Abstract
Background
As a kind of flavonoid, baicalin (C21H18O11) is extracted from Scutellaria baicalensis Georgi, the extract of which can be added to animal feed in China.
Objectives
The present review will describe the current understanding of the pharmacological effects of baicalin in the regulation of inflammation, oxidative stress anti‐virus and anti‐tumour responses.
Methods
We highlight emerging literature that the application in livestock health and performance, the biological activities, the molecular mechanisms and the dosage forms of baicalin by analysing and summarising the main points of the cited literatures.
Results
It is found that baicalin can improve the functions of multiple physiological systems. Baicalin has a strong anti‐inflammatory effect by regulating TLR4‐NFκB‐MAPK signalling pathway; it also can reduce oxidative stress by regulating Nrf2–Keap1 pathway; it can inhabit many kinds of virus such as influenza virus, respiratory virus, hepacivirus and others; it can also inhibit the growth of tumour cells by blocking the cell cycle or inducing apoptosis; and new dosage forms such as cationic solid lipid nanoparticles, cyclodextrin inclusion complexes or nanocrystalline can be applied to improve the deficiency of baicalin.
Conclusions
In summary, these studies have elucidated a comprehensive report on the anti‐inflammatory, anti‐oxidant, anti‐virus and anti‐tumour of baicalin, these findings thus indicated that baicalin can be used effectively to the field of animal production in future when the appropriate dosage form is determined.
Keywords: anti‐inflammatory, anti‐oxidant, anti‐tumour, anti‐viral, baicalin
This current review offers a comprehensive report on the anti‐inflammatory, anti‐oxidant, anti‐viral and anti‐tumor of baicalin, the application of new dosage forms of baicalin to the development progress which would be an effective medicine for clinical application.
Baicalin (C21H18O11) is a flavonoid extracted from the dried roots of Scutellaria baicalensis Georgi. The species can be planted and produced in most provinces of northern China with relatively sufficient resources. Baicalin is the main active component of Scutellaria baicalensis, and it possesses several biological effects, such as anti‐inflammatory (Yanqiu et al., 2019), anti‐oxidant (Yutao et al., 2017), anti‐viral (Jia et al., 2016) and anti‐tumour (Yuan et al., 2015) activity. Other pharmacological actions of the compound include anti‐bacterial activity, heat clearing, and detoxification (Bin et al., 2019). In this paper, the molecular mechanisms of the anti‐inflammatory, anti‐oxidant and anti‐tumour effects of baicalin, as well as the relationships among inflammation, oxidative stress, and cancer metastasis, are summarised to clarify the targets of baicalin and facilitate its clinical use.
1. APPLICATION OF BAICALIN IN LIVESTOCK HEALTH AND PERFORMANCE
Baicalin is a natural compound with multiple biological functions, such as anti‐inflammatory, anti‐oxidant, anti‐viral and so on. Though the effects and mechanisms of baicalin playing biological functions have been explicated successfully, there were few review about the application of baicalin for animal husbandry production. At present, it has been proved that baicalin can improve the functions of multiple physiological systems (respiratory system, digestive system, cardiovascular system, etc.) by the ways of anti‐inflammatory, anti‐oxidant or anti‐viral to protect pigs, cattle, birds, fish and others. Moreover, according to regulations of Ministry of Agriculture and Rural Development of the People's Republic of China, the extract of Scutellaria baicalensis Georgi (the content of baicalin is more than 85%) plays the role of anti‐inflammatory, bacteriostatic and growth promotion, which can be added to animal feed as a medicine to improve the growth performance of broilers and weaned piglets. So, it can be the anticipated that baicalin would be used effectively to the field of animal production in future. The therapeutic effects of baicalin on animal husbandry production are shown below (Table 1).
TABLE 1.
Therapeutic effect of baicalin on livestock and poultry diseases
| Species | Pathogen infections | Pathological system | Action mechanism | Treatment dose and time |
|---|---|---|---|---|
| Pig | G. parasuis | Cardiovascular system |
Anti‐inflammation Anti‐apoptotic (Fu et al., 2020) |
50–100 mg/kg for 7 days |
| Peritoneal system | Protect the integrity of peritoneal tight junction protein (Liu et al., 2021) | 50–100 mg/kg for 8 days | ||
| Deoxynivalenol | Intestinal system |
Anti‐oxidative stress Anti‐inflammation Protect the structural integrity of Intestinal mucosa (Liao et al., 2020) |
1% of basic diet for 14 days | |
| Chicken | Mycoplasma gallisepticum |
Respiratory system (lung and tracheal) |
Anti‐inflammatory Restoring energy or phenylalanine metabolism regulating the intestinal microbiota (Wang et al., 2021; Zou et al., 2021; Ishfaq et al., 2019; Ishfaq et al., 2021) |
450 mg/kg for 5–7 days |
|
Immune system (bursa of fabricius, thymus, spleen etc.) |
Anti‐inflammation Anti‐oxidative stress Anti‐autophagy Anti‐apoptosis (Li et al., 2019; Ishfaq et al., 2019; Zhang et al., 2020) |
450 mg/kg for 5–7 days | ||
| Zearalenone | Metabolic system (liver and kidney) |
Anti‐inflammation Anti‐oxidative stress (Xu et al., 2021) |
20–80 mg/kg for 7 days | |
| Avian pathogenic Escherichia coli |
Respiratory system (lung and tracheal) |
Anti‐inflammation Regulating microorganism (Cheng et al., 2022, Peng et al., 2021) |
50–200 mg/kg for 7 days | |
| Liver system | Anti‐inflammation | 50–200 mg/kg for 7 days | ||
| Digestive system (intestine) |
Anti‐inflammation Improve intestinal barrier function (Peng et al., 2019; Peng et al., 2019) |
10–20 mg/kg for 15 days | ||
| H9N2 |
Respiratory system (lung and tracheal) |
Anti‐bacterial Anti‐inflammation (Wu et al., 2019) |
0.05% of basic diet for 12 days | |
| Cow | Milk quality | Lactation system |
Anti‐inflammation Reduce the number of Somatic cells Increase lactation (Olagaray et al., 2019) |
3.3 g/day for 120 days |
| Duck | Duck virus hepatitis | Liver system |
Anti‐inflammation Anti‐oxidative stress Inhibit virus replication (Su et al., 2021; Chen et al., 2019; Chen et al., 2018; Chen et al., 2018) |
3 mg/kg for 3 days |
| Fish | H2O2 | Tilapia liver system |
Anti‐oxidative stress Promote growth (Jia et al., 2021) |
0.8–1.6 mg/kg for 60 days |
| Thioacetamide | Zebrafish liver system |
Anti‐inflammation Anti‐oxidative stress Anti‐apoptotic Promote liver growth and development (Zhang et al., 2020) |
10 mg/kg for 2 days |
2. ANTI‐INFLAMMATORY EFFECTS OF BAICALIN AND ITS MOLECULAR MECHANISMS
Inflammation is a process by which the body resists foreign pathogens. However, excessive inflammation can lead to tissue damage. Inflammatory reactions have roles in several diseases in organisms. Acute inflammatory reaction is generally the attack period of the course of disease. This process involves the release of a large number of inflammatory mediators, causing symptoms such as swelling and pain (Zeng et al., 2020).The acute aggravation of allergic respiratory tract inflammation caused by particulate matter results in extensive inflammatory factor secretion, which leads to respiratory oedema (Dai et al., 2020). Chronic low‐grade inflammation may occur in various aging tissues (Franceschi & Campisi, 2014). The types of tissue damage associated with excessive inflammation include intestinal immune cell damage following excessive tumour necrosis factor alpha (TNF‐α) release, which leads to intestinal barrier dysfunction (Soderholm, 2002).
Baicalin has a strong anti‐inflammatory effect. In mice, baicalin can protect the intestinal epithelium of rats by inhibiting the secretion of inflammatory factors (Jian et al., 2015). Baicalin can suppress the inflammatory response in the liver and reduce cell death (Huilian et al., 2020). Baicalin can also effectively suppress lipopolysaccharide (LPS)‐induced liver inflammation in poultry (Cheng et al., 2017). At the cellular level, baicalin can enhance the cytotoxic effect of neutrophils on Staphylococcus aureus and effectively suppress the inflammatory response in bovine mammary epithelial cells (Jia et al., 2020). Baicalin can protect the intestinal mucosal barrier by blocking the adhesion of pathogenic Escherichia coli to porcine small intestinal epithelial cells, and reducing the inflammatory response in cells (Liu et al., 2019). Baicalin can exert inhibitory effects on E. coli by inhibiting the biological activity of ATP synthase of E. coli. This function is important for maintaining the integrity of the intestinal mucosal barrier (Chinnam et al., 2010). Baicalin improved cell viability, suppress apoptosis (Guo et al., 2014), and inhibit the production of the inflammatory factors TNF‐α, interleukin (IL‐6 and IL‐8) in a mouse mammary epithelial cell mastitis model (Yang et al., 2018). These studies illustrated that baicalin, as a natural plant extract, has a good therapeutic effect on inflammation induced by inflammatory diseases or in vitro treatments.
The Toll‐like receptor 4 (TLR4) NF‐κB signalling pathway plays an extremely important role in the regulation of inflammation in the body. TLR4, a member of the TLR family that is expressed on the surface of cell membranes, can recognise cascades of extracellular stimuli that ultimately lead to inflammation (Wada & Makino, 2016). NF‐κB is a pleiotropic regulator of a variety of damage response genes. In normal cells, NF‐κB binds to inhibitor of kappa B (IκB) and exists in the cytoplasm. Following stimulation, IκB is phosphorylated and degraded, which leads to NF‐κB activation. Activated NF‐κB binds to p65/p50 proteins and enters the nucleus in the form of heterodimers to bind to corresponding target sites on DNA, thereby regulating downstream pro‐inflammatory factors (TNF‐α, IL‐1β, chemokines and adhesion factors) (Sumner et al., 2014).
In vivo, baicalin can reduce the mRNA and protein expression of TLR2/4 and reduce the expression of NF‐κB, p65, cyclooxygenase‐2, TNF‐α and IL‐6 in serum (Tu et al., 2011). Baicalin (Yan et al., 2019) can inhibit the protein expression of p‐p65 and p‐extracellular signal‐related kinase (ERK) and promote the expression of p‐AKT and p‐endothelial nitric oxide synthase in rats. Baicalin can inhibit the TLR–NF‐κB signalling pathway to alleviate experimental colitis and hepatic inflammation in chicken (Heng Zhai, 2019; Zhang et al., 2013). Baicalin can improve chronic gastritis and cerebral ischaemic inflammation in rats (Wanli et al., 2019). In vitro, baicalin can suppress the inflammatory injury of porcine intestinal epithelial cells by inhibiting the NF‐κB signalling pathway (Zhongqing et al., 2019). Baicalin has obvious therapeutic effect on the inflammatory response induced by LPS in human cardiomyocytes. Baicalin significantly downregulated TLR4, p‐NF‐κB p65, p‐p38 MAPK and p‐IκB protein expression in prior research (Xiangyu et al., 2020). Cui et al. (2014) found that baicalin can block TLR4–NF‐κB pathway activation and inhibit the inflammatory response to LPS in RAW264.7 cells. Baicalin can significantly inhibit inflammatory responses in vivo and at the cellular level in animals. Baicalin can inhibit the upregulation of TLR4 signalling on the cell surface and thus block the production of inflammatory mediators. From these findings, it can be stated that the main target of baicalin as an anti‐inflammatory agent is TLR4, and inhibition of p38 phosphorylation (Liu et al., 2020) might have a key anti‐inflammatory role.
The MAPK signalling pathway is a key signalling pathway that promotes inflammation, and p38 is one of the main effectors of this pathway (Gu et al., 2017). Phosphorylated p38 activates Janus kinase (JNK) and NF‐κB (Wang et al., 2018); thus, p38 phosphorylation can be an important indicator of inflammation. Baicalin exerts anti‐inflammatory effects by inhibiting the phosphorylation of p38 and thereby inhibiting the expression of related inflammatory factors (Winkler et al., 2017). For example, baicalin can reduce chronic inflammation in atherosclerosis by inhibiting p38 expression (Wu et al., 2018). Therefore, baicalin can regulate downstream signalling factors to exert anti‐inflammatory effects through the TLR4–NF‐κB and p38 MAPK signalling pathways, and its possible target sites are TLR4 and p38.
Baicalin has been proven to have anti‐microbial effects in particular. some studies illustrated that baicalin can alleviate the pulmonary symptoms caused by S. aureus in mice (Luo et al., 2017). Baicalin can significantly inhibit the biological activity of E. coli cultured in vitro (Luo et al., 2017). Baicalin also has a significant inhibitory effect on drug‐resistant S. aureus (Zhang et al., 2020) and Helicobacter pylori (Wu et al., 2013). For drug‐resistant bacteria, baicalin can be used as an SOS inhibitor, which can significantly reduce the resistance mutation rate of Staphylococcus aureus to rifampicin induced by ciprofloxacin (Peng et al., 2011). For fungi, baicalin has a good inhibitory effect on Candida albicans (Dai et al., 2009). The invasion of bacterial pathogens into the body has been proved to be one of the important factors leading to inflammatory response (Ikuse et al., 2019), The antibacterial activity of baicalin is an important factor for baicalin to play a role in the treatment of inflammatory response.
3. ANTI‐OXIDANT EFFECTS OF BAICALIN AND ITS MOLECULAR MECHANISMS
Oxidative stress can cause tissue damage, and it occurs when free radicals attack tissue or cells (Kattoor et al., 2017). Oxidative stress is significantly associated with tumourigenesis (Chikara et al., 2018), aging (Zhang et al., 2018), pulmonary fibrosis (Gross & Hunninghake, 2001), Alzheimer's disease (Kamat et al., 2016), diabetes (Mccracken et al., 2018), depression (Sumiao & Xingbing, 2019) and other diseases. Under normal conditions, the levels of free radicals and anti‐oxidants are in a dynamic state of balance. When this balance is destroyed by some cause (e.g., acute hypoxia, drug toxicity, acute inflammatory reaction), the increased content of oxygen‐rich active factors can lead to damage in the internal environment, including damage to DNA mitochondrial organelles (Fandy et al., 2014). Baicalin can scavenge free radicals and reactive oxygen species (ROS), inhibit oxidase activity, and enhance the expression of anti‐oxidant and detoxification enzymes (Shi et al., 2018). Baicalin can reduce oxidative stress injury in trabecular meshwork cells in vitro by reducing oxidative stress, suppressing the activity of senescence‐associated beta‐galactosidase, and downregulating carboxylated proteins (Gong & Zhu, 2018). Baicalin can improve oxidative stress induced by acute lung injury in mice through Nrf2‐mediated haeme oxidase (HO‐1) signalling (Meng et al., 2019).
The nuclear factor erythroid 2‐related factor 2 (Nrf2)–Kelch like‐ECH‐associated protein (Keap1) pathway, a key signalling pathway in oxidative stress responses, has a significant anti‐oxidant effect that is critical for cellular protection and cell survival. Under normal conditions, the Nrf2–Keap1 complex binds to actin in the cytoplasm. Nrf2–Keap1 is dissociated when oxidative stress occurs, and Nrf2 translocates to the nucleus, in which it binds anti‐oxidative response elements and induces the synthesis of corresponding detoxification and anti‐oxidant enzymes (Li et al., 2014; Tong et al., 2006). Baicalin has strong anti‐oxidant effects, and it induces the nuclear translocation of Nrf2 (Draheim et al., 2016; Xue et al., 2017). Baicalin has a good effect against diseases caused by oxidative stress in animals. For example, baicalin can improve liver fibrosis in mice by regulating oxidative stress and Nrf2 (Cui et al., 2014). The compound can inhibit oxidative stress through Nrf2‐mediated HO‐1 signalling, thereby alleviating acute lung injury induced by LPS in mice. The primary effect of baicalin on lung injury involves its direct activation of Nrf2, which regulates downstream anti‐oxidant factors (Meng et al., 2019). Baicalin can improve insulin resistance in the livers of obese mice by increasing Nrf2 expression and reducing oxidative stress (Cui et al., 2014). Baicalin can improve oxidative damage in the oviduct in mice under heat stress (Xue et al., 2017). Therefore, these studies indicated that baicalin can reduce damage associated with diseases caused by oxidative stress by improving the oxidative stress response.
P38 MAPK phosphorylates IκB (Shen et al., 2019). There are important relationships between oxidative stress and inflammatory responses. The in vitro stimulation of inflammatory responses, such as those induced by LPS through TLRs, leads to NF‐κB activation. Activated NF‐κB forms a complex with p65 and p50 in the nucleus to regulate the secretion of anti‐inflammatory factors. In the presence of oxidative stress, the increased production of ROS promotes the activation of p38 MAPK and its downstream factors in the nucleus, which regulate the secretion of related signalling factors and form a negative feedback regulation to reduce intracellular ROS levels (Zhangzhao, 2019). Therefore, the p38 MAPK signalling pathway may be an important cascade for the interaction between inflammatory responses and oxidative stress. As a member of the MAPK family, p38 MAPK regulates a variety of intracellular signalling factors. The protein levels of the phosphorylated forms of NF‐κB, p38 MAPK, and IκB are significantly increased and those of IκB are decreased during the inflammatory response. Therefore, the inflammatory response is considered important in the regulation of MAPK signalling molecules.
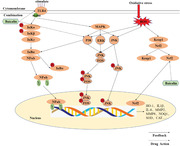
Baicalin reduces inflammatory and oxidative stress responses through the p38 MAPK signalling pathway, thereby improving insulin resistance in the livers of obese mice (Fang et al., 2019). As a therapeutic agent, baicalin can reduce both inflammatory responses and oxidative stress. Baicalin can treat liver fibrosis in mice by regulating inflammatory responses, oxidative stress and Nrf2 (Cui et al., 2014). Baicalin can inhibit oxidative stress and inflammation through the Nrf2‐mediated HO‐1 signalling pathway, which leads to improvements of acute lung injury induced by LPS in mice (Meng et al., 2019). Baicalin can improve insulin resistance in the livers of obese mice by increasing Nrf2 expression and reducing oxidative stress and inflammatory responses (Cui et al., 2014). In summary, the pharmacological action of baicalin involves the combination of multiple pathways targeting both inflammatory responses and oxidative stress. Its molecular pathways interact with each other through MAPK, NF‐κB and other signalling factors (Figure 1).
FIGURE 1.
Molecular mechanism of the effects of baicalin on inflammation and oxidation
Baicalin can play a therapeutic role on cardiovascular diseases through antioxidant biological function. Baicalin can ameliorate acute myocardial infarction in rats induced by isoproterenol by inhibiting nitric oxide synthase, oxidative stress, and inflammation (Sun et al., 2015). Baicalin can reduce myocardial ischaemic injury in rats by reversing the inflammatory response through inducing the inactivation of aryl hydrocarbon receptor (Xue et al., 2015). In a rabbit atherosclerosis model, baicalin reduced lesion size and improved arterial elasticity (He et al., 2016). At present, the effects of baicalin on cardiovascular disease are being increasingly studied, but little research has clarified its mechanism of action.
4. ANTI‐VIRAL EFFECTS OF BAICALIN
Baicalin has been proven to have and anti‐viral effects (Xuexue et al., 2020). In particular, baicalin can effectively inhibit the growth of human herpes virus type 6 (Chen & Zhang, 2012). Baicalin has obvious therapeutic effects on viral myocarditis in humans, and its therapeutic mechanism may be related to miR21 (Liu et al., 2020). Baicalin can inhibit the activity of H1N1/H3N2 influenza viruses in mice at the cellular level and in vivo (Ding et al., 2014) and its mechanism may involve the suppression of neuraminidase activity (Jin et al., 2018). In terms of anti‐microbial activity for hepacivirus, the combination of baicalin and ETV exhibited significant efficacy in HepG2 cells infected with anti Na HBVrtM204V/rtLI80M, which blocked the transcription of HBV‐RNAs by inhibiting the transcription and expression of HNF1α and HNF4α, which are required for HBV replication, thereby inhibiting the synthesis of HBV RNAs, viral protein templates and HBV‐DNA (Huang et al., 2017). For coxsackievirus, in the CVB3 constructed mouse myocarditis model, baicalin could exert biological functions of inhibiting the replication of CVB3 by inhibiting the activation of Akt and p38 (Fu et al., 2019). Baicalin could significantly inhibit the formation of autophagosomes and increase lipid content in HeLa cells induced by CVB3 infection (Wang et al., 2020). For respiratory synthetic virus (RSV), in a mouse model of RSV infection, baicalin can reduce T lymphocyte infiltration and pro‐inflammatory factor gene expression caused by RSV infection, inhibiting RSV through anti‐inflammatory and antiviral effects (Shi et al., 2016).
Studies have shown that baicalin can inhibit neuraminidase activity, so it has obvious anti influenza virus activity in cells and mouse models and shows dose‐dependent tolerance (Ding et al., 2014). Baicalin can promote the production of IFN‐ γ. Thus it showed anti influenza virus activity in mice (Hai‐Yan et al., 2015). For human immunodeficiency virus 1 (HIV 1), baicalin can inhibit the replication of HIV‐1 virus by inhibiting the expression of HIV‐1 specific core antigen p24 and the activity of reverse transcriptase (Li et al., 1993; Zhang et al., 1991). Baicalin can stimulate the body to produce nonspecific antibodies to vascular stomatitis virus (VSV) by regulating the release of cytokines and innate immune translation (Orzechowska et al., 2014). In addition, baicalin has inhibitory effects on human cytomegalovirus (Evers et al., 2006), Zika virus (Oo et al., 2019), chikungunya virus (Oo et al., 2017), tick borne encephalitis virus (Leonova et al., 2020) and respiratory syncytial (Shi et al., 2016) virus in vitro.
Baicalin can effectively protect against viruses through direct virucidal, inhibition of viral replication, regulation of functional protein expression in host cells, and anti‐inflammatory mechanisms. At present, there are few studies about baicalin as a clinical anti‐viral treatment, and the specific mechanism of its biological function has not been extensively studied. Therefore, further research is needed to clarify the anti‐viral properties of baicalin.
5. ANTI‐TUMOUR EFFECTS OF BAICALIN
Tumours are masses of abnormal cells in the body that grow in large numbers, and they can be benign or malignant. Malignant tumours cause serious damage, and they are the second leading cause of death globally (Siegel et al., 2019). Increasing attention has been paid to the anti‐tumour effects of baicalin (Kim & Lee, 2012). The anti‐tumour effects of baicalin mainly include inhibiting the proliferation of tumour cells by blocking the cell cycle, inducing apoptosis in tumour cells by producing cytotoxicity (Chao et al., 2007; Qinfeng, 2016), and suppressing the erosion and metastasis of tumour cells (Wang et al., 2017).
The cell cycle refers to the process of eukaryotic cells from the end of one round of mitosis to the next, and it is generally divided into G1, S, G2, M and other stages. Cancerous cells usually have abnormal cell division cycles (Champeris Tsaniras et al., 2014). Cell cycle proteins (cyclins) are important in the normal regulation of the cell cycle. Reports indicated that baicalin can block cell cycle progression in S phase by suppressing cyclin A, whereas in SK‐LU1, SK‐MES‐1 and DU145 cells, baicalin suppresses the expression of cyclin D1, which leads to cell cycle arrest in G1 phase (Gao et al., 2011; Narushima et al., 2016). Cyclin‐dependent kinase 2 (CDK2) is an important protein regulating cell cycle progression. It has been demonstrated that CDK2 can inhibit the phosphorylation of apoptotic proteins, thereby promoting the apoptosis of tumour cells (Wall et al., 2003). Meanwhile, baicalin inhibited the proliferation of T‐GH8301 and BFTC905 bladder cancer cells by suppressing the activity of the CDK2–cyclin B1 complex (Chao et al., 2007) (Figure 2).
FIGURE 2.
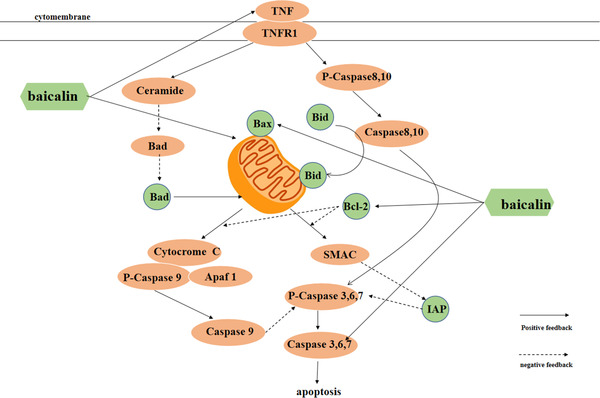
Molecular mechanism of the effects of baicalin on the mitochondrial apoptosis pathway
The JAK–signal transducer and activator of transcription (STAT) signalling pathway plays an important role in cell growth and apoptosis by promoting cell cycle progression and inhibiting cell apoptosis (Booz et al., 2002). Abnormal activation of the signalling pathway occurs in a variety of tumour cells, and it is believed that the JAK–STAT signalling pathway may be related to the abnormal cell cycle regulation of tumour cells (Wang et al., 2019). Baicalin can also participate in the regulation of the cell cycle in tumours through a variety of other regulatory signalling pathways. For example, baicalin can inhibit tumour growth through the phosphoinositide 3‐kinase (PI3K)–AKT signalling pathway (Zhaoqin et al., 2017) and the JAK‐STAT signalling pathway (Guoli & Jinfeng, 2011). Baicalin inhibits the activity of P13K (Kong et al., 2011), thereby suppressing the phosphorylation of AKT in tumour cells (Xu et al., 2017). Activation of the P13K–AKT signalling pathway might lead to cell cycle dysregulation or inhibition of apoptosis, and this signalling pathway is abnormally activated in a variety of tumour cells (Kong et al., 2011). Baicalin can significantly inhibit the activation of STAT signalling factors in human liver cancer cells SMMC‐7721 (Guoli & Jinfeng, 2011); therefore, it is believed that baicalin may inhibit the growth of tumour cells through the JAK–STAT signalling pathway.
Apoptosis is a complex physiological process that is influenced and regulated by many factors, including the death receptor‐mediated exogenous and mitochondria‐mediated endogenous pathways. The exogenous pathway mainly induces apoptosis by through the death acceptor Fas and TNF. Mitochondrial pathways are regulated by multiple factors. For example, pro‐apoptotic factors such as Bad (Xiao‐Ping et al., 2016) and cytochrome c (Li et al., 2000) can induce a series of cascade reactions of cysteine proteases (caspases) leading to apoptosis, and caspases are generally the ultimate inducers of cell apoptosis. The two pathways of apoptosis are not independent of each other, and they can both influence each other and eventually induce caspase‐3 as the final factor that induces apoptosis (Choudhary et al., 2015). It has been reported that baicalin can downregulate the expression of the anti‐apoptotic protein Bcl‐2 in various cancer cells (Shasha et al., 2019) and upregulate Bad expression and promote the release of cytochrome c in mitochondria to promote apoptosis in cancer cells (Zheng et al., 2012). The expression of caspase‐3 is significantly increased in gastric cancer cells treated with baicalin, which significantly elevates the apoptosis rate (Qinfeng, 2016). Baicalin can inhibit QBC939 cell growth by regulating the expression of caspase‐3 (Yang, 2020). These findings suggest that baicalin can synergistically increase the expression of caspase‐3 as an apoptotic factor through exogenous and mitochondrial pathways, thereby promoting apoptosis in tumour cells (Figure 3).
FIGURE 3.

Molecular mechanism of the effects of baicalin on cell cycle pathways
The invasion and metastasis of tumour cells to other normal tissues are important processes in tumour development. Matrix metalloproteinases (MMPs) and their inhibitors metalloproteinase tissue inhibitors (TIMPs) play important roles in these processes. Abnormal expression of MMPs and TIMPs may lead to the metastasis of cancer cells and poor prognoses (Huang et al., 2019; Kosaka et al., 2016). It has been reported that baicalin inhibits the metastasis of HeLa cervical cancer cells by downregulating the expression of MMP‐2/MMP‐9 through the p38 MAPK signalling pathway (Yue et al., 2016).
NF‐κB has been revealed to inhibit tumour cell metastasis and prevent immune avoidance (Dolcet et al., 2005; Hoesel & Schmid, 2013). Baicalin has a significant activating effect on NF‐κB, and dose‐dependent reductions of NF‐κB p65 phosphorylation induced by baicalin significantly inhibit breast cancer progression (Zhao et al., 2019). There is evidence that NF‐κB has an important influence on the formation of tumour cells. At the same time, NF‐κB influences cell cycle progression and apoptosis in tumour cells (Greten, 2004). Baicalin can inhibit the effects of TLR4–NF‐κB pathway activation (Jin et al., 2019) thereby affecting tumour cells. However, research on the effects of baicalin on tumour cells through the NF‐κB pathway is relatively sparse.
As a phytochemical extract, baicalin can influence the tumour cell cycle through the JAK–STAT and P13K–AKT signalling pathways, activate caspase‐3 to promote the apoptosis of tumour cells, and inhibit the migration and invasion of tumour cells through MMP‐2, MMP‐9 and other factors. These findings indicate that baicalin has great applicability as an anti‐cancer agent.
6. NEW DOSAGE FORM OF BAICALIN
Flavonoids have the characteristics of poor water solubility and low bioavailability (Badshah et al., 2021). Baicalin, as a flavonoid, also has the characteristics of poor water solubility and low bioavailability (Li et al., 2019) (Ikuse et al., 2019); therefore, the modification or coating of baicalin has important application value. Baicalin has a good neuroprotective effect, but it is eliminated quickly in the body and does not easily reach the brain (Cao et al., 2011). The research shows that cationic baicalin solid lipid nanoparticles modified with OX26 antibody and polyethylene glycol (PEG) can significantly increase the content of Baicalin in mouse spine. The principle is that the binding of antibody, surface PEGylation and the reduction of drug loaded particle size are conducive to improve the bioavailability of baicalin, and the cationic polymer is used as the carrier to make the surface of SLN positively charged, which can promote drug uptake by cells. In addition, because there are many transferrin receptors at the top of the blood–brain barrier, SLN binding OX26 has more advantages in the ability to pass through BBB (Liu et al., 2015). Borneol–Baicalin–liposomes can significantly improve the pharmacokinetic parameters and half‐life time of bacialin compared with bacialin alone, and can significantly promote the drug effect (Zhang et al., 2020). Baicalin's cyclodextrin inclusion complexes have the characteristics of improving its water solubility and bioavailability and also have research prospects (Jakab et al., 2019). The inclusion process of baicalin and cyclodextrin only has physical changes without changing its chemical properties. In the process of forming the inclusion, the hydrophobic benzene ring of baicalin is embedded into the cavity of cyclodextrin, which greatly improves the water solubility of baicalin (Jing et al., 2017). Nanocrystalline treatment of baicalin can significantly improve the bioavailability and water solubility of oral baicalin (Xie et al., 2019). Baicalin through a novel mesoporous carbon nanopowder drug carrier has better water solubility and bioavailability than baicalin monomer in rats (Li et al., 2016). Baicalin phospholipid complex has better cell transfer performance and can significantly improve its bioavailability (Li et al., 2012). At the same time, studies have shown that baicalin phospholipid complex has good antiviral performance for duck hepatitis A virus type 1 (Chen et al., 2018). Baicalin gel delivery system triggered by pH can be used to improve the bioavailability of ophthalmic drug because of its slow release effect. After topical injection of the drug delivery system, the local pH environment (35°C, pH 6.8) will change the liquid from the liquid to the viscous gel to prolong the contact time, thereby improving the pharmacokinetic parameters such as AUC, Cmax and t1/2 of baicalin (Wu et al., 2012).
Researchers have prepared it into new drug‐delivery systems such as solid lipid nanoparticles, nanocrystals, solid dispersions and phospholipid complexes. β‐Cyclodextrin inclusion complex has attracted extensive attention in improving the water solubility, bioavailability and stability of insoluble drugs. Its liposomes have the advantages of controlling drug release, prolonging drug half‐life and improving drug bioavailability. However, there are few studies on how to combine the new drug delivery system with various inclusion complexes to further improve the bioavailability of baicalin, which may be the focus of the next research.
7. CONCLUSION
This review offers a comprehensive report on the anti‐inflammatory, anti ‐oxidant, anti‐virus and anti‐tumour of baicalin. Baicalin has a strong anti‐inflammatory effect, and it may regulate the inflammation reaction through TLR4/NF‐κB signalling pathway. Baicalin has a strong anti‐oxidative effect, while the Nrf2‐ HO1 signalling pathway maybe an important target. In the process of inflammation and oxidative stress, MAPKs may function as an crosstalk target to mediate NF‐κB and Nrf2‐HO‐1 signalling pathways. Baicalin can also inhibit the growth of tumour cells, suppress tumour metastasis, play anti‐viral and anti‐bacterial effects and so on. The water solubility and bioavailability of baicalin can be significantly improved after preparation treatment or coating treatment, so as to give better play to its biological activity in organisms. It can be expected that baicalin would be an effective medicine for clinical application.
AUTHOR CONTRIBUTIONS
M‐LB: Conceptualisation, writing original draft and writing review & editing. M‐YF: Writing review & editing. LM and S‐XY: Investigation. J‐XH and Y‐YH: Project administration and supervision. L‐XX: Conceptualisation, writing original draft, writing review & editing, funding acquisition and resources.
CONFLICT OF INTEREST
All of the authors declare no conflict of interests.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journals author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.960.
ACKNOWLEDGEMENTS
Thank Dr. Xiaoxi Liu for his guidance and supervision of this article. Thank Professor Yunfei Ma for her suggestions on the revision and language polish of the article. Thank Professor Xianghong Ju for his team support. This work was supported by grants from the National Natural Science Foundation of China (No. 31902314), the Nanhai Scholars Program of Guangdong Ocean University (No. 002029002005) and the Program for scientific research start‐up funds of Guangdong Ocean University (101402/R17088), the Natural Science Foundation of Guangdong Province, China (No. 2019A1515011142), Shenzhen Projects for Basic Research (JCYJ20190813142005766) and Project of Enhancing School with Innovation of Guangdong Ocean University (GDOU230419057).
Bao, M. , Ma, Y. , Liang, M. , Sun, X. , Ju, X. , Yong, Y. , & Liu, X. (2022). Research progress on pharmacological effects and new dosage forms of baicalin. Veterinary Medicine and Science, 8, 2773–2784. 10.1002/vms3.960
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research.
REFERENCES
- Badshah, S. L. , Faisal, S. , Muhammad, A. , Poulson, B. G. , Emwas, A. H. , & Jaremko, M. (2021). Antiviral activities of flavonoids. Biomedicine & Pharmacotherapy, 140, 111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin, G. , Qi, W. , Yong‐Qing, S. , Wen‐Hua, X. , Wei, L. , Yi‐Jun, Z. , & Jian‐Ya, Y. (2019). Effect of Baicalin on expression of inflammatory factors in rats with acute lung injury. Anatomy Research (China), 41(2), 119–123. [Google Scholar]
- Booz, G. W. , Day, J. N. E. , & Baker, K. M. (2002). Interplay between the cardiac renin angiotensin system and JAK‐STAT signaling: Role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. Journal of Molecular and Cellular Cardiology, 34(11), 1443–1453. [DOI] [PubMed] [Google Scholar]
- Cao, Y. , Mao, X. , Sun, C. , Zheng, P. , Gao, J. , Wang, X. , Min, D. , Sun, H. , Xie, N. I. , & Cai, J. (2011). Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti‐oxidative and anti‐apoptotic pathways. Brain Research Bulletin, 85(6), 396–402. [DOI] [PubMed] [Google Scholar]
- Champeris Tsaniras, S. , Kanellakis, N. , Symeonidou, I. E. , Nikolopoulou, P. , Lygerou, Z. , & Taraviras, S. (2014). Licensing of DNA replication, cancer, pluripotency and differentiation: An interlinked world? Seminars in Cell & Developmental Biology, 30, 174–180. [DOI] [PubMed] [Google Scholar]
- Chao, J.‐I. , Su, W.‐C. , & Liu, H.‐F. (2007). Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen‐activated protein kinase and AKT. Molecular Cancer Therapeutics, 6(11), 3039. [DOI] [PubMed] [Google Scholar]
- Chen, X. I. , & Zhang, C. (2012). Inhibitory role of baicalin on human herpes virus type 6 in Vitro. Procedia Engineering, 37, 75–78. [Google Scholar]
- Chen, Y. , Yang, Y. , Wang, F. , Yang, X. , Yao, F. , Ming, K. , Yuan, W. , Zeng, L. , & Liu, J. (2018). Antiviral effect of baicalin phospholipid complex against duck hepatitis A virus type 1. Poultry Science, 97(8), 2722–2732. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Yao, F. , Ming, K. , Shi, J. , Zeng, L. , Wang, D. , Wu, Y. , Hu, Y. , & Liu, J. (2019). Assessment of the effect of baicalin on duck virus hepatitis. 19, 376–386. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Yuan, W. , Yang, Y. , Yao, F. , Ming, K. E. , & Liu, J. (2018). Inhibition mechanisms of baicalin and its phospholipid complex against DHAV‐1 replication. Poultry Science, 97(11), 3816–3825. [DOI] [PubMed] [Google Scholar]
- Cheng, P. , Wang, T. , Li, W. , Muhammad, I. , Wang, H. E. , Sun, X. , Yang, Y. , Li, J. , Xiao, T. , & Zhang, X. (2017). Baicalin alleviates lipopolysaccharide‐induced liver inflammation in chicken by suppressing TLR4‐mediated NF‐κB Pathway. Frontiers in Pharmacology, 8, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Cao, Z. , Luo, J. , Hu, R. , Cao, H. , Guo, X. , Xing, C. , Yang, F. , Zhuang, Y. U. , & Hu, G. (2022). Baicalin ameliorates APEC‐induced intestinal injury in chicks by inhibiting the PI3K/AKT‐mediated NF‐κB signaling pathway. Poultry Science, 101(1), 101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikara, S. , Nagaprashantha, L. D. , Singhal, J. , Horne, D. , Awasthi, S. , & Singhal, S. S. (2018). Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Letters, 413, 122–134. [DOI] [PubMed] [Google Scholar]
- Chinnam, N. , Dadi, P. K. , Sabri, S. A. , Ahmad, M. , Kabir, M. A. , & Ahmad, Z. (2010). Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. International Journal of Biological Macromolecules, 46(5), 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary, G. S. , Al‐Harbi, S. , & Almasan, A. (2015). Caspase‐3 activation is a critical determinant of genotoxic stress‐induced apoptosis. Methods in Molecular Biology, 1219, 1–9. [DOI] [PubMed] [Google Scholar]
- Cui, L. , Feng, L. , Zhang, Z. , & Jia, X. B. (2014). The anti‐inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF‐κB pathway activation. International Immunopharmacology, 23. [DOI] [PubMed] [Google Scholar]
- Dai, B. D. , Cao, Y. Y. , Shan, H. , Xu, Y. G. , Gao, P. H. , Wang, Y. , & Jiang, Y. Y. (2009). Baicalein induces programmed cell death in Candida albicans . Journal of Microbiology and Biotechnology, 19(8), 803–809. [PubMed] [Google Scholar]
- Dai, M. , Chen, F. , Wang, Y. , Wang, M. Z. , Lv, Y. X. , & Liu, R. Y. (2020). Particulate matters induce acute exacerbation of allergic airway inflammation via the TLR2/NF‐κB/NLRP3 signaling pathway. Toxicology Letters, 321, 146–154. [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Dou, J. , Teng, Z. , Yu, J. , Wang, T. , Lu, N. , Wang, H. , & Zhou, C. (2014). Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Archives of Virology, 159(12), 3269–3278. [DOI] [PubMed] [Google Scholar]
- Dolcet, X. , Llobet, D. , Pallares, J. , & Matias‐Guiu, X. (2005). NF‐kB in development and progression of human cancer. Virchows Archiv, 446(5), 475–482. [DOI] [PubMed] [Google Scholar]
- Draheim, T. , Liessem, A. , Scheld, M. , Wilms, F. , WeiãŸFlog, M. , Denecke, B. , Kensler, T. W. , Zendedel, A. , Beyer, C. , Kipp, M. , Wruck, C. J. , Fragoulis, A. , & Clarner, T. (2016). Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia, 64(12), 2219–2230. [DOI] [PubMed] [Google Scholar]
- Evers, D. L. , Chao, C.‐F. , Wang, X. , Zhang, Z. , Huong, S.‐M. , & Huang, E.‐S. (2006). Human cytomegalovirus‐inhibitory flavonoids: Studies on antiviral activity and mechanism of action. Antiviral Research, 68(3), 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandy, T. E. , Jiemjit, A. , Thakar, M. , Rhoden, P. , Suarez, L. , & Gore, S. D. (2014). Decitabine induces delayed reactive oxygen species (ROS) accumulation in leukemia cells and induces the expression of ROS generating enzymes. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 20(5), 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, P. , Sun, Y. , Gu, X. , Shi, M. , Bo, P. , Zhang, Z. , & Bu, L. (2019). Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC‐1α pathway. Phytomedicine, 64, 153074. [DOI] [PubMed] [Google Scholar]
- Franceschi, C. , & Campisi, J. (2014). Chronic inflammation (Inflammaging) and its potential contribution to age‐associated diseases. The Journals of Gerontology: Series A, 69(Suppl 1), S4–S9. [DOI] [PubMed] [Google Scholar]
- Fu, Q. , Gao, L. , Fu, X. , Meng, Q. , & Lu, Z. (2019). Scutellaria baicalensis inhibits coxsackievirus B3‐induced myocarditis via AKT and p38 pathways. Journal of Microbiology and Biotechnology, 29(8), 1230–1239. [DOI] [PubMed] [Google Scholar]
- Fu, S. , Yin, R. , Zuo, S. , Liu, J. , Zhang, Y. , Guo, L. , Qiu, Y. , Ye, C. , Liu, Y. U. , Wu, Z. , Hou, Y. , & Hu, C.‐A. A. (2020). The effects of baicalin on piglets challenged with Glaesserella parasuis . Veterinary Research, 51(1), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , Morgan, W. A. , Sanchez‐Medina, A. , & Corcoran, O. (2011). The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and bax in human lung cancer cells. Toxicology and Applied Pharmacology, 254(3), 221–228. [DOI] [PubMed] [Google Scholar]
- Gong, L. , & Zhu, J. (2018). Baicalin alleviates oxidative stress damage in trabecular meshwork cells in vitro. Naunyn‐Schmiedeberg's Archives of Pharmacology, 391(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Greten, F. (2004). The IKK/NF‐κB activation pathway – A target for prevention and treatment of cancer. Cancer Letters, 206(2), 193–199. [DOI] [PubMed] [Google Scholar]
- Gross, T. J. , & Hunninghake, G. W. (2001). Idiopathic pulmonary fibrosis. New England Journal of Medicine, 345(7), 517–525. [DOI] [PubMed] [Google Scholar]
- Gu, X. , Zhang, Q. , Du, Q. , Shen, H. , & Zhu, Z. (2017). Pinocembrin attenuates allergic airway inflammation via inhibition of NF‐κB pathway in mice. International Immunopharmacology, 53, 90–95. [DOI] [PubMed] [Google Scholar]
- Guo, M. , Cao, Y. , Wang, T. , Song, X. , Liu, Z. , Zhou, E. , Deng, X. , Zhang, N. , & Yang, Z. (2014). Baicalin inhibits Staphylococcus aureus‐induced apoptosis by regulating TLR2 and TLR2‐related apoptotic factors in the mouse mammary glands. European Journal of Pharmacology, 723, 481–488. [DOI] [PubMed] [Google Scholar]
- Guo, Q. , Xuan, M. F. , Luo, Z. B. , Wang, J. X. , Jin, S. S. , Yin, X. J. , & Kang, J. D. (2019). Baicalin improves IVM of pig oocytes and subsequent preimplantation embryo development by inhibiting apoptosis. Reproduction Fertility & Development, 31(5), 983–992. [DOI] [PubMed] [Google Scholar]
- Guo, Q. , Xuan, M.‐F. , Luo, Z.‐B. , Wang, J.‐X. , Han, S.‐Z. , Ri, M.‐H. , Choe, Y.‐G. , Hwang, K.‐M. , Yin, X.‐J. , & Kang, J.‐D. (2019). Baicalin improves the in‐vitro developmental capacity of pig embryos by inhibiting apoptosis, regulating mitochondrial activity and activating sonic hedgehog signaling. Molecular Human Reproduction, 25(9), 538–549. [DOI] [PubMed] [Google Scholar]
- Guoli, R. H. , & Jinfeng, Y. (2011). Effect of baicalin on the JAKSTAT3 signaling pathway in human hepatocellular carcinoma cell line SMMC‐7721. World Chinese Journal of Digestology, 19(22), 2363–2367. [Google Scholar]
- Hai‐Yan, Z. , Lv, B. , Zhang, W. , Zhao, Y. , Tan, R. X. , & Li, E. (2015). Baicalin inhibits autophagy induced by influenza A virus H3N2. Antiviral Research, 113, 62–70. [DOI] [PubMed] [Google Scholar]
- He, X. , Yu, D. , Li, W. , Zheng, Z. , Lv, C. L. , Li, C. , Liu, P. , Xu, C. Q. , Hu, X. F. , & Jin, X. P. (2016). Anti‐atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ‐LXRα‐ABCA1/ABCG1 pathway. Biomedicine & Pharmacotherapy, 83, 257–264. [DOI] [PubMed] [Google Scholar]
- Heng Zhai, Z. K. H. Z. (2019). Baicalin attenuated substantia nigra neuronal apoptosis in Parkinson's disease rats via the mTOR/AKT/GSK‐3β pathway. Journal of Integrative Neuroscience, 18(4), 423–429. [DOI] [PubMed] [Google Scholar]
- Hoesel, B. , & Schmid, J. A. (2013). The complexity of NF‐κB signaling in inflammation and cancer. Molecular Cancer, 12(1), 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Zhou, W. , Zhu, H. , Zhou, P. , & Shi, X. (2017). Baicalin benefits the anti‐HBV therapy via inhibiting HBV viral RNAs. Toxicology & Applied Pharmacology, 323, 36–43. [DOI] [PubMed] [Google Scholar]
- Huang, Q. , Zhang, J. , Peng, J. , Zhang, Y. , Wang, L. , Wu, J. , Ye, L. , & Fang, C. (2019). Effect of baicalin on proliferation and apoptosis in pancreatic cancer cells. American Journal of Translational Research, 11(9), 5645–5654. [PMC free article] [PubMed] [Google Scholar]
- Huilian, S. , Yanliang, Z. , Jing, X. , Liu, L. , Qiao, F. , Li, J. , & Chen, Y. (2020). Baicalin attenuates hepatic injury in non‐alcoholic steatohepatitis cell model by suppressing inflammasome‐dependent GSDMD‐mediated cell pyroptosis. International Immunopharmacology, 81, 106195. [DOI] [PubMed] [Google Scholar]
- Ikuse, T. , Blanchard, T. G. , & Czinn, S. J. (2019). Inflammation, immunity, and vaccine development for the gastric pathogen Helicobacter pylori . Current Topics in Microbiology and Immunology, 421, 1–19. [DOI] [PubMed] [Google Scholar]
- Ishfaq, M. , Chen, C. , Bao, J. , Zhang, W. , Wu, Z. , Wang, J. , Liu, Y. , Tian, E. , Hamid, S. , Li, R. , Ding, L. , & Li, J. (2019). Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF‐κB and nrf2/ho‐1 signaling pathway during Mycoplasma gallisepticum infection. Poultry Science, 98(12), 6296–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishfaq, M. , Zhang, W. , Hu, W. , Waqas Ali Shah, S. , Liu, Y. , Wang, J. , Wu, Z. , Ahmad, I. , & Li, J. (2019). Antagonistic effects of baicalin on Mycoplasma gallisepticum ‐induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infection and Drug Resistance, 12, 3075–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishfaq, M. , Zhang, W. , Liu, Y. , Wang, J. , Wu, Z. , Shah, S. W. A. , Li, R. , Miao, Y. , Chen, C. , & Li, J. (2021). Baicalin attenuated Mycoplasma gallisepticum ‐induced immune impairment in chicken bursa of fabricius through modulation of autophagy and inhibited inflammation and apoptosis. Journal of the Science of Food and Agriculture, 101(3), 880–890. [DOI] [PubMed] [Google Scholar]
- Jakab, G. , Bogdán, D. , Mazák, K. , Deme, R. , Mucsi, Z. , Mándity, I. M. , Noszál, B. , Kállai‐Szabó, N. , & Antal, I. (2019). Physicochemical profiling of baicalin along with the development and characterization of cyclodextrin inclusion complexes. AAPS PharmSciTech, 20(8), 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, F. , Ma, W. , Zhang, X. , Wang, D. , & Zhou, X. (2020). Matrine and baicalin inhibit apoptosis induced by panton‐valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. Journal of Dairy Science, 103(3), 2731–2742. [DOI] [PubMed] [Google Scholar]
- Jia, R. , Du, J. , Cao, L. , Feng, W. , Xu, P. , & Yin, G. (2021). Effects of dietary baicalin supplementation on growth performance, antioxidative status and protection against oxidative stress‐induced liver injury in GIFT tilapia (Oreochromis niloticus). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 240, 108914. [DOI] [PubMed] [Google Scholar]
- Jia, Y. , Ruiguang, X. U. , Yanchun, H. U. , Zhu, T. , Ma, T. , Wu, H. , & Hu, L. (2016). Anti‐NDV activity of baicalin from a traditional Chinese medicine in vitro. Journal of Veterinary Medical Science, 78(5), 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian, C. , Ren, Z. , Jian, W. , Yu, P. , Liu, Q. , Zeng, D. , Song, H. , & Kuang, Z. (2015). Protective effects of baicalin on LPS‐induced injury in intestinal epithelial cells and intercellular tight junctions. Canadian Journal of Physiology and Pharmacology, 93(4), 233–7. [DOI] [PubMed] [Google Scholar]
- Jin, J. , Chen, Y. , Wang, D. , Ma, L. , Guo, M. , Zhou, C. , & Dou, J. (2018). The inhibitory effect of sodium baicalin on oseltamivir‐resistant influenza a virus via reduction of neuraminidase activity. Archives of Pharmacal Research, 41(6), 664–676. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Yuan, Y. , Gong, X. , Zhang, L. , Zhou, Q. , Wu, S. , Zhang, X. , Hu, J. , Kuang, G. , Yin, X. , Wan, J. , & Yuan, Y. (2020). Baicalin and its nanoliposomes ameliorates nonalcoholic fatty liver disease via suppression of TLR4 signaling cascade in mice. International Immunopharmacology, 80, 106208. [DOI] [PubMed] [Google Scholar]
- Jin, X. , Liu, M. , Zhang, D. , Zhong, X. , Du, K. , Qian, P. , Yao, W. , Gao, H. , & Wei, M. (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia‐mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF‐κB signaling pathway. CNS Neuroscience & Therapeutics, 25(5), 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, L. , Jiang, Q. , Deng, P. , Chen, Q. , Yu, M. , Shang, J. , & Li, W. (2017). The formation of a host‐guest inclusion complex system between β‐cyclodextrin and baicalin and its dissolution characteristics. Journal of Pharmacy and Pharmacology, 69(6), 663–674. [DOI] [PubMed] [Google Scholar]
- Kamat, P. K. , Kalani, A. , Rai, S. , Swarnkar, S. , Tota, S. , Nath, C. , & Tyagi, N. (2016). Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer's disease: Understanding the therapeutics Strategies. Molecular Neurobiology, 53(1), 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattoor, A. J. , Pothineni, N. V. K. , Palagiri, D. , & Mehta, J. L. (2017). Oxidative stress in atherosclerosis. Current Atherosclerosis Reports, 19(11), 42. [DOI] [PubMed] [Google Scholar]
- Kim, S.‐J. , & Lee, S.‐M. (2012). Effect of baicalin on toll‐like receptor 4‐mediated ischemia/reperfusion inflammatory responses in alcoholic fatty liver condition. Toxicology and Applied Pharmacology, 258(1), 43–50. [DOI] [PubMed] [Google Scholar]
- Kong, D. , Zhang, Y. , Yamori, T. , Duan, H. , & Jin, M. (2011). Inhibitory activity of flavonoids against class I phosphatidylinositol 3‐kinase isoforms. Molecules (Basel, Switzerland), 16(6), 5159–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka, N. , Yoshioka, Y. , Fujita, Y. U. , & Ochiya, T. (2016). Versatile roles of extracellular vesicles in cancer. The Journal of Clinical Investigation, 126(4), 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonova, G. N. , Shutikova, A. L. , Lubova, V. A. , & Maistrovskaya, O. S. (2020). Inhibitory activity of Scutellaria baicalensis flavonoids against tick‐borne encephalitis virus. Bulletin of Experimental Biology and Medicine, 168(5), 665–668. [DOI] [PubMed] [Google Scholar]
- Li, B. Q. , Fu, T. , Yan, Y. D. , Baylor, N. W. , Ruscetti, F. W. , & Kung, H. F. (1993). Inhibition of HIV infection by baicalin – A flavonoid compound purified from Chinese herbal medicine. Cellular & Molecular Biology Research, 39(2), 119. [PubMed] [Google Scholar]
- Li, C. , Long, X. Y. , Huang, S. H. , Wu, H. Y. , & Pan, S. J. (2012). Drug delivery systems of baicalin, baicalin‐phospholipid complex and self‐microemulsifying drug across caco‐2 cell model. Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 35(5), 757. [PubMed] [Google Scholar]
- Li, C. , Sune, E. , Jie, S. , Wang, J. , Jia, X. B. , & Zhang, Z. H. (2016). Characterization and bioavailability study of Baicalin‐mesoporous carbon nanopowder solid dispersion. Pharmacognosy Magazine, 12(48), 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Chen, Q. , Zhang, S. , Jiang, Q. , Shang, J. , Zhou, L. , Li, Q. , Li, S. , Shi, S. , Li, Y. , & Li, W. (2019). Maltosyl‐β‐cyclodextrin mediated supramolecularhost‐guest inclusion complex used for enhancing baicalin antioxidant activity and bioavailability. Journal of Drug Delivery Science and Technology, 54, 101346. [Google Scholar]
- Li, J. , Qiao, Z. , Hu, W. , Zhang, W. , Shah, S. W. A. , & Ishfaq, M. (2019). Baicalin mitigated Mycoplasma gallisepticum ‐induced structural damage and attenuated oxidative stress and apoptosis in chicken thymus through the nrf2/ho‐1 defence pathway. Veterinary Research, 50(1), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , Li, Y. , Shelton, J. M. , Richardson, J. A. , Spencer, E. , Chen, Z. J. , Wang, X. , & Williams, R. S (2000). Cytochrome c deficiency causes embryonic lethality and attenuates stress‐induced apoptosis. Cell, 101(4), 389–399. [DOI] [PubMed] [Google Scholar]
- Li, L. , Dong, H. , Song, E. , Xu, X. , Liu, L. , & Song, Y. (2014). Nrf2/ARE pathway activation, HO‐1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chemico‐Biological Interactions, 209, 56–67. [DOI] [PubMed] [Google Scholar]
- Liao, P. , Li, Y. , Li, M. , Chen, X. , Yuan, D. , Tang, M. , & Xu, K. (2020). Baicalin alleviates deoxynivalenol‐induced intestinal inflammation and oxidative stress damage by inhibiting NF‐κB and increasing mTOR signaling pathways in piglets. Food and Chemical Toxicology, 140, 111326. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Liu, F. , Ma, Y. , Li, H. , Ju, X. , & Xu, J. (2019). Effect of puerarin, baicalin and berberine hydrochloride on the regulation of IPEC‐J2 cells infected with enterotoxigenic Escherichia coli . Evidence‐Based Complementary and Alternative Medicine, 2019, 7438593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Wang, S. , & Zhao, G. (2020). Baicalin relieves lipopolysaccharide‐evoked inflammatory injury through regulation of miR‐21 in H9c2 cells. Phytotherapy Research, 34(5), 1134–1141. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Li, X. , Zhang, Z. , Zhang, J. , Xu, J. , Qiu, Y. , Ye, C. , Fu, S. , Wu, Z. , & Hu, C.‐A. A (2021). Baicalin protects vascular tight junctions in piglets during Glaesserella parasuis infection. Frontiers in Veterinary Science, 25(8), 671936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Zhao, H. , Shu, L. , Zhang, Y. , Okeke, C. , Zhang, L. , Li, J. , & Li, N. (2015). Preparation and evaluation of Baicalin‐loaded cationic solid lipid nanoparticles conjugated with OX26 for improved delivery across the BBB. Drug Development & Industrial Pharmacy, 41(3), 353. [DOI] [PubMed] [Google Scholar]
- Luo, J. , Dong, B. , Wang, K. E. , Cai, S. , Liu, T. , Cheng, X. , Lei, D. , Chen, Y. , Li, Y. , Kong, J. , & Chen, Y. (2017). Baicalin inhibits biofilm formation, attenuates the quorum sensing‐controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One, 12(4), e176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccracken, E. , Monaghan, M. , & Sreenivasan, S. (2018). Pathophysiology of the metabolic syndrome. Clinics in Dermatology, 36(1), 14–20. [DOI] [PubMed] [Google Scholar]
- Meng, X. , Hu, L. , & Li, W. (2019). Baicalin ameliorates lipopolysaccharide‐induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2‐mediated HO‐1 signaling pathway. Naunyn‐Schmiedeberg's Archives of Pharmacology, 392(11), 1421–1433. [DOI] [PubMed] [Google Scholar]
- Narushima, Y. , Kozuka‐Hata, H. , Koyama‐Nasu, R. , Tsumoto, K. , Inoue, J. , Akiyama, T. , & Oyama, M. (2016). Integrative network analysis combined with quantitative phosphoproteomics reveals transforming growth Factor‐beta receptor type‐2 (TGFBR2) as a novel regulator of glioblastoma stem cell Properties. Molecular & Cellular Proteomics, 15(3), 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagaray, K. E. , Brouk, M. J. , Mamedova, L. K. , Sivinski, S. E. , Liu, H. , Robert, F. , Dupuis, E. , Zachut, M. , & Bradford, B. J. (2019). Dietary supplementation of Scutellaria baicalensis extract during early lactation decreases milk somatic cells and increases whole lactation milk yield in dairy cattle. PLos One, 4(1), e0210744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo, A. , Rausalu, K. , Merits, A. , Higgs, S. , Vanlandingham, D. , Bakar, S. A. , & Zandi, K. (2017). Deciphering the potential of baicalin as an antiviral agent for chikungunya virus infection. Antiviral Research, 101–111. [DOI] [PubMed] [Google Scholar]
- Oo, A. , Teoh, B. T. , Sam, S. S. , Bakar, S. A. , & Zandi, K. (2019). Baicalein and baicalin as Zika virus inhibitors. Archives of Virology, 164(2), 585–593. [DOI] [PubMed] [Google Scholar]
- Orzechowska, B. , Chaber, R. , Wiå›Niewska, A. , Pajtasz‐Piasecka, E. , Jatczak, B. , Siemieniec, I. , Gulanowski, B. , Chybicka, A. , & BÅ‚Ach‐Olszewska, Z. (2014). Baicalin from the extract of Scutellaria baicalensis affects the innate immunity and apoptosis in leukocytes of children with acute lymphocytic leukemia. International Immunopharmacology, 23(2), 558–567. [DOI] [PubMed] [Google Scholar]
- Peng, L. , Shi, H. , Tan, Y. , Shen, S. Y. , Yi, P. F. , Shen, H. Q. , & Fu, B. D. (2021). Baicalin inhibits APEC‐induced lung injury by regulating gut microbiota and SCFA production. Food & Function, 12(24), 12621–12633. [DOI] [PubMed] [Google Scholar]
- Peng, L.‐Y. , Yuan, M. , Song, K. , Yu, J.‐L. , Li, J.‐H. E. , Huang, J.‐N. I. , Yi, P.‐F. , Fu, B.‐D. , & Shen, H.‐Q. (2019). Baicalin alleviated APEC‐induced acute lung injury in chicken by inhibiting NF‐κB pathway activation. International Immunopharmacology, 72, 467–472. [DOI] [PubMed] [Google Scholar]
- Peng, L.‐Y. , Yuan, M. , Wu, Z.‐M. , Song, K. E. , Zhang, C.‐L. , An, Q. , Xia, F. , Yu, J.‐L. , Yi, P.‐F. , Fu, B.‐D. , & Shen, H.‐Q. (2019). Anti‐bacterial activity of baicalin against APEC through inhibition of quorum sensing and inflammatory responses. Scientific Reports, 9(1), 4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Q. , Zhou, S. , Yao, F. , Hou, B. , Huang, Y. , Hua, D. , Zheng, Y. , & Qian, Y. (2011). Baicalein suppresses the SOS response system of Staphylococcus aureus induced by ciprofloxacin. Cellular Physiology & Biochemistry, 28(5), 1045–1050. [DOI] [PubMed] [Google Scholar]
- Qinfeng, C. (2016). Baicalin induces apoptosis of gastric cancer MGC‐803 and BGC‐823 cells through death receptor pathway. China Medical Abstracts (Internal Medicine), 33(01), 39–40. [Google Scholar]
- Shasha, L. , Yang, Z. , & Xingbin, S. (2019). Inhibition of baicalin on the growth of esophageal cancer cells and related mechanism. Hebei Medicine, 25(11), 1887–1891. [Google Scholar]
- Shen, J. , Cheng, J. , Zhu, S. , Zhao, J. , Ye, Q. , Xu, Y. , Dong, H. , & Zheng, X. (2019). Regulating effect of baicalin on IKK/IKB/NF‐kB signaling pathway and apoptosis‐related proteins in rats with ulcerative colitis. International Immunopharmacology, 73, 193–200. [DOI] [PubMed] [Google Scholar]
- Shi, H. , Ren, K. , Lv, B. , Zhang, W. , Zhao, Y. , Tan, R. X. , & Li, E. (2016). Baicalin from Scutellaria baicalensis blocks respiratory syncytial virus (RSV) infection and reduces inflammatory cell infiltration and lung injury in mice. Scientific Reports, 21(6), 35851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Hao, Z. , Zhang, S. , Wei, M. , Lu, B. , Wang, Z. , & Ji, L. (2018). Baicalein and baicalin alleviate acetaminophen‐induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochemical Pharmacology, 150, 9–23. [DOI] [PubMed] [Google Scholar]
- Siegel, R. L. , Miller, K. D. , & Jemal, A. (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34. [DOI] [PubMed] [Google Scholar]
- Soderholm, J. D. (2002). Augmented increase in tight junction permeability by luminal stimuli in the non‐inflamed ileum of Crohn's disease. Gut, 50(3), 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, L. , Wang, R. , Qiu, T. , Wang, J. , Meng, J. , Zhu, J. , Wang, D. , Wu, Y. I. , & Liu, J. (2021). The protective effect of baicalin on duck hepatitis A virus type 1‐induced duck hepatic mitochondria dysfunction by activating nuclear erythroid 2‐related factor 2/antioxidant responsive element signaling pathway. Poultry Science, 100(5), 101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiao, Z. , & Xingbing, H. (2019). Research progress on the mechanism of oxidative stress involved in cognitive dysfunction of depression patients. Journal of New Medicine, 50(12), 877–880. [Google Scholar]
- Sumner, R. P. , Maluquer De Motes, C. , Veyer, D. L. , & Smith, G. L. (2014). Vaccinia virus inhibits NF‐κB‐Dependent gene expression downstream of p65 translocation. Journal of Virology, 88(6), 3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, S. , Wu, X. , Song, H. , & Li, G. Q. (2015). Baicalin ameliorates isoproterenol‐induced acute myocardial infarction through iNOS, inflammation, oxidative stress and P38MAPK pathway in rat. International Journal of Clinical and Experimental Medicine, 8(12), 22063–22072. [PMC free article] [PubMed] [Google Scholar]
- Tong, K. I. , Katoh, Y. , Kusunoki, H. , Itoh, K. , Tanaka, T. , & Yamamoto, M. (2006). Keap1 recruits neh2 through binding to ETGE and DLG motifs: Characterization of the two‐site molecular recognition Model. Molecular and Cellular Biology, 26(8), 2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, X.‐K. , Yang, W.‐Z. , Shi, S.‐S. , Chen, Y. E. , Wang, C.‐H. , Chen, C.‐M. , & Chen, Z. (2011). Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral Ischemia. Inflammation, 34(5), 463–470. [DOI] [PubMed] [Google Scholar]
- Wada, J. , & Makino, H. (2016). Innate immunity in diabetes and diabetic nephropathy. Nature Reviews Nephrology, 12(1), 13–26. [DOI] [PubMed] [Google Scholar]
- Wall, N. R. , O'Connor, D. S. , Plescia, J. , Pommier, Y. , & Altieri, D. C. (2013). Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer research, 63(1), 230–235. [PubMed] [Google Scholar]
- Wang, C. , Choi, Y. H. O. , Xian, Z. , Zheng, M. , Piao, H. , & Yan, G. (2018). Aloperine suppresses allergic airway inflammation through NF‐κB, MAPK, and nrf2/ho‐1 signaling pathways in mice. International Immunopharmacology, 65, 571–579. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Ishfaq, M. , & Li, J. (2021). Baicalin ameliorates Mycoplasma gallisepticum‐induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism. Food & Function, 12(9), 4092–4104. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Wang, C. , Tao, Z. , Zhao, L. , Zhu, Z. , Wu, W. , He, Y. , Chen, H. , Zheng, B. , Huang, X. , Yu, Y. , Yang, L. , Liang, G. , Cui, R. , & Chen, T. (2019). Curcumin derivative WZ35 inhibits tumor cell growth via ROS‐YAP‐JNK signaling pathway in breast cancer. Journal of Experimental & Clinical Cancer Research, 38(1), 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M.‐J. , Yang, C.‐H. , Jin, Y. , Wan, C.‐B. , Qian, W.‐H. E. , Xing, F. , Li, X. , & Liu, Y.‐Y. (2020). Baicalin inhibits coxsackievirus B3 replication by reducing cellular lipid synthesis. The American Journal of Chinese Medicine, 48(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Wang, H. , Zhou, R. , Zhong, W. , Lu, S. , Ma, Z. , & Chai, Y. (2017). Baicalin inhibits human osteosarcoma cells invasion, metastasis, and anoikis resistance by suppressing the transforming growth factor‐β1‐induced epithelial‐to‐mesenchymal transition. Anti‐Cancer Drugs, 28(6), 581–587. [DOI] [PubMed] [Google Scholar]
- Wanli, J. , Kun, L. , Rui, A. , & Wang, X. (2019). Baicalin protects against ethanol‐induced chronic gastritis in rats by inhibiting Akt/NF‐κB pathway. Life Sciences, 15(239), 117064. [DOI] [PubMed] [Google Scholar]
- Winkler, C. , Ferdous, F. , Dimmick, M. , & Scott, T. (2017). Lipopolysaccharide induced interleukin‐6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFκB in chicken thrombocytes. Developmental & Comparative Immunology, 73, 124–130. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Liu, Z. , Peng, J. , Li, L. , Li, N. , Li, J. , & Pan, H. (2012). Design and evaluation of baicalin‐containing in situ pH‐triggered gelling system for sustained ophthalmic drug delivery. International Journal of Pharmaceutics, 410(1–2), 31–40. [DOI] [PubMed] [Google Scholar]
- Wu, M.‐H. , Huang, Y.‐Q. , Huang, Z.‐S. , Zhou, X. I.‐H. , Yu, W.‐Q. , & Su, J.‐W. (2013). In vitro bacteriostatic effect of berberine, emodin,schisandra, and baicalin on multidrug resistant strains of Helicobacter pylori . World Chinese Journal of Digestology, 21(30), 3247–3251. [Google Scholar]
- Wu, Y. , Wang, F. , Fan, L. , Zhang, W. , Wang, T. , Du, Y. , & Bai, X. (2018). Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF‐κB and p38 MAPK signaling pathways. Biomedicine & Pharmacotherapy, 97, 1673–1679. [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Chen, C. , Miao, Y. , Liu, Y. , Zhang, Q. , Li, R. , Ding, L. , Ishfaq, M. , & Li, J. (2019). Baicalin attenuates Mycoplasma gallisepticum‐induced inflammation via inhibition of the TLR2‐NF‐κB pathway in chicken and DF‐1 Cells. Infection and Drug Resistance, 12, 3911–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiangyu, L. , Shengli, W. , & Guoan, Z. (2020). Baicalin relieves lipopolysaccharide‐evoked inflammatory injury through regulation of miR‐21 in H9c2 cells. Phytotherapy Research: PTR, 34(5), 1134–1141. [DOI] [PubMed] [Google Scholar]
- Xiao‐Ping, J. , Wei‐Wei, C. , & Li, H. E. (2016). Effect of resveratrol on expression of bad, p‐Bad and caspase‐3 protein in MGC803 Cells. Chinese Journal of Experimental Traditional Medical Formula, 22(5), 147–150. [Google Scholar]
- Xie, J. , Luo, Y. , Liu, Y. , Ma, Y. , Yue, P. , & Yang, M. (2019). Novel redispersible nanosuspensions stabilized by co‐processed nanocrystalline cellulose–Sodium carboxymethyl starch for enhancing dissolution and oral bioavailability of baicalin. International Journal of Nanomedicine, 14, 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Li, S. , Jiang, L. , Gao, X. , Liu, W. , Zhu, X. , Huang, W. , Zhao, H. , Wei, Z. , Wang, K. , & Yang, Z. (2021). Baicalin protects against zearalenone‐induced chicks liver and kidney injury by inhibiting expression of oxidative stress, inflammatory cytokines and caspase signaling pathway. International Immunopharmacology, 100, 108097. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Mei, J. , & Tan, Y. (2017). Baicalin attenuates DDP (cisplatin) resistance in lung cancer by downregulating MARK2 and p‐Akt. International Journal of Oncology, 50(1), 93–100. [DOI] [PubMed] [Google Scholar]
- Xue, W. , Zhongling, J. , Xin, W. , Aimin, J. , Xia, C. , Rongfeng, C. , Huatao, L. , & Wenru, T. (2017). Effect of baicalin on oxidative damage of mouse oviduct induced by heat stress. Acta Agriculturae Boreali‐Sinica, 32(6), 147–153. [Google Scholar]
- Xue, Y. , Shui, X. , Su, W. , He, Y. , Lu, X. , Zhang, Y. U. , Yan, G. , Huang, S. , Lei, W. , & Chen, C. (2015). Baicalin inhibits inflammation and attenuates myocardial ischaemic injury by aryl hydrocarbon receptor. Journal of Pharmacy and Pharmacology, 67(12), 1756–1764. [DOI] [PubMed] [Google Scholar]
- Xuexue, H. , Li, C. , & Lishuang, Y. (2020). Advances in research on the composition and pharmacological effects of Astragalus. Journal of GuiZhou University of Traditional Chinese Medicine, 42(02), 79–82. [Google Scholar]
- Yan, G. , Wang, J. , Yi, T. , Cheng, J. , Guo, H. , He, Y. , Shui, X. , Wu, Z. , Huang, S. , & Lei, W. (2019). Baicalin prevents pulmonary arterial remodeling in vivo via the AKT/ERK/NF‐κB signaling pathways. Pulmonary circulation, 9(4), 766669113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. (2020). Functional and mechanism analysis of baicalin on human cholangiocarcinom A cells. Southwest Medical University. [Google Scholar]
- Yang, X. , Yao, W. , Shi, H. , Liu, H. , Li, Y. , Gao, Y. , Liu, R. , & Xu, L. (2016). Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis. Journal of Ethnopharmacology, 185, 361–369. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Zhang, Q. , Gao, Z. , Yu, C. , & Zhang, L. (2018). Baicalin alleviates IL‐1β‐induced inflammatory injury via down‐regulating miR‐126 in chondrocytes. Biomedicine & Pharmacotherapy, 99, 184–190. [DOI] [PubMed] [Google Scholar]
- Yanqiu, H. , Mengyan, S. , Xuefang, Y. , Ma, A. , Ma, Y. , & Zhao, A. (2019). Baicalin relieves inflammation stimulated by lipopolysaccharide via upregulating TUG1 in liver cells. Journal of Physiology and Biochemistry, 75(4), 463–473. [DOI] [PubMed] [Google Scholar]
- Yuan, Z. , Xing, L. , Bogoljub, C. , Ma, C. G. , Gran, B. , Rostami, A. , & Zhang, G. X. (2015). Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Scientific Reports, 30(5), 17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, Z. , Qiao‐Shan, Q. , Wei, L. , & Qing, G. (2016). Inhibitory effect of baicalin on invasion of cervical cancer Hela cells and its mechanism. Journal of Xi'an Jiaotong University (Medical Sciences) (China), 37(4), 599–603. [Google Scholar]
- Yutao, P. , Di, C. , Qingyou, L. , Liu, L. , Li, X. , & Li, Z. (2017). Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Molecular Medicine Reports, 16(3), 2985–2991. [DOI] [PubMed] [Google Scholar]
- Zeng, M. , Qi, M. , Wang, Y. , Xu, R. , Wu, Y. , Li, M. , Zheng, X. , & Feng, W. (2020). 5‐O‐methyldihydroquercetin and cilicicone B isolated from Spina Gleditsiae ameliorate lipopolysaccharide‐induced acute kidney injury in mice by inhibiting inflammation and oxidative stress via the TLR4/MyD88/TRIF/NLRP3 signaling pathway. International Immunopharmacology, 80, 106194. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Deng, Y. , Cheng, B. O. , Huang, Y. , Meng, Y. , Zhong, K. , Xiong, G. , Guo, J. , Liu, Y. I. , & Lu, H. (2020). Protective effects and molecular mechanisms of baicalein on thioacetamide‐induced toxicity in zebrafish larvae. Chemosphere, 256, 127038. [DOI] [PubMed] [Google Scholar]
- Zhang, P. , Hou, J. , Fu, J. , Li, D. , Zhang, C. , & Liu, J. (2013). Baicalin protects rat brain microvascular endothelial cells injured by oxygen‐glucose deprivation via anti‐inflammation. Brain Research Bulletin, 97, 8–15. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Hu, B. , Xu, J. , Ren, Q. , Wang, Z. , Wang, S. , Dong, Y. , & Yang, G. (2020). Baicalin suppress growth and virulence‐related factors of methicillin‐resistant Staphylococcus aureus in vitro and vivo. Microbial Pathogenesis, 139, 103899. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Tang, X. , & Chen, H. C. (1991). Inhibition of HIV replication by baicalin and S. baicalensis extracts in H9 cell culture. Chinese Medical Sciences Journal, 6(4), 230–232. [PubMed] [Google Scholar]
- Zhang, X. , Zhao, Q. , Ci, X. , Chen, S. , Chen, L. , Lian, J. , Xie, Z. , Ye, Y. , Lv, H. , Li, H. , Lin, W. , Zhang, H. , & Xie, Q. (2020). Effect of baicalin on bacterial secondary infection and inflammation caused by H9N2 AIV infection in Chickens. BioMed Research International, 2(18), 2524314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Liu, S. , Wan, J. , Yang, Q. , Xiang, Y. , Ni, L. I. , Long, Y. U. , Cui, M. , Ci, Z. , Tang, D. , & Li, N. (2020). Preparation, characterization and in vivo study of borneol‐baicalin‐liposomes for treatment of cerebral ischemia‐reperfusion Injury. International Journal of Nanomedicine, 15, 5977–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Jiang, F. , Zeng, L. , Wang, X. , & Tu, S. (2018). PHACTR1 regulates oxidative stress and inflammation to coronary artery endothelial cells via interaction with NF‐κB/p65. Atherosclerosis, 278, 180–189. [DOI] [PubMed] [Google Scholar]
- Zhangzhao . (2019). Baicalin attenuates hypoxia/reoxygenation‐induced renal tubular epithelial cell apoptosis by inhibiting ROS‐ER stress PERK pathway. Chongqing Medical University. [Google Scholar]
- Zhao, R. , Han, X. , & Jia, B. (2019). Apoptotic mechanism of baicalin on human breast cancer MCF‐7 cells. Journal of Inner Mongolia Medical University, 41(05), 508–510. [Google Scholar]
- Zhaoqin, B. , Huixia, W. , & Hongwei, W. (2017). The effects of baicalin on the expression of TLR8, HIF‐1α, PDGFβ and pten of gastric cancer SGC‐7901 Cells. Western Journal of Traditional Chinese Medicine, 30(08), 12–16. [Google Scholar]
- Zheng, J. , Hu, J.‐D. , Chen, Y.‐Y. , Chen, B.‐Y. , Huang, Y. , Zheng, Z. H. , & Liu, T.‐B. (2012). Baicalin induces apoptosis in leukemia HL‐60/ADR cells via possible down‐regulation of the PI3K/Akt signaling pathway. Asian Pacific Journal of Cancer Prevention, 13(4), 1119–1124. [DOI] [PubMed] [Google Scholar]
- ZhongQing, W. , ChunFa, L. , WenJie, Z. , YiChen, L. , ZhaoRong, Z. , & Juan, L. (2019). Effect of zhu qin extractive fluid on the proliferation and transcription of inflammatory factors in LPS‐injured IPEC‐J2 Cells. Chinese Journal of Animal and Veterinary Sciences, 50(07), 1500–1508. [Google Scholar]
- Zou, M. , Yang, L. , Niu, L. , Zhao, Y. , Sun, Y. , Fu, Y. , & Peng, X. (2021). Baicalin ameliorates Mycoplasma gallisepticum‐induced lung inflammation in chicken by inhibiting TLR6‐mediated NF‐κB signalling. British Poultry Science, 62(2), 199–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated, or the article describes entirely theoretical research.



