Abstract
Background
Multiple studies have shown that patients with severe emphysema can significantly benefit from bronchoscopic lung volume reduction endobronchial valve (EBV) treatment up to 1 year after treatment. However, hardly any data exist on longer term follow-up, especially on quality of life. Our aim was to investigate long-term follow-up after EBV treatment up to 3 years including quality of life in a real-life routine clinical setting.
Methods
We retrospectively included patients who underwent EBV treatment in our hospital in the Netherlands at least 3 years prior. Patients were invited for annual visits to our hospital, and spirometry, body plethysmography, 6-min walk distance (6MWD) test and St George's Respiratory Questionnaire (SGRQ) were performed during these visits.
Results
At 1-, 2- and 3-year follow-up, data were available from 189, 146 and 112 patients, respectively. Forced expiratory volume in 1 s, residual volume and SGRQ total score significantly improved up to 3 years after treatment compared with baseline, and 6MWD up to 2 years after treatment. In general, the magnitude of improvements gradually decreased over time.
Conclusions
Our results show that patients can benefit at least up to 3 years after EBV treatment. For the first time we found that patients can also benefit in terms of quality of life in the long term, which is an important outcome for this group of patients with end-stage COPD.
Short abstract
A substantial number of patients still experience benefit 3 years after lung volume reduction treatment with endobronchial valves. This benefit includes quality of life, which is an important outcome for patients with end-stage COPD. https://bit.ly/3wqEZER
Introduction
The development of bronchoscopic endobronchial valve treatment (EBV) for emphysema started ∼20 years ago and the treatment was included in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines in 2017 [1–3]. This inclusion in treatment guidelines was based on positive outcomes of the treatment on lung function, exercise capacity and quality of life in multiple randomised controlled trials [4].
So far a handful of studies have published the results up to 1 year after treatment and all showed sustained positive effects of the treatment [5–8]. However, less is known about the longer term follow-up. With the exception of the LIBERATE trial (ClinicalTrials.gov: NCT01796392) [7], which has an ongoing long-term follow-up of 5 years, the follow-up duration of the other randomised clinical trials was 6 or 24 months. Therefore, data registries from regular care settings are needed for long-term outcomes [7, 9–11]. To the best of our knowledge, the only longer term follow-up analysis with a substantial amount of patients originates from a German data registry [12]. The study included 256 treated patients and showed that although clinical benefit gradually declined over time, a high number of patients still had a clinically significant response in hyperinflation and exercise capacity at 3-year follow-up [12]. Due to the retrospective database design of the study and the high number of dropouts due to a study population with severe disease, additional data on long-term results after EBV treatment would be useful. Furthermore, the study did not include any information on quality of life, which is an important outcome, especially in the long term, for this patient population with limited life expectancy.
Therefore, the aim of our study was to investigate the long-term follow-up after bronchoscopic lung volume reduction EBV treatment (up to 3-year follow-up) including quality of life in a regular care setting.
Methods
Study population
We retrospectively included patients with severe emphysema who were treated in our hospital (University Medical Centre Groningen, Groningen, The Netherlands) with EBVs for lung volume reduction at least 3 years prior. Patients were either treated in clinical trials (LIBERATE, STELVIO, TRANSFORM, IMPACT or CHARTIS [7, 9–11, 13]), for compassionate use or in our regular care programme BREATHE-NL (as of 2016; ClinicalTrials.gov: NCT02815683). All trials were approved by our local ethics committee and all patients signed written informed consent for use of their data.
Study design and measurements
After treatment, all patients were invited for a voluntary follow-up visit in our hospital after 6 weeks, 6 months and afterwards yearly. During most of the visits, spirometry, body plethysmography, diffusing capacity of the lung for carbon monoxide and 6-min walk distance (6MWD) were measured according to European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines [14–17]. In addition, the following questionnaires were completed: St George's Respiratory Questionnaire (SGRQ), COPD Assessment Test (CAT), modified Medical Research Council (mMRC) scale and EuroQol-5D questionnaire (EQ5D) [18–21]. Furthermore, blood gas measurement was performed at baseline, and a chest computed tomography (CT) scan, on which a quantitative analysis was undertaken using LungQ software (Thirona, Nijmegen, The Netherlands), was performed at baseline and 2 or 6 months of follow-up.
Statistical analyses
Differences in clinical outcomes between baseline and follow-up time-points were tested with a paired sample t-test. To calculate the number of responders the following established minimal important differences (MIDs) were used: FEV1 100 mL [22], 6MWD 26 m [23], residual volume (RV) 310 mL [24], SGRQ total score 7.1 units [25], and target lobe volume reduction (TLVR) 563 mL and 22.4% [26]. The number of pneumothoraces, revision bronchoscopies and deaths were calculated together with the median time between the occurrence of the event and the date of treatment. All statistical analyses were performed using SPSS statistics version 23 (IBM, Armonk, NY, USA). p-values <0.05 were considered statistically significant.
Results
Study population
In total, 322 patients underwent bronchoscopy, of which 280 patients were actually treated with EBVs between June 2008 and June 2018 in our hospital. The main reason for no treatment was presence of collateral ventilation. Table 1 shows patient characteristics; patient flow during the 3-year follow-up can be found in the flowchart in figure 1 (and supplementary table S1). Procedure details are shown in supplementary table S2.
TABLE 1.
Baseline patient characteristics (n=280)
| Valid | ||
| Male | 89 (32) | 280 |
| Age, years | 60±8.5 | 280 |
| BMI, kg·m−2 | 23.9±3.6 | 280 |
| Pack-years | 39 (0–148) | 263 |
| LTOT use | 75 (32) | 235 |
| FEV1, L | 0.75±0.27 | 280 |
| FEV1, % pred | 27.8±8.0 | 280 |
| RV, L | 4.90±1.06 | 278 |
| RV, % pred | 234±41 | 278 |
| RV/TLC, % | 63.5±7.9 | 278 |
| DLCO, % pred | 34.4±11.3 | 219 |
| PaO2, kPa | 9.08±1.3 | 252 |
| PaCO2, kPa | 5.32±0.76 | 252 |
| 6MWD, m | 325±95 | 266 |
| mMRC score | 3.0 (1–4) | 258 |
| EQ5D VAS score | 48.5±16.7 | 243 |
| SGRQ | ||
| Impact score | 47.5±16.8 | 258 |
| Activity score | 85.9 (38.7–100) | 258 |
| Symptoms score | 48.4±18.7 | 258 |
| Total score | 59.3±12.1 | 258 |
| CAT total score | 22.2±5.4 | 147 |
| Target lobe volume, mL | 1905±638 | 273 |
| Emphysema score # , target lobe, % | 48.7±9.8 | 273 |
Data are presented as n (%), mean±sd, median (range) or n. BMI: body mass index; LTOT: long-term oxygen therapy; FEV1: forced expiratory volume in 1 s; RV: residual volume; TLC: total lung capacity; DLCO: diffusing capacity of the lung for carbon monoxide; PaO2: arterial oxygen tension; PaCO2: arterial carbon dioxide tension; 6MWD: 6-min walk distance; mMRC: modified Medical Research Council; EQ5D VAS: EuroQol-5D questionnaire visual analogue scale; SGRQ: St George's Respiratory Questionnaire, CAT: COPD Assessment Test. #: percentage of voxels < −950 HU threshold.
FIGURE 1.
Flowchart of patients through study follow-up. CV+: presence of collateral ventilation; EBV: endobronchial valve.
Long-term follow-up
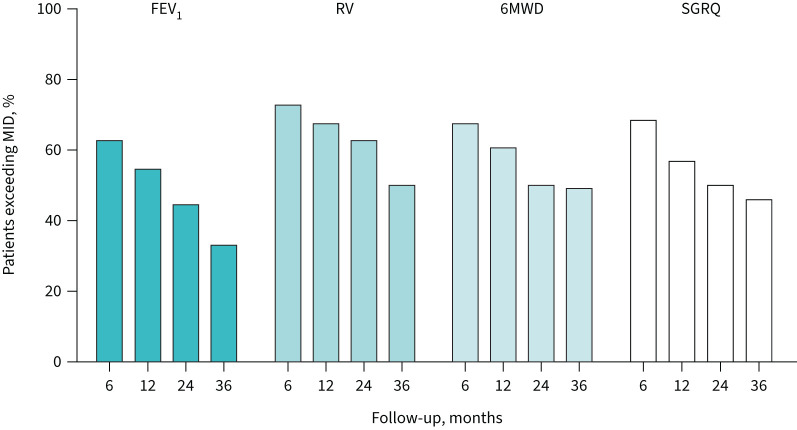
FEV1, RV, SGRQ, mMRC score and EQ5D visual analogue scale score significantly improved at all time-points compared with baseline (table 2 and supplementary table S3 show the results in all patients who completed the baseline and 3-year follow-up visits; supplementary table S7 shows the median values). 6MWD and CAT score were significantly higher compared with baseline up to 2-year follow-up but not at 3-year follow-up. In general, the magnitude of improvements decreased over time. The numbers of responders are shown in figure 2 and supplementary table S4.
TABLE 2.
Changes in clinical outcomes compared with baseline up to 3 years after treatment
| 6-week follow-up | n | p-value | 6-month follow-up | n | p-value | 12-month follow-up | n | p-value | 24-month follow-up | n | p-value | 36-month follow-up | n | p-value | |
| ΔFEV1, L | 0.21±0.18 | 251 | <0.001* | 0.19±0.19 | 196 | <0.001* | 0.15±0.19 | 189 | <0.001* | 0.10±0.20 | 146 | <0.001* | 0.04±0.18 | 110 | 0.033* |
| ΔRV, L | −0.80±0.64 | 238 | <0.001* | −0.69±0.69 | 189 | <0.001* | −0.62±0.63 | 177 | <0.001* | −0.44±0.71 | 123 | <0.001* | −0.33±0.69 | 90 | <0.001* |
| Δ6MWD, m | ND | 57.9±64 | 197 | <0.001* | 45.6±74 | 173 | <0.001* | 36.1±83 | 94 | <0.001* | 8.8±96 | 86 | 0.397 | ||
| ΔSGRQ total score | −18.0±15.4 | 220 | <0.001* | −15.3±16.3 | 194 | <0.001* | −11.0±17.0 | 184 | <0.001* | −8.0±15.7 | 141 | <0.001* | −7.1±14.7 | 112 | <0.001* |
| ΔCAT total score | −4.88±6.46 | 122 | <0.001* | −3.42±6.08 | 115 | <0.001* | −2.36±6.63 | 113 | <0.001* | −1.5±6.00 | 72 | 0.037* | −1.40±5.50 | 60 | 0.053 |
| ΔEQ5D VAS score | ND | ND | 12.56±20.8 | 170 | <0.001* | 8.95±19.6 | 131 | <0.001* | 6.63±21.3 | 107 | 0.002* | ||||
| ΔmMRC score | −0.64±0.76 | 211 | <0.001* | −0.54±0.74 | 198 | <0.001* | −0.46±0.76 | 170 | <0.001* | −0.38±0.86 | 126 | <0.001* | −0.23±0.81 | 88 | 0.010* |
Changes (Δ) in outcome are presented as mean±sd. FEV1: forced expiratory volume in 1 s; RV: residual volume; 6MWD: 6-min walk distance; SGRQ: St George's Respiratory Questionnaire; CAT: COPD Assessment Test; EQ5D VAS: EuroQol-5D questionnaire visual analogue scale; mMRC: modified Medical Research Council; ND: not done. Changes between baseline and follow-up measurement were tested with a paired t-test. *: significant values (p<0.05) using the Holm–Bonferroni method to adjust for multiple comparisons.
FIGURE 2.
Percentage of patients who exceeded the established minimal important difference (MID) per time-point (6-, 12-, 24- and 36-month follow-up). MIDs used: forced expiratory volume in 1 s (FEV1) 100 mL, residual volume (RV) 310 mL, 6-min walk distance (6MWD) 26 m and St George's Respiratory Questionnaire (SGRQ) total score 7.1 units.
Target lobe volume reduction
A follow-up CT scan after 2 or 6 months was performed in 226 patients and mean±sd TLVR was −1281±675 mL (relative reduction compared with baseline: −71±32%). 86 patients (38%) had a complete atelectasis (100% TLVR) and 193 patients (85%) had a clinically relevant reduction in target lobe volume according to the established MID of −563 mL (supplementary table S4). At 12-month follow-up, patients with a complete atelectasis had a significantly larger improvement in RV, FEV1, SGRQ, CAT and 6MWD compared with patients who did not have a complete atelectasis (supplementary table S5).
Pneumothorax, revision bronchoscopy and survival
Pneumothorax occurred in 60 patients (21%), with a median (range) of 1 (0–660) days after treatment. In 13 patients (22%) no treatment was needed, in 43 patients (72%) a chest tube was placed and four patients needed surgery. There were no differences in outcomes at all time-points between patients who developed pneumothorax versus patients who did not. 124 patients (44%) underwent at least one revision bronchoscopy during follow-up, with a median (interquartile range (IQR)) of 140 (49–425) days after treatment. During the 3-year follow-up 50 patients died (17.9%), with a median (IQR) of 692 (39–1079) days after the treatment (supplementary figure S6).
Discussion
Our results show that patients can benefit up to 3 years from bronchoscopic lung volume reduction treatment with EBVs. At 3-year follow-up, we still found significant improvements in terms of lung function, TLVR, dyspnoea severity and quality of life, and up to 2 years after treatment in exercise capacity.
In line with the results of the German registry study by Gompelmann et al. [12], we found significant improvements in exercise capacity up to 2 years after treatment and similar responder rates for the outcomes up to 3 years. Furthermore, we also found significant improvements in dyspnoea severity up to 3 years after treatment. In contrast, we found a persistent significant improvement in FEV1 up to 3 years after treatment, while in the German registry FEV1 was only significantly higher up to 1 year. Additionally, the number of FEV1 responders was higher in our population. Also, in our patients, RV was still significantly improved at 3-year follow-up, in contrast to the German registry in which significant improvements were found until 2-year follow-up. Remarkably, the responder rates of RV were higher in the German registry population while even using a higher MID. To the best of our knowledge, there are no reports with large sample sizes on the long-term follow-up after other bronchoscopic lung volume reduction techniques. Therefore, we were unable to compare our outcomes with other lung volume reduction techniques.
For the first time we investigated quality of life after EBV treatment in the long term. Our results show that quality of life measured using the SGRQ was still significantly higher up to 3 years after treatment. Moreover, at each time-point the change in SGRQ was on or above the MID of −7.1 units. When comparing our results to lung volume reduction surgery (National Emphysema Treatment Trial (NETT) trial [27]) our responder rates were higher: 57%, 50% and 46% versus 40%, 32% and 20%, respectively, at 1-, 2- and 3-year follow-up. It should be noted that the NETT trial used a slightly higher MID cut-off (−8 versus −7.1 units in our study), and the NETT trial was performed >20 years ago and surgical treatment options further developed afterwards. In a previous study in which we investigated a small subgroup of patients treated with lung volume reduction coils we also found that on average the SGRQ was still higher than the MID of −7.1 units [28].
Our results show that the size of the clinical improvement gradually declines over time, which could be an indication of a diminishing treatment effect. On the other hand, EBV treatment does not stop the natural disease progression of COPD, which may also be a likely explanation for the decline over time. Previously, we collected pre-treatment spirometry results in a small group of patients treated with lung volume reduction coil treatment to investigate pre-treatment decline [28]. The decline before treatment was −0.08 L per year, indicating that without treatment FEV1 would even be lower. However, the rate of decline will probably slow down when FEV1 decreases. The NETT trial, investigating lung volume reduction surgery, was able to include a nontreated control group for the long term [27]. Their results showed that functional outcomes in the survivors in the control group worsened after ∼6 months below baseline and continued to deteriorate afterwards [27]. For example, the SGRQ total score was +4.6 units after 3-year follow-up (compared with −7.1 units in our population treated with EBV). Thus, although the benefit gradually declines, it is likely that patients still have a clinical advantage compared with patients who did not undergo EBV treatment. Another important note is that the MIDs used were in general calculated for the short term (up to 1 year), and it is questionable whether these are applicable and not too strict to evaluate long-term results.
Pneumothorax rate and number of revision bronchoscopies were comparable with previously reported results in the literature. Furthermore, survival rate at 3-year follow-up was quite high (82%). Previously, we [29] as well as Garner et al. [30] found that bronchoscopically reducing lung volume in patients with severe hyperinflation can lead to a survival benefit as well, which can also be an important indicator of long-term treatment efficacy.
A limitation of our study is the high number of patients who were lost to follow-up over time. This can be attributed to the retrospective data registry study design with voluntary follow-up visits and also to the study population with very severe disease who already had a limited life expectancy, were not able to travel to our hospital or underwent other treatments, e.g. lung transplantation. Our number lost to follow-up is in line or even slightly better than the German registry study of Gompelmann et al. [12] and underlines the difficulty of performing real clinical practice studies in comparison with controlled trials. However, we realise that patients who did complete the visits were more likely to be the better treatment responders, which could have led to an overestimation of our results.
Unfortunately, it is difficult and expensive to perform clinical trials with long-term follow-up. The LIBERATE trial is the only (still ongoing) trial with a follow-up of 5 years after treatment [7]. However, the follow-up visits only include spirometry and safety reporting, and therefore will not provide insight into changes in lung volumes, exercise capacity or quality of life in the long term. Furthermore, in the LIBERATE trial we still expect a substantial amount of patients will be lost to follow-up and additionally the patients were only randomised up to 1 year.
To conclude, our results indicate that a substantial number of patients can still benefit from bronchoscopic lung volume reduction EBV treatment 3 years after treatment. The magnitude of improvements gradually decreased over time, which is probably also a consequence of the natural disease progression of COPD. For the first time we also showed that quality of life of the patients is still better 3 years after treatment, which is an important outcome for this group of patients with end-stage COPD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00235-2022.SUPPLEMENT (434KB, pdf)
Acknowledgements
We would like to thank Gea Zwart (University Medical Centre Groningen, Groningen, The Netherlands) for scheduling all the patient visits, and Sonja W.S. Augustijn (University Medical Centre Groningen, Groningen, The Netherlands) for performing lung function and 6-min walk tests.
Provenance: Submitted article, peer reviewed.
Conflict of interest: K. Klooster reports payment or honoraria for lectures from PulmonX and Boehringer. D-J. Slebos reports grants or contracts from PulmonX, PneumRx/BTG/Boston Scientific, FreeFlowMedical and Nuvaira (principal investigator and advisor, to institution); consulting fees, payment or honoraria for lectures, support for attending meetings and/or travel, and receipt of study material and medical devices to institution from PulmonX, PneumRx and Nuvaira. All other authors have nothing to disclose.
Support statement: This research was supported by a grant from the Lung Foundation Netherlands (5.1.17.171.0). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003; 361: 931–933. doi: 10.1016/S0140-6736(03)12762-6 [DOI] [PubMed] [Google Scholar]
- 2.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 3.Snell G, Holsworth L, Borrill Z, et al. The potential for bronchoscopic lung volume reduction using bronchial prostheses: a pilot study. Chest 2003; 124: 1073–1080. doi: 10.1378/chest.124.3.1073 [DOI] [PubMed] [Google Scholar]
- 4.van Geffen WH, Slebos DJ, Herth FJ, et al. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med 2019; 7: 313–324. doi: 10.1016/S2213-2600(18)30431-4 [DOI] [PubMed] [Google Scholar]
- 5.Eberhardt R, Slebos DJ, Herth FJF, et al. Endobronchial valve (Zephyr) treatment in homogeneous emphysema: one-year results from the IMPACT randomized clinical trial. Respiration 2021; 100: 1174–1185. doi: 10.1159/000517034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klooster K, Hartman JE, Ten Hacken NHT, et al. One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration 2017; 93: 112–121. doi: 10.1159/000453529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criner GJ, Sue R, Wright S, et al. A multicenter RCT of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018; 198: 1151–1164. doi: 10.1164/rccm.201803-0590OC [DOI] [PubMed] [Google Scholar]
- 8.Dransfield MT, Garner JL, Bhatt SP, et al. Effect of Zephyr endobronchial valves on dyspnea, activity levels, and quality of life at one year. Results from a randomized clinical trial. Ann Am Thorac Soc 2020; 117: 829–838. doi: 10.1513/AnnalsATS.201909-666OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klooster K, ten Hacken NHT, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325–2335. doi: 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]
- 10.Kemp SV, Slebos DJ, Kirk A, et al. A multicenter RCT of Zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535–1543. doi: 10.1164/rccm.201707-1327OC [DOI] [PubMed] [Google Scholar]
- 11.Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med 2016; 194: 1073–1082. doi: 10.1164/rccm.201607-1383OC [DOI] [PubMed] [Google Scholar]
- 12.Gompelmann D, Heinhold T, Rötting M, et al. Long-term follow up after endoscopic valve therapy in patients with severe emphysema. Ther Adv Respir Dis 2019; 13: 1753466619866101. doi: 10.1177/1753466619866101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herth FJF, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J 2013; 41: 302–308. doi: 10.1183/09031936.00015312 [DOI] [PubMed] [Google Scholar]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 15.MacIntyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26: 720–735. doi: 10.1183/09031936.05.00034905 [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 17.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26: 511–522. doi: 10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 18.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991; 85: Suppl. B, 25–31. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 20.EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34: 648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 22.Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2: 111–124. doi: 10.1081/COPD-200053377 [DOI] [PubMed] [Google Scholar]
- 23.Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91: 221–225. doi: 10.1016/j.apmr.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 24.Hartman JE, Ten Hacken NHT, Klooster K, et al. The minimal important difference for residual volume in patients with severe emphysema. Eur Respir J 2012; 40: 1137–1141. doi: 10.1183/09031936.00219111 [DOI] [PubMed] [Google Scholar]
- 25.Welling JBA, Hartman JE, Ten Hacken NHT, et al. The minimal important difference for the St George's Respiratory Questionnaire in patients with severe COPD. Eur Respir J 2015; 46: 1598–1604. doi: 10.1183/13993003.00535-2015 [DOI] [PubMed] [Google Scholar]
- 26.Welling JBA, Hartman JE, van Rikxoort EM, et al. Minimal important difference of target lobar volume reduction after endobronchial valve treatment for emphysema. Respirology 2018; 23: 306–310. doi: 10.1111/resp.13178 [DOI] [PubMed] [Google Scholar]
- 27.Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006; 82: 431–443. doi: 10.1016/j.athoracsur.2006.05.069 [DOI] [PubMed] [Google Scholar]
- 28.Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015; 20: 319–326. doi: 10.1111/resp.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman J, Welling J, Klooster K, et al. Survival in COPD patients treated with bronchoscopic lung volume reduction. Respir Med 2022; 196: 106825. doi: 10.1016/j.rmed.2022.106825 [DOI] [PubMed] [Google Scholar]
- 30.Garner J, Kemp SV, Toma TP, et al. Survival after endobronchial valve placement for emphysema: a 10-year follow-up study. Am J Respir Crit Care Med 2016; 19: 519–521. doi: 10.1164/rccm.201604-0852LE [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00235-2022.SUPPLEMENT (434KB, pdf)