Abstract
A gene encoding a fibrinogen binding protein from Staphylococcus epidermidis was previously cloned, and the nucleotide sequence was determined. A portion of the gene encompassing the fibrinogen binding domain has now been subcloned in an expression-fusion vector. The fusion protein can bind to fibrinogen in a capture enzyme-linked immunosorbent assay and can be purified by fibrinogen affinity chromatography. This protein can completely inhibit the adherence of S. epidermidis to immobilized fibrinogen, suggesting that the adherence of S. epidermidis to fibrinogen is mainly due to this protein. Antibodies against this fibrinogen binding protein were also found to efficiently block the adherence of S. epidermidis to immobilized fibrinogen. Despite homology with clumping factors A and B from S. aureus (cell surface-associated proteins binding to fibrinogen), binding involved the β chain of fibrinogen rather than the γ chain, as in clumping factor A.
Staphylococcus epidermidis is considered an important pathogen. It is a common etiologic agent for infections associated with implanted devices and at sites of surgery (32). S. epidermidis is often found in cases of peritonitis among patients undergoing peritoneal dialysis (31) and in neonatal infections (24).
The adherence of S. epidermidis to biomaterials seems to be a process composed of two stages: the initial attachment is mainly mediated by hydrophobic interactions, whereas biofilm formation and intercellular interactions are important for the second stage (8, 10, 15). The proteinaceous nature of the process of adherence to biomaterials has been demonstrated (29, 30), whereas a polysaccharide is required for biofilm formation (15).
A foreign material implanted into the body is quickly coated with various plasma proteins, such as fibrinogen (Fg), fibronectin, and vitronectin (11, 34). Several reports have described the capacity of S. epidermidis to adhere to immobilized Fg (5, 11, 22, 25, 27, 34). However, precoating of surfaces in vitro with various plasma proteins has also been shown to have a blocking effect on early adhesion for several strains of S. epidermidis (8); in addition, decreased binding of S. epidermidis to Dacron coated with Fg was found (35).
In a screening of 40 strains from an S. epidermidis strain collection, both characteristics were found: some strains adhered strongly to immobilized Fg, whereas the adherence of others was blocked by Fg (14). It has been suggested that different levels of slime production result in differences in Fg binding (1). S. epidermidis is heterogeneous, and generalizations concerning adherence behavior cannot easily be made.
The adherence of S. aureus to Fg has been well characterized; a surface-associated Fg binding protein termed clumping factor (ClfA) mediates S. aureus adherence to immobilized Fg (19) and contributes to virulence in an experimental endocarditis model (23). A second Fg binding protein, ClfB, is expressed only during the early exponential phase (6). In addition to these proteins, no less than three extracellular Fg binding proteins are released from S. aureus into the growth medium (2).
Fg consists of six polypeptide chains forming a symmetric structure with two chains each of the α, β, and γ chains with the N termini in the central part. Calcium can bind to the C termini of the γ chain and to the central nodule of Fg and accelerates fibrin formation (33).
We have recently shown that the adhesion of S. epidermidis to Fg is protease sensitive and, using a phage display system, we have cloned the gene (fbe) encoding the Fg binding protein (Fbe) (25). This protein shows partial homology in the A region with clumping factors A and B from S. aureus and has the characteristic SD repeats found in clumping factors A and B (6, 17).
In this report, we have further characterized the nature of the interaction between Fg and Fbe from S. epidermidis.
MATERIALS AND METHODS
Bacteria and growth conditions.
Escherichia coli TG1 was grown in Luria broth and used as a host for the recombinant plasmid. It was made competent by the CaCl2 method. S. epidermidis HB was used as a source of template DNA for PCR amplification, and S. epidermidis 19, which showed strong adherence to Fg (25), was used in the assay of adherence of bacteria to immobilized Fg. When appropriate, ampicillin was added to 100 μg/ml.
PCR amplification of the fbe gene.
Chromosomal DNA from S. epidermidis HB was used as template DNA. By PCR, a DNA fragment encoding a portion of Fbe was amplified. The upper primer was 5′-GCGGATCCAATCAGTCAATAAACACCGACGAT, and the lower primer was 5′-CGGAATTCTGTTCGGACTGATTTGGAAGTTCC; extending BamHI and EcoRI sites, respectively, are underlined. The amplified fragment corresponded to amino acids 87 to 646, as numbered in reference 25. Amplification was done at 94°C for 4 min; for 25 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 2 min; and at 72°C for 4 min. The amplified fragment was digested with EcoRI and BamHI and ligated into plasmid pGEX-4T3 (Pharmacia, Uppsala, Sweden) that had been digested with EcoRI and BamHI. This fusion expression system utilizes an affinity tag consisting of glutathione S-transferase (GST) at the N-terminal end of the recombinant protein; this tag can bind to glutathione-Sepharose 4B (Pharmacia) and be eluted from the affinity matrix under mild elution conditions. Plasmid pGEX-4T3 encodes a thrombin protease recognition site between the 29-kDa GST and the N-terminal fused peptide. The ligated DNA was transformed into E. coli TG1. A transformant was isolated with plasmid pPL46, encoding a fusion protein composed of GST and a portion of Fbe corresponding to the DNA fragment originally cloned in the phage display system (25). This protein is called GST-Fbe. Restriction enzymes, T4 DNA ligase, Taq DNA polymerase, and DNA purification kits were purchased from Promega.
Protein purification.
E. coli TG1 with plasmid pPL46 was grown and induced with isopropyl-β-d-thiogalactopyranoside (IPTG) (1 mM). The supernatant of the sonicated cells was applied to a glutathione-Sepharose 4B column, washed with phosphate-buffered saline (PBS), and then incubated with elution buffer 1 (10 mM reduced glutathione [Sigma Chemical Co., St. Louis, Mo.] in 50 mM Tris [pH 8.0]) at room temperature for 3 h to collect the eluent.
Fg-Sepharose was prepared by coupling 70 mg of human Fg (Sigma) to 3,500 mg of CNBr-activated Sepharose 4B (Pharmacia) by following the procedure recommended by the manufacturer. The supernatant of the sonicated IPTG-induced bacterial cells was applied to the column, washed with running buffer (40 mM Tris [pH 8.0], 5 mM EDTA), and then eluted with elution buffer 2 (40 mM Tris [pH 7.4], 5 mM EDTA, 500 mM NaCl).
The proteins purified by affinity chromatography, glutathione-Sepharose and Fg-Sepharose, were dialyzed against buffer A (40 mM Tris [pH 7.4]) at 4°C overnight, applied to MonoQ HR5/5 (Pharmacia), washed with buffer A, and eluted by the gradual addition of buffer B (40 mM Tris [pH 7.4], 1 M NaCl) in a fast protein liquid chromatography (FPLC) system (Pharmacia).
Thrombin cleavage.
Cleavage at the thrombin site between GST and Fbe was done by the addition of 10 ml of thrombin solution (10 cleavage units in PBS) per mg of fusion protein and incubation at room temperature for 5 h. GST was removed by glutathione-Sepharose 4B affinity chromatography or by FPLC as described above.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed by use of the Phast system with 8 to 25% gradient gels (Pharmacia) that were stained with Coomassie blue. Western blotting was performed as described earlier (3). The nitrocellulose membranes were blocked with PBS–1% Tween 20 for 20 min at room temperature. After three washes in PBS–0.05% Tween 20 (PBST), the membranes were incubated with Fg (5 μg/ml in PBST) at room temperature for 1 h, washed three times with PBST, incubated with rabbit anti-human Fg–horseradish peroxidase (HRP)-conjugated antibodies diluted 1:1,000 with PBST (Dakopatts, Copenhagen, Denmark) for 1 h, washed with PBST, and substrated by using a 4-chloronaphthol tablet (Sigma) as recommended by the manufacturer.
In experiments to determine the chain of Fg to which Fbe binds, Fg was run on 7.5% homogeneous Phast gels, transferred to nitrocellulose membranes, and probed with GST-Fbe or clumping factor (ClfA) (10 μg/ml). Bound GST-Fbe or ClfA was detected with rat antibodies against GST-Fbe or ClfA, respectively (diluted 1,000-fold), followed by HRP-conjugated anti-rat IgG antibodies. Also, purified chains of Fg were subjected to Western affinity blotting and probed with GST-Fbe. Rats were immunized with three doses of 20 mg of GST-Fbe or ClfA, with 2 weeks between doses, and serum samples were taken 2 weeks after the last immunization. Freund’s complete adjuvant was used with the first dose, and Freund’s incomplete adjuvant was used with the second and third doses.
ClfA was produced as a poly-His fusion protein from plasmid pQE33, encoding amino acids 221 to 550 of ClfA (18). This clone was kindly provided by T. Foster (Dublin, Ireland).
Capture of GST-Fbe by stationary-phase Fg.
Microtiter plate wells were coated with human Fg at concentrations ranging from 1.3 to 20 mg/ml in PBS at room temperature overnight. The plates were then coated with 2% bovine serum albumin (BSA) for 1 h at 37°C. The microtiter plates were washed three times, GST-Fbe was added to the wells at 25 μg/ml, and the plates were incubated for 2 h at 37°C. The plates were washed, and capture of GST-Fbe by immobilized Fg was detected with antibodies (diluted 1,000-fold) raised against GST-Fbe in a rat. The plates were washed again, and binding of antibodies was detected with rabbit anti-rat immunoglobulin G (IgG) antibodies conjugated with HRP. The substrate for HRP was o-phenylenediamine tablets (Dakopatts) with H2O2. The color reaction was measured at 492 nm. The binding of GST-Fbe (10 μg/ml) to purified β and γ chains of Fg was measured with the same procedure. The chains were purified by preparative SDS–8% PAGE on a 491 Prep Cell (Bio-Rad) overnight at 200 V.
Capture of Fg by stationary-phase GST-Fbe.
Microtiter plate wells were coated with GST-Fbe at concentrations ranging from 3 to 200 μg/ml at room temperature overnight and then coated with BSA and washed as described above. Fg was added at 20 μg/ml, and the plates were incubated for 2 h at 37°C. Captured Fg was detected as described above but with HRP-conjugated anti-Fg antibodies (Dakopatts).
Determination of the effect of Ca2+ on the interaction between Fbe and Fg.
Microtiter plate wells were coated with Fg (1 μg/ml) which had been dialyzed against 10 mM EDTA and then against 10 mM Tris–100 mM NaCl to remove Ca2+ trapped by the protein. The plates were then coated with 2% BSA. After the plates were washed, serially diluted CaCl2 (in H2O) was added to the wells together with GST-Fbe or Fbe, each at 10 μg/ml in Tris-HCl (pH 7.4)–0.05% Tween 20, and incubated for 1 h at 37°C. GST-Fbe and Fbe had been dialyzed against EDTA to remove Ca2+. The binding of GST-Fbe or Fbe was detected with anti–GST-Fbe antiserum.
The same kind of enzyme-linked immunosorbent assay (ELISA) was performed to assess the function of Ca2+ in the binding of soluble Fg to immobilized GST-Fbe or Fbe, i.e., as described above but with the components in the reverse order.
Competition in a capture ELISA.
Microtiter plate wells were coated as described above with 1 μg of GST-Fbe or ClfA per ml and then with 2% BSA. Serially diluted GST-Fbe or ClfA (each ranging from 0 to 20 mg/ml in PBST) together with Fg (1 μg/ml in PBST) was added, and the plates were incubated for 1 h at 37°C. The binding of Fg was detected with anti-Fg antibodies.
3H labeling of S. epidermidis.
Five milliliters of Luria broth was inoculated with a fresh colony of S. epidermidis 19 from a blood agar plate and incubated for 2 h at 37°C. 3H-thymidine was added (100 μCi/ml; specific activity, 80 Ci/mmol) and incubation was continued for another 3 h. The bacteria were washed with PBS and kept frozen. Specific incorporation was usually about 600 CFU/cpm.
Adherence of S. epidermidis to immobilized Fg in the presence of GST-Fbe, Fbe, or GST.
Fg at 15 μg/ml was used to coat microtiter plate wells overnight at room temperature; the plates were then coated with BSA as described above. GST-Fbe fusion protein, GST, or Fbe obtained by thrombin cleavage was added at concentrations ranging from 0.4 to 50 μg/ml, and the plates were incubated for 1 h at 37°C. Radioactively labeled bacteria (100 μl at 5 × 107 CFU/ml) were added, and incubation was continued for 1 h. Nonadherent bacteria were washed away, and adherent bacteria were released by the addition of 2% SDS for 30 min. The released bacteria were added to scintillation fluid, and the radioactivity was determined.
Adherence of S. epidermidis to immobilized Fg in the presence of IgG against GST-Fbe, Fbe, or GST.
Radiolabeled bacteria were pretreated with various concentrations of IgG, starting at 40 μg/ml, for 1 h at room temperature. The IgG-treated bacteria were transferred to Fg-coated microtiter plate wells, and adherence was assayed as described above.
RESULTS
Western blotting analysis of Fbe.
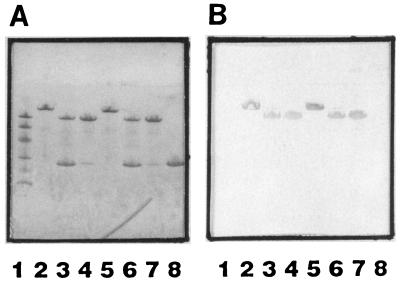
The recombinant GST-Fbe fusion protein expressed in TG1 was isolated and purified by affinity chromatography and ion-exchange chromatography by FPLC. The protein of interest, Fbe, was also purified by affinity chromatography and ion-exchange chromatography after thrombin digestion. These proteins were analyzed by Coomassie blue stain SDS-PAGE and Western affinity blotting, where probing was done with Fg followed by HRP-conjugated anti-Fg antibodies. Figure 1 shows that both GST-Fbe and Fbe were recognized by the soluble form of Fg, whereas GST was not. GST-Fbe could be purified by affinity for either glutathione or Fg, with qualitatively equal results. However, the yield with Fg-Sepharose was lower than that with glutathione-Sepharose (data not shown).
FIG. 1.
(A) SDS-PAGE. Lane 1, Molecular size markers (94, 67, 43, 30, 20, and 14 kDa); lane 2, GST-Fbe purified by glutathione-Sepharose 4B affinity chromatography; lane 3, GST-Fbe purified by glutathione-Sepharose 4B affinity chromatography and cleaved with thrombin; lane 4, GST-Fbe purified by glutathione-Sepharose 4B affinity chromatography, cleaved with thrombin, and recovered as Fbe by FPLC; lane 5, GST-Fbe purified by Fg-Sepharose 4B affinity chromatography; lane 6, GST-Fbe purified by Fg-Sepharose 4B affinity chromatography and cleaved with thrombin; lane 7, GST-Fbe purified by Fg-Sepharose 4B affinity chromatography, cleaved with thrombin, and recovered as Fbe by FPLC; lane 8, GST purified from a culture induced with plasmid pGEX-4T3. (B) Western affinity blotting. Proteins from SDS-PAGE were transferred to nitrocellulose filters. The filters were probed with Fg followed by HRP-conjugated anti-Fg antibodies. Lanes are as in panel A.
Demonstration of interactions between Fg and GST-Fbe in a capture ELISA.
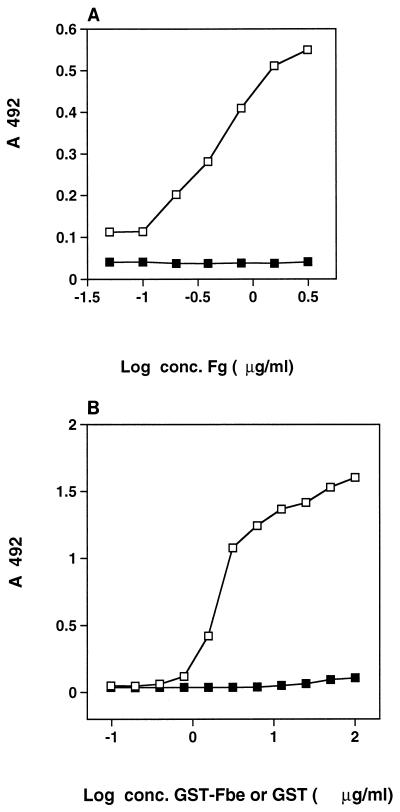
In separate experiments, either GST-Fbe or Fg was immobilized in microtiter plate wells, and the corresponding ligand was added afterward. Figure 2A shows that GST-Fbe bound to immobilized Fg, and Fig. 2B shows that Fg bound to immobilized GST-Fbe, thus demonstrating that both immobilized and soluble forms of Fg are recognized by Fbe. Figure 2 also shows that purified GST does not interact with Fg.
FIG. 2.
(A) Capture of GST-Fbe or GST by Fg. Microtiter plate wells were coated with the indicated concentrations (conc.) of Fg and then coated with BSA. GST-Fbe (open squares) or purified GST (closed squares) was added at 25 μg/ml and incubated. Detection of GST-Fbe or GST capture was done with antibodies raised against GST-Fbe in a rat, followed by HRP-conjugated antibodies against rat IgG. (B) Capture of Fg by GST-Fbe or GST. Microtiter plate wells were coated with the indicated concentrations of GST-Fbe (open squares) or purified GST (closed squares) and then coated with BSA. Fg was added at 20 μg/ml and incubated. Detection of Fg capture was done with HRP-conjugated antibodies against Fg.
The interaction between GST-Fbe or Fbe and Fg was positively influenced by the presence of Ca2+, as shown in Fig. 3, when Fg was in a soluble or an immobilized form.
FIG. 3.
Effect of Ca2+ on the interaction between GST-Fbe and Fg. The binding of GST-Fbe and of Fbe to Fg was measured at different concentrations (conc.) of Ca2+ essentially as described in the legend to Fig. 2A. Likewise, the binding of Fg to GST-Fbe and to Fbe was measured at different concentrations of Ca2+. All proteins were dialyzed against EDTA to remove endogenous Ca2+. Symbols: ■, binding of Fg to GST-Fbe; □, binding of Fg to Fbe; ●, binding of GST-Fbe to Fg; ○, binding of Fbe to Fg. The experiment shown is representative of at least three experiments.
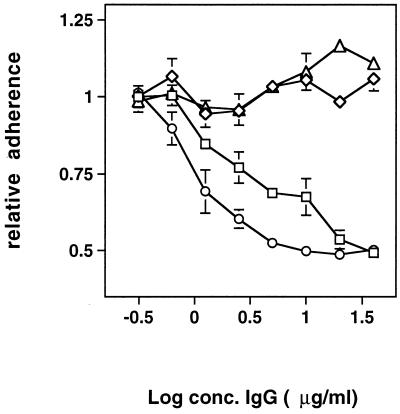
Blocking of bacterial adherence by Fbe.
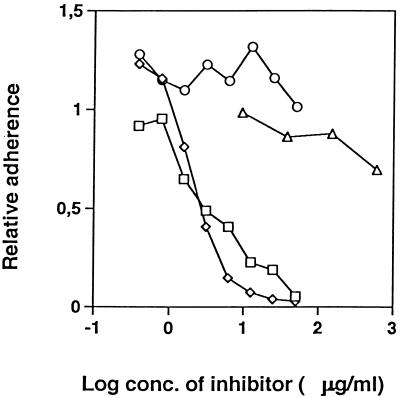
Radiolabeled S. epidermidis cells were added to microtiter plate wells coated with Fg and containing GST-Fbe, Fbe, or GST. Bacterial adherence was completely blocked by the addition of GST-Fbe or Fbe but was not affected by GST, as shown in Fig. 4. The addition of ClfA resulted in only a slight inhibition at high concentrations, whereas the adherence of S. aureus to Fg was blocked about 50% by ClfA (data not shown).
FIG. 4.
Inhibition of S. epidermidis adherence to immobilized Fg. To microtiter plate wells coated with Fg, inhibiting proteins at the indicated concentrations (conc.) were added. Immediately thereafter, radiolabeled cells of S. epidermidis were added and allowed to adhere. After the removal of nonadherent bacteria, bound bacteria were quantified by scintillation counting. Symbols: ○, GST; □, GST-Fbe; ◊, Fbe FPLC purified after thrombin cleavage; ▵, ClfA. The experiment shown is representative of at least three experiments.
Comparison between ClfA and Fbe.
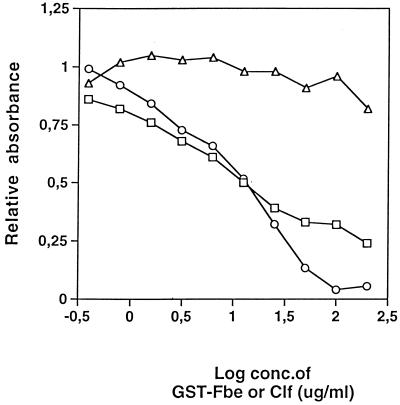
ClfA is a cell surface-associated Fg binding protein of S. aureus shown to be responsible for the adherence of S. aureus to immobilized Fg (19). A comparison between ClfA and Fbe was done by testing for inhibition in a capture ELISA. The binding of Fg to ClfA immobilized on microtiter plate wells could be inhibited by soluble ClfA but not by GST-Fbe. Similarly, the binding of Fg to immobilized GST-Fbe could be inhibited by soluble GST-Fbe (Fig. 5), whereas ClfA did not have any blocking effect (data not shown). The data imply different binding sites on Fg for ClfA and Fbe.
FIG. 5.
Competition in a capture ELISA with ClfA or GST-Fbe. Microtiter plate wells were coated with GST-Fbe or ClfA and then with BSA. The indicated concentrations (conc.) of GST-Fbe or ClfA as an inhibitor were added immediately before the addition of Fg. The relative amount of bound Fg was determined with HRP-conjugated antibodies against Fg. Symbols: □, GST-Fbe inhibition of Fg binding to GST-Fbe; ○, ClfA inhibition of Fg binding to ClfA; ▵, inability of GST-Fbe to inhibit Fg binding to ClfA. The experiment shown is representative of at least three experiments.
To further demonstrate different binding sites for ClfA and Fbe on Fg, a Western affinity blotting experiment was done. α, β, and γ chains of Fg were separated by SDS-PAGE and transferred to nitrocellulose filters. The filters were probed with GST-Fbe or ClfA separately. The binding of ClfA or GST-Fbe was detected with antibodies against ClfA or GST-Fbe, respectively. Purified α, β, and γ chains were also run in separate lanes and probed with GST-Fbe. Figure 6 shows that Fbe bound to the β chain of Fg, whereas ClfA bound to the g chain, as expected from earlier studies (20, 28). When ClfA or GST-Fbe in Fig. 6a was omitted, as a negative control, no bands were displayed, indicating that antibodies against GST-Fbe or ClfA do not cross-react with Fg.
FIG. 6.
(a) Western blot of separated Fg chains. Fg from SDS-PAGE was transferred to nitrocellulose filters. The filters were probed with either GST-Fbe followed by rat anti–GST-Fbe antibodies or ClfA followed by rat anti-ClfA antibodies and, in both cases, by anti-rat IgG antibodies conjugated with HRP. Lanes (Coomassie blue-stained gel in lanes 1 and 2 and Western blot strips in lanes A to D): 1, molecular size markers (94, 67, and 43 kDa, from the top); 2, α, β, and γ chains of Fg; A, membrane probed with GST-Fbe and anti–GST-Fbe serum; B, as in A, but without GST-Fbe; C, membrane probed with ClfA and anti-ClfA serum; D, as in C, but without ClfA. (b) Western blot of purified Fg chains. Purified α, β, and γ chains (lanes 2, 3, and 4, respectively) from SDS-PAGE were transferred to nitrocellulose filters. (B) The filters were probed with GST-Fbe followed by rat anti–GST-Fbe antibodies and anti-rat IgG antibodies conjugated with HRP. (C) As in B, but without GST-Fbe. (A) Coomassie blue-stained gel. Lanes 1, molecular size markers.
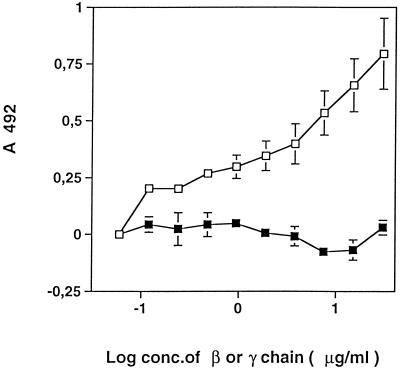
The binding of GST-Fbe to the β chain, rather than to the γ chain, was confirmed in a capture ELISA, as shown in Fig. 7. The binding of GST-Fbe increased with increasing coating concentrations of the β chain, whereas coating with the γ chain did not support the binding of GST-Fbe.
FIG. 7.
Binding of GST-Fbe to the β chain of Fg. Microtiter plate wells were coated with the indicated concentrations (conc.) of the β (□) or γ (■) chain of Fg and then coated with BSA. GST-Fbe was added at 10 μg/ml and incubated. Detection of GST-Fbe capture was done with antibodies raised against GST-Fbe in a rat, followed by HRP-conjugated antibodies against rat IgG. Mean values from quadruplicate experiments are shown, with bars indicating standard errors.
Blocking of bacterial adherence by antisera.
In the experiment shown in Fig. 8, purified IgG was incubated with radiolabeled cells of S. epidermidis prior to addition to microtiter plate wells coated with Fg. IgG against GST-Fbe or against Fbe cleaved from the fusion protein was able to inhibit adhesion. Serum taken before immunization was not able to block adhesion, nor was IgG from a rat immunized only with GST.
FIG. 8.
Inhibition of S. epidermidis adherence to immobilized Fg by antibodies. To microtiter plate wells coated with Fg, radiolabeled cells of S. epidermidis which had been preincubated with purified IgG at the indicated concentrations (conc.) were added and allowed to adhere. After the removal of nonadherent bacteria, bound bacteria were quantified by scintillation counting. Mean values from triplicate experiments are shown, with bars indicating standard errors. Symbols: □, IgG against GST-Fbe; ○, IgG against Fbe cleaved from GST-Fbe by thrombin; ◊, IgG against GST; ▵, serum taken before immunization.
DISCUSSION
The purpose of the present study was to further characterize Fbe originating from a clinical isolate of S. epidermidis. We had previously cloned the gene for this protein (25); here, a high-level expression system was used to obtain a sufficient amount of this protein in the form of a fusion protein for analysis.
Bacterial adhesion to extracellular components is assumed to be an essential step in the pathogenesis of staphylococcal infections. Although the production of extracellular slime by coagulase-negative staphylococci appears to be an important determinant of the capacity of these bacteria to adhere and cause clinical infection (4), slime production is a late phenomenon in the process of adherence of coagulase-negative staphylococci in vitro (12). The capsular polysaccharide (adhesin) has been shown to mediate initial adherence to biomaterials and intercellular adhesion (21). A correlation between the production of adherent biofilm and the expression of a polysaccharide intercellular adhesin has been found (16). Furthermore, the ability to form a biofilm was found significantly more often in isolates from blood cultures than in skin isolates, implying its clinical importance (36). Evidence for the essential role of a surface protein of S. epidermidis in the initial binding to biomaterials has been obtained, and it has been demonstrated that the adhesion of S. epidermidis to biomaterials is mediated by some protease-sensitive surface constituents (29). Another protein (AtlE) has been shown to be involved in attachment to polystyrene (9). It is obvious that the adherence of S. epidermidis to biomaterials and to host tissue components is multifactorial and relies on several different factors, presumably involved at different stages of the infection. We propose that Fbe, described here, is involved at a stage when Fg is deposited on foreign materials, i.e., in vivo. The adherence of contaminating S. epidermidis in vitro to implant materials is more likely to depend on other factors.
The recombinant Fbe fusion protein was purified by glutathione-Sepharose 4B affinity chromatography. The protein could also be purified by Fg-Sepharose 4B affinity chromatography (Fig. 1A). The results of Western blotting showed that no matter how the protein was purified, the property of the protein did not change, although the yield with Fg-Sepharose was lower. In addition, thrombin digestion was also performed, and the results of Western blotting showed that fusion to GST, a 29-kDa protein, did not change the Fg-binding property of the protein of interest (Fig. 1B). In capture ELISAs, both soluble and immobilized forms of Fg were used, and in both cases, binding between Fg and Fbe was obtained (Fig. 1).
It has been reported that the addition of Ca2+ to ClfA (26) and ClfB (6) inhibits their respective binding to Fg. An EF-hand motif, often found in Ca2+ binding proteins (26), is located within the Fg binding region of ClfA. It was thus hypothesized that Ca2+ binding competes with binding to Fg. In contrast to inhibition, we have found here that the addition of Ca2+ stimulates the interaction between Fg and Fbe (Fig. 3). Fbe is similar to ClfA and ClfB from S. aureus (25). In the region of Fbe spanning from amino acids 300 to 515, there is ca. 30% identity with the corresponding regions of both ClfA (18) and ClfB (6). An even higher degree of homology is found between Fbe and another protein from S. aureus, SdrE, with an as-yet-unknown function (13). However, the sequence in Fbe corresponding to the sequence in ClfA with a putative EF-hand motif (amino acids 310 to 321) deviates too much from a consensus EF-hand motif to serve as a Ca2+ binding domain. Ca2+ binds to Fg and is required for blood clotting (7). One of the binding sites for Ca2+ in Fg is at the C-terminal part of the g chain (29), which is also the portion of Fg to which ClfA binds (20). Although Fbe, like ClfB (6), binds to the β chain of Fg, it is evident that the interactions of Ca2+ are of a different nature in ClfB and Fbe. The stimulatory effect of Ca2+ in the interaction between Fg and Fbe observed here might thus be a result of Ca2+ binding to Fg rather than to Fbe. The stimulatory effect on binding between Fbe and Fg occurs at a physiological concentration of Ca2+ (1.5 to 2.5 mM).
In the bacterial adherence assay, inhibition was obtained with both GST-Fbe and Fbe released from the fusion protein. The inhibition was dose dependent, and bacterial adhesion to immobilized Fg was blocked to near completion (Fig. 4). The GST protein alone is not inhibitory, implying that the dose-dependent inhibition is specific and that adherence of S. epidermidis to Fg might depend mainly on this protein (Fbe). Additional mechanisms for adherence to Fg may exist. However, such binding would be similar in nature and would involve the same domain of Fg, as explained by the complete blocking of adherence to Fg by Fbe.
ClfA has been shown to bind to the C-terminal end of the γ chain of Fg (20, 28), and ClfB interacts with both the α and the β chains of Fg (6). Fbe was shown here to bind to the β chain of Fg and is thus more similar to ClfB than to ClfA. This finding was demonstrated both in a Western blotting experiment (Fig. 6) and in a capture ELISA with purified β and γ chains of Fg (Fig. 7). In binding competition experiments, it was shown that ClfA and Fbe do not interfere with one another for binding to Fg, thus confirming that the binding sites on Fg are different for ClfA and Fbe. Furthermore, ClfA was unable to block the adhesion of S. epidermidis to Fg (Fig. 4).
Antibodies raised against GST-Fbe or Fbe cleaved from the fusion protein were shown to block the adhesion of S. epidermidis to immobilized Fg (Fig. 8). Preimmune serum or serum against GST was unable to block adhesion. This result implies the possibility of using antibodies against Fbe as a prophylactic measure against implant-associated infections.
In conclusion, we have found that Fbe is able to fully block the adherence of S. epidermidis to immobilized Fg, suggesting a single type of interaction. Antibodies against Fbe were also able to block adherence, implying that antibodies against Fbe might protect against foreign body-associated infections caused by coagulase-negative staphylococci, which are largely dependent on adherence to Fg. Fbe is more similar to ClfB than to ClfA from S. aureus.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (K98-16X-12218-02B and K97-16X-03778-26A) and Biostapro AB.
We thank David Wade for fruitful discussions and comments.
REFERENCES
- 1.Baldassarri L, Donelli G, Gelosa A, Simpson A W, Christensen G D. Expression of slime interferes with in vitro detection of host protein receptors of Staphylococcus epidermidis. Infect Immun. 1997;65:1522–1526. doi: 10.1128/iai.65.4.1522-1526.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodén M, Flock J-I. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain Newman. Microb Pathog. 1992;12:289–298. doi: 10.1016/0882-4010(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 3.Bodén M, Flock J-I. Fibrinogen binding protein/clumping factor from Staphylococcus aureus. Infect Immun. 1989;57:2358–2363. doi: 10.1128/iai.57.8.2358-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen G D, Simpson W A, Bisno A L, Beachy E H. Adherence of slime-producing Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai N P, Hossainy S F, Hubbell J A. Surface-immobilized polyethylene oxide for bacterial repellence. Biomaterials. 1992;13:417–420. doi: 10.1016/0142-9612(92)90160-p. [DOI] [PubMed] [Google Scholar]
- 6.Eidhin D N, Perkins S, Francois P, Vaudaux P, Hook M, Foster T J. Clumping factor B (ClfB), a new surface-located fibrinogen binding adhesin of Staphylococcus aureus. Mol Microbiol. 1998;30:245–257. doi: 10.1046/j.1365-2958.1998.01050.x. [DOI] [PubMed] [Google Scholar]
- 7.Furlan M, Stucki B, Steinmann C, Jungo M, Lämmle B. Normal binding of calcium to five fibrinogen variants with mutations in the carboxy terminal part of the γ-chain. Thromb Haemost. 1996;76:377–383. [PubMed] [Google Scholar]
- 8.Galliani S, Viot M, Cremieux A, Van der Auwera P. Early adhesion of bacteremic strains of Staphylococcus epidermidis to polystyrene: influence of hydrophobicity, slime production, plasma, albumin, fibrinogen, and fibronectin. J Lab Clin Med. 1994;123:685–692. [PubMed] [Google Scholar]
- 9.Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann M, Peters G. Catheter-associated infections caused by coagulase-negative staphylococci: clinical and biological aspects. In: Seifert H, Jansen B, Farr B M, editors. Catheter-related infections. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 79–109. [Google Scholar]
- 11.Herrmann M, Vaudaux P, Pittet D, Auckenthaler R, Lew P D, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josefsson E, McCrea K W, NiEdhin D, O’Connell D, Cox J, Hook M, Foster T J. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology. 1998;144:3387–3395. doi: 10.1099/00221287-144-12-3387. [DOI] [PubMed] [Google Scholar]
- 14.Jung, K., and J.-I. Flock. Unpublished data.
- 15.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulations of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglucan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881–884. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- 17.McDevitt D, Foster T J. Variation in the size of the repeat region of the fibrinogen receptor (clumping factor) of Staphylococcus aureus strains. Microbiology. 1995;141:937–943. doi: 10.1099/13500872-141-4-937. [DOI] [PubMed] [Google Scholar]
- 18.McDevitt D, Francois P, Vaudaux P, Foster T. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol Microbiol. 1995;16:895–907. doi: 10.1111/j.1365-2958.1995.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 19.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 20.McDevitt D, Nanavaty T, House-Pompeo K, Bell E, Turner N, McIntire L, Foster T, Höök M. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem. 1997;247:416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 21.McKenney D, Hubner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammad S F, Topham N S, Burns G L, Olsen D B. Enhanced bacterial adhesion on surfaces pretreated with fibrinogen and fibronectin. ASAIO Trans. 1988;34:573–577. [PubMed] [Google Scholar]
- 23.Moreillon P, Entenza J, Francioli P, McDevitt D, Foster T, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumeister B, Kastner S, Conrad S, Klotz G, Bartmann P. Characterization of coagulase-negative staphylococci causing nosocomial infections in preterm infants. Eur J Clin Microbiol Infect Dis. 1995;14:856–863. doi: 10.1007/BF01691491. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson M, Frykberg L, Flock J-I, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell D, Nanavaty T, McDevitt D, Gurusiddappa S, Höök M, Foster T J. The fibrinogen-binding MSCRAMM (clumping factor) of Staphylococcus aureus has a Ca2+-dependent inhibitory site. J Biol Chem. 1998;273:6821–6829. doi: 10.1074/jbc.273.12.6821. [DOI] [PubMed] [Google Scholar]
- 27.Paulsson M, Kober M, Freij-Larsson C, Stollenwerk M, Wesslen B, Ljungh Å. Adhesion of staphylococci to chemically modified and native polymers, and the influence of preadsorbed fibronectin, vitronectin, and fibrinogen. Biomaterials. 1993;14:845–853. doi: 10.1016/0142-9612(93)90006-n. [DOI] [PubMed] [Google Scholar]
- 28.Strong D D, Laudano A P, Hawiger J, Doolittle R F. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982;21:1414–1420. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- 29.Timmerman C P, Fleer A, Besnier J M, de Graaf L, Cremers F, Verhoef J. Characterization of a proteinaceous adhesin of Staphylococcus epidermidis which mediates attachment to polystyrene. Infect Immun. 1991;59:4187–4192. doi: 10.1128/iai.59.11.4187-4192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veenstra G J, Cremers F F, van Dijk H, Fleer A. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J Bacteriol. 1996;178:537–541. doi: 10.1128/jb.178.2.537-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Graevenitz A, Amsterdam D. Microbiological aspects of peritonitis associated with continuous amulatory peritoneal dialysis. Clin Microbiol Rev. 1992;5:36–48. doi: 10.1128/cmr.5.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong E S. Surgical site infections. In: Mayhall C G, editor. Hospital epidemiology and infection control. Baltimore, Md: Williams & Wilkins; 1996. p. 154. [Google Scholar]
- 33.Yee V C, Pratt K P, Cote H C F, Trong I L, Chung D W, Davie E W, Stenkamp R E, Teller D C. Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure. 1997;5:125–138. doi: 10.1016/s0969-2126(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Montelius M N, Paulsson M, Gouda I, Larm O, Montelius L, Ljungh Å. Adhesion of coagulase-negative staphylococci and adsorption of plasma proteins to heparinized polymer surfaces. Biomaterials. 1994;15:805–814. doi: 10.1016/0142-9612(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 35.Zdanowski Z, Ribbe E, Schalen C. Influence of some plasma proteins on in vitro bacterial adherence to PTFE and Dacron vascular prosthesis. APMIS. 1993;101:926–932. doi: 10.1111/j.1699-0463.1993.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 36.Ziebuhr W, Heilmann C, Götz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun. 1997;65:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]