Abstract
Acute lymphoblastic leukemia (ALL) in children and adolescents can involve the testes at diagnosis or upon relapse. The testes were long considered pharmacologic sanctuary sites, presumably because of the blood–testis barrier that prevents the entry of large-molecular-weight compounds into the seminiferous tubule. Patients with testicular involvement were historically treated with testicular irradiation or orchiectomy. With the advent of contemporary intensive chemotherapy, including high-dose methotrexate, vincristine/glucocorticoid pulses, and cyclophosphamide, testicular leukemia present at diagnosis can be eradicated, with the risk of testicular relapse being 2% or lower. However, the management of testicular leukemia is not well described in the recent literature and remains relevant in low- and middle-income countries where testicular relapse is still experienced. Chemotherapy can effectively treat late isolated testicular B-ALL relapses without the need for irradiation or orchiectomy in patients with early response, thereby preserving testicular function. For refractory or early relapsed testicular leukemia, newer treatment approaches such as chimeric antigen receptor–modified T (CAR-T) cell therapy are under investigation. The control of testicular relapse with CAR-T cells and their penetration of the blood–testis barrier have been reported. The outcome of pediatric ALL has been improved remarkably by controlling the disease in the bone marrow, central nervous system, and testes, and such success should be extended globally.
Keywords: Acute lymphoblastic leukemia, testicular leukemia, children, adolescents, diagnosis, biology, management
Lay Summary
Acute lymphoblastic leukemia (ALL) in children and adolescents can involve the testes at diagnosis or upon relapse. Modern intensive chemotherapy has largely eradicated testicular relapse in high-income countries. Consequently, most current clinicians are not familiar with how to manage it if it does occur, and testicular relapse continues to be a significant problem in low- and middle-income countries that have not had access to modern intensive chemotherapy. We review the historical progress made in eradicating testicular ALL and use the lessons learned to make recommendations for treatment.
Precis
Although testicular involvement of acute lymphoblastic leukemia (ALL) has been largely eradicated with intensive contemporary chemotherapy in high-income countries, the management of testicular leukemia is not well described in the recent literature, and this issue remains relevant in low- and middle-income countries where testicular relapse is still frequently experienced because of inadequate access to intensive chemotherapy. This review discusses the incidence, clinical presentation, and biology of testicular leukemia, and it describes the sequential improvements in its management and recommendations for treatment.
Introduction
As a result of improved risk-directed therapy and supportive care, the 5-year survival for pediatric acute lymphoblastic leukemia (ALL) has been increased to more than 90% in high-income countries.1 The testes were long regarded as pharmacologic sanctuary sites, with the blood–testis barrier reducing the efficacy of systemic chemotherapy.2 This was considered to account, at least in part, for the worse outcomes in boys than in girls with ALL, and an international meta-analysis of ALL studies showed that longer duration of maintenance therapy was associated with lower relapse rates, including those in the testes.3 Boys were, therefore, given maintenance therapy for longer periods.4 The introduction of high-dose methotrexate and overall improved systemic chemotherapy have eliminated the need for local irradiation or orchiectomy for testicular leukemia at diagnosis and have reduced the risk of testicular relapse.5,6 Consequently, overt testicular leukemia at diagnosis has therapeutic implications but little prognostic significance, with testicular relapse now being a rare event.6,7 However, the management of testicular leukemia is not well described in the recent literature and remains relevant in low- and middle-income countries where testicular relapse is still experienced. Here, we review the incidence, clinical presentation, and biology of testicular leukemia and the sequential improvements in and recommendations for its management.
Testicular involvement in patients with newly diagnosed leukemia
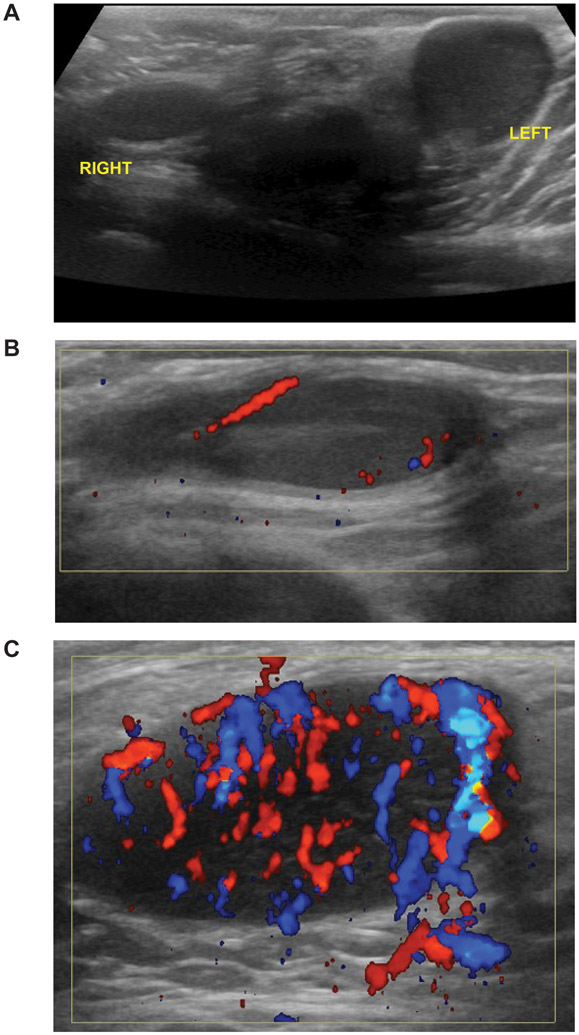
Overt testicular involvement is found in 1.1% to 2.4% of boys at the time of diagnosis of pediatric ALL but is very rare in adults.4,6,7 Testicular involvement can be diagnosed based on increased size, irregular swelling, and/or firm consistency of the testes on physical examination. Ultrasonography with color Doppler is useful for confirming testicular involvement and can be used to evaluate other causes of scrotal enlargement, such as hydrocele, varicocele, extratesticular mass, and torsion, and for monitoring response to therapy.8 Gray-scale sonograms typically show enlargement of one or both of the testes with diffuse or focal regions of decreased echogenicity, and Doppler examination shows increased intralesional blood flow in areas of ALL involvement (Figure 1). Patients with testicular involvement at diagnosis are more likely than others to have higher-risk clinical or biological features: age less than 1 year or 10 years or older at presentation; high initial white blood cell counts; the T-cell phenotype; and other extramedullary involvement, such as central nervous system (CNS) leukemia, mediastinal mass, and/or hepatosplenomegaly.4,6,7 Although the information on genetic subtype is limited, patients with high-risk genetic features, such as BCR-ABL1 and KMT2A rearrangement, can present with testicular disease at diagnosis and/or relapse.4,7
Figure 1. Ultrasound imaging of testicular leukemia.
(A) Asymmetric testes (left > right). (B) Doppler sagittal imaging of the right testis shows normal vascularity at the epididymis. (C) Doppler sagittal imaging of the left testis shows hypervascularity.
Although overt testicular leukemia at diagnosis is rare, a small study of bilateral wedge testicular biopsy at diagnosis of ALL revealed occult testicular involvement without overt changes being noted on physical examination in four of 20 boys with non-T-ALL and in one of four boys with T-ALL.9 No clusters of blasts were seen on repeated biopsies after remission-induction therapy in four of these five boys, suggesting that occult testicular leukemia is sensitive to chemotherapy and that pretreatment testicular biopsy is not indicated.
At St. Jude Children’s Research Hospital (St. Jude), patients with overt testicular disease who were enrolled in the Total Therapy X and XI studies (1979 to 1988) had lower event-free survival (EFS) and overall survival (OS) estimates and a higher cumulative incidence of relapse than did those patients without testicular disease.4 However, no significant differences in outcome were observed between patients with and without overt testicular disease treated in the later Total Therapy XII–XIV studies (1988 to 2000).6 Similarly, in the multicenter European Organization for Research and Treatment of Cancer (EORTC) 58881 trial (1989 to 1998), testicular involvement at diagnosis was associated with the presence of worse prognostic features but had no independent adverse prognostic significance.7 Therefore, most current frontline ALL treatment protocols consider overt testicular involvement only as a criterion for higher-risk ALL, for which more intensive chemotherapy is administered.
Testicular relapse in the 1970s and the use of testicular biopsy to demonstrate residual disease
In the 1970s, isolated overt testicular relapses occurred in 1% to 4% of boys during chemotherapy and in approximately 10% of those patients who completed 24 to 36 months of therapy (Figure 2 and supplemental Table 1).5,10-16 Therefore, testicular biopsy was often performed during and at the completion of chemotherapy and revealed occult testicular leukemia in 8% to 33% of the patients in hematologic remission.16-20 However, a search for other occult extramedullary disease in 29 children with ALL in continuous complete remission at the end of therapy by using kidney and liver biopsies, pelvic ultrasonography, intravenous pyelograms, skeletal surveys, cranial computed axial tomography scans, electroencephalography, and ophthalmologic examinations failed to find occult disease.18 Patients with occult testicular leukemia in otherwise continuous remission in the bone marrow had significantly worse outcomes when compared to patients with negative biopsies.16,17 Testicular irradiation and/or orchiectomy were commonly used for local control in patients with occult testicular leukemia. However, those patients often experienced subsequent relapse in their bone marrow and CNS without further relapse in the testes, a finding that led to additional intensified systemic chemotherapy in the salvage therapy.
Figure 2. Decreasing incidences of isolated testicular relapses.
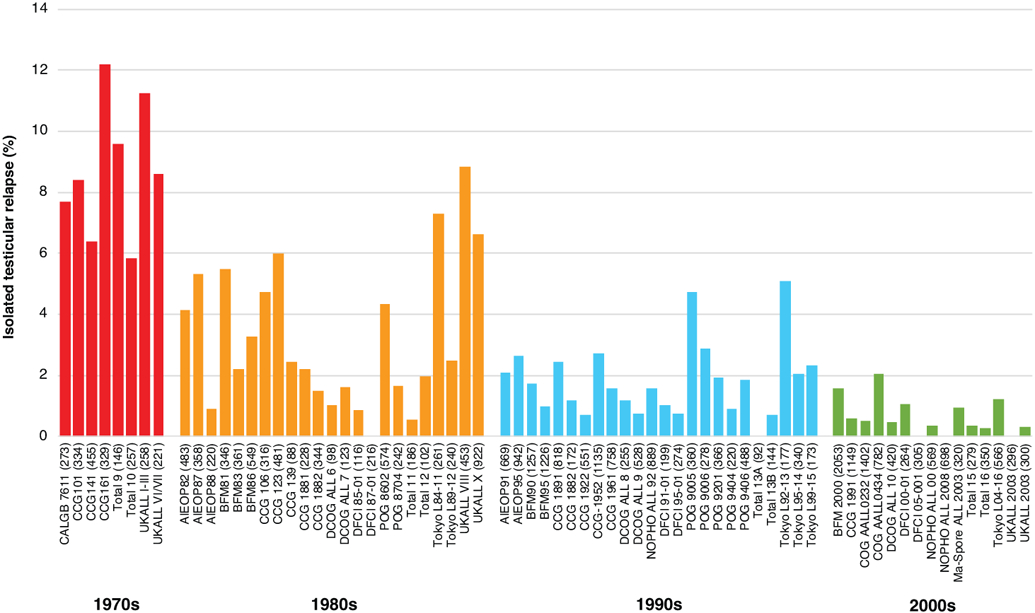
The percentages of isolated testicular relapse are shown according to the decade in which the treatment protocols were initiated. As the testicular relapses were rarely presented as survival curves, the percentages of relapses represent proportions (total isolated testicular relapses/total male enrollments) during the median follow-up period of each treatment protocol. The numbers in parentheses show the total male enrollments in the study. References for the treatment studies are provided in the supplemental information.
Abbreviations: CALGB, Cancer and Leukemia Group B; CCG, Children’s Cancer Group; UKALL, United Kingdom Acute Lymphoblastic Leukemia; AIEOP, Associazione Italiana di Ematologia e Oncologia Pediatrica; BFM, Berlin–Frankfurt–Münster; DCOG, Dutch Childhood Oncology Group; DFCI, Dana-Farber Cancer Institute; POG, Pediatric Oncology Group; NOPHO, Nordic Society of Pediatric Hematology and Oncology; COG, Children’s Oncology Group
In the St. Jude Total Therapy X study, which featured intensive chemotherapy, only one of 120 biopsies (0.8%) revealed occult testicular disease during continuation therapy or at the end of therapy.13 In this study, 13 boys, including the one with occult disease, developed testicular relapses; six of the boys had had a negative biopsy finding 12 to 28 months before their testicular relapse, and six did not have a biopsy. The false-negative biopsy results were probably due to an overall improvement in systemic chemotherapy and/or to focal or scanty leukemic testicular infiltration that escaped microscopic detection. Consequently, elective testicular biopsy was considered not clinically beneficial and has now been discontinued, a decision supported by the low frequency of testicular relapse observed in subsequent clinical trials.
Biology of testicular involvement/relapse
A mutational profiling study in a patient with combined bone marrow and testicular ALL relapse suggested that the leukemia in the testes represented a different subclone from that in the bone marrow, although both subclones were derived from the same ancestral clone.21 Specifically, the leukemia cells in the testes of this patient were found to have an NT5C2 mutation (NT5C2-D407V) that was different from the NT5C2 mutation in the bone marrow leukemia cells (NT5C2-R367Q). The NT5C2 mutation confers 6-mercaptopurine resistance on leukemia cells.22 These findings suggest that the chemotherapy exposure can differ between the bone marrow and the testes, which in turn suggests that the testes are indeed pharmacologic sanctuary sites. Patients with isolated testicular relapses or combined testicular/bone marrow relapses (bone marrow disease in this setting is presumed to be due to seeding from testicular disease) have better survival than do patients with isolated bone marrow relapses, which also supports the concept of sanctuary sites.23,24 Isolated bone marrow relapses are probably caused by selected drug-resistant clones, whereas testicular and combined relapses are caused by clones that are protected from selection pressure and remain susceptible to salvage therapy.
One possible explanation for the testes being sanctuary sites is the existence of a physiologic blood–testis barrier that prevents the entry of large-molecular-weight compounds into the seminiferous tubule (Figure 3).2 Anatomically, this barrier is believed to comprise the tight junctions of the myoid cells and Sertoli cells that line the seminiferous tubule. Whether a second physiologic barrier exists between the blood and the interstitial space has not been well defined. One or both barriers could modify the therapeutic efficacy and/or toxicity of the commonly used chemotherapeutic agents. The decreased ability of methotrexate to pass from the blood into the interstitial space (where the levels are lower than those in the serum by a factor of two to four) and the seminiferous tubule (where the levels are lower than those in the serum by a factor of 18 to 50) has been demonstrated in a rat model.2 ALL cells were usually found in the interstitial space, and infiltration of seminiferous tubules occurred only in the advanced stages of disease.25 Although the difference in methotrexate penetration between the blood and the interstitium was relatively small, these findings suggested that ALL cells in the testes might be exposed to lower concentrations of chemotherapeutic agents. However, higher doses of chemotherapy could conceivably overcome the blood–testis barrier,5,26,27 and cyclophosphamide and vincristine have been found to cross the barrier.28
Figure 3. Possible mechanisms of chemotherapy resistance in the testes.
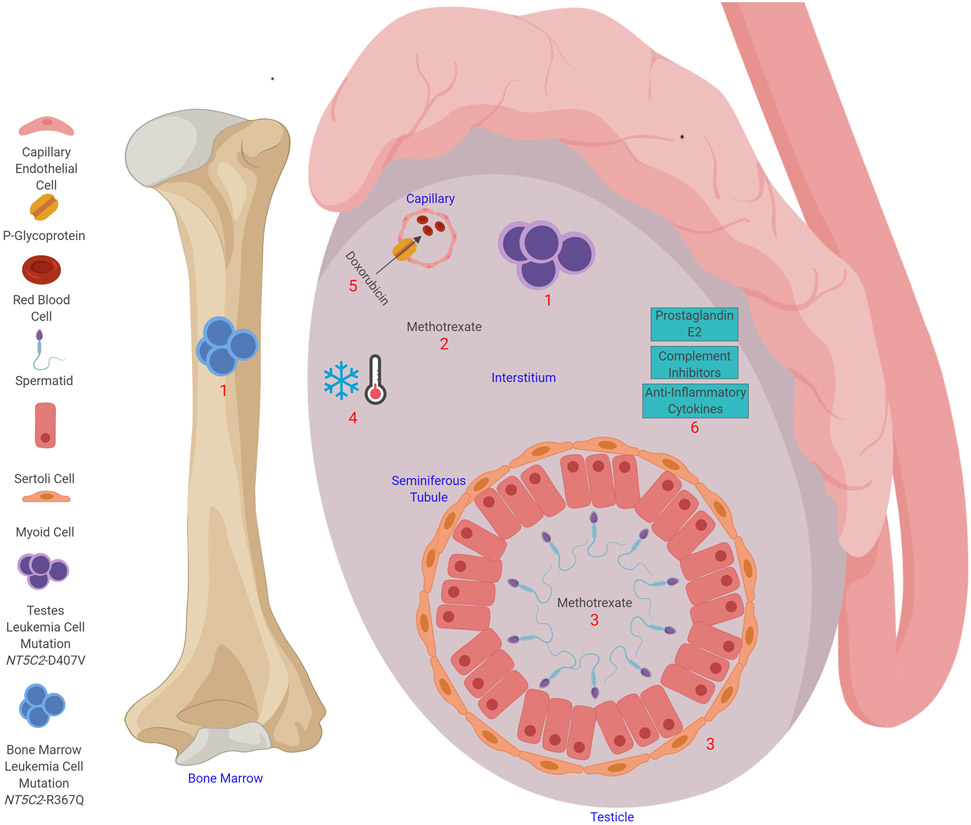
Testicular leukemia relapse is considered to occur as a result of insufficient exposure to chemotherapy. (1) Leukemia relapse in the testes can develop from a clone that is independent of the one seen in the bone marrow relapse; in a patient with relapsed ALL, the testicular leukemia cells were found to have an NT5C2-D407V mutation, whereas the bone marrow cells had an NT5C2-R367Q mutation. (2) The chemotherapy concentration can be reduced in the interstitium; for example, methotrexate levels in the interstitium are lower than those in the serum by a factor of two to four. (3) Tight junctions between the myoid cells and Sertoli cells prevent the entry of large molecules into the seminiferous tubule. Methotrexate levels in seminiferous tubule are lower than those in the serum by a factor of 18 to 50. (4) The testes are 2.5°C colder than the body temperature, which could decrease the efficacy of chemotherapy. (5) P-glycoprotein can export chemotherapy agents (e.g., doxorubicin) from the testes into capillaries. (6) Prostaglandin E2, anti-inflammatory cytokines, and complement inhibitors in the testes help to make the testes an immune-privileged site.
Another possible cause of testicular relapse is the lower temperature of the testes compared to that of the rest of the body (Figure 3), which might decrease the cytotoxic effects of chemotherapy.29 This could explain why ovarian relapse is rarer than testicular relapse: the ovaries are located inside the abdomen, where the temperature is higher than in the scrotum. Furthermore, it has been reported that treatment with estradiol significantly decreases the occurrence of leukemic infiltrates in rat testes.30 Estrogen may prevent Leydig cells from binding to leukemia cells and may directly inhibit lymphoblast infiltration. P-glycoprotein present in the capillary endothelium of the testes could also lead to decreased chemotherapy exposure for leukemia cells by serving as an energy-dependent pump for the efflux of various chemotherapeutic agents (Figure 3).31
The immune-privileged status of the testes could contribute to leukemia cell survival. The testes contain macrophages producing prostaglandin E2, which inhibits T-cell proliferation and natural killer cell function; testicular cells also produce anti-inflammatory cytokines and complement inhibitors (Figure 3).32 Leukemia cells themselves have mechanisms of immune tolerance. It has been postulated that a relatively low mutational burden results in fewer neoantigens being available for recognition by host T cells.33 Similarly, danger-associated molecular patterns may not be present in concentrations sufficient to mediate dendritic cell (DC) maturation, and leukemia antigen presentation by immature DCs results in T-cell tolerance.34
There are several possible explanations for the incidence of testicular involvement of ALL at diagnosis and relapse being lower in adults than in pediatric patients. Intraperitoneal injection of T-ALL cells induced testicular infiltrates in 100% of sexually immature (25-day-old) rats but in only 42% of sexually mature (50-day-old) rats.35 Infiltrates were located in the perivascular interstitial tissue. The testis extracts from the sexually matured rats, but not those from the immature rats, inhibited the proliferation of Con A–stimulated normal lymphoblasts and leukemia cells. This suggests that the permeability of vascular endothelium and the immunosuppressive effect of the testes change with physiologic pubertal development. Also, the tight junctions of the blood–testis barrier diminish with age through reduced expression of genes and proteins involved in cell adhesion of the seminiferous epithelium.36 This allows better chemotherapy penetration into the testes of adults, who typically receive intensive regimens with high-dose methotrexate and cytarabine.
Frontline therapy to control testicular leukemia
In the 1970s, pre-symptomatic testicular irradiation (12–18 Gy) after remission-induction therapy was used to reduce testicular relapse and prevent possible reseeding of the testicular leukemia to the bone marrow.10,15 The United Kingdom (UK) ALL studies VI and VII found that testicular irradiation completely prevented testicular relapse, whereas the incidence in patients who received no irradiation was 13.8% (Table 1).15 However, adding testicular irradiation to the treatment regimen did not improve the rates of bone marrow remission or survival and was associated with infertility and gonadal dysfunction.10,15 Therefore, prophylactic testicular irradiation was discontinued.
Table 1.
Randomized or historical-comparison studies that benefited for testicular disease control.
| Protocol | Risk | Steroid (mg/m2) | Methotrexate (g/m2) |
Cranial irradiation |
Patient (N) |
Male (N) |
Relapse (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induction | Delayed intensification |
Maintenance | Isolated testicular |
Isolated CNS |
BM | Any | ||||||
| Testicular radiation | ||||||||||||
| UKALL VI/VII | VI:HR, VII:SR | Pred 40 | No | Pred 40 | No | Yes | NA | 221 | 8.6 | NA | NA | NA |
| (Testicular irradiation) | VI:HR, VII:SR | Pred 40 | No | Pred 40 | No | Yes | NA | 83 | 0.0* | NA | NA | NS |
| (No testicular irradiation) | VI:HR, VII:SR | Pred 40 | No | Pred 40 | No | Yes | NA | 138 | 13.8* | NA | NA | NS |
| Methotrexate dosing | ||||||||||||
| CALGB 7611 | SR/HR | Pred 40 | No | Pred 40 | 0.5/No [R] | R | 525 | 273 | NA | NA | NA | NA |
| (IDMTX/ITMTX) | SR/HR | Pred 40 | No | Pred 40 | 0.5 | No | 266 | 131 | 2.0* | 28.0* | 27.0* | NS |
| (CRI/ITMTX) | SR/HR | Pred 40 | No | Pred 40 | No | Yes | 259 | 142 | 13.0* | 8.0* | 43.0* | NS |
| BFM81, no MTX | SR/MR/HR | Pred 60 | Dex 10 | No | 0 | Yes | NA | 241 | 6.7* | NA | NA | 30.3 |
| BFM83, MTX 0.5 | SR/MR/HR | Pred 60 | Dex 10 | No | 0.5 | SR-H/MR/HR | NA | 313 | 2.5* | NA | NA | 35.8 |
| BFM86, MTX 5 | SR/Ri/E | Pred 60 | Dex 10 | No | 5 | Ri/E | NA | 485 | 2.3* | NA | NA | 25.8 |
| Total 10 | SR/HR | Pred 40 | No | No | 1 [R for SR] | Yes [R for SR] | 427 | 257 | 5.8 | 8.2 | 22.2 | 38.4 |
| (SR, HD-MTX) | SR | Pred 40 | No | No | 1 | No | 154 | 89 | 2.2* | 11.0* | 17.5* | 36.4* |
| (SR, CRI) | SR | Pred 40 | No | No | No | Yes | 155 | 90 | 11.1* | 3.9* | 32.3* | 45.2* |
| (HR) | HR | Pred 40 | No | No | No | Yes | 101 | 68 | 4.4 | 11.9 | 17.8 | 37.6 |
| CCG 1882 | HR/SER | Pred 60 | Dex 10 | Pred 40 | Escalating [R] | Yes | 311 | 172 | 1.2 | 2.6 | 23.5 | 28.6 |
| (No MTX) | HR/SER | Pred 60 | Dex 10 | Pred 40 | No | Yes | 156 | 89 | 2.2 | 5.1 | 27.6 | 37.2* |
| (Escalating MTX) | HR/SER | Pred 60 | Dex 10 | Pred 60 | Escalating | Yes | 155 | 83 | 0.0 | 0.0 | 19.4 | 20.0* |
| CCG 1961 | HR | Pred 60 | Dex 10 | Pred 40 | Capizzi [R] | CNS+ | 1299 | 758 | 1.6 | 4.7 | 10.2 | 3.2 |
| (No MTX) | HR | Pred 60 | Dex 10 | Pred 40 | No | CNS+ | 649 | 392 | 2.6 | 4.9 | 12.9 | 4.0* |
| (Capizzi MTX) | HR | Pred 60 | Dex 10 | Pred 40 | Capizzi | CNS+ | 650 | 366 | 0.5 | 4.5 | 7.5 | 2.3* |
| CCG 1991 | B-SR | Dex 6 | Dex 10 | Dex 6 | Escalating [R] | CNS+ | 2078 | 1149 | 0.6 | 1.8 | 4.5 | 8.1 |
| (No MTX) | B-SR | Dex 6 | Dex 10 | Dex 6 | No | CNS+ | 1036 | 561 | 1.2* | 2.5* | 4.2 | 9.3* |
| (Escalating MTX) | B-SR | Dex 6 | Dex 10 | Dex 6 | Escalating | CNS+ | 1042 | 588 | 0.0* | 1.1* | 4.9 | 6.9* |
| Intrathecal therapy | ||||||||||||
| CCG-1952 | SR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 2027 | 1135 | 2.7 | 4.4 | 7.0 | 16.0 |
| (ITMTX) | SR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 1018 | 570 | 1.9* | 5.7* | 5.3* | 14.8 |
| (ITTriple) | SR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 1009 | 565 | 3.5* | 3.1* | 8.7* | 17.2 |
| Glucocorticoids | ||||||||||||
| CCG 161# | SR | Pred 40 | No | Pred/No [R] | No | R | 605 | 329 | 12.2 | 5.3 | 17.7 | 25.6 |
| (VCR/Pred) | SR | Pred 40 | No | Pred 40 | No | R | 302 | 163 | 6.1* | 6.3 | 12.6* | 18.9* |
| (No VCR/Pred) | SR | Pred 40 | No | No | No | R | 303 | 166 | 18.1* | 4.3 | 22.8* | 32.3* |
| CCG 1922 | SR | Pred/Dex [R] | Dex 10 | Pred/Dex [R] | No | CNS+ | 1060 | 551 | 0.7 | 5.4 | 10.6 | 16.4 |
| (Pred) | SR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 530 | 281 | 1.1 | 7.0 | 12.1 | 19.8* |
| (Dex) | SR | Dex 6 | Dex 10 | Dex 6 | No | CNS+ | 530 | 270 | 0.4 | 3.8 | 9.1 | 13.0* |
| BFM 2000 | SR/MR/HR | Pred/Dex [R] | Dex 10 | No | 5 | HR/T/CNS+ | 3720 | 2053 | 1.6 | 1.5 | 9.7 | 14.8 |
| (Pred) | SR/MR/HR | Pred 60 | Dex 10 | No | 5 | HR/T/CNS+ | 1867 | 864 | 2.7* | 2.0* | 10.9* | 17.3* |
| (Dex) | SR/MR/HR | Dex 10 | Dex 10 | No | 5 | HR/T/CNS+ | 1853 | 803 | 1.1* | 1.0* | 8.5* | 12.4* |
| Asparaginase | ||||||||||||
| POG 8704 | T | Pred 40 | Pred 40 | Pred 120 | No | WBC >50000 | 317 | 242 | 1.7 | 3.5 | 26.5 | 41.6 |
| (No asparaginase) | T | Pred 40 | Pred 40 | Pred 120 | No | WBC >50000 | 157 | 124 | 3.2 | 4.5 | 34.4 | 55.4* |
| (Asparaginase) | T | Pred 40 | Pred 40 | Pred 120 | No | WBC >50000 | 160 | 118 | 0.0 | 2.5 | 18.8 | 28.1* |
| Chemotherapy intensity | ||||||||||||
| CCG 1881 | LR | Pred 40 | Dex 10 [R] | Pred 40 | No | CNS+ | 700 | 228 | 2.2 | 5.3 | 10.3 | 18.9 |
| (No DI) | LR | Pred 40 | No | Pred 40 | No | CNS+ | 351 | 113 | 3.5 | 6.0 | 11.7 | 22.2 |
| (DI) | LR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 349 | 115 | 0.9 | 4.6 | 8.9 | 15.5 |
| CCG 1891 | IR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 1204 | 818 | 2.4 | 6.5 | 8.1 | 18.4 |
| (DI) | IR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 405 | 279 | 2.5 | 7.9 | 9.9 | 21.7* |
| (DDI) | IR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 402 | 264 | 1.9 | 4.7 | 5.7 | 13.7* |
| (DIVPI) | IR | Pred 40 | Dex 10 | Pred 40 | No | CNS+ | 397 | 275 | 2.9 | 6.8 | 8.8 | 19.9* |
Abbreviations: N, number; CNS, central nervous system; BM, bone marrow; UK, United Kingdom; ALL, acute lymphoblastic leukemia; SR, standard-risk; HR, high-risk; Pred, prednisone; NA, not available; y, year; min, minimum; DFS, disease-free survival; NS, not significant; CALGB; Cancer and Leukemia Group B; R, randomization; CCR, continuous complete remission; IDMTX, intermediate-dose methotrexate; CRI, cranial irradiation; ITMTX; intrathecal MTX; BFM; Berlin–Frankfurt–Münster; MR, medium-risk; Dex, dexamethasone; SR-H, SR-high; Ri, risk group; E, experimental group; HD-MTX, high-dose MTX; CCG, Children's Cancer Group; SER, slow early response; mo, month; VCR, vincristine; POG, Pediatric Oncology Group; DI, delayed intensification; IR, intermediate-risk; DDI, double DI; DIVPI, DI with an increased number of vincristine and prednisone pulses
Parentheses indicate randomized groups.
Significant difference between randomized groups.
No data for isolated relapses are available; data for combined relapses are shown.
As the testicular relapses were rarely presented as survival curves, the relapse percentages represent the proportions (total of the specified event/total protocol enrollments [total male enrollments for testicular relapse]) during the median follow-up period of each treatment protocol.
References for the treatment studies are provided in the supplemental information.
Improvements in systemic therapy have been most efficacious in overcoming the pharmacologic sanctuary status of the testes. Methotrexate is given at a wide range of doses (e.g., 20 mg/m2 to 5000 mg/m2) in ALL therapy. The Cancer and Leukemia Group B (CALGB) 7611 protocol for children and adolescents with ALL randomized patients to receive either three courses of intermediate-dose methotrexate (500 mg/m2 over 24 hours) plus intrathecal methotrexate or cranial irradiation plus intrathecal methotrexate (Table 1).5 Patients who received the intermediate-dose methotrexate had a significantly lower incidence of testicular relapse (2% ± 1% vs. 13% ± 3% at 12 years after diagnosis) and a lower incidence of bone marrow relapse (27% ± 3% vs. 43% ± 3%) when compared to those treated with cranial irradiation. The higher methotrexate plasma concentration and the increased penetration of methotrexate into the interstitium of the testes probably eradicated sequestered residual leukemia cells and prevented the emergence of drug-resistant clones. However, the CNS relapse rate was significantly higher in the intermediate-dose methotrexate group than in the cranial irradiation group as a result of insufficient CNS-directed therapy (28% ± 3% vs. 8% ± 2%).
Intermediate-dose (500 mg/m2) and high-dose (5000 mg/m2) methotrexate were introduced in the Berlin–Frankfurt–Münster (BFM) 83 and 86 studies, respectively (Table 1).37 Patients treated in those studies had equally low rates of testicular relapse (2.5% and 2.3%, respectively) when compared with patients treated in the BFM 81 study without the increased dose of methotrexate (for whom the testicular relapse rate was 6.7%). In the Children’s Cancer Group (CCG) 1882 (high risk and slow early response), 1961 (high risk and rapid early response), and 1991 (standard risk) studies, patients who received augmented post-induction therapy including escalated intermediate doses of methotrexate without leucovorin rescue had fewer relapses, including isolated testicular relapses, when compared with those who did not receive it (Table 1).38,39 Therefore, most current frontline regimens/protocols for patients with National Cancer Institute (NCI) high-risk ALL include high-dose methotrexate or escalating doses of methotrexate and asparaginase without leucovorin rescue (the Capizzi regimen).
In the CCG 1952 study, which compared triple (methotrexate, hydrocortisone, and cytarabine) and single (methotrexate alone) intrathecal therapy in patients with standard-risk ALL who did not receive systemic high-dose methotrexate, triple intrathecal therapy was associated with better CNS control but also with significantly higher incidences of bone marrow and testicular relapse (Table 1).38 These results, especially those of the CALGB 7611, UK ALL VI and VII, and CCG 1952 studies,5,15,38 suggest that bone marrow, CNS, and testicular relapses are competing events. Therefore, treatment regimens should provide effective systemic (and testicular) therapy and CNS-directed therapy.
Vincristine and glucocorticoids have been used in remission induction and as pulses during continuation therapy. The CCG 161 study showed that monthly administrations of vincristine and prednisone pulses in patients with low-risk ALL significantly reduced relapses in the marrow and testes (both overt and occult) as compared to those in patients not receiving the pulses (Table 1).11 However, the vincristine/prednisone pulses did not improve OS; the patients receiving the vincristine/prednisone pulses had increased mortality in remission due to viral or Pneumocystis jirovecii infections. Vincristine is probably useful for preventing testicular relapse because of its ability to cross the blood–testis barrier.28 Currently, dexamethasone is often used during delayed intensification and continuation therapy because the extramedullary tissue penetration of dexamethasone is superior to that of prednisone, especially in the CNS. The CCG 1922 study demonstrated that dexamethasone was more efficacious than prednisone for controlling systemic leukemia (including testicular relapse) in patients with standard-risk ALL.38 The AIEOP-BFM ALL 2000 study also compared dexamethasone and prednisone during induction therapy and found that the incidences of overall relapse and isolated testicular relapse were significantly reduced in the dexamethasone arm.40 Nevertheless, dexamethasone needs to be used judiciously, as it was associated with an increased risk of severe infection and death.40
Asparaginase is routinely used to treat ALL. The Pediatric Oncology Group (POG) 8704 study for T-ALL demonstrated that intensive E. coli L-asparaginase administration during consolidation resulted in outcomes superior to those obtained with the control regimen without asparaginase (Table 1).41 No testicular relapse was seen in the 118 male patients who received asparaginase, whereas four of 124 patients (3.2%) in the control arm experienced testicular relapse. CNS relapses were also less frequent in the asparaginase arm (2.5% vs. 4.5%). Systemic administration of native or polyethylene glycol (PEG)–conjugated E. coli asparaginase reduced asparagine levels in the cerebrospinal fluid (CSF), albeit completely in only 28% of the patients who received the latter agent.42 Asparaginase would also be effective against ALL cells by depleting asparagine in the testes.
Cyclophosphamide is an important component of ALL therapy and can penetrate and exert anti-leukemia effects in the testes.28 However, cumulative doses of 4000 mg/m2 or greater are associated with Leydig cell dysfunction/failure and infertility (Table 2).43,44
Table 2.
Impact on fertility of various treatments for testicular leukemia
| Therapy | Impact on fertility |
|---|---|
| Chemotherapy | Alkylating agents: cumulative cyclophosphamide equivalent doses of ≥4000 mg/m2 are associated with Leydig cell dysfunction/failure and infertility. |
| Testicular irradiation | 24 Gy: severe gonadal dysfunction and azoospermia is expected. 15 Gy: Leydig cell function can be preserved to allow spontaneous pubertal development. Testicular irradiation with ≥4 Gy is associated with infertility. |
| Orchiectomy | Even unilateral orchiectomy is associated with infertility. |
| Hematopoietic cell transplantation | Total body irradiation is a significant risk factor for infertility. |
| Chimeric antigen receptor T cells | Unknown but expected to be minimal. |
The incidence of testicular relapse has been significantly decreased with recent protocols
In the 1980s, the reported incidence of isolated testicular relapses ranged from 0.0% to 7.3%, which was an improvement over the incidence of 5.8% to 12.2% in the 1970s (Figure 2 and supplemental Table 1).38,41,45-51 Higher incidences of testicular relapse were seen with treatment protocols such as BFM 81 (5.5%), CCG 123 (6.0%), Tokyo L84-11 (7.3%), and UKALL VIII and X (8.8% and 6.6%, respectively).38,46,50,51 In those studies, routine use of intermediate- or high-dose methotrexate was not incorporated. Other protocols had very low incidences of testicular relapse during this period: 0.9% in Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) 88, 0.9% in Dana-Farber Cancer Institute (DFCI) 85-01, 0.0% in DFCI 87-01, and 0.5% in St. Jude Total Therapy XI.45,48,49 All of these protocols included high-dose methotrexate (2 g/m2 to 5 g/m2), and the DFCI protocols used high-dose prednisone (120 mg/m2) for high-risk patients during the intensification and maintenance therapy phases. During this period, the incidences of isolated CNS relapse and bone marrow relapse were still generally high, ranging from 0.2% to 14.6% and from 10.1% to 26.5%, respectively. The addition of delayed intensification to lower-risk ALL therapy in the CCG 1881 study decreased all bone marrow, CNS, and testicular relapses (Table 1).38,41
In the 1990s, the incidence of isolated testicular relapse was decreased to between 0.0% and 2.9% (Figure 2 and supplemental Table 1) in most studies,38,41,45-50,52-55 although the incidence remained high in the POG 9005 and Tokyo L92-13 studies (4.7% and 5.1%, respectively);41,50 POG 9005 used prednisone only during induction without delayed intensification or routine use of high-dose methotrexate, and Tokyo L92-13 was characterized by a short duration of therapy (a total of 1 year) and was also associated with a high incidence of any relapse (32.3%). Most of the groups, other than the CCG, used high-dose methotrexate (1 g/m2 to 8 g/m2), and most protocols incorporated delayed intensification. Furthermore, improved risk stratification based on age, white blood cell count at diagnosis, immunophenotype, and cytogenetics was applied. In addition to isolated testicular relapse, the incidences of isolated CNS relapse and bone marrow relapses improved in the 1990s, ranging from 0.6% to 6.7% for CNS relapses and from 4.0% to 24.3% for bone marrow relapses. Two delayed intensification phases, as opposed to a single phase, in patients with intermediate-risk ALL (in CCG 1891) and more intensive post-induction therapy in higher-risk patients with slow early response (in CCG 1882) or rapid early response (in CCG 1961), compared with the control arms, resulted in improved systemic control, including better control of testicular relapse (Table 1).38
Since 2000, testicular relapses have been substantially reduced and the incidences have ranged from 0.0% to 2.0% (Figure 2 and supplemental Table 1).39,40,56-67 As a result of the improvements in systemic and intrathecal chemotherapy, the use of cranial irradiation therapy for CNS-directed treatment has been markedly reduced. In most protocols, dexamethasone during either induction or post-induction therapy, high-dose methotrexate with close pharamacodynamics monitoring, and intensive asparaginase have been used for high-risk disease. The Children’s Oncology Group studies showed improved systemic control with high-dose methotrexate in high-risk B-ALL and with Capizzi methotrexate in T-ALL.56,57 Furthermore, many protocols improved risk stratification by incorporating genetic/cytogenetic classification and minimal residual disease (MRD). In addition to testicular relapses being better controlled, the rates of relapse in both CNS and bone marrow decreased during this period, ranging from 1.2% to 3.2% and from 1.0% to 11.0%, respectively.
Currently, for patients with overt testicular disease at diagnosis, most groups monitor the response to induction therapy by physical examination and/or ultrasound and administer testicular irradiation only if persistent testicular involvement is confirmed by biopsy, unless the involvement is considered unequivocal (Table 3). Because of the efficacy of multi-agent induction therapy, such cases are rare. The practice of administering longer maintenance therapy to boys than to girls has been discontinued in most studies.68
Table 3.
Recommended management of testicular involvement of acute lymphoblastic leukemia
| Evaluation at diagnosis and relapse | ||
|---|---|---|
| Physical examination of testes | ||
| Ultrasound examination of suspected cases | ||
| Biopsy to confirm testicular disease for patients with isolated testicular involvement at diagnosis or relapse or for those with equivocal testicular involvement at the time of concurrent bone marrow or central nervous system relapse | ||
| Treatment | ||
| Patients with newly diagnosed ALL | ||
| Induction chemotherapy | ||
| Biopsy for patients with persistent enlargement or abnormal ultrasound imaging after induction therapy (if involvement is unequivocal, biopsy may not be necessary) | ||
| Testicular irradiation (24 Gy) for persistent leukemia involvement | ||
| Isolated testicular relapse | ||
| –Early relapse (<18 months after diagnosis) in B-ALL and relapse at any time in T-ALL | ||
| Intensive chemotherapy* with hematopoietic cell transplantation | ||
| Testicular boost (12 Gy) with total body irradiation (12 Gy)** | ||
| Chimeric antigen receptor T cells for patients with B-ALL# | ||
| –Intermediate relapse (18–36 months after diagnosis) and late relapse (≥36 months after diagnosis) in B-ALL | ||
| Systemic chemotherapy*†‡ | ||
| Bilateral: testicular irradiation (24 Gy) or orchiectomy | ||
| Unilateral: orchiectomy of the affected testis and prophylactic irradiation (15 Gy) of the biopsy-negative contralateral testis OR testicular irradiation (24 Gy) | ||
| Chimeric antigen receptor T cells for patients with B-ALL# | ||
| Combined testicular and bone marrow relapse | ||
| –Early relapse (<18 months after diagnosis) and intermediate relapse (18–36 months after diagnosis) in B-ALL and relapse at any time in T-ALL | ||
| Intensive chemotherapy* with hematopoietic cell transplantation | ||
| Testicular boost (12 Gy) with total body irradiation (12 Gy)** | ||
| Chimeric antigen receptor T cells for patients with B-ALL# | ||
| –Late relapse (≥36 months after diagnosis) in B-ALL | ||
| Systemic chemotherapy*†‡ | ||
| Bilateral: testicular irradiation (24 Gy) or orchiectomy | ||
| Unilateral: orchiectomy of the affected testis and prophylactic irradiation (15 Gy) of the biopsy-negative contralateral testis OR testicular irradiation (24 Gy) | ||
| Chimeric antigen receptor T cells for patients with B-ALL# | ||
Immunotherapy (e.g., blinatumomab) can be used in B-ALL although the efficacy of blinatumomab for treatment of testicular relapse is not established.
For patients who do not receive total body irradiation, testicular radiation (24 Gy) or orchiectomy can be considered.
The number of patients with testicular relapse who have been treated with chimeric antigen receptor T cells is limited.
When intensified chemotherapy including high-dose methotrexate is given, some treatment regimens omit testicular irradiation or orchiectomy if no residual disease is observed after the induction regimen.
Patients with MRD of ≥0.1% at the end of induction therapy should be treated in the same way as those with early relapse. Even in cases of isolated testicular relapse, MRD in the bone marrow can be positive at relapse.
The radiation dose, schedule, and timing may vary between treatment regimens.
Abbreviation: ALL, acute lymphoblastic leukemia; MRD, minimal residual disease
Management of testicular relapse
Clinically isolated testicular leukemia should be considered a systemic disease because treatment by testicular irradiation or orchiectomy alone is usually followed by a bone marrow relapse within a few months.16,17 Similarly, if no CNS treatment is given after a testicular relapse, 10% to 20% of the patients will subsequently develop CNS relapse. Indeed, an MRD assay using PCR or flow cytometry analysis can detect bone marrow disease in more than 50% of patients in whom a so-called isolated testicular relapse has been diagnosed by morphologic exam.69 Therefore, systemic chemotherapy with effective CNS prophylaxis is required for boys with isolated testicular relapse and MRD should be monitored (Table 3).
As shown in patients with bone marrow and isolated CNS relapses, the prognosis for patients with isolated testicular relapse depends on the time of relapse.23 The 5-year OS in patients who had an early testicular relapse (<18 months after diagnosis) (13.6%) was significantly poorer than in patients who had an intermediate relapse (18–36 months after diagnosis) (52.2%) or a later relapse (≥36 months after diagnosis) (60.0%). Furthermore, post-relapse survival is significantly worse in patients with T-ALL than in those with B-ALL.23 Therefore, for patients with B-ALL and early testicular relapse and for those with T-ALL and relapse at any time, very aggressive treatment including hematopoietic cell transplantation (HCT) has been recommended (Table 3).70-72 When total body irradiation (~12 Gy) is included in the conditioning regimen, a testicular boost (an additional ~12 Gy) is typically administered. Patients with combined bone marrow and testicular relapse are treated similarly to those with isolated bone marrow relapse.
Patients with B-ALL and an intermediate or late isolated testicular relapse can generally be cured by salvage chemotherapy (Table 3).27,71 In addition to systemic chemotherapy, many salvage regimens have used testicular irradiation or orchiectomy of the affected testis, and it has been suggested that at least 24 Gy of scrotal irradiation is needed unless orchiectomy is performed.71 Although there is insufficient evidence to favor one treatment modality over the other, orchiectomy can be justified if the patient has bulky testicular disease, if unilateral disease is probable, or if radiation is refused by the patient/family. The AIEOP-BFM group suggests orchiectomy for patients with unilateral testicular relapse, with biopsy of the contralateral testis to confirm that there is no involvement before giving prophylactic radiotherapy (15 Gy).71 For patients with bilateral testicular involvement, either radiotherapy (24 Gy) or orchiectomy can be considered. Severe gonadal dysfunction and azoospermia are expected after 24 Gy of testicular radiation, but Leydig cell function can be preserved at 15 Gy to allow spontaneous pubertal development (Table 2).73 Alkylating agents with cumulative cyclophosphamide equivalent doses of ≥4000 mg/m2, testicular irradiation with ≥4 Gy, unilateral orchiectomy, and total body irradiation in HCT recipients are also associated with infertility (Table 2).43,44,74,75 Patients should be offered the option of fertility preservation through semen banking before testicular irradiation or orchiectomy. If patients are pre-pubertal, testicular tissue harvesting can be considered, although this is experimental and should be performed under a protocol approved by the institutional review board or research ethics committee.
To avoid the late effects on fertility and hormonal function resulting from testicular irradiation or orchiectomy, the Dutch Late Effects Study Group successfully treated five boys with late isolated testicular relapse with only systemic chemotherapy, including high-dose methotrexate (5 g/m2 to 12 g/m2).26 Encouraged by this study, the Children’s Oncology Group developed a study for patients with first isolated testicular relapse of B-ALL and an initial remission of at least 18 months. Patients were treated with intensive multi-agent chemotherapy without testicular irradiation if their testicular leukemia resolved (as indicated by resolution of enlargement or negative testicular biopsies) after induction therapy.27 Multi-agent chemotherapy included drugs known to penetrate the blood–testis barrier, such as high-dose methotrexate (5 g/m2, nine courses), cyclophosphamide (a maximum cumulative dose of 6.4 g/m2, including pre-relapse therapy), dexamethasone (10 mg/m2/day during induction, reinduction, and maintenance), vincristine, and high-dose cytarabine (3 g/m2, eight doses). At the end of induction, 26 of 40 patients had persistent testicular enlargement and 12 (30.0%) had confirmed residual leukemia by biopsy and were offered 24 Gy of bilateral testicular irradiation. The 5-year OS was 73.1% and did not differ between those patients who received bilateral testicular irradiation and those who did not. However, this study could not address the necessity of testicular irradiation for patients with T-ALL or for those with B-ALL and early relapse.
Rarely, isolated testicular relapse can occur after HCT for refractory or relapsed ALL. Although treatment options are limited in these cases, considering the previous high cumulative exposure of the patients to chemotherapy and radiation, some patients with very late relapses after HCT have been successfully treated with orchiectomy.76
Recently, chimeric antigen receptor–modified T (CAR-T) cells targeting B-cell antigens (e.g., CD19, CD22, or both) have shown efficacy in patients with relapsed/refractory B-ALL.77 Although CAR-T cells have mostly been used to treat bone marrow relapse, CAR-T therapy has been effective for CNS leukemia, and CAR-T cells have been observed beyond the blood–brain barrier. Similarly, the control of testicular relapse with CAR-T cells and their penetration of the blood–testis barrier have been reported.78-80 If other studies confirm CAR-T therapy to be effective for treating testicular relapse, this approach will be quite attractive, as CAR-T cells can control both medullary and extramedullary disease and are expected to have less gonadal toxicity. It has not yet been shown whether other immunotherapeutic approaches, such as blinatumomab and inotuzumab ozogamicin, or molecularly targeted agents are effective against testicular leukemia.
Testicular relapse in low- and middle-income countries
Although the treatment and survival rates for ALL have improved significantly in high-income countries, clinicians and patients in low- and middle-income countries face many obstacles to successful treatment, including late presentation/delayed diagnosis, coexisting debilitating conditions such as malnutrition and infections, suboptimal treatment adherence, abandonment of treatment, shortages of chemotherapeutic agents, inadequate supportive care, and a lack of specialists.81 Therefore, patients in low- and middle-income countries still experience testicular relapse. Salvage therapy is not always available, and when it is, salvage rates are dismal. A single institution study in India showed that the rates for isolated and any (isolated and combined) testicular relapse were similar for 93 boys treated from 1984 to 1986 (3.2% and 6.5%, respectively) and for 361 boys treated from 1986 to 1993 (3.3% and 6.1%).82,83 Another single-institution study in India reported 17 patients (4.2%) with isolated testicular relapses and 30 patients (7.4%) with any testicular relapse among 407 boys treated with a regimen based on the UKALLX study from 1990 to 2006.84 A salvage regimen (reinduction, testicular irradiation, and maintenance therapy) was given to 12 of the 17 boys with isolated testicular relapses but to only one of the 13 boys with combined relapses. At the Hue Central Hospital in Viet Nam, 156 children with ALL (including 93 boys) were treated with the CCG 1881 or CCG 1882 regimen from 2012 to 2018. Four patients (4.3%) developed any testicular relapses, including two (2.2%) with isolated testicular relapses.85 Neither study used intermediate- or high-dose methotrexate.
It has been shown that administration of high-dose methotrexate is feasible with extended hydration and leucovorin rescue, along with monitoring of serum creatinine and urine pH without measuring methotrexate levels.86 High-dose methotrexate (3 g/m2 for low-risk disease and 5 g/m2 for intermediate- and high-risk disease) was incorporated into the Shanghai Children’s Medical Center (SCMC) ALL-2005 protocol.87 At the SCMC, the 1085 patients (including 667 boys) treated between 2005 and 2014 had 5-year EFS and OS of 68.3% and 80.0%, respectively. The cumulative incidence of relapse was 24.5% at 10 years, and any and isolated testicular relapses were seen in 38 (5.7%) and 30 (4.5%), respectively, of the 667 boys. In addition, 165 of the 1085 patients (15.2%) experienced isolated bone marrow relapses and 29 (2.7%) had any CNS relapse. This suggests that merely incorporating high-dose methotrexate is insufficient.
It is important to develop centers of excellence and study groups with an adequate number of trained professionals who can adapt well-designed protocols to the local conditions.81,88 Twinning or global collaboration between centers or groups in low-/middle-income countries and those in high-income countries will facilitate the advancement of treatment. To overcome socioeconomic hardships, charitable organizations, philanthropists, and government agencies should be involved to enable better access to medical resources and patient/family support.88 In this regard, 20 major hospitals in the China Children’s Cancer Group, including the SCMC, collaborated with St. Jude to launch the ALL-2015 protocol.89 Between 2015 and 2019, 7640 patients (including 4521 boys) were enrolled and the 5-year EFS and OS were 80.3% and 91.1%, respectively. Of the 7640 patients, 658 (8.6%) experienced relapse, with 470 experiencing (6.2%) isolated bone marrow relapses and 136 (1.8%) experiencing any CNS relapse. Remarkably, any and isolated testicular relapses were limited to 46 (1.0%) and 27 (0.6%) of the 4521 boys, respectively. This excellent result validates the effectiveness of a comprehensive collaborative approach between low-/middle-income countries and high-income countries, leading to improved survival and a reduction in both medullary and extramedullary relapses.81
Conclusion
As a result of the introduction of improved systemic combination chemotherapy regimens that eradicate testicular leukemia by penetrating the blood–testis barrier, the incidence of testicular relapse has significantly decreased. Recent advances in immunotherapy and molecularly targeted therapy are expected to result in further improvements in the survival of patients with ALL and to decrease the intensity and toxicity of treatment with conventional chemotherapeutic agents. ALL is cured by controlling disease in the three major body compartments: the bone marrow, the CNS, and the testes. It is important to confirm that newer treatment strategies do not compromise the leukemia control in any of these compartments. Furthermore, successful treatment strategies employed in high-income countries need to be adapted for use in low- and middle-income countries through comprehensive collaborations so that all patients have a chance for cure regardless of their geographic location.
Supplementary Material
Acknowledgments
The authors thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript and Sue C. Kaste, DO, for providing ultrasound imaging. Figure 3 was created with BioRender.com.
Funding
This work was supported by the National Cancer Institute (Cancer Center Support CORE Grant CA21765 to M.A.T., D.M.G, C.H.P, and H.I.); and by the American Lebanese Syrian Associated Charities (ALSAC) to M.A.T., D.M.G, C.H.P, and H.I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
All authors have no conflicting interests to declare.
References
- 1.Pui CH, Nichols KE, Yang JJ. Somatic and germline genomics in paediatric acute lymphoblastic leukaemia. Nat Rev Clin Oncol. 2019;16:227–240. [DOI] [PubMed] [Google Scholar]
- 2.Riccardi R, Vigersky RA, Barnes S, Bleyer WA, Poplack DG. Methotrexate levels in the interstitial space and seminiferous tubule of rat testis. Cancer Res. 1982;42:1617–1619. [PubMed] [Google Scholar]
- 3.Childhood ALL Collaborative Group. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Lancet. 1996;347:1783–1788. [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Ribeiro RC, Mahmoud HH, et al. Overt testicular disease at diagnosis is associated with high risk features and a poor prognosis in patients with childhood acute lymphoblastic leukemia. Cancer. 1996;78:2437–2442. [PubMed] [Google Scholar]
- 5.Freeman AI, Boyett JM, Glicksman AS, et al. Intermediate-dose methotrexate versus cranial irradiation in childhood acute lymphoblastic leukemia: a ten-year follow-up. Med Pediatr Oncol. 1997;28:98–107. [DOI] [PubMed] [Google Scholar]
- 6.Hijiya N, Liu W, Sandlund JT, et al. Overt testicular disease at diagnosis of childhood acute lymphoblastic leukemia: lack of therapeutic role of local irradiation. Leukemia. 2005;19:1399–1403. [DOI] [PubMed] [Google Scholar]
- 7.Sirvent N, Suciu S, Bertrand Y, Uyttebroeck A, Lescoeur B, Otten J. Overt testicular disease (OTD) at diagnosis is not associated with a poor prognosis in childhood acute lymphoblastic leukemia: results of the EORTC CLG Study 58881. Pediatr Blood Cancer. 2007;49:344–348. [DOI] [PubMed] [Google Scholar]
- 8.Hamm B Differential diagnosis of scrotal masses by ultrasound. Eur Radiol. 1997;7:668–679. [DOI] [PubMed] [Google Scholar]
- 9.Kim TH, Hargreaves HK, Brynes RK, et al. Pretreatment testicular biopsy in childhood acute lymphocytic leukaemia. Lancet. 1981;2:657–658. [DOI] [PubMed] [Google Scholar]
- 10.Nesbit ME, Sather H, Robison LL, et al. Sanctuary therapy: a randomized trial of 724 children with previously untreated acute lymphoblastic leukemia: a report from Children's Cancer Study Group. Cancer Res. 1982;42:674–680. [PubMed] [Google Scholar]
- 11.Bleyer WA, Sather HN, Nickerson HJ, et al. Monthly pulses of vincristine and prednisone prevent bone marrow and testicular relapse in low-risk childhood acute lymphoblastic leukemia: a report of the CCG-161 study by the Childrens Cancer Study Group. J Clin Oncol. 1991;9:1012–1021. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Aur RJ, Bowman WP, et al. Failure of late intensification therapy to improve a poor result in childhood lymphoblastic leukemia. Cancer Res. 1984;44:3593–3598. [PubMed] [Google Scholar]
- 13.Pui CH, Dahl GV, Bowman WP, et al. Elective testicular biopsy during chemotherapy for childhood leukaemia is of no clinical value. Lancet. 1985;2:410–412. [DOI] [PubMed] [Google Scholar]
- 14.Testicular disease in acute lymphoblastic leukaemia in childhood. Report on behalf of the Medical Research Council's Working Party on leukaemia in childhood. Br Med J. 1978;1:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eden OB, Lilleyman JS, Richards S, on behalf of the Medical Research Council Working Party on Leukaemia in Childhood. Testicular irradiation in childhood lymphoblastic leukaemia. Br J Haematol. 1990;75:496–498. [DOI] [PubMed] [Google Scholar]
- 16.Miller DR, Leikin SL, Albo VC, Palmer NF, Sather HN, Hammond GD. The prognostic value of testicular biopsy in childhood acute lymphoblastic leukemia: a report from the Childrens Cancer Study Group. J Clin Oncol. 1990;8:57–66. [DOI] [PubMed] [Google Scholar]
- 17.Nachman J, Palmer NF, Sather HN, et al. Open-wedge testicular biopsy in childhood acute lymphoblastic leukemia after two years of maintenance therapy: diagnostic accuracy and influence on outcome—a report from Children's Cancer Study Group. Blood. 1990;75:1051–1055. [PubMed] [Google Scholar]
- 18.Mahoney DHJ, Gonzales ET, Ferry GD, Sanjad SA, von Noorden GK, Fernbach DJ. Childhood acute leukemia: a search for occult extramedullary disease prior to discontinuation of chemotherapy. Cancer. 1981;48:1964–1966. [DOI] [PubMed] [Google Scholar]
- 19.Ortega JJ, Javier G, Torán N. Testicular infiltrates in children with acute lymphoblastic leukemia: a prospective study. Med Pediatr Oncol. 1984;12:386–393. [DOI] [PubMed] [Google Scholar]
- 20.Wong KY, Ballard ET, Strayer FH, Kisker CT, Lampkin BC. Clinical and occult testicular leukemia in long-term survivors of acute lymphoblastic leukemia. J Pediatr. 1980;96:569–574. [DOI] [PubMed] [Google Scholar]
- 21.Ding LW, Sun QY, Mayakonda A, et al. Mutational profiling of acute lymphoblastic leukemia with testicular relapse. J Hematol Oncol. 2017;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzoneva G, Perez-Garcia A, Carpenter Z, et al. Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat Med. 2013;19:368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahnukainen K, Salmi TT, Kristinsson J, Müller J, Madsen B, Gustafsson G. The clinical indications for identical pathogenesis of isolated and non-isolated testicular relapses in acute lymphoblastic leukaemia. Acta Paediatr. 1998;87:638–643. [DOI] [PubMed] [Google Scholar]
- 25.Kuo TT, Tschang TP, Chu JY. Testicular relapse in childhood acute lymphocytic leukemia during bone marrow remission. Cancer. 1976;38:2604–2612. [DOI] [PubMed] [Google Scholar]
- 26.van den Berg H, Langeveld NE, Veenhof CH, Behrendt H. Treatment of isolated testicular recurrence of acute lymphoblastic leukemia without radiotherapy. Report from the Dutch Late Effects Study Group. Cancer. 1997;79:2257–2262. [DOI] [PubMed] [Google Scholar]
- 27.Barredo JC, Hastings C, Lu X, et al. Isolated late testicular relapse of B-cell acute lymphoblastic leukemia treated with intensive systemic chemotherapy and response-based testicular radiation: a Children's Oncology Group study. Pediatr Blood Cancer. 2018;65:e26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrest JB, Turner TT, Howards SS. Cyclophosphamide, vincristine, and the blood testis barrier. Invest Urol. 1981;18:443–444. [PubMed] [Google Scholar]
- 29.Van Eys J, Sullivan MP. Letter: Testicular leukaemia and temperature. Lancet. 1976;2:256–257. [PubMed] [Google Scholar]
- 30.Jahnukainen K, Saari T, Morris ID, Salmi TT, Pöllänen P. Regulation of testicular infiltration in acute lymphoblastic leukaemia of the rat. Leukemia. 1994;8:458–464. [PubMed] [Google Scholar]
- 31.Cordon-Cardo C, O'Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur G, Mital P, Dufour JM. Testisimmune privilege—assumptions versus facts. Anim Reprod. 2013;10:3–15. [PMC free article] [PubMed] [Google Scholar]
- 33.Gröbner SN, Worst BC, Weischenfeldt J, et al. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321–327. [DOI] [PubMed] [Google Scholar]
- 34.Curran EK, Godfrey J, Kline J. Mechanisms of immune tolerance in leukemia and lymphoma. Trends Immunol. 2017;38:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahnukainen K, Morris I, Roe S, Salmi TT, Mäkipernaa A, Pöllänen P. A rodent model for testicular involvement in acute lymphoblastic leukaemia. Br J Cancer. 1993;67:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul C, Robaire B. Impaired function of the blood-testis barrier during aging is preceded by a decline in cell adhesion proteins and GTPases. PLoS One. 2013;8:e84354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dördelmann M, Reiter A, Zimmermann M, et al. Intermediate dose methotrexate is as effective as high dose methotrexate in preventing isolated testicular relapse in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1998;20:444–450. [DOI] [PubMed] [Google Scholar]
- 38.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the Children's Cancer Group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group report. Leukemia. 2010;24:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011;118:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–2112. [DOI] [PubMed] [Google Scholar]
- 41.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the Pediatric Oncology Group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the Children's Oncology Group. Leukemia. 2010;24:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzari C, Lanvers-Kaminsky C, Valsecchi MG, et al. Asparagine levels in the cerebrospinal fluid of children with acute lymphoblastic leukemia treated with pegylated-asparaginase in the induction phase of the AIEOP-BFM ALL 2009 study. Haematologica. 2019;104:1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chemaitilly W, Liu Q, van Iersel L, et al. Leydig cell function in male survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2019;37:3018–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conter V, Aricò M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. [DOI] [PubMed] [Google Scholar]
- 46.Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. [DOI] [PubMed] [Google Scholar]
- 47.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, Fiocco M, Bierings M, Pieters R. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–319. [DOI] [PubMed] [Google Scholar]
- 48.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000). Leukemia. 2010;24:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuchida M, Ohara A, Manabe A, et al. Long-term results of Tokyo Children's Cancer Study Group trials for childhood acute lymphoblastic leukemia, 1984–1999. Leukemia. 2010;24:383–396. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell C, Richards S, Harrison CJ, Eden T. Long-term follow-up of the United Kingdom Medical Research Council protocols for childhood acute lymphoblastic leukaemia, 1980–2001. Leukemia. 2010;24:406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–354. [DOI] [PubMed] [Google Scholar]
- 53.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404). Blood. 2011;118:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tower RL, Jones TL, Camitta BM, et al. Dose intensification of methotrexate and cytarabine during intensified continuation chemotherapy for high-risk B-precursor acute lymphoblastic leukemia: POG 9406: a report from the Children's Oncology Group. J Pediatr Hematol Oncol. 2014;36:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato M, Koh K, Manabe A, et al. No impact of high-dose cytarabine and asparaginase as early intensification with intermediate-risk paediatric acute lymphoblastic leukaemia: results of randomized trial TCCSG study L99-15. Br J Haematol. 2014;164:376–383. [DOI] [PubMed] [Google Scholar]
- 56.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children's Oncology Group study AALL0232. J Clin Oncol. 2016;34:2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter SS, Dunsmore KP, Devidas M, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children's Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36:2926–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34:2591–2601. [DOI] [PubMed] [Google Scholar]
- 59.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. [DOI] [PubMed] [Google Scholar]
- 61.Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–615. [DOI] [PubMed] [Google Scholar]
- 62.Yeoh AEJ, Ariffin H, Chai ELL, et al. Minimal residual disease–guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30:2384–2392. [DOI] [PubMed] [Google Scholar]
- 63.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeha S, Pei D, Choi J, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37:3377–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi H, Kajiwara R, Kato M, et al. Treatment outcome of children with acute lymphoblastic leukemia: the Tokyo Children's Cancer Study Group (TCCSG) Study L04-16. Int J Hematol. 2018;108:98–108. [DOI] [PubMed] [Google Scholar]
- 66.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14:199–209. [DOI] [PubMed] [Google Scholar]
- 67.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–818. [DOI] [PubMed] [Google Scholar]
- 68.Teachey DT, Hunger SP, Loh ML. Optimizing therapy in the modern age: differences in length of maintenance therapy in acute lymphoblastic leukemia. Blood. 2021;137:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagedorn N, Acquaviva C, Fronkova E, et al. Submicroscopic bone marrow involvement in isolated extramedullary relapses in childhood acute lymphoblastic leukemia: a more precise definition of "isolated" and its possible clinical implications, a collaborative study of the Resistant Disease Committee of the International BFM study group. Blood. 2007;110:4022–4029. [DOI] [PubMed] [Google Scholar]
- 70.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120:2807–2816. [DOI] [PubMed] [Google Scholar]
- 72.Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136:1803–1812. [DOI] [PubMed] [Google Scholar]
- 73.Castillo LA, Craft AW, Kernahan J, Evans RG, Aynsley-Green A. Gonadal function after 12-Gy testicular irradiation in childhood acute lymphoblastic leukaemia. Med Pediatr Oncol. 1990;18:185–189. [DOI] [PubMed] [Google Scholar]
- 74.Wasilewski-Masker K, Seidel KD, Leisenring W, et al. Male infertility in long-term survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2014;8:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borgmann-Staudt A, Rendtorff R, Reinmuth S, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transplant. 2012;47:271–276. [DOI] [PubMed] [Google Scholar]
- 76.Bhadri VA, McGregor MR, Venn NC, et al. Isolated testicular relapse after allo-SCT in boys with ALL: outcome without second transplant. Bone Marrow Transplant. 2010;45:397–399. [DOI] [PubMed] [Google Scholar]
- 77.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu J, Hu Y, Pu C, et al. Successful chimeric Ag receptor modified T cell therapy for isolated testicular relapse after hematopoietic cell transplantation in an acute lymphoblastic leukemia patient. Bone Marrow Transplant. 2017;52:1065–1067. [DOI] [PubMed] [Google Scholar]
- 79.Chen X, Wang Y, Ruan M, et al. Treatment of testicular relapse of B-cell acute lymphoblastic leukemia with CD19-specific chimeric antigen receptor T cells. Clin Lymphoma Myeloma Leuk. 2020;20:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubinstein JD, Krupski C, Nelson AS, O'Brien MM, Davies SM, Phillips CL. Chimeric antigen receptor T cell therapy in patients with multiply relapsed or refractory extramedullary leukemia. Biol Blood Marrow Transplant. 2020;26:e280–e285. [DOI] [PubMed] [Google Scholar]
- 81.Pui CH, Yang JJ, Bhakta N, Rodriguez-Galindo C. Global efforts toward the cure of childhood acute lymphoblastic leukaemia. Lancet Child Adolesc Health. 2018;2:440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Advani SH, Iyer RS, Pai SK, et al. Four-agent induction/consolidation therapy for childhood acute lymphoblastic leukemia: an Indian experience. Am J Hematol. 1992;39:242–248. [DOI] [PubMed] [Google Scholar]
- 83.Advani S, Pai S, Venzon D, et al. Acute lymphoblastic leukemia in India: an analysis of prognostic factors using a single treatment regimen. Ann Oncol. 1999;10:167–176. [DOI] [PubMed] [Google Scholar]
- 84.Kulkarni KP, Marwaha RK, Trehan A, Bansal D. Testicular relapse in childhood acute lymphoblastic leukemia: the challenges and lessons. Indian J Cancer. 2010;47:134–138. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen H, Tran K, Chau V, Watanabe K. Relapse analysis of childhood acute lymphoblastic leukaemia at Hue Central Hospital in Vietnam. Pediatr Blood Cancer. 2018;65:e27455.30240102 [Google Scholar]
- 86.Vaishnavi K, Bansal D, Trehan A, Jain R, Attri SV. Improving the safety of high-dose methotrexate for children with hematologic cancers in settings without access to MTX levels using extended hydration and additional leucovorin. Pediatr Blood Cancer. 2018;65:e27241. [DOI] [PubMed] [Google Scholar]
- 87.Shen S, Cai J, Chen J, et al. Long-term results of the risk-stratified treatment of childhood acute lymphoblastic leukemia in China. Hematol Oncol. 2018;36:679–688. [DOI] [PubMed] [Google Scholar]
- 88.Tang JY, Pui CH. The international collaboration to save children with cancer. JAMA Oncol. 2021. doi: 10.1001/jamaoncol.2020.6187. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 89.Tang J, Yu J, Cai J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. 2021. doi: 10.1182/blood.2020010438. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.