable number of participants had missing data (or were not assessed) for TDP-43 pathology in the amygdala (as noted in footnote to Table 3). Given this, plus the fact that amygdala-only (Stage 1) LATE-NC may not have significant cognitive consequences, we conducted an additional sensitivity analysis in which we reran the major analyses for cognitive outcomes excluding the 69 Stage 1 LATE-NC cases. There were only minor differences compared with the main analysis (Tables 5–7 vs Supplementary Data Tables S4a, S4b, and S5). On multivariable analysis (Supplementary DataTable S4b), all symptoms that had been significantly associated with ADNC + LATE-NC in the main analysis remained significantly associated in the sensitivity analysis. In addition, a few other symptoms became significant in the sensitivity analysis. In the low/intermediate ADNC strata, irritability became significantly associated with ADNC + LATE-NC. In the high ADNC strata, executive function and visuospatial symptoms became significantly associated with ADNC + LATE-NC. There was no difference in the multivariable analysis for cognitive domain z-scores compared with the main analysis (Table 7 vs Supplementary DataTable S5).
DISCUSSION
In this series, a third of participants with ADNC had comorbid LATE-NC. On average, these participants were older, had higher ADNC burden, and had worse cognitive performance than those with ADNC without LATE-NC. When stratified by degree of ADNC, differences in cognitive outcomes were most notable in the strata with low/intermediate ADNC. In this group, participants with comorbid LATE-NC had worsened cognitive status and a tendency to manifest behavioral and neuropsychiatric symptoms (Tables 5 and 6). Some of these symptomatic differences may be partly attributable to increases of copathologies, such as HS and arteriolosclerosis, which are associated with LATE-NC (26, 27). Differences in clinical features between ADNC with and without comorbid LATE-NC narrowed in the high ADNC strata, with minimal differences in cognitive or behavioral symptoms, and with worsened performance in only episodic memory in the group with comorbid LATE-NC.
Multiple studies have confirmed worsened cognitive decline when LATE is comorbid with ADNC (1, 4, 8, 10, 11, 13, 14). Using data from the Rush University autopsy cohorts, Kapasi et al (8) found that cognitive decline was most severe in the presence of ADNC + LATE-NC, followed by ADNC alone, followed by LATE-NC alone, followed by neither pathology. The effect of combined neuropathologies (ADNC + LATE-NC) was attenuated after age 90 years. Harrison et al (4) evaluated the independent effects of ADNC and LATE in a cohort of persons above 90 years, adjusting for the presence of each other when they were comorbid. Intermediate to high ADNC had an independent odds ratio (OR) of 19.8 and LATE-NC had an independent OR of 8.7 for increased risk of dementia. LATE-NC has been shown to be associated with worsened cognitive decline across the spectrum of ADNC, with worsened MMSE scores at each Braak NFT stage (11).
In terms of domains, Kapasi et al (8) looked at 16 neuropsychological tests in 5 domains (episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability), showing that LATE-NC with ADNC had worsened performance in all 5 domains compared to ADNC alone. Wilson et al looked at the independent effects of several neuropathologies (ADNC, LATE-NC, Lewy body, and HS) on these domains, also using data from the Rush cohorts. Each neuropathology independently decreased episodic memory 10 to 16 years before death. As time to death got closer, ADNC, Lewy body, and HS all were associated with decreased function in all other domains. However, LATE-NC’s effect was confined to decreased episodic memory (15). Not directly related to the effect of LATE-NC on ADNC, several reports have addressed domain-specific effects of increased stage of LATE-NC, showing decreases in the same 5domains noted above (1, 28).
Several studies have looked at neuropsychiatric symptoms. Using data from the Brains for Dementia Research cohort in the United Kingdom, Liu et al (12) found that ADNC with LATE-NC was not associated with greater burden of neuropsychiatric symptoms than ADNC alone. On the other hand, using NACC data in 2015 before LATE-NC had been formally defined, Sennik et al found that higher ADNC burden was associated with higher proportion of TDP-43 pathology in participants. The group with the high ADNC burden had increased delusions, hallucinations, and depression, but not other symptoms. The independent effect of TDP-43 pathology was not addressed (29).
Several authors have addressed the potential mechanisms of LATE-NC’s potentiation of ADNC’s effects. McAleese et al (14) showed that the presence of LATE-NC in ADNC was not associated with increased burden of tau or amyloid and that the effect of LATE-NC on cognition was independent of tau pathology. Robinson et al (13) postulated that the longer duration of dementia symptoms when ADNC was comorbid with LATE-NC implied that LATE-NC’s interactions occurred after plaques and tangles had already accumulated.
Although the effect of comorbid LATE-NC in the current study was primarily detected among participants who died with low/intermediate ADNC, it is interesting to note that LATE-NC was associated with higher ADNC stage and that these participants had worsened cognitive outcomes. Similarly, Robinson et al (13), using a combination of data from NACC and the Center for Neurodegenerative Disease Research at the University of Pennsylvania, found that increased Braak NFT stage was associated with LATE-NC, Lewy body, and cerebral amyloid angiopathy. Likewise, the lack of differences between ADNC and ADNC + LATE-NC in the high ADNC strata may be due to a ceiling effect in which functioning has deteriorated to a level at which the tests can no longer discriminate between the groups. This is evidenced in Table 5, in which many of the cognitive symptoms are present in greater than 80% and even greater than 90% of participants in both groups.
LATE-NC has been associated with comorbid HS (1, 7, 27, 30). In the current study, a low percent (6%–7%) of participants with ADNC had HS, which did not change with ADNC severity. The presence of comorbid LATE-NC was associated with increased HS (over 37%), which also did not change with ADNC severity. The association of comorbid LATE-NC with increased proportion of HS in people with ADNC is well documented (27). For example, in the University of Kentucky autopsy series, HS increased from 4% in ADNC alone to 68% in ADNC + LATE-NC (11). HS appears likely to be part of the causal pathway of the effect of TDP-43 pathology in clinical LATE (11). Kapasi et al investigated the role of HS in further detail, comparing participants with ADNC + LATE-NC with and without HS; most (75%) had HS. The presence of HS was associated with more rapid declines in global cognition, episodic memory and semantic memory (8). We previously found that HS is associated with worse cognitive performance (but not with a history of either seizures or strokes), in persons with LATE-NC in the NACC data set (31).
We performed analysis stratified by extent of ADNC, using 2 groups: low/intermediate and high ADNC. The decision to combine low and intermediate ADNC was based on a number of factors, including clinical-pathological correlations and neuropathologic findings. Primarily, the pathological features of ADNC are most confidently associated with cognitive impairment in Braak NFT Stages V and VI (32, 33). Whereas Braak NFT stage III is considered “intermediate ADNC severity” (34), the great majority of Braak NFT stage III brains are in people who are not demented (32, 35). Further, many cases with Braak NFT stages III or IV are actually primary age-related tauopathy (PART) (36). By contrast, if a brain has Braak NFT stages V or VI, it is almost always an example of severe ADNC (i.e. Aβ plaques are present) (5, 34).
We ran a sensitivity analysis to evaluate the differences between ADNC + LATE-NC participants in the analytic sample and FTLD-TDP participants who had been excluded from the main analysis. The 2 groups differed in their clinical symptoms and neuropsychological test scores, consistent with prior work, which supported the validity of excluding FTLD-TDP cases from the main analysis (1, 24, 25). There were also interesting neuropathologic differences between the 2 groups, with the ADNC + LATE-NC group having more arteriosclerosis and with the FTLD-TDP group having a higher percentage of people with neocortical TDP-43 inclusions. Teylan et al (25) used a NACC cohort sample that overlapped with the current study, but which included a larger number of FTLD-TDP participants. The Teylan study also showed a higher percentage of cerebral arteriosclerosis among the LATE-NC group. This difference may in part be due to the higher average age of people with LATE-NC. However, even factoring in age, LATE-NC (and its frequent concomitant pathology, HS) appear to be associated rather specifically with increased brain arteriolosclerosis (4, 20, 26, 37, 38). In addition to finding evidence for more neocortical TDP-43 pathology in FTLD-TDP in comparison to LATE-NC, Robinson et al reported differences in distribution of different subtypes of TDP-43 inclusions (alpha vs beta inclusions). However, there were several cases (<2% of the cohort) with clinical and pathological features that overlapped between LATE-NC and FTLD-TDP (24).
The present study identifies several priorities for future research. As noted, LATE-NC and FTLD-TDP may have some pathogenetic overlap, but the great majority of cases can be differentiated based on pathology alone and the conditions also have distinct clinical, genetic, and epidemiologic characteristics (1, 24). More work is needed to delineate the “frontal” symptoms of non-FTLD cases (e.g. disinhibition and language problems) that fall short of clinical features seen in full-blown FTD cases, and also to distinguish the clinical characteristics of FTLD-TDP and FTLD-tau. Second, future work may more precisely define associations between “pure” LATE-NC and neurobehavioral outcomes. The NACC cohort is enriched for ADNC, given the nature of the contributory research clinics and their fundamental mission of AD research. One obvious manifestation of this selection bias is that the APOE ε4 prevalence was almost 50% among the participants included in this study, as compared to a prevalence of approximately 25% in the general population (39). Hence, relatively few cases (with or without LATE-NC) lack ADNC. In recent years, NACC contributory ADRCs have recruited and autopsied more “normal” participants (20). As TDP-43 pathologic assessment increases at the research centers and as the number of cases with LATE-NC increase, the NACC database will allow greater understanding of LATE-NC without accompanying ADNC.
This study has several limitations. First, study participants were more likely to be white and highly educated than the general US population, limiting generalizability (40). Second, the study used cross-sectional data, which are less sensitive than longitudinal data in detecting subtle changes, especially early in the course of cognitive decline (41, 42). Third, some of the neuropsychological tests were changed when the UDS version changed from UDS 2 to UDS 3. However, the tests in both versions reflect the same domains and there is no a priori reason that the tests would perform differently in people with ADNC alone versus ADNC with LATE-NC. Fourth, we excluded participants with neuropathologic diagnosis of FTLD-TDP. There could have been misclassification in either direction between FTLD-TDP and LATE-NC. However, the clinical syndrome of frontotemporal dementia was present in only a small percent of participants and was not associated with ADNC + LATE-NC (3.3%) more than it was with pure ADNC (5.0%). Fifth, in the sensitivity analysis, the number of participants with FTLD-TDP is low in the high ADNC strata. Sixth, TDP-43 pathologic assessment is relatively new and not all centers are at the same level of development for its use. This is reflected in a wide range among the centers in the percent of cases that were excluded due to missing TDP-43 pathology data. Nonetheless, even the center that contributed the most cases (n = 178) only accounted for 14.7% of cases and there were only 2 centers that did not contribute any cases. Thus, the TDP-43 pathology data (and other data) used in the study come from a wide range of centers.
Despite these limitations, the study has several important strengths. Data were derived from over 30 centers across the United States, representing the state-of-the-art in neuropathologic practice; this increases the generalizability of the findings. Data collection methods were standardized and autopsies were performed at all centers using standardized up-to-date methods (20, 43). Further, all of the autopsies were performed after 2014, which entails some advantages by minimizing potential cohort effects, and because the neuropathologic methodologies have become more standardized over time.
We conclude that the co-occurrence of LATE-NC with ADNC is associated with a different pattern of behavior and cognitive performance than ADNC alone. The effect is strongly modified by the extent of ADNC, and possibly with other copathologies associated with LATE-NC. The associations between LATE-NC and cognitive symptoms are most readily detected in participants who died with low/intermediate ADNC, whereas the differences are more subtle (or nonexistent) among those with severe ADNC. These findings contribute to a better understanding of the public health impact of the highly prevalent condition of LATE-NC.
Supplementary Material
Contributor Information
Kathryn Gauthreaux, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA.
Charles Mock, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA.
Merilee A Teylan, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA.
Jessica E Culhane, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA.
Yen-Chi Chen, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Kwun C G Chan, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Yuriko Katsumata, Sanders-Brown Center on Aging, University of Kentucky, Lexington, Kentucky, USA; Department of Biostatistics, University of Kentucky, Lexington, Kentucky, USA.
Peter T Nelson, Sanders-Brown Center on Aging, University of Kentucky, Lexington, Kentucky, USA; Division of Neuropathology, Department of Pathology, University of Kentucky, Lexington, Kentucky, USA.
Walter A Kukull, From the Department of Epidemiology, National Alzheimer’s Coordinating Center, University of Washington, Seattle, Washington, USA.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
PTN is supported by NIA/NIH grants R01 AG061111, RF1 NS118584. YK is supported by NIA/NIH grants R56AG057191, R01AG057187, R21AG061551, R01AG054060, and the UK-ADC P30AG028383 from the National Institute on Aging. The NACC database is funded by NIA/NIH grant U24 AG072122. NACC data are contributed by the NIA-funded ADCs: P50 AG005131 (PI James Brewer, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG005138 (PI Mary Sano, PhD), P50 AG005142 (PI Helena Chui, MD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005681 (PI John Morris, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG008051 (PI Thomas Wisniewski, MD), P50 AG008702 (PI Scott Small, MD), P30 AG010124 (PI John Trojanowski, MD, PhD), P30 AG010129 (PI Charles DeCarli, MD), P30 AG010133 (PI Andrew Saykin, PsyD), P30 AG010161 (PI David Bennett, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG013854 (PI Robert Vassar, PhD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P30 AG019610 (PI Eric Reiman, MD), P50 AG023501 (PI Bruce Miller, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG028383 (PI Linda Van Eldik, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P30 AG035982 (PI Russell Swerdlow, MD), P50 AG047266 (PI Todd Golde, MD, PhD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG049638 (PI Suzanne Craft, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Marwan Sabbagh, MD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), and P30 AG072959 (PI James Leverenz, MD)
REFERENCES
- 1. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019;142:1503–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katsumata Y, Abner EL, Karanth S, et al. Distinct clinicopathologic clusters of persons with TDP-43 proteinopathy. Acta Neuropathol 2020;140:659–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nag S, Barnes LL, Yu L, et al. Limbic-predominant age-related TDP-43 encephalopathy in Black and White decedents. Neurology 2020;95:e2056–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harrison WT, Lusk JB, Liu B, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol 2021;142:917–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer’s disease neuropathology: Combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol 2022;144:27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Josephs KA, Martin PR, Weigand SD, et al. Protein contributions to brain atrophy acceleration in Alzheimer’s disease and primary age-related tauopathy. Brain 2020;143:3463–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Besser LM, Teylan MA, Nelson PT.. Limbic predominant age-related TDP-43 encephalopathy (LATE): Clinical and neuropathological associations. J Neuropathol Exp Neurol 2020;79:305–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kapasi A, Yu L, Boyle PA, et al. Limbic-predominant age-related TDP-43 encephalopathy, ADNC pathology, and cognitive decline in aging. Neurology 2020;95:e1951–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karanth S, Nelson PT, Katsumata Y, et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol 2020;77:1299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karanth SD, Schmitt FA, Nelson PT, et al. Four common late-life cognitive trajectories patterns associate with replicable underlying neuropathologies. J Alzheimers Dis 2021;82:647–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson PT. LATE neuropathologic changes with little or no Alzheimer disease is common and is associated with cognitive impairment but not frontotemporal dementia. J Neuropathol Exp Neurol 2021;80:649–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu KY, Reeves S, McAleese KE, et al. Neuropsychiatric symptoms in limbic-predominant age-related TDP-43 encephalopathy and Alzheimer’s disease. Brain 2020;143:3842–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson JL, Richardson H, Xie SX, et al. The development and convergence of co-pathologies in Alzheimer’s disease. Brain 2021;144:953–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McAleese KE, Walker L, Erskine D, et al. Concomitant LATE-NC in Alzheimer’s disease is not associated with increased tau or amyloid-β pathological burden. Neuropathol Appl Neurobiol 2020;46:722–34 [DOI] [PubMed] [Google Scholar]
- 15. Wilson RS, Yang J, Yu L, et al. Postmortem neurodegenerative markers and trajectories of decline in cognitive systems. Neurology 2019;92:e831–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beekly DL, Ramos EM, van Belle G, et al. ; NIA-Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: An Alzheimer disease database. Alzheimer Dis Assoc Disord 2004;18:270–7 [PubMed] [Google Scholar]
- 17. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 2006;20:210–6 [DOI] [PubMed] [Google Scholar]
- 18. Beekly DL, Ramos EM, Lee WW, et al. ; NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 2007;21:249–58 [DOI] [PubMed] [Google Scholar]
- 19. Besser L, Kukull W, Knopman DS, et al. ; Neuropsychology Work Group, Directors, and Clinical Core Leaders of the National Institute on Aging-Funded US Alzheimer’s Disease Centers. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 2018;32:351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Besser LM, Kukull WA, Teylan MA, et al. The revised National Alzheimer’s Coordinating Center’s neuropathology form-available data and new analyses. J Neuropathol Exp Neurol 2018;77:717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer disease centers’ neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 2018;32:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayden KM, Jones RN, Zimmer C, et al. Factor structure of the National Alzheimer’s Coordinating Centers Uniform Dataset neuropsychological battery: An evaluation of invariance between and within groups over time. Alzheimer Dis Assoc Disord 2011;25:128–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robinson JL, Porta S, Garrett FG, et al. Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain 2020;143:2844–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teylan MA, Mock C, Gauthreaux K, et al. Differences in symptomatic presentation and cognitive performance among participants with LATE-NC compared to FTLD-TDP. J Neuropathol Exp Neurol 2021;80:1024–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 2014;137:255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 2007;61:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nag S, Yu L, Boyle PA, et al. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun 2018;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sennik S, Schweizer TA, Fischer CE, et al. Risk factors and pathological substrates associated with agitation/aggression in Alzheimer’s Disease: A preliminary study using NACC data. J Alzheimers Dis 2017;55:1519–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 2013;126:161–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gauthreaux KM, Teylan MA, Katsumata Y, et al. Limbic-predominant age-related TDP-43 encephalopathy: Medical and pathologic factors associated with comorbid hippocampal sclerosis. Neurology 2022;98:e1422–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson PT, Braak H, Markesbery WR.. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol 2009;68:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abner EL, Kryscio RJ, Schmitt FA, et al. “End-stage” neurofibrillary tangle pathology in preclinical Alzheimer’s disease: Fact or fiction? J Alzheimers Dis 2011;25:445–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montine TJ, Phelps CH, Beach TG, et al. ; Alzheimer’s Association. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jicha GA, Abner EL, Schmitt FA, et al. Preclinical AD Workgroup staging: Pathological correlates and potential challenges. Neurobiol Aging 2012;33:622.e1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang SJ, Guo Y, Ervin JF, et al. Neuropathological associations of limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) differ between the oldest-old and younger-old. Acta Neuropathol 2022;144:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agrawal S, Yu L, Kapasi A, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol 2021;31:e12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corbo RM, Scacchi R.. Apolipoprotein E (APOE) allele distribution in the world. Is APOE4 a ‘thrifty’ allele? Ann Hum Genet 1999;63:301–10 [DOI] [PubMed] [Google Scholar]
- 40. Mock C, Teylan M, Beecham G, et al. The utility of the National Alzheimer’s Coordinating Center’s Database for the rapid assessment of evolving neuropathologic conditions. Alzheimer Dis Assoc Disord 2020;34:105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knopman DS, Caselli RJ.. Appraisal of cognition in preclinical Alzheimer’s disease: A conceptual review. Neurodegener Dis Manag 2012;2:183–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riley KP, Jicha GA, Davis D, et al. Prediction of preclinical Alzheimer’s disease: Longitudinal rates of change in cognition. J Alzheimers Dis 2011;25:707–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montine TJ, Monsell SE, Beach TG, et al. Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer’s disease. Alzheimers Dement 2016;12:164–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.