Abstract
Gastrointestinal pythiosis is a severe, progressive and often a fatal disease, which is caused by the aquatic pathogen Pythium insidiosum. Treatment is challenging due to the disease's resistance to antifungal drugs. Surgical resection is frequently attempted in cases of pythiosis; however, it can be technically challenging. This report presents two dogs with decreased appetite, abdominal pain, progressive haematochezia, tenesmus and significant weight loss. With the medical histories of both being young canines, living in areas with access to natural water resources and with the main chronic gastrointestinal symptoms having not responded to symptomatic treatment, pythiosis was taken into consideration. Abdominal ultrasound revealed severe, diffuse thickening and loss of normal layering of the colonic wall. These findings led to a differential diagnosis between intestinal neoplasia and fungal disease. Full‐thickness biopsies were later performed, and immunohistochemistry staining was suggested for colonic pythiosis. Medical treatment for pythiosis was successful with a combination of oral terbinafine and prednisolone. However, therapy with itraconazole in case 1 did not improve the clinical signs, and in case 2, itraconazole was used after all clinical signs have improved for clinical control. Since then, there has been no recurrence of clinical signs until the time of preparing this report (19 months for case 1, 11 months for case 2 since the cessation of treatment). The treatment was successful based on clinical signs and ultrasonographic data, and the disease remission was not confirmed by advance imaging, monitoring of pythiosis enzyme‐linked immunosorbent essay concentration or repeat sampling.
Keywords: dog disease, eosinophilic enteritis, prednisolone, Pythium insidiosum, terbinafine
Successful management of colonic pythiosis in two dogs in Thailand using antifungal therapy.

1. INTRODUCTION
Pythiosis is a chronic infectious disease caused by a fungus‐like aquatic pathogen. Pythium insidiosum belongs to the class of oomycetes; unlike fungi, P. insidiosum produces motile, flagellates zoospores and has cell walls that contain cellulose and β‐glucan but not chitin. Globally, pythiosis is most often found in southeast Asia (especially Thailand and Indonesia), eastern coastal Australia, New Zealand and the Gulf coast region of the United States, but it has recently reported as far west as California (Hummel et al., 2011). It has been reported, more particularly, in swamps or flooded areas (Pal & Mahendra, 2014).
Pythiosis commonly affects young large dog breeds that engage in outdoor activity and have access to standing water. However, some infections have been reported in suburban house dogs with no known access to any of water sources (Grooters & Foil, 2012). Affected animals are typically immunocompetent but otherwise clinically healthy (Grooters & Foil, 2012). In dogs, gastrointestinal pythiosis is commonly characterized by pyogranulomatous or eosinophilic inflammation of the gastrointestinal tract, predominately in the colon (Berryessa et al., 2008). Pythiosis is generally presumptive diagnosed based on medical history, clinical presentation and histopathological findings, which is confirmed with one or more of the following immunohistochemistry, serological methods (immunodiffusion test, enzyme‐linked immunosorbent essay, immunoblot analysis, immunochromatographic test and haemagglutination test) and culture of the pathogen followed by morphologic as well as molecular identification by species‐specific polymerase chain reaction (Berryessa et al., 2008; Keeratijarut et al., 2009).
The severity of pythiosis in mammals and the absence of a drug of choice for treating it are the challenges in treatment (Loreto et al., 2014). Therefore, success in treating with medical therapy has questionable efficacy. An aggressive radical surgical excision of 5 cm margins is the current recommendation for gastrointestinal pythiosis (Grooters, 2017). However, most of the cases of gastrointestinal pythiosis are diagnosed at a late stage of the disease, when complete excision is usually not possible (Grooters, 2003). As for the drug of choice, antifungal drugs are generally inefficient for pythiosis treatment due to the lack of ergosterol in pythium cell walls, which are the primary target of antifungal drugs (Gaastra et al., 2010). Nonetheless, the use of ergosterol–targeting drugs such as itraconazole and terbinafine has been reported as the successful treatment of pythiosis (Reagan et al., 2019; Fischer et al., 1994). The combination of prednisolone and antifungal treatment has also previously reported with a complete recovery from gastrointestinal pythiosis without surgical procedure (Reagen et al., 2019). Hummel et al. (2011) and Cridge et al. (2020) also reported cases of canine pythiosis, which is resolved with itraconazole, terbinafine and mefenoxam. Mefenoxam is an agricultural fungicide traditionally used to control oomycetes in plants. The drug has been found to markedly inhibit radial growth of P. insidiosum in vitro, whereas terbinafine and itraconazole have only limited effects on the radial growth of P. insidiosum. Therefore, this off‐label drug must be used with cautious.
The purpose of this report is to describe the successful therapeutic management of colonic pythiosis in two dogs. To our knowledge, this is the first report on the successful management of colonic pythiosis in Thailand using terbinafine as antifungal monotherapy and prednisolone without surgical procedure. However, medical therapy in the absence of surgery has been successful in multiple other geographic regions.
2. CASE HISTORY
2.1. Case 1
A 3‐year‐old intact female, mixed breed dog weighing 15.5 kg was presented at the animal hospital with a history of inappetence, weakness, depression, tenesmus, haematochezia and diarrhoea for several months. Upon examination, the dog was depressed, had 5% dehydration with a 4/9 body condition score (BCS). The body temperature was 103°F (100.5–102.5°F). An erythematous rash was presented in the dog's perineum. Rectal examination revealed an irregular thickening of the rectal wall. A complete blood count, serum biochemistry profile and urinalysis revealed that apart from a mild decrease in blood urea nitrogen (BUN), other parameters were within the normal range (Table 1) and faecal flotations performed were unremarkable.
TABLE 1.
Laboratory findings
| Case 1 | Case 2 | ||||
|---|---|---|---|---|---|
| Parameter | First visit | Start terbinafine | Finish terbinafine | First visit | Reference range b |
| Haematology a | |||||
| RBC (1012/L) | 6.49 | 6.18 | 6.73 | 6.75 | 5.1–7.6 |
| MCV (fl) | 69 | 75.9 | 71.8 | 65.2 | 60–71 |
| MCH (pg) | 22.5 | 24.3 | 23.3 | 21.2 | 22–26 |
| MCHC (g/L) | 326 | 320 | 325 | 325 | 344–381 |
| HCT (L/L) | 0.44 | 0.46 | 0.48 | 0.44 | 0.35–0.52 |
| TP (g/L) | 100 | ‐ | 72 | 90 | 53–78 |
| WBC (109/L) | 11.35 | 11.75 | 10.5 | 12.0 | 5.6–20.4 |
| Neutrophils (109/L) | 9.53 | 8.93 | 8.71 | 9.12 | 2.9–13.6 |
| Lymphocytes (109/L) | 1.58 | 2.82 | 1.78 | 1.8 | 1.1–5.3 |
| Monocytes (109/L) | 0 | 0 | 0 | 0.48 | 0.4–1.6 |
| Eosinophils (109/L) | 0.22 | 0 | 0 | 0.6 | 0.1–3.1 |
| Platelet (109/L) | 172 | 168 | 119 | 193 | 108–562 |
| Blood morphology | Normal | Normal | Normal | Few anisocytosis/polychromasia | |
| Blood parasite | Not found | Not found | |||
| Blood chemistry b | |||||
| ALT (IU/L) | 32 | 28 | 35 | 40 | 10–125 |
| ALP (IU/L) | 217 | 539 | ‐ | 251 | 23–212 |
| Creatinine (μmol/L) | 53 | 70.7 | 97.3 | 87.6 | 44.2–123.9 |
| BUN (mmol/L) | 2.67 | 4.25 | 4.96 | 6.17 | 2.5–9.6 |
| Albumin (g/L) | 32 | – | 33 | 25 | 23–40 |
| Glucose (mmol/L) | 4.1 | 5.8 | 4.1–7.93 | ||
| Sodium (mmol/L) | 146 | 144–160 | |||
| Potassium (mmol/l) | 4.2 | 3.5–5.8 | |||
| Chloride (mmol/L) | 114 | 109–122 | |||
| Others | |||||
| Folate (nmol/L) | 7.8 | 1.7–11.8 | |||
| Vitamin B12 (pmol/L) | 656 | 269–1056 | |||
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; BUN, blood urea nitrogen; HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell; TP, total protein; WBC, white blood cell.
Canine automated haematology reference values for Sysmex XT‐2000iv instruments (Tvedten, 2012).
Reference ranges for the IDEXX Catalyst Chemistry One Analyzer.
Abdominal ultrasonography revealed a diffuse circumferential thickening of the descending colon wall, ranging from 5 to 11.5 mm (mean reference value 1.5 mm) with obliterated layers (Figure 1). The mesenteric lymph nodes were enlarged with hypoechogenicity, whereas perimesenteric fat was hyperechoic. Colonoscopy was later performed, leaving out intestinal biopsy with the intention to obtain full thickness biopsy from exploratory laparotomy, and revealed a narrowing of the colonic lumen due to multiple nodules with irregular surface throughout the colon (Figure 2).
FIGURE 1.

Abdominal ultrasound revealed the descending colonic wall to be moderately thickened, measuring 11.5 mm, with a loss of the layering.
FIGURE 2.

Colonoscopy disclosed multiple nodules with irregular surface throughout in the rectum and descending colon. The nodule was approximately 1.5 cm in diameter (arrow), locating at 5 cm from the anus.
An exploratory laparotomy found mild thickening of the ileum wall through the rectum with enlarged colonic lymph nodes. Incisional biopsy from the colonic lymph node and full‐thickness biopsy from the ileum and descending colon were performed for histopathological examination. The dog recovered from anaesthesia and underwent surgery without complications. Postoperatively, the dog received fentanyl (3 μg/kg/h) for analgesia. Metronidazole (13 mg/kg every 12 h) and tylosin (10 mg/kg every 12 h) were prescribed for 3 weeks for antibacterial and immunomodulatory effects. The histopathological examination revealed moderate‐to‐severe multifocal areas of pyogranulomatous inflammation characterized by the central necrosis and infiltration of mixed inflammatory cells, predominately neutrophils, macrophages and multinucleated giant cells. A few number of plasma cells, lymphocytes and eosinophils were also present. In addition, the intralesional poorly stained hyphal elements were infrequently noticed on haematoxylin and eosin stains (Figure 3). The hyphae were demonstrated by Grocott methenamine silver (GMS) stain as non‐parallel walls, 5–8 μm width with 90° occasional branching. Lymphoid hyperplasia was seen in the colonic lymph nodes without any hyphae (Figure 4).
FIGURE 3.

Pyogranulomatous formation with extensive inflammatory cell infiltration and poorly stained fungal hyphae (arrow) in the descending colon (haematoxylin and eosin stain, 400×)
FIGURE 4.

Lymphoid hyperplasia was seen in the colonic lymph node without any fungal hyphae (haematoxylin and eosin stain, 400×).
In the presented cases, surgical excision was not considered practical due to the extensive expansion of the lesion (from the ascending colon to the rectum), so itraconazole (5 mg/kg PO every 24 h) was tried and prescribed for 8 weeks. After treatment with the antifungal drugs, the clinical signs did not improve. The ultrasonography showed that the distal descending colon had continually thickened to approximately 17 mm with a loss of layering (Figure 5). Due to the poor response to the treatments and the high prevalence of human pythiosis in Thailand which facilitates the laboratory expertise, the tissue sample was submitted for immunohistochemistry. Immunohistochemical staining was performed with an immunoperoxidase staining (IPS) assay using rabbit polyclonal anti‐P. insidiosum antibodies as primary antibody (Keeratijarut et al., 2009). Immunoreactivity to P. insidiosum was observed on the hyphae, indicating that the intestinal lesion was affected by pythiosis as in human (Figure 6) (Keeratijarut et al., 2009).
FIGURE 5.

Abdominal ultrasound revealed the descending colonic wall to be moderately thickened, measuring 17.1 mm, with a loss of the layering.
FIGURE 6.

Positive immunoperoxidase staining (IPS) assay result showing brownish‐stained hyphae Pythium insidiosum (arrow) (IPS, 400×)
After colonic pythiosis was confirmed by the lab (Anatomical Pathology Unit, Ramathibodi Hospital, Bangkok), terbinafine was prescribed for 14 weeks (30 mg/kg PO every 24 h) in combination with prednisolone for 6 weeks (0.6 mg/kg PO every 24 h for 4 weeks and reduced to 0.3 mg/kg PO every 24 h for 2 weeks).
The results of a complete blood count and a serum biochemistry profile after treatment were within the normal range (Table 1). The clinical signs improved within 6 weeks of treatment with terbinafine and prednisolone. The patient regained normal appetite, gained weight and had normal defecation. Ultrasonographic findings at 4 weeks after the cessation of medical treatment showed a mild thickening of the descending colonic wall of about 4.3 mm (Figure 7). At the follow‐up at 9 months, the dog was in a good condition and had no clinical abnormalities. Abdominal ultrasonography showed that the descending colonic wall remained within the normal range (Figure 8). The owner reported that the clinical signs remained normal for about 19 months after the cessation of medical treatment. There was no follow‐up advanced imaging, serology, cytology or histopathology to confirm successful treatment.
FIGURE 7.

The ultrasonographic findings at 4 weeks after the cessation of medical treatment (terbinafine and prednisolone) showed that the descending colonic wall was mildly thickened by up to 4.3 mm.
FIGURE 8.
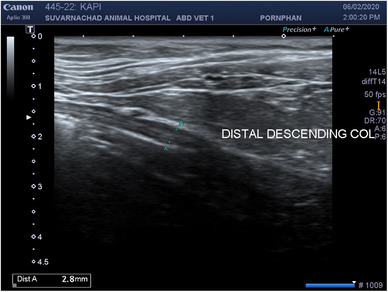
The ultrasonographic findings at 9 months after the cessation of medical treatment showed that descending colonic wall thickness was within the normal range (2.8 mm).
2.2. Case 2
A 4‐year‐old intact male mixed breed dog weighing 20 kg was presented at the animal hospital with a history of large bowel diarrhoea characterized by haematochezia, tenesmus inappetence and weight loss for 1 month. The dog lived in a rural area where it had frequent visited flooded rice fields. Upon examination, the dog was bright, alert and responsive but thin with a BCS of 4/9. The body temperature was normal. The perianal area disclosed erythematous rash. Rectal examination found thickened rectal wall.
A complete blood count and serum biochemistry profile were within the normal range (Table 1). Abdominal ultrasound revealed that the colonic wall was circumferentially severely thickened ranging up to 24 mm with a complete loss of layering (Figure 9). The colonic lymph nodes showed homogeneous hypoechogenicity and were enlarged to 13.5 mm (Figure 10). However, the rectum and descending colon within the pelvic canal could not be assessed by ultrasonography. The remainder of the intestinal tract was unremarkable. Note that computed tomography was not available at the hospital, treating this present case.
FIGURE 9.

Abdominal ultrasound revealed diffuse and circumferential thickening of the descending colonic wall by 24 mm and loss of the layering.
FIGURE 10.

Abdominal ultrasound revealed mildly enlarged and homogeneous hypoechogenicity of the colonic lymph node.
An exploratory laparotomy found a diffuse thickening of the descending colonic wall. The colonic lymph nodes were enlarged to approximately 25 mm in diameter (Figure 11a,b). The remainder of the intestinal tract appeared normal. A full‐thickness biopsy was obtained from the descending colon. The histopathological examination revealed multifocal to coalescing foci of inflammation characterized by the presence of eosinophils, neutrophils and necrosis of the muscular layer and surrounded by macrophages and epithelioid cells with intralesional poorly stained hyphal elements (Figure 12). Non‐septate hyphae with non‐parallel walls and occasional branching hyphae were demonstrated by GMS stain in the inflammatory area. Colonic pythiosis (Figure 13) was confirmed by immunohistochemistry as mentioned before.
FIGURE 11.

(a) The colonic lymph nodes were enlarged to approximately 25 mm in diameter. (b) Diffuse thickening of the descending colonic wall was observed (arrow).
FIGURE 12.

Pyogranulomatous formation with extensive inflammatory cell infiltration and intralesional hyphae (arrow) in the descending colon (haematoxylin and eosin stain, 400×)
FIGURE 13.

Positive immunoperoxidase staining (IPS) assay result showing brownish‐stained hyphae Pythium insidiosum (arrow) (IPS, 400×)
Surgical treatment was not considered a viable option due to the extent of the lesion. Medical treatment was initiated with a combination of oral terbinafine (20 mg/kg PO every 24 h) and prednisolone (0.7 mg/kg PO every 24 h) for 4 weeks.
At the follow‐up, 4 weeks after starting medical treatment, the clinical signs, including diarrhoea, haematochezia and tenesmus, had been resolved. The dog's body weight gradually increased. With a normal clinical presentation, an increase in weight to 22.8 kg was observed. Because of the owner's financial situation, itraconazole at 10 mg/kg every 24 h for 1 month was prescribed PO to replace terbinafine in order to maintain the clinical signs. Rectal examination found a smooth rectal wall. A follow‐up abdominal ultrasound at about 10 weeks after the cessation of treatment was unremarkable (Figure 14). The owner reported no recurrence of clinical signs after 11 months of discontinuing medication. There was no follow‐up advanced imaging, serology, cytology or histopathology to confirm successful treatment.
FIGURE 14.

Abdominal ultrasound revealed no remarkable lesions of the descending colonic wall.
3. DISCUSSION
Diagnosis of pythiosis is based on epidemiology, signalment, clinical signs and ultrasonographic findings, which is confirmed by histopathological examination with serology tests (Pal & Mahendra, 2014). It has been reported that human pythiosis has been found all over Thailand, suggesting that the pathogen is widely distributed throughout the country (Krajaejun et al., 2006; Susaengrat et al., 2019). This report presents the first two confirmed canine colonic pythiosis cases in Thailand. The pathogen is generally found in unmoving water and oospores in the soil and field of dry land (Pal & Mahendra, 2014). In this study, the affected dogs have been frequently exposed to outdoor water sources in rice fields, a potential risk factor for pythiosis. The main clinical signs include weight loss, vomiting, diarrhoea and haematochezia (Grooters, 2017).
Physical examination typically reveals a patient with a thin body condition, the same as in this case report. Common haematological and biochemical findings include anaemia, eosinophilia, hypoalbuminemia and hyperglobulinemia (Berryessa et al., 2008). In both cases, a few eosinophils were found in histological section of the descending colonic wall and without eosinophilia. In this study, most of the laboratory parameters remained within the reference level except in case 1, which had low BUN that could have been associated with the patient's malnutrition. The ultrasonographic appearance of gastrointestinal pythiosis appeared nonspecific and resembled to descriptions of neoplastic lesions and severe inflammatory disease. Although eosinophilia was found in most of the case reports on pythiosis, there were also cases that reported no abnormality in haematological profiles (Dycus et al., 2015; Schmiedt et al., 2012).
Histopathological findings from colonic tissues in both cases were characterized by eosinophilic granulomatous and pyogranulomatous inflammation associated with hyphae‐liked elements. Due to the histopathological similarities among fungal organisms, immunohistochemistry was required for differentiation (Parambeth et al., 2019). The definitive diagnosis of pythiosis in this study was made on the basis of immunohistochemistry and IPS. With IPS assay using rabbit anti‐P. insidiosum, antibodies directly detected P. insidiosum in infected tissues. IPS assay was developed for a direct detection of P. insidiosum in tissue samples eliminating cross‐reactive staining issues. The assay had high sensitivity (100%) and specificity (94%); theoretically, there is a possibility of cross‐reacting with other oomycetes; however, other oomycetes such as Lagenidium have never been found in gastrointestinal tract. Statistical analysis indicated that IPS assay requires routine IPS reagents and laboratory equipment and can be performed easily by pathologists in rural hospitals where the disease is more prevalent (Keeratijarut et al., 2009).
The treatment of choice for gastrointestinal pythiosis by aggressive surgical resection was not considered applicable to either dog in this study because of the extensive progressed lesions.
There have been reports on improvements of clinical signs, ultrasonographic findings and serologic cures for pythiosis following the medical therapy of ergosterol targeting drugs such as itraconazole and terbinafine in dogs (Reagen et al., 2019; Pereira et al., 2013) regardless of the common understanding that pythiosis does not respond well to standard antifungal agents (Loveto et al., 2014). Itraconazole was prescribed prior to the diagnosis of pythiosis in case 1, but clinical signs did not improve. After the colonic pythiosis was confirmed, terbinafine and prednisolone were prescribed. With the treatment for 14 weeks of terbinafine and 6 weeks of prednisolone, the dog showed a good response with medication and no recurrence of clinical signs in 19 months after cessation. Noted that prednisolone was not prescribed with itraconazole as it was with terbinafine. In case 2, a combination of oral terbinafine and prednisolone was prescribed for 4 weeks. At the follow‐up, the dog's clinical signs had resolved. Our results correspond to that of Brown et al. (2008), which mentioned that P. insidiosum showed no remarkable susceptibility to itraconazole as was shown to terbinafine, which may be as a consequence of the intracellular accumulation of squalene rather than the effect of ergosterol inhibition. Boothe (2012) has also written that allylamines such as terbinafine competitively inhibit squalene epoxidase, blocking the conversion of squalene to lanosterol, leading to squalene accumulation and ergosterol depletion in the cell membrane. The accumulation of intracellular squalene concentration to toxic levels interferes with cell membrane function and cell wall synthesis leading to cell death (Krishnan‐Natesan, 2009).
Terbinafine recommended dose varies from 5.9 to 40 mg/kg PO every 24 h (Cridge et al., 2020; Papich, 2016); a dose of 30 mg/kg PO every 24 h used in this report, case 1, can be considered a higher dose, which was used to control the extent of the lesion. Prednisolone was also prescribed in both dogs for anti‐inflammation purposes and we found that the lesion in the colon improved within 2 weeks. This may have been the effect of prednisolone on eosinophilic inflammation, which is a hallmark of gastrointestinal pythiosis contributing to the severe clinical signs. Our findings correspond to preceding reports that lower anti‐inflammatory dosages of prednisolone may improve the outcome of treatment (Reagan et al., 2019; Berryessa et al., 2008).
However, the prognosis for pythiosis is not favourable, especially in the gastrointestinal disease form which often involves large segments of the gastrointestinal tract (Frade et al., 2017). The successful treatment of the presented cases strengthens the prospect of treating extended stage pythiosis with medicines. In addition to the efficacy of terbinafine and prednisolone playing important roles in controlling the disease, the dogs’ immunocompetence from being young and healthy could also be a great support to the successful treatment.
In this case report, advanced imaging or serologic testing was not performed to confirm successful treatment due to the availability of the laboratory and facility of the hospital treating the cases. Therefore, the treatment success was based predominantly on clinical signs, physical examination and abdominal ultrasound examination.
One of the main intentions of this paper is to emphasize that colonic pythiosis should be included in differential diagnoses in young dogs that have access to any natural water resource or standing water and exhibit weight loss and chronic diarrhoea that is unresponsive to symptomatic medical treatment. Moreover, terbinafine is proved to efficiently control the disease as antifungal monotherapy in these cases.
AUTHOR CONTRIBUTIONS
Pornphan Sukanan: resources; conceptualization; project administration; writing–original draft; writing–review and editing. Bongkot Suparp: resources; software; writing–original draft. Supattra Yongsiri: methodology; resources; supervision; writing–review and editing. Piyarat Chansiripornchai: resources; validation; writing‐original draft; writing‐review and editing. Sawang Kesdangsakonwut: data curation; resources; writing–original draft.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
FUNDING INFORMATION
The authors receive no financial support for the research, authorship and/or publication of this article.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this study did not include any experimentation on animals, and all data were generated from a part of daily clinical activities.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.955.
ACKNOWLEDGEMENTS
We would like to thank the owners of the dogs, veterinarians and staffs at Suvarnachad Animal Hospital and the Department of Veterinary Pathology, Faculty of Veterinary Science, Chulalongkorn University for their assistances and contributions. We also thank assistant professor Simon Wright, Faculty of Arts, Chulalongkorn University for his assistance with language correction.
Sukanan, P. , Suparp, B. , Yongsiri, S. , Chansiripornchai, P. , & Kesdangsakonwut, S. (2022). Successful management of colonic pythiosis in two dogs in Thailand using antifungal therapy. Veterinary Medicine and Science, 8, 2283–2291. 10.1002/vms3.955
DATA AVAILABILITY STATEMENT
All data underlying the results of this case report are available as part of the articles and its Supporting Information files.
REFERENCES
- Boothe, D. M. (2012). Treatment of fungal infection. In: Small animal clinical pharmacology and therapeutics (2nd ed., pp. 388–394). Saunders Elsevier. [Google Scholar]
- Berryessa, N. A. , Marks, S. L. , Pesavento, P. A. , Krasnansky, T. , Yoshimoto, S. K. , Johnson, E. G. , & Grooters, A. M. (2008). Gastrointestinal pythiosis in 10 dogs from California. Journal of Veterinary Internal Medicine, 22, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Brown, T. A. , Grooters, A. M. , & Hosgood, G. L. (2008). In vitro susceptibility of Pythium insidiosum and a Lagenidium sp. to itraconazole, posaconazole, voriconazole, terbinafine, caspofungin, and mefenoxam. American Journal of Veterinary Research, 69, 1463–1468. [DOI] [PubMed] [Google Scholar]
- Cridge, H. , Hughes, S. M. , Langston, V. C. , & Mackin, A. J. (2020). Mefenoxam, itraconazole, and terbinafine combination therapy for management of pythiosis in dogs (Six Cases). Journal American Animal Hospital Association, 56, 307–314. [DOI] [PubMed] [Google Scholar]
- Dycus, D. L. , Fisher, C. , & Butler, R. (2015). Surgical and medical treatment of pyloric and duodenal pythiosis in a dog. Journal American Animal Hospital Association, 51, 385–391. [DOI] [PubMed] [Google Scholar]
- Fischer, J. R. , Pace, L. W. , Turk, J. R. , Kreeger, J. M. , Miller, M. A. , & Gosser, H. S. (1994). Gastrointestinal pythiosis in Missouri dogs: Eleven cases. Journal of Veterinary Diagnostic Investigation, 6, 380–382. [DOI] [PubMed] [Google Scholar]
- Frade, M. T. S. , Diniz, P. V. N. , Olinda, R. G. , Maia, L. A. , Galiza, G. J. N. , de Souza, A. P. , Nóbrega Neto, P. I. , & Dantas, A. F. M. (2017). Pythiosis in dogs in the semiarid region of Northeast Brazil. Pesquisa Veterinaria Brasileira, 37(5), 485–490. [Google Scholar]
- Gaastra, W. , Lipman, L. J. , De Cock, A. W. , Exel, T. K. , Pegge, R. B. G. , Scheurwater, J. , Vilela, R. , & Mendoza, L. (2010). Pythium insidiosum: An overview. Veterinary Microbiology, 146, 1–16. [DOI] [PubMed] [Google Scholar]
- Grooters, A. M. (2003). Pythiosis, lagenidiosis, and zygomycosis in small animals. Veterinary Clinics of North America: Small Animal Practice, 33(4), C95–C720. [DOI] [PubMed] [Google Scholar]
- Grooters, A. M. (2017). Miscellaneous fungal infection. In: Ettinger S. T., Feldman E. C., & Cote E. (Eds.), Textbook of veterinary internal medicine (8th ed., pp. 1044–1050). Elsevier. [Google Scholar]
- Grooters, A. M. , & Foil, C. S. (2012). Miscellaneous fungal infection. In: Greene C. E. (Ed.), Textbook of canine and feline infectious diseases (4th ed., pp. 677–678). Elsevier. [Google Scholar]
- Hummel, J. , Grooters, A. , Davidson, G. , Jennings, S. , Nicklas, J. , & Birkenheuer, A. (2011). Successful management of gastrointestinal pythiosis in a dog using itraconazole, terbinafine, and mefenoxam. Medical Mycology, 49, 539–542. [DOI] [PubMed] [Google Scholar]
- Keeratijarut, A. , Karnsombut, P. , Aroonroch, R. , Srimuang, S. , Sangruchi, T. , Sansopha, L. , Mootsikapun, P. , Larbcharoensub, N. , & Krajae, T. (2009). Evaluation of an in‐house immunoperoxidase staining assay for histodiagnosis of human pythiosis. The Southeast Asian Journal of Tropical Medicine and Public Health, 40(6), 1297–1305. [PubMed] [Google Scholar]
- Krajaejun, T. , Sathapatayavongs, B. , Pracharktam, R. , Nitiyanant, P. , Leelachaikul, P. , Wanachiwanawin, W. , Chaiprasert, A. , Assanasen, P. , Saipetch, M. , Mootsikapun, P. , Chetchotisakd, P. , Lekhakula, A. , Mitarnun, W. , Kalnauwakul, S. , Supparatpinyo, K. , Chaiwarith, R. , Chiewchanvit, S. , Tananuvat, N. , Srisiri, S. , … Somkaew, S. (2006). Clinical and epidemiological analyses of human pythiosis in Thailand. Clinical Infectious Diseases, 43, 569–576. [DOI] [PubMed] [Google Scholar]
- Krishnan‐Natesan, S. (2009). Terbinafine: A pharmacological and clinical review. Expert Opinion on Pharmacotherapy, 10, 2723–2733. [DOI] [PubMed] [Google Scholar]
- Loreto, E. S. , Tondolo, J. S. M. , & Zanette, R. A. (2014). Update on pythiosis immunobiology and immunotherapy. World Journal of Immunology, 4, 88–97. [Google Scholar]
- Pal, M. , & Mahendra, R. (2014). Pythiosis: An emerging oomycetic disease of humans and animals. International Journal of Livestock Research, 4(6), 1–9. [Google Scholar]
- Papich, M. G. (2016). Terbinafine hydrochloride. In: Papich M. G. (Ed.), Saunders handbook of veterinary drug small and large animal (4th ed., pp. 764–765). Elsevier. [Google Scholar]
- Parambeth, J. C. , Lawhon, S. D. , Mansell, J. , Wu, J. , Clark, S. D. , Sutton, D. , Gibas, C. , Wiederhold, N. P. , Myers, A. N. , Johnson, M. C. , Levine, G. J. , Schlemmer, S. , Ruoff, C. , Isaiah, A. , & Creevy, K. E. (2019). Gastrointestinal pythiosis with concurrent presumptive gastrointestinal basidiobolomycosis in a Boxer dog. Veterinary Clinical Pathology, 48, 83–88. [DOI] [PubMed] [Google Scholar]
- Pereira, D. I. , Botton, S. A. , Azevedo, M. I. , Motta, M. A. A. , Lobo, R. R. , Soares, M. P. , Fonseca, A. O. S. , Jesus, F. P. K. , Alves, S. H. , & Santurio, J. M. (2013). Canine gastrointestinal pythiosis treatment by combined antifungal and immunotherapy and review of published studies. Mycopathologia, 176, 309–315. [DOI] [PubMed] [Google Scholar]
- Reagan, K. L. , Marks, S. L. , Pesavento, P. A. , Della Maggiore, A. , Zhu, B. Y. , & Grooters, A. M. (2019). Successful management of 3 dogs with colonic pythiosis using itraconazole, terbinafine, and prednisolone. Journal of the American Veterinary Medical Association, 33, 1434–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt, C. W. , Phelps, M. S. , Torres, B. T. , Bell, D. , Uhl, E. W. , Zimmerman, S. , Epstein, J. , & Cornell, K. K. (2012). Treatment of intestinal pythiosis in a dog with a combination of marginal excision chemotherapy, and immunotherapy. Journal of the American Veterinary Medical Association, 241, 358–363. [DOI] [PubMed] [Google Scholar]
- Susanengrat, N. , Torvorapanit, P. , Plongla, R. , Chuleerarux, N. , Manothummetha, K. , Tuangsirisup, J. , Worasilchai, N. , Chindamporn, A. , & Permpalung, N. (2019). Adjunctive antibacterial agents as a salvage therapy in relapsed vascular pythiosis patients. International Journal of Infectious Disease, 88, 27–30. [DOI] [PubMed] [Google Scholar]
- Tvedten, H. (2012). Listing of selected reference values. In: Willard M. D., & Tvedten H. (Eds.), Small animal clinical diagnosis by laboratory methods (5th ed., pp. 399–400). Elsevier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results of this case report are available as part of the articles and its Supporting Information files.


