Abstract
Lichen simplex chronicus is a form of chronic localized pruritus with a secondary dermatitis, and one of the most common types of chronic itch conditions, estimated to affect more than 10% of the general population. However, despite its prevalence and burden, there has been limited research into the pathogenesis and aetiology of lichen simplex chronicus, which, historically, made it a challenging condition to treat. In recent years, our understanding of this condition, along with that of pruritus and the itch-scratch cycle, has increased greatly, enabling a substantial increase in treatment options. In addition, there are several new promising treatments currently in development and trials. This article discusses the definition, epidemiology, clinical characteristics, pathophysiology, and current therapeutic options for lichen simplex chronicus, in order to highlight recent advancements in this field.
Key words: itch, lichen simplex chronicus, physiopathology, pruritus
Chronic itch is a highly burdensome disease with severe implications for the health and quality of life of affected individuals. Chronic itch conditions can be as debilitating as chronic pain conditions, and lead to mood disturbances and loss of sleep (1). Of chronic itch conditions, lichen simplex chronicus (LSC) is one of the most common; in some populations it is even the most common dermatological condition resulting in consultation (2). LSC is characterized by thick, scaly plaques caused by constant manipulation of an area with intractable, chronic itch (3).
Although LSC is usually a non-life-threatening condition, the severe pruritus associated with the condition can severely impact patients’ well-being (1). Adequate treatment of itch is paramount to the management of LSC. In addition, frequent scratching can cause increased skin barrier disruption and the release of inflammatory mediators, leading to sensory dysfunction and activation of itch-scratch cycles, further exacerbating itch and causing more scratching (3). Constant scratching not only leads to more severe LSC, but may also cause infection and, in rare cases, malignant transformation (4). Evaluation of the underlying causes of pruritus in LSC is also important to detect diseases that may otherwise be hidden. A variety of diseases can present with localized pruritus, including neurological, psychiatric and malignant diseases (1). Therefore, understanding LSC, its underlying pathophysiology, and treatment is necessary for the everyday practice of many clinicians.
SIGNIFICANCE
Lichen simplex chronicus is a common chronic itchy skin condition that seriously affects the quality of life in many people and is characterized by symmetrically thickened skin from constant scratching. Recent breakthroughs in research have identified potential causes of lichen simplex chronicus and expanded our treatment options for this condition. Therefore, a review on the latest findings is needed to maximize the clinical benefits for patients. Here, we update readers with this comprehensive review.
DEFINITION, EPIDEMIOLOGY, AND CLINICAL CHARACTERISTICS
LSC is a localized skin disorder clinically characterized by lichenified plaques of skin often accompanied by overlying excoriations. These plaques can become discoloured, with varying shades of erythema ranging from pink to dark brown. Over a longer course, it may transform into a hypopigmented plaque with a darker border. Rather than a diffuse distribution, they are localized to specific areas of the body as 1 or a few skin plaques that are frequently manipulated (Fig. 1) (4). This is in contrast to prurigo nodularis (PN), another chronic pruritic condition, and secondary dermatitis, which is frequently more broadly distributed across multiple regions of the body as nodules. While LSC may sometimes be referred to as a neurodermatitis, which encompasses other chronic itchy conditions, such as PN, care should be taken when referring to it with this terminology, as neurodermatitis may also refer to atopic dermatitis, a separate condition with a different aetiology.
Fig. 1.
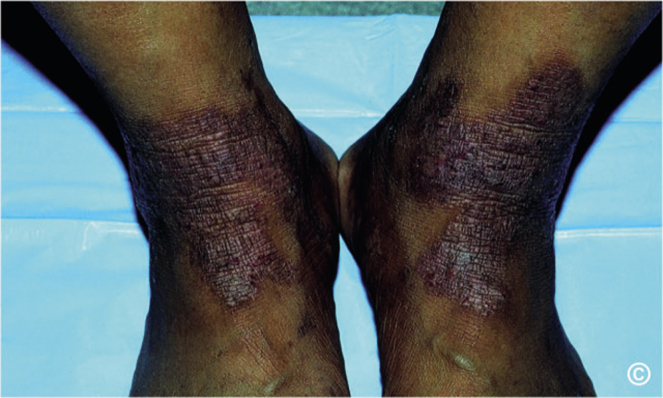
Bilateral lichen simplex chronicus plaques on the flexural surfaces of the feet.
LSC is a highly prevalent condition, affecting an estimated 12% of the general population, and is particularly prevalent in middle-aged patients between the ages of 30 and 50 years (4). Women are more frequently affected than men, with a 2-to-1 ratio (4). The condition is also common in Asia, particularly in elderly patients (2). Those with a family or personal history of atopy may be more susceptible to LSC. Approximately 20–90% of people affected by LSC report a personal or immediate family history of atopic dermatitis, allergic rhinitis, and/or asthma (5). Psychosocial stress is also linked to severely pruritic dermatoses such as LSC, and patients demonstrate a significantly higher prevalence of psychosomatic and psychiatric comorbidities, such as clinical depression and anxiety. LSC often presents in high achievers with stressful and competitive lifestyles (6).
As a secondary dermatitis, LSC is caused by mechanical manipulation of an intractable itch leading to a thickening and scaling of the skin. Since it is induced by habitual scratching and rubbing, LSC is often found in areas of the body that are self-accessible, such as the ankles, shins, elbows, dorsal hands, upper back, neck and anogenital regions. Lesions are often symmetrically distributed, particularly on the extensor surfaces of the extremities (4).
A study on the scratch pleasurability of various pruritic dermatological conditions found that LSC has one of the highest ratings for pleasure from scratching, behind only notalgia paraesthetica. In addition, higher scratch pleasurability scores were found to correspond with areas where LSC is frequently present. Areas such as the scrotum and vulva, where scratch pleasurability were found to be high, are a common location for LSC, while areas such as the scalp, where scratch pleasurability was found to be low, is rarely involved in LSC, with the exception of the posterior scalp (7).
The histopathology of LSC shows a hyperkeratotic plaque, sometimes with foci of parakeratosis, a prominent and thickened granular layer, marked acanthosis, elongated and thick rete ridges, pseudoepitheliomatosus hyperplasia, papillary dermal fibrosis and mild spongiosis. Examination of the superficial dermis may reveal vertically oriented, thickened collagen bundles with perivascular and interstitial inflammation characterized by histiocytes, lymphocytes and eosinophils in the superficial dermis (Fig. 2) (4).
Fig. 2.
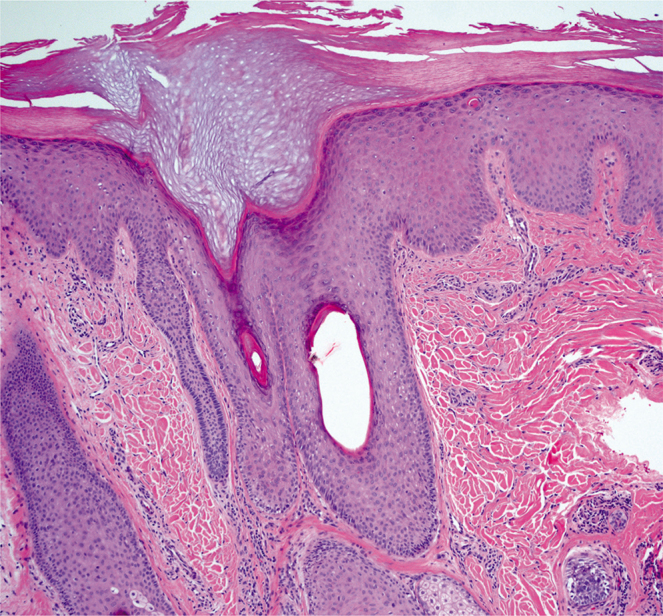
Lichen simplex chronicus of the posterior scalp shows irregular acanthosis with hypergranulosis and pronounced hyperkeratosis reminiscent of the acral skin. There is an increased number of fibroblasts in the upper dermis, and mild perivascular lymphocytic infiltrate (haematoxylin and eosin, ×10). Credit to and permission obtained to publish from Dr Mariya Miteva.
PATHOPHYSIOLOGY
While the exact pathophysiology of LSC is unknown, LSC has been thought of as a chronic process, resulting from primary psychological or environmental factors or secondary to other dermatoses evoking itch and leading to a vigorous itch-scratch cycle (Fig. 3). Pruritogens are any molecules that, when introduced into the skin, induce itching. These pruritogenic molecules bind to their respective receptors on C-nerve fibres, leading to neuronal activation and transmission of an itch signal to the dorsal root ganglion and spinal cord. Overall, pruritus can be classified as histaminergic (often associated with acute itch) and non-histaminergic (often associated with chronic itch), with each having their own neuronal pathway. The particular pruritogens implicated in LSC have yet to be identified; however, because LSC is a chronic process, the pathophysiology is thought to be mainly non-histaminergic itch mediated by binding of pruritogens to G-protein coupled receptors (GPCR) and/or ion channels, especially transient receptor potential (TRP) channels. TRPs may respond to capsaicin and to temperature. Two calcium-permeable ion channels expressed in sensory nerve fibres, transient receptor potential vanilloid 1 (TRPV1) and ankyrin-1 (TRPA1), were discovered to play a significant role in the interaction with pruritogens and nociceptive stimuli, and may even be necessary in T-cell mediated IL-31-induced itch (8). TRPA1 acts as the downstream molecule to various histamine-independent, chronic itch pathways. A study analysing 21 biopsies from patients with LSC and 28 healthy controls demonstrated significantly down-regulated expression of TRPA1 in LSC lesions, indicating that TRPA1 may have a role in pathogenesis of the disease (9).
Fig. 3.
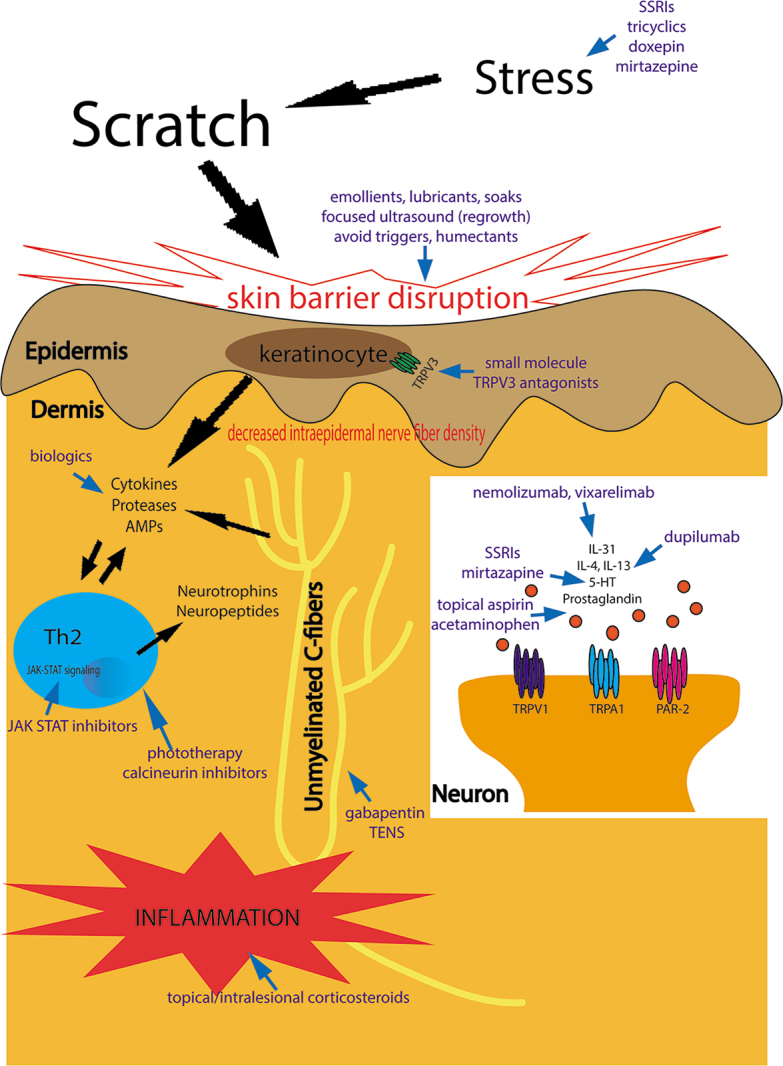
Pathophysiology of lichen simplex chronicus and its therapeutic targets. AMPs: antimicrobial peptides; JAK-STAT: janus kinase-signal transducer and activator of transcription; IL: interleukin; PAR-2: protease-activated receptor 2; SSRIs: selective serotonin reuptake inhibitors; TENS: transcutaneous electrical nerve stimulation; Th: T-helper cell; TRPV: transient receptor potential vanilloid; 5-HT: serotonin.
TRPV3 are warm temperature-sensitive channels, which are expressed abundantly in keratinocytes. They are associated with itch signalling via protease-activated receptor-2 (PAR-2), a GPCR receptor found in sensory nerve fibres and keratinocytes (10). TRPV3 and PAR-2 are key players in other pruritic conditions including atopic dermatitis. Activation of TRPV3 coupled with PAR-2 leads to the release of several cytokines and chemokines (11), while inhibition results in reduced inflammation and itch attenuation (10). LSC pathophysiology is likely to involve some aspect of these TRPV channels and GPCRs.
In addition, increasing evidence suggests that LSC involves neuroimmune interactions. Skin is a richly innervated organ, and where nerve fibres contact cells of the immune system, there are opportunities for localized neuroimmune interaction, especially through the release of neurotrophins and neuropeptides from immune cells. During times of stress and chronic inflammation, neurotrophin levels and the number of neuropeptidergic nerve fibres in the skin as well as their contacts with immune cells usually increase (12). This response results in cutaneous inflammation and development of pruritus. This may correspond to the higher rates of LSC in patients with heightened levels of stress, such as high achievers (6). Several studies have demonstrated increased levels of the neurotrophins nerve growth factor (NGF) or brain-derived neurotrophic factor (BDNF) in atopic dermatitis and uraemic patients with pruritus (13). However, a recent study of 36 patients showed decreased levels of serum neurotrophin-3 (NT-3), NGF, glial cell line-derived neurotrophic factor (GDNF), and BDNF in patients with LSC compared with healthy controls, although levels were not correlated with disease severity (14). Another study of 33 patients with LSC demonstrated a significant reduction in intraepidermal nerve fibre density in sample tissue from the lesion site compared with a contralateral control site (15). This was associated with reduced sensitivity to warm and cool stimuli. These injured fibres in the epidermis were postulated to become abnormally sensitive and develop pathological spontaneous activity perceived as itch (15). Chronic scratching could then further exacerbate epidermal denervation. Sandoval et al. (15) showed that the small fibre neuropathy in these patients with LSC can be improved with lidocaine plasters, either through reduced transmission of action potentials or decreased neurogenic inflammation, although inflammatory mediators were not significantly altered.
Scratching temporarily relieves the uncomfortable itch sensation by activating pain-sensory fibres that inhibit itch at the level of the spinal cord. However, scratching can lead to a pathological process, known as the itch-scratch cycle. Scratching causes epithelial damage, resulting in the release of cytokines, proteases, and antimicrobial peptide, which activate immune cells and stimulate itch sensory neurones and channels, as mentioned above. This leads to a vicious circle of itch and scratch (3).
DIAGNOSTIC APPROACH
As with most dermatological conditions, a thorough patient history is essential to establishing a diagnosis of LSC. A full dermatological examination should be conducted to exclude purely primary inflammatory dermatoses and assess for lesions that are secondary to scratching, such as the characteristic solitary lichenified plaques. A biopsy could be helpful in distinguishing LSC from other conditions with similar clinical presentations, such as hypertrophic lichen planus, psoriasiform rashes, contact dermatitis, squamous cell carcinoma, and mycosis fungoides. The intensity of the pruritus may be evaluated with subjective scales, such as the visual analogue scale. As a secondary dermatitis, understanding the origin of itch in LSC, whether psychological, environmental, or secondary to other dermatoses, may be highly useful in the treatment of the condition.
THERAPEUTICS
There are numerous therapeutic approaches to managing LSC (Table I). Treatments are often targeted towards identifying and treating the underlying disease, repairing the barrier layer function, reducing inflammation, and breaking the itch-scratch cycle. However, treatment for LSC can be challenging, as we have only recently begun to understand its pathophysiology.
Table I.
Recommended treatments for lichen simplex chronicus based on disease severity
| Mild disease | Moderate disease | Severe disease |
|---|---|---|
| Emollients | Gabapentinoids (gabapentin) | Nemolizumab |
| Topical calcineurin inhibitors | Antidepressants (SSRI, TCAs i.e. imipramine) | Dupilumab |
| High-potency topical steroids | For night-time itch: Mirtazapine, sedating antihistamines | Janus kinase inhibitors (i.e. tofacitinib) |
| Combination of topical steroids and salicylates | Topical acetaminophen | Transcutaneous electrical nerve stimulation |
| Menthol | Cyclosporine | Focused ultrasound (in vulvar areas) |
| Pramoxine | Methotrexate | |
| Topical doxepin | Narrow-band ultraviolet B | |
| Topical aspirin with dichloromethane | ||
| Topical ketamine/amitriptyline/lidocaine |
SSRI: selective serotonin uptake inhibitors; TCA: tricyclic antidepressants.
During the initial evaluation, clinicians should consider and address any potential non-dermatological aetiologies of LSC (1). To repair the barrier layer function to reduce the exposure of nerve endings, patients should be advised to avoid any triggering factors, such as heat or irritants, and to wear looser, cotton blend clothing to avoid exacerbating their disease. Emollients with ceramides, soaks, lubricants such as sitz baths and use of wetted towelling should be encouraged. Low pH emollients have been shown to reduce the activation of proteases. Emollients containing supplementary treatment, such as N-palmitoylethanolamine, or with anaesthetic properties, such as polidocanol, have been shown to improve chronic itch in clinical studies. Emollients containing pilodocanol, in particular, have been found to reduce non-histaminergic itch in double-blind, placebo-controlled clinical trials (16). Humectants, such as lactic acid, glycolic acid and urea, are not recommended as they can irritate the skin.
Reducing inflammation is another cornerstone to the treatment of LSC. High-potency topical corticosteroids are usually well-tolerated for short-term use and should be considered first-line in treating LSC. Occlusive dressings may be used in conjunction with topical steroids; however, few studies evaluating their efficacy exist. A combination of topical steroids and salicylates also may be used for treatment (4). Depending on the severity of the disease, intralesional steroids, such as triamcinolone acetonide, may also be considered (17). Other non-steroidal options include topical immunomodulators, such as tacrolimus and pimecrolimus, which block the release of inflammatory pruritic cytokines from T lymphocytes in the skin and promote cutaneous host defences. These topical immunomodulators avoid the adverse effects of long-term steroid use, such as atrophy, irreversible striae, and telangiectasia, and should be used in more sensitive areas, such as the vulva (18). However, they can cause transient burning sensation, which should be communicated to patients (19).
As LSC is typically the result of habitual scratching; hence, breaking the itch-scratch cycle is paramount to treatment. Occlusion, often used in conjunction with pharmacological treatment, such as corticosteroids, can provide a physical barrier that prevents further scratching, improves skin barrier damage and reduce pruritogenic stimuli on top of enhancing drug absorption. Some commonly employed occlusions in LSC are occlusive plastic film and hydrocolloid dressings (20). Menthol, which causes a cooling sensation via activation of TRP channels, and pramoxine, a local anaesthetic, can be used to control pruritus in these patients (17, 21). It should be noted that capsaicin, despite being a popular over-the-counter anti-pruritic, has not been found to be efficacious for the treatment of LSC pruritus (17). Topical doxepin as an adjuvant therapy to topical steroids and topical aspirin with dicloromethane have also been shown to be effective in several studies (22, 23). Topical analgesic mixture of ketamine-amitriptyline-lidocaine (KAL) in a lipoderm base has been found to be efficacious in treating patients with chronic pruritus, including those with prurigo nodularis. It is theorized to work on c-fibre nerves through blockage of N-methyl-D-aspartate receptor and sodium channels (24). Topical acetaminophen, which acts along the arachidonic pathway and has metabolites that activate and sensitize TRPV channels, may ameliorate chronic itch (25). More recently, Janus kinase (JAK) inhibitors, such as topical tofacitinib, have also been found to reduce pruritus in patients with LSC and PN in a small case series (26).
As LSC is usually localized, topical treatments are often employed. This contrasts with similar, but more broadly distributed, dermatological conditions, such as PN. However, systemic treatments should be considered if topical treatments fail. One study reported 5 patients with severe LSC who responded to the anticonvulsant gabapentin, a neuroleptic agent that reduces central neural hypersensitization, although its exact anti-pruritic mechanism is unclear (27). While aprepitant and naltrexone have been reported to ameliorate other chronic pruritic diseases, research is needed to determine whether these agents will be valuable in treating patients with LSC (28). Because of the association between LSC and stress, anxiety and depression, some clinicians suggest a potential benefit of anti-depressants in patients with comorbid anxiety or depression. Daytime scratching can be managed with selective serotonin reuptake inhibitors (SSRIs) and night-time scratching with sedating antihistamines and tricyclics to help induce sleep (29). Mirtazapine also reduces night-time itch in LSC and helps with sleep (30). A double-blind randomized control trial found that treatment with chlorpheniramine or imipramine significantly reduced itch severity in patients with LSC (31). Methotrexate and cyclosporine have been shown to be effective in PN, and are likely to be effective in LSC, although there are no well-known studies of their efficacy in LSC (32, 33).
A number of small studies have reported successful treatment of LSC with non-traditional therapies. Many of these have focused on the neuroimmune aspects of the disease. One study that included 26 patients with LSC found that targeted narrowband UVB (NB-UVB) phototherapy was safe and effective, especially in patients who were unresponsive to topical treatments. Mean pruritus scores improved by 75% (34). Transcutaneous electrical nerve stimulation (TENS), another non-traditional treatment, ameliorates itch by sending pulsed electrical currents to inhibit nociceptive A delta and C fibres. When used in 22 patients with LSC unresponsive to topical corticosteroids, TENS effectively reduced itching by greater than 50% in 80% of patients (35). Focused ultrasound therapy for the treatment of vulva can also promote reconstruction and growth of tissue affected by LSC. A retrospective observational study showed 41 out of 85 patients achieved complete resolution of itch and restoration of skin elasticity (36).
There are several novel therapies that are promising treatments for LSC, which may be employed in more intractable, severe forms of LSC. Nemolizumab, a monoclonal antibody targeting the IL-31 receptor, was found to be effective in reducing pruritus and severity of skin lesions in PN in a randomized clinical trial of 70 patients (37). Several clinical trials on the efficacy of other biologic therapies, such as dupilumab for PN, are currently underway and may be applicable to LSC (38). In addition, a current unpublished phase 2 clinical trial on the efficacy of vixarelimab, a fully-human monoclonal antibody that targets the oncostatin M receptor beta subunit (OSMRβ) of the IL-31 receptor, shows promising initial data on the treatment of chronic pruritus, including within a small sample of patients with LSC (39). Given the association of TRPV3 with itch signalling in keratinocytes, a novel topical small molecular inhibitor of TRPV3 channel (KM001) is currently undergoing phase 2 trials for LSC (40).
Overall, studies on existing and novel treatment options for LSC are limited. A recent review of LSC treatments found only 9 randomized clinical trials in the literature that included more than 25 patients (33). Thus, more robust and innovative research on therapeutic options for LSC is needed.
Conclusion
Despite being a highly common and troublesome disease, historically, little was known about LSC and its underlying mechanisms. Traditionally thought of as a secondary dermatitis resulting from chronic scratching in pruritic conditions, much of our understanding of LSC is rooted in our understanding of itch and its pathogenesis, which has seen many recent advances in the field. However, our understanding of LSC and its treatment options are still limited. Further research is needed to evaluate and provide a more comprehensive picture of this intractable, challenging disease, which affects many people worldwide.
ACKNOWLEDGEMENTS
Conflicts of interest: GY has served as an advisory board member, investigator and/or received consulting fees from Galderma, Pfizer, Sanofi Regeneron, Novartis, Eli Lilly, Kiniksa, Vellus and Cell Dex.
REFERENCES
- 1.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013; 368: 1625–1634. [DOI] [PubMed] [Google Scholar]
- 2.Tianco EA, Buendia-Teodosio G, Alberto NL. Survey of skin lesions in the Filipino elderly. Int J Dermatol 1992; 31: 196–198. [DOI] [PubMed] [Google Scholar]
- 3.Mack MR, Kim BS. The itch-scratch cycle: a neuroimmune perspective. Trends Immunol 2018; 39: 980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charifa A, Badri T, Harris BW. Lichen simplex chronicus. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 5.Crone AM, Stewart EJ, Wojnarowska F, Powell SM. Aetiological factors in vulvar dermatitis. J Eur Acad Dermatol Venereol 2000; 14: 181–186. [DOI] [PubMed] [Google Scholar]
- 6.Leow Y-H, Yosipovitch G. Pruritus in lichen simplex chronicus and lichen amyloidosis. Basic Clin Dermatol 2004; 27: 255–258. [Google Scholar]
- 7.Golpanian RS, Fourzali K, Fowler E, Kursewicz CD, Lipman Z, Chan YH, et al. The pleasurability of scratching an itch amongst different pruritic conditions. Acta Derm Venereol 2020; 100: adv00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014; 133: 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu Y, Tang N, Zhang W, Xiong JX, Hu L, Cai T. Down-regulated expression of transient receptor potential ankyrin 1 in lichen simplex chronicus. Ann Palliat Med 2020; 9: 3757–3765. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Munanairi A, Liu XY, Zhang J, Hu L, Hu M, et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J Invest Dermatol 2020; 140: 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013; 155: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui S, Xiao T, Wang Y, Lu H, Wang Y, Gao XH, et al. Morphological relationship between nerve fibers and Langerhans cells in the epidermis of psoriasis vulgaris and lichen simplex chronicus. J Dermatol Sci 2009; 56: 132–134. [DOI] [PubMed] [Google Scholar]
- 13.Sorour NE, Elesawy FM, Tabl HA, Ibrahim ME, Akl EM. Evaluation of serum levels of neurotrophin 4 and brain-derived nerve growth factor in uremic pruritus patients. Clin Cosmet Investig Dermatol 2019; 12: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altunay İ K, Özkur E, Uğurer E, Baltan E, Aydın Ç, Serin E. More than a skin disease: stress, depression, anxiety levels, and serum neurotrophins in lichen simplex chronicus. An Bras Dermatol 2021; 96: 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval M, Parra J, Reyna-Jeldes M, Curi-Tuma M, Espinoza F, Muñoz D, et al. Itch in lichen simplex chronicus is associated with localized small fiber neuropathy. J Invest Dermatol 2022; 142: 731–735.e3. [DOI] [PubMed] [Google Scholar]
- 16.Yosipovitch G, Misery L, Proksch E, Metz M, Ständer S, Schmelz M. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol 2019; 99: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 17.Burgin S. Chapter 15. Nummular eczema, lichen simplex chronicus, and prurigo nodularis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick’s dermatology in general medicine, 8th edn. New York, NY: The McGraw-Hill Companies; 2012. [Google Scholar]
- 18.Goldstein AT, Thaçi D, Luger T. Topical calcineurin inhibitors for the treatment of vulvar dermatoses. Eur J Obstet Gynecol Reprod Biol 2009; 146: 22–29. [DOI] [PubMed] [Google Scholar]
- 19.Remitz A, Harper J, Rustin M, Goldschmidt WF, Palatsi R, van der Valk PG, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. Acta Derm Venereol 2007; 87: 54–61. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I. Treatment of skin disease e-book: comprehensive therapeutic strategies. Elsevier Health Sciences, Amsterdam; 2013. [Google Scholar]
- 21.Greaves MW. Recent advances in pathophysiology and current management of itch. Ann Acad Med Singap 2007; 36: 788–792. [PubMed] [Google Scholar]
- 22.Drake LA, Millikan LE. The antipruritic effect of 5% doxepin cream in patients with eczematous dermatitis. Doxepin Study Group. Arch Dermatol 1995; 131: 1403–1408. [PubMed] [Google Scholar]
- 23.Yosipovitch G, Sugeng MW, Chan YH, Goon A, Ngim S, Goh CL. The effect of topically applied aspirin on localized circumscribed neurodermatitis. J Am Acad Dermatol 2001; 45: 910–913. [DOI] [PubMed] [Google Scholar]
- 24.Lee HG, Grossman SK, Valdes-Rodriguez R, Berenato F, Korbutov J, Chan YH, et al. Topical ketamine-amitriptylinelidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol 2017; 76: 760–761. [DOI] [PubMed] [Google Scholar]
- 25.Nattkemper LA, Zhi K, Romero KE, Shah SM, Ju T, Fourzali K, et al. Antipruritic effect of topical acetaminophen gel in histaminergic and non-histaminergic itch provocation: a double-blind, vehicle-controlled pilot study. Acta Derm Venereol 2022; 102: adv00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju T, Labib A, Vander Does A, Yosipovitch G. Topical Janus kinase-signal transducers and activators of transcription inhibitor tofacitinib is effective in reducing nonatopic dermatitis chronic itch: a case series. J Am Acad Dermatol 2022; 87: 400–403. [DOI] [PubMed] [Google Scholar]
- 27.Gencoglan G, Inanir I, Gunduz K. Therapeutic hotline: treatment of prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther 2010; 23: 194–198. [DOI] [PubMed] [Google Scholar]
- 28.More on mu-opioid receptor antagonists in PD-1 blockade–induced pruritus. N Engl J Med 2019; 380: 601–602. [DOI] [PubMed] [Google Scholar]
- 29.Lynch PJ. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatol Ther 2004; 17: 8–19. [DOI] [PubMed] [Google Scholar]
- 30.Hundley JL, Yosipovitch G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: a pilot study. J Am Acad Dermatol 2004; 50: 889–891. [DOI] [PubMed] [Google Scholar]
- 31.Sanjana V, Fernandez R. Evaluation of an antihistamine and an antidepressant for the treatment of lichen simplex chronicus. Ind J Dermatol Venereol Leprol 1992; 58: 384–387. [Google Scholar]
- 32.Qureshi AA, Abate LE, Yosipovitch G, Friedman AJ. A systematic review of evidence-based treatments for prurigo nodularis. J Am Acad Dermatol 2019; 80: 756–764. [DOI] [PubMed] [Google Scholar]
- 33.Juarez MC, Kwatra SG. A systematic review of evidence based treatments for lichen simplex chronicus. J Dermatolog Treat 2021; 32: 684–692. [DOI] [PubMed] [Google Scholar]
- 34.Esen Salman K, Kıvanç Altunay İ, Salman A. The efficacy and safety of targeted narrowband UVB therapy: a retrospective cohort study. Turk J Med Sci 2019; 49: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engin B, Tufekci O, Yazici A, Ozdemir M. The effect of trans-cutaneous electrical nerve stimulation in the treatment of lichen simplex: a prospective study. Clin Exp Dermatol 2009; 34: 324–328. [DOI] [PubMed] [Google Scholar]
- 36.Wu C, Zou M, Xiong Y, Wang L, Chen H, Fan Y, et al. Short-and long-term efficacy of focused ultrasound therapy for non-neoplastic epithelial disorders of the vulva. BJOG 2017; 124: 87–92. [DOI] [PubMed] [Google Scholar]
- 37.Ständer S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med 2020; 382: 706–716. [DOI] [PubMed] [Google Scholar]
- 38.Study of Dupilumab for the Treatment of Patients With Prurigo Nodularis, Inadequately Controlled on Topical Prescription Therapies or When Those Therapies Are Not Advisable (LIBERTY-PN PRIME). ClinicalTrials.gov identifier: NCT 04183335. Updated February 17, 2022. [Accessed April 3, 2022] Available from https://clinicaltrials.gov/ct2/show/NCT04183335.
- 39.A Study to Assess the Efficacy, Safety, and Tolerability of KPL-716 in Reducing Pruritus in Chronic Pruritic Diseases. ClinicalTrials.gov identifier: NCT03858634. Updated December 28, 2021. [Accessed April 3, 2022] Available from https://ClinicalTrials.gov/show/NCT03858634.
- 40.Kamari Pharma L, Bioskin Gmb H. KM-001 Cream for Treatment of Pruritus in Adult Patients With Lichen Simplex Chronicus (LSC). ClinicalTrials.gov identifier: NCT05454462. Updated July 12, 2022. [Accessed July 13, 2022] Available from https://clinicaltrials.gov/ct2/show/NCT05454462.


