Abstract
Objective:
Radiation-induced lung injury (RILI) is one of the serious complications of radiotherapy. We have recently demonstrated that nicaraven can effectively mitigate RILI in healthy mice. Here, we further tried to optimize the dose and time of nicaraven administration for alleviating the side effects of radiotherapy in tumor-bearing mice.
Methods and results:
A subcutaneous tumor model was established in the back of the chest in C57BL/6N mice by injecting Lewis lung cancer cells. Therapeutic thoracic irradiations were done, and placebo or different doses of nicaraven (20, 50, 100 mg/kg) were administrated intraperitoneally pre-irradiation (at almost 5–10 min before irradiation) or post-irradiation (within 5 min after irradiation). Mice that received radiotherapy and nicaraven were sacrificed on the 30th day, but control mice were sacrificed on the 15th day. Serum and lung tissues were collected for evaluation. Nicaraven significantly decreased the level of CCL8, but did not clearly change the levels of 8-OHdG, TGF-β, IL-1β, and IL-6 in serum. Besides these, nicaraven effectively decreased the levels of TGF-β, IL-1β, and SOD2 in the lungs, especially by post-irradiation administration with the dose of 20 mg/kg. Although there was no significant difference, the expression of SOD1, 53BP1, and caspase 3 was detected lower in the lungs of mice received nicaraven post-irradiation than that of pre-irradiation.
Conclusion:
According to our data, the administration of nicaraven at a relatively low dose soon after radiotherapy will be recommended for attenuating the side effects of radiotherapy.
Keywords: DNA damage, fibrosis, inflammatory response, lung injury, radiation
Introduction
Radiotherapy is an essential tool for the treatment of intrathoracic malignancies, including lung, breast, and esophageal cancers.1,2 Exposure of healthy tissues to radiation and the toxicity it causes often limits its effectiveness and decreases the survival benefit of radiotherapy. Beyond the systemic side effects, radiation-induced lung injury (RILI) is a serious obstacle to patients receiving radiotherapy for thoracic malignant tumors.3,4 RILI occurs in 5–20% of lung cancer patients receiving radiotherapy, which may lead to the discontinuation of treatment. However, there are still no effective drugs and protective strategies to prevent radiation side effects in cancer patients undergoing radiotherapy.
It is well known that ionizing radiation induces directly DNA double-strand breaks and triggers the release of ROS.5 The level of ROS overwhelms can cause oxidative damage to DNA, lipids, and proteins.6 Although radiotherapy is a local therapy, it has systemic effects mainly influencing immune and inflammation processes.7 Moreover, it has been demonstrated that radiation-induced injuries to tissue cells can promote the release of a multitude of inflammatory cytokines and chemokines, which indirectly contribute to the consequent damage to cells and tissues and eventually culminate in fibrotic changes.8–10 Therefore, the scavenging of ROS and the suppression of the inflammatory response are thought to be potential pharmacological interventions for mitigating the side effects of radiotherapy.
Many past studies have challenged to develop radioprotective agents. Thiol-synthetic compounds, such as amifostine has been approved to use clinically for protecting against radiation injury, however, amifostine has the disadvantages of toxicity and limited route of administration in the clinic.11 Nitrogen oxides, such as Tempol, have also been tested as a radioprotectant, but its application is limited due to problems on producing hypotension and increasing heart rate.12 Some natural antioxidants, such as vitamin E and selenium have also shown radioprotective effects, but the benefit of antioxidants for cancer radiotherapy is asked to be further confirmed because of the probable effect on radiosensitivity of cancer cells.12,13 Therefore, there is still required to develop an ideal agent for mitigating the side effects of radiotherapy for cancer patients.
Nicaraven, a chemically synthesized hydroxyl radical-specific scavenger,14 has previously been reported to protect against radiation-induced cell death.14,15 Nicaraven can also reduce the radiation-induced recruitment of macrophages and neutrophils into irradiated lungs.16 Moreover, we have recently demonstrated that nicaraven can also effectively protect against RILI by suppressing the inflammatory response.17 To further develop for clinical application, we herein aim to optimize the dose and time of nicaraven administration for attenuating the side effects of radiotherapy.
Using a preclinical tumor-bearing mice model, we administered different doses of nicaraven, before or soon after thoracic irradiations. We then evaluated the systemic side effects and RILI, mainly by focusing on oxidative stress and inflammatory responses. According to our experimental data, the administration of nicaraven at a relatively low dose soon after radiotherapy will be recommended for attenuating the side effects of radiotherapy.
Materials and methods
Cancer cells and animals
Mouse Lewis lung cancer (LLC) cells were used for the experiments. The cells were maintained in DMEM (FUJIFILM Wako Pure Chemical Corporation), supplemented with 10% fetal bovine serum (Cytiva) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.), and cultured at 37°C in a humidified incubator with 5% CO2.
Male C57BL/6N mice (8 weeks old) were used for the study. Mice were housed in a pathogen-free room with a controlled environment under a 12-h light-dark cycle and maintained on laboratory chow, with free access to food and water. This study was approved by the Institutional Animal Care and Use Committee of Nagasaki University (No.1608251335-12). All animal procedures were performed in accordance with institutional and national guidelines.
Tumor-bearing mouse model, radiotherapy, and nicaraven administration
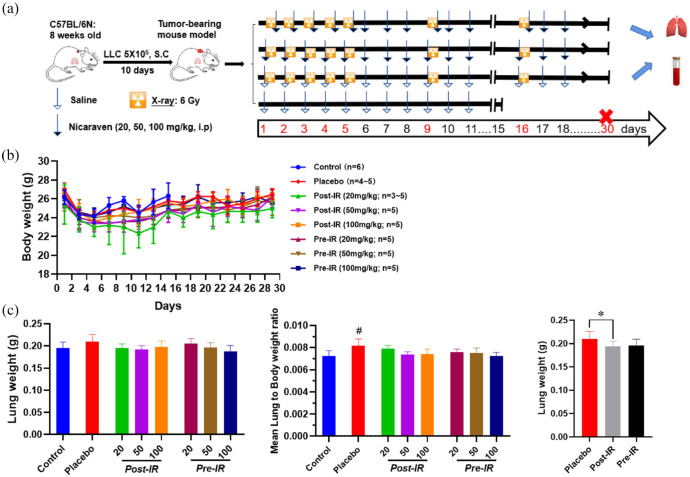
To match the pathological status of cancer patients, we used a preclinical tumor-bearing model for the experiment. Briefly, mice were subcutaneously inoculated with 5 × 105 LLC cells/0.1 ml of saline in the back of the chest. At 10 days after cancer cell inoculation, mice had randomly received radiotherapy and nicaraven administration as indicated in Figure 1(a). Considering the common clinical radiotherapy regimen for lung cancer and breast cancer,18,19 thoracic irradiations (including the heart and lungs) were delivered to mice at a dosage rate of 1.0084 Gy/min (200 kV, 15 mA, 5 mm Al filtration, ISOVOLT TITAN320, General Electric Company, United States). Mice were intraperitoneally injected with 0 (placebo), 20, 50, 100 mg/kg nicaraven pre-irradiation (almost 5–10 min before irradiation) or post-irradiation (within 5 min after irradiation), respectively. Six mice without irradiation exposure were used as control (n = 6, Control group).
Figure 1.
Changes of body weight and lung weight in mice. (a) Schematic diagram about the experimental timeline and protocol. (b) Quantitative data on the changes of body weights through 30 days. (c) Quantitative data on the changes of lung weight and lung to body weight ratio in mice. Data are represented as the means ± SD, n = 3~6 in per group. #p < 0.05 versus Control group, *p < 0.05 versus Placebo group.
IR, irradiation; Post-IR, post-irradiation; Pre-IR, pre-irradiation.
We measured the body weights of mice every other day. Mice that received radiotherapy and nicaraven were sacrificed on the 30th day, but the control mice were sacrificed on the 15th day. To collect serum, we took the blood from the inferior vena cava of mice under general anesthesia before sacrifice. Lung tissues were then excised and weighed. The collected serum and lung tissue samples were stored under −80°C, and used for experimental evaluations as follows.
ELISA
We measured the concentrations of 8-oxo-29-deoxyguanosine (8-OHdG), a marker of DNA oxidation in serum using an ELISA kit (Nikken SEIL Corporation, Shizuoka, Japan) according to the manufacturer’s instructions. The mean values of duplicate assays with each sample were used for the statistical analyses.
ELISA kits (R&D Systems) were used to detect the contents of transforming growth factor β (TGF-β), interleukin-1beta (IL-1β), interleukin-6 (IL-6), C-C Motif Chemokine Ligand 8 (CCL8) in serum and lung tissues according to the manufacturer’s instructions. Briefly, the lung tissues were homogenized using Multi-beads shocker® and added to the T-PER reagent (Thermo Fisher Scientific) consisting of proteinase and dephosphorylation inhibitors (Thermo Fisher Scientific). Then, lung lysates and serum were added to each well and measured per the manufacturer’s instructions. The optical density of each well was measured at 450 nm using a microplate reader (Multiskan Fc, Thermo Fisher Scientific).
Western blot
Western blot was performed as previously described.20 Briefly, total protein from the lung tissues was separated by SDS-PAGE gels and then transferred to 0.22-μm PVDF membranes (Bio-Rad). After blocking, the membranes were incubated with primary antibodies against SOD1 (1:500 dilution; cat. no. sc11407; Santa Cruz), SOD2 (1:500 dilution; cat. no. sc30080; Santa Cruz), β-actin (1:1,000 dilution; cat. no. 8457S; CST), 53BP1 (1:1,000 dilution; cat. no. ab36823; Abcam), caspase 3 (1:1,000 dilution; cat. no. 9662; CST), α-SMA (1:1,000 dilution; cat. no. 19245S; CST), collagen Ⅰ (1:1,000 dilution; cat. no. ab34710; Abcam), or α-Tubulin (1:1,000 dilution; cat. no. 3873S; CST) overnight at 4°C, respectively, followed by the appropriate horseradish peroxidase-conjugated secondary antibodies (Dako). The expression was visualized using an enhanced chemiluminescence detection kit (Thermo Scientific). Semiquantitative analysis was done using ImageQuant LAS 4000 mini (GE Healthcare Life Sciences).
Statistical analysis
All the values were presented as the mean ± SD. For comparison of multiple sets of data, one-way analysis of variance (ANOVA) followed by Tukey’s test (Dr. SPSS II, Chicago, IL) was used for statistical analyses. All analyses were carried out with the SPSS19.0 statistical software (IBM SPSS Co., USA). A p-value less than 0.05 was accepted as significant.
Results
Nicaraven for mitigating the systemic side effects of radiotherapy is not very clearly detectable in tumor-bearing mice under our experimental treatment regimens
Mice had well tolerated the therapeutic regimens, but two mice in the post-irradiation administration with the dose of 20 mL/kg group died on the 9th and 10th days, and one mouse in the placebo group died on the 19th day during the follow-period (Figure 1(a)). Thoracic irradiation was delivered to mice using lead shielding sheets, and we found the death of mice at the next morning after radiation exposure. Radiation exposure to the brain stem may happen even only 1-mm positioning error. As a single 6 Gy exposure to the brain stem can kill some mice,21 we speculated the death of mice should be an error exposure to the brain due to some positioning or shadowing problems of mice during thoracic exposure. The body weights of mice in all groups were decreased temporarily during radiotherapy, but tended to increase a few days after the stopping of irradiation exposures (Figure 1(b)). There was no significant difference on the body weight changes of mice among groups. Compared with the control mice, the lung weights were slightly increased in mice received placebo treatment after radiotherapy. However, the lung weights showed significantly lower in mice received nicaraven post-irradiation than that of placebo treatment (p < 0.05, Figure 1(c)).
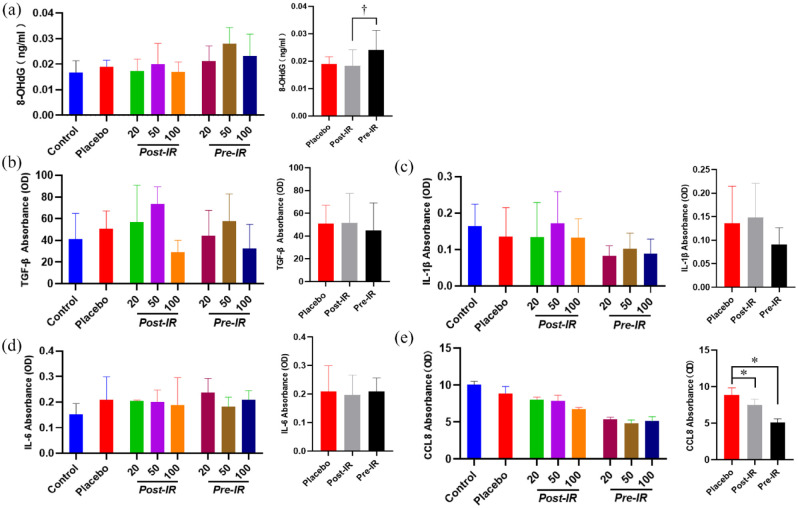
ELISA was performed to detect the levels of 8-OHdG, TGF-β, IL-1β, IL-6, CCL8 in serum. The level of 8-OHdG in serum was not significantly different among groups (Figure 2(a)). However, the serum level of 8-OHdG was detected significantly lower in mice received nicaraven post-irradiation than that of pre-irradiation (p < 0.05, Figure 2(a)). Our results also showed that the administration of nicaraven with any dose before or after irradiation did not clearly change the levels of TGF-β, IL-1β, IL-6 in serum. However, the level of CCL8 in serum was significantly lower in mice received nicaraven either post-irradiation or pre-irradiation when compared with mice received placebo treatment (p < 0.05, Figure 2(e)).
Figure 2.
The effect of nicaraven on levels of 8-OHdG and inflammatory factors in serum.
Quantitative data on the levels of 8-OHdG (a), TGF-β (b), IL-1β (c), IL-6 (d), CCL8 (e). Data are represented as the means ± SD, n = 3~6 in per group. *p < 0.05 versus Placebo group, †p < 0.05 versus post-IR group.
IR: irradiation; Post-IR: post-irradiation; Pre-IR: pre-irradiation.
The administration of nicaraven at a relatively low dose after radiotherapy shows partial attenuation of RILI in a preclinical tumor-bearing mouse model
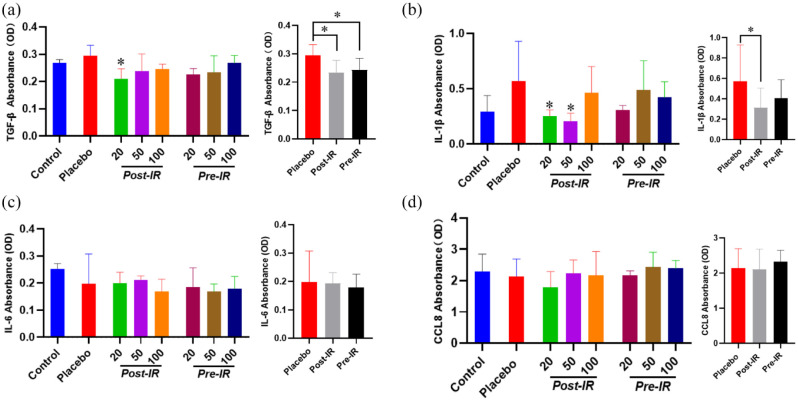
To evaluate the inflammatory responses in lungs, ELISA analysis indicated that the TGF-β level in lungs was slightly increased in mice received placebo treatment after thoracic radiation, but was effectively attenuated by post-irradiation administration with 20 mg/kg nicaraven (p < 0.05, Figure 3(a)). Similarly, the IL-1β level in the lungs was also increased in mice that received placebo treatment after thoracic radiation, but significantly decreased by post-irradiation administration with 20 or 50 mg/kg nicaraven (p < 0.05, Figure 3(b)). Strangely, it seems that post-irradiation administration with relatively lower doses of nicaraven more effectively alleviates the enhancement of TGF-β and IL-1β in lung tissues. However, the levels of IL-6 and CCL8 in the lungs were not significantly different among all groups (Figure 3(c), (d)).
Figure 3.
ELISA analysis on the inflammatory response in irradiated lungs. Quantitative data on the levels of TGF-β (a), IL-1β (b), IL-6 (c), CCL8 (d) in irradiated lung tissues were shown. Data are represented as the means ± SD, n = 3~6 in per group. *p < 0.05 versus Placebo group.
IR: irradiation; Post-IR: post-irradiation; Pre-IR: pre-irradiation.
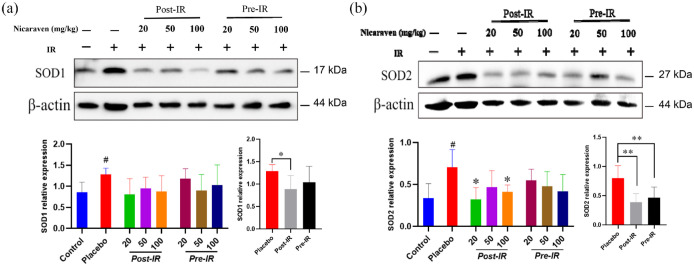
The expression of SOD1 in lungs was detected higher by Western blot in the placebo group than the control group (p < 0.05, Figure 4(a)). Although there was no significant difference among all groups (Figure 4(a)), the increased expression of SOD1 in the lungs was effectively attenuated by post-irradiation administration (p < 0.05, Figure 4(a)). The expression of SOD2 was also significantly increased in the placebo group, but the increased expression of SOD2 was clearly decreased by post-irradiation administration with 20 or 100 mg/kg nicaraven (p < 0.05, Figure 4(b)). The enhanced expression of SOD2 in the lungs was effectively decreased by either post-irradiation or pre-irradiation administration (p < 0.01, Figure 4(b)).
Figure 4.
Nicaraven on attenuating oxidative response in irradiated lungs. Representative blots (up), and quantitative data (down) on the expression of SOD1 (a), SOD2 (b). Data are represented as the means ± SD, n = 3~6 in per group. #p < 0.05 versus Control group, *p < 0.05, **p < 0.01 versus Placebo group.
IR: irradiation; Post-IR: post-irradiation; Pre-IR: pre-irradiation.
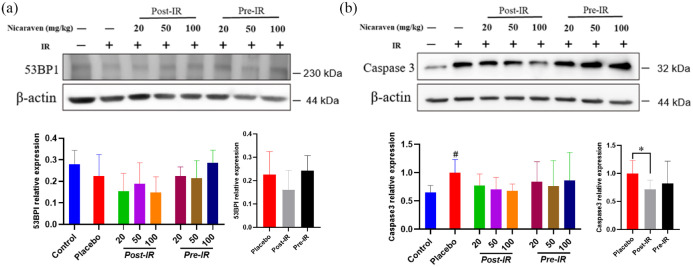
We also measured the expression of 53BP1, a marker for DNA damage in lungs by Western blot. Post-irradiation administration of nicaraven showed to slightly decrease the 53BP1 expression in lungs (Figure 5(a)). Caspase 3 has been considered a key effector in inducing cell apoptosis. Compared with the control group without irradiation, Western blot analysis showed a significant enhancement on the expression of caspase 3 in lungs of mice from the placebo group (p < 0.05, Figure 5(b)), but the enhanced expression of caspase 3 in irradiated lungs was effectively attenuated only by post-irradiation administration of nicaraven (p < 0.05, Figure 5(b)).
Figure 5.
Western blot analysis on the expression of the DNA damage and cell apoptosis in irradiated lungs. Representative blots (up) and quantitative data (down) on the expression of 53BP1 (a), caspase 3 (b). Data are normalized to β-actin. Data are represented as the means ± SD, n = 3~6 in per group. #p < 0.05 versus Control group, *p < 0.05 versus Placebo group.
IR: irradiation; Post-IR: post-irradiation; Pre-IR: pre-irradiation.
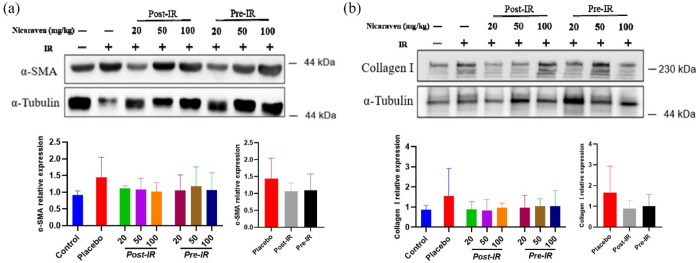
We finally investigated the expression of α-SMA and collagen Ⅰ, the common markers of fibrosis in lungs. Compared with the control group, Western blot analysis showed higher expression of α-SMA and collagen Ⅰ in lungs of mice from the placebo group (Figure 6). Although there was no significant difference among groups, the enhanced expression of α-SMA and collagen Ⅰ in irradiated lungs was partially attenuated in mice that received post-irradiation administration of nicaraven (Figure 6).
Figure 6.
The fibrotic changes in irradiated lungs.
Representative blots (up) and quantitative data (down) on the expression of α-SMA (a), collagen Ⅰ (b) were shown. Data are normalized to α-Tubulin. Data are represented as the means ± SD, n = 3~6 in per group.
IR: irradiation; Post-IR: post-irradiation; Pre-IR: pre-irradiation.
Discussion
Radiotherapy for cancer is known to accompany side effects, which may lead to the discontinuation of treatment and decrease the quality of life of patients.22 In this study, we investigated the optimal dose and time of nicaraven administration for attenuating the side effects of radiotherapy in tumor-bearing mice. We could not clearly detect significant changes on body weight and the levels of inflammatory cytokines in serum. However, nicaraven administration, especially with a relatively lower dose at the time soon after thoracic irradiations partially decreased the levels of TGF-β, IL-1β, SOD1, SOD2, and caspase 3 in lungs, suggesting the effectiveness of nicaraven for attenuating the side effects of radiotherapy.
Nicaraven has been well recognized on radical-specific scavenging property.14 The 8-OHdG, an oxidized nucleoside of DNA has been frequently used as a marker for detecting oxidative stress.23 However, consistent with our previous study,24 nicaraven administration did not effectively decrease the level of 8-OHdG in serum in this study.
Radiation exposure results in the release of pro-inflammatory cytokines and chemokines.8–10 Radiation-induced systemic and local inflammatory responses can be detected in the blood by an increased level of circulatory cytokines and the activation of immune cells.7,25 However, we found that nicaraven administration did not significantly change the levels of TGF-β, IL-1β, and IL-6 in serum. Several reasons can be considered for it. First, the sample number in each group was too small to detect a statistical significance. Second, the initial sizes of tumors were widely varied among animals and groups, which also resulted in large variations in measuring data. Third, we collected lungs and serum at 14 days after the last irradiation, which will be not a suitable time window for sensitive detection about the changes of 8-OHdG and inflammatory factors in serum. Otherwise, tumor-secreted factors might also be considered to affect the levels of cytokines and chemokines in serum,26 but we did not find significant correlations between tumor weights and the levels of systemic inflammatory factors (data not shown).
Increasing evidence has shown that the release of a multitude of cytokines in response to radiation exposure can contribute to the damage to the cells/tissues.22 Previous studies have demonstrated that RILI could be alleviated by blocking pro-inflammatory factors.27,28 In this study, we observed that nicaraven partially attenuated the enhanced expression of TGF-β and IL-1β in the lungs, especially by post-irradiation administration with relatively low doses. We have not yet found a clear reason why post-irradiation administration with relatively lower doses of nicaraven even more effectively alleviates the enhancement of TGF-β and IL-1β in the irradiated lungs. Previous studies have well documented the harmless of nicaraven at the dose of 100 mg/kg in mice.29 As VEGF level may increase in tumor-bearing mice, it is a possibility that a high dose of nicaraven increases the permeability of alveolar capillaries and cause edema of lungs in these tumor-bearing mice.
SOD1 and SOD2 are antioxidant enzymes, but their expression generally increases in response to oxidative stresses and various types of injuries.6 Nicaraven partially attenuated the enhanced expression of SOD1 and SOD2 in irradiated lungs, especially by post-irradiation administration. Radiation can directly lead to cell death and apoptosis.4,17 Nicaraven also partially decreased the expression of 53BP1 and caspase 3 in irradiated lungs, especially by post-irradiation administration. The main manifestations of the late stage of RILI are fibroblast proliferation and collagen deposition.25 Post-irradiation administration of nicaraven partially decreased the expression of α-SMA and collagen Ⅰ in irradiated lungs.
This study has several limitations. First, we did not perform histopathological analysis on lungs. Second, we only used male mice for the experiment, but gender difference may affect radiation-induced outcomes.30,31 Third, we could not provide data on the 8-OHdG level in lungs because of our technical mistake. Otherwise, due to the small sample size and large individual variation, there was no statistically significance on the expression of α-SMA and collagen I in lungs among groups. Although we have already planned a phase I-II clinical trial in esophageal cancer patients who receiving radiotherapy, the benefit of nicaraven administration will be needed to be further confirmed before clinical application for cancer radiotherapy.
According to our experimental evaluations in a preclinical tumor-bearing mouse model, nicaraven seems to effectively attenuate the side effects of radiotherapy. As nicaraven has a very limited effect on the growth of established tumors,16 nicaraven may be useful for mitigating the side effects of radiotherapy in cancer patients, and post-irradiation administration with a relatively low dose will be highly recommended.
Acknowledgments
None.
Footnotes
ORCID iD: Tao-Sheng Li  https://orcid.org/0000-0002-7653-8873
https://orcid.org/0000-0002-7653-8873
Contributor Information
Yong Xu, Department of Stem Cell Biology, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan; Department of Stem Cell Biology, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan.
Lina Abdelghany, Department of Stem Cell Biology, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan; Department of Stem Cell Biology, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan.
Reiko Sekiya, Department of Stem Cell Biology, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan.
Da Zhai, Department of Stem Cell Biology, Atomic Bomb Disease Institute, Nagasaki University, Nagasaki, Japan; Department of Stem Cell Biology, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan.
Keiichi Jingu, Department of Radiation Oncology, Graduate School of Medicine, Tohoku University, Sendai, Japan.
Tao-Sheng Li, Department of Stem Cell Biology, Atomic Bomb Disease Institute, Nagasaki University, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan; Department of Stem Cell Biology, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan.
Declarations
Ethics approval and consent to participate: The animal experiments were approved by the Institutional Animal Care and Use Committee of Nagasaki University (Approval no.1608251335-12) and all animal procedures were performed in accordance with institutional and national guidelines.
Consent for publication: The authors give their consent for publication.
Author contributions: Yong Xu: Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Lina Abdelghany: Data curation; Investigation; Writing – review & editing.
Reiko Sekiya: Data curation; Investigation.
Da Zhai: Data curation; Writing – review & editing.
Keiichi Jingu: Conceptualization.
Tao-Sheng Li: Conceptualization; Data curation; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was mainly supported by the Japan Agency for Medical Research and Development (JP20lm0203081), a Grant-in-Aid from the Ministry of Education, Science, Sports, Culture and Technology, Japan. The funder played no role in the study design, data collection, and analysis, decision to publish, or manuscript preparation.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Giuranno L, Ient J, De Ruysscher D, et al. Radiation-induced lung injury (RILI). Front Oncol 2019; 9: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim H, Park SH, Han SY, et al. LXA4-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-β/Smad signaling. Cell Death Dis 2020; 11: 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bickelhaupt S, Erbel C, Timke C, et al. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J Natl Cancer Inst 2017; 109: 8. [DOI] [PubMed] [Google Scholar]
- 4. McBride WH, Schaue D. Radiation-induced tissue damage and response. J Pathol 2020; 250: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang L, Snyder AR, Morgan WF. Radiation-induced genomic instability and its implications for radiation carcinogenesis. Oncogene 2003; 22: 5848–5854. [DOI] [PubMed] [Google Scholar]
- 6. Nakane M. Biological effects of the oxygen molecule in critically ill patients. J Intensive Care 2020; 8: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009; 10: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin H, Yoo Y, Kim Y, et al. Radiation-induced lung fibrosis: preclinical animal models and therapeutic strategies. Cancers (Basel) 2020; 12: 1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kainthola A, Haritwal T, Tiwari M, et al. Immunological aspect of radiation-induced pneumonitis, current treatment strategies, and future prospects. Front Immunol 2017; 8: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Im J, Lawrence J, Seelig D, et al. FoxM1-dependent RAD51 and BRCA2 signaling protects idiopathic pulmonary fibrosis fibroblasts from radiation-induced cell death. Cell Death Dis 2018; 9: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol 2009; 85: 539–573. [DOI] [PubMed] [Google Scholar]
- 12. Hahn SM, Krishna MC, DeLuca AM, et al. Evaluation of the hydroxylamine Tempol-H as an in vivo radioprotector. Free Radic Biol Med 2000; 28: 953–958. [DOI] [PubMed] [Google Scholar]
- 13. Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today 2007; 12: 794–805. [DOI] [PubMed] [Google Scholar]
- 14. Akimoto T. Quantitative analysis of the kinetic constant of the reaction of N, Nʹ-propylenedini-cotinamide with the hydroxyl radical using dimethyl sulfoxide and deduction of its structure in chloroform. Chem Pharm Bull (Tokyo) 2000; 48: 467–476. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe M, Akiyama N, Sekine H, et al. Inhibition of poly (ADP-ribose) polymerase as a protective effect of nicaraven in ionizing radiation- and ara-C-induced cell death. Anticancer Res 2006; 26(5A): 3421–3427. [PubMed] [Google Scholar]
- 16. Yan C, Luo L, Urata Y, et al. Nicaraven reduces cancer metastasis to irradiated lungs by decreasing CCL8 and macrophage recruitment. Cancer Lett 2018; 418: 204–210. [DOI] [PubMed] [Google Scholar]
- 17. Xu Y, Zhai D, Goto S, et al. Nicaraven mitigates radiation-induced lung injury by downregulating the NF-κB and TGF-β/Smad pathways to suppress the inflammatory response. J Radiat Res 2022; 2022; rrab112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kepka L, Socha J. Dose and fractionation schedules in radiotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2021; 10: 1969–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah BA, Xiao J, Oh C, et al. Five-fraction prone accelerated partial breast irradiation: long-term oncologic, dosimetric, and cosmetic outcome. Pract Radiat Oncol 2022; 12: 106–112. [DOI] [PubMed] [Google Scholar]
- 20. Doi H, Kitajima Y, Luo L, et al. Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep 2016; 6: 18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang L, Yang J, Li G, et al. Pathophysiological responses in rat and mouse models of radiation-induced brain injury. Mol Neurobiol 2017; 54: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanania AN, Mainwaring W, Ghebre YT, et al. Radiation-induced lung injury: assessment and management. Chest 2019; 156: 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2ʹ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009; 27: 120–139. [DOI] [PubMed] [Google Scholar]
- 24. Kawakatsu M, Urata Y, Imai R, et al. Nicaraven attenuates radiation-induced injury in hematopoietic stem/progenitor cells in mice. PLoS ONE 2013; 8: e60023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mavragani IV, Laskaratou DA, Frey B, et al. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol Res (Camb) 2015; 5: 12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh M, Tian XJ, Donnenberg VS, et al. Targeting the temporal dynamics of hypoxia-induced tumor-secreted factors halts tumor migration. Cancer Res 2019; 79: 2962–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W, Lu L, Liu B, et al. Effects of phycocyanin on pulmonary and gut microbiota in a radiation-induced pulmonary fibrosis model. Biomed Pharmacother 2020; 132: 110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen B, Na F, Yang H, et al. Ethyl pyruvate alleviates radiation-induced lung injury in mice. Biomed Pharmacother 2017; 92: 468–478. [DOI] [PubMed] [Google Scholar]
- 29. Zhang X, Moriwaki T, Kawabata T, et al. Nicaraven attenuates postoperative systemic inflammatory responses-induced tumor metastasis. Ann Surg Oncol 2020; 27: 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Özdemir BC, Csajka C, Dotto GP, et al. Sex differences in efficacy and toxicity of systemic treatments: an undervalued issue in the era of precision oncology. J Clin Oncol 2018; 36: 2680–2683. [DOI] [PubMed] [Google Scholar]
- 31. Narendran N, Luzhna L, Kovalchuk O. Sex difference of radiation response in occupational and accidental exposure. Front Genet 2019; 10: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]