Abstract
Background
The skin has several functions, one of the most important of which is to protect the internal organs from external damage and the entry of germs. Since skin and wound healing is one of the mostly concerned issues worldwide, the development of wound healing remedies is one of the main fields in modern medical research.
Objectives
To evaluate the effect of the hydroalcoholic extract of Cumin carvi L. seed as one of the traditional medicinal plants used for wound healing through an in vivo model.
Methods
Wide circular skin wounds (2 × 2 cm) were created on the dorsal area of 50 Sprague–Dawley male rats following ethical principles. The animals were divided into five groups including no treatment, base gel, tetracycline treatment, 10% v/v hydroalcoholic extract of Cumin carvi L. seed treatment and 20% v/v hydroalcoholic extract of Cumin carvi L. seed treatment group. Treatment was performed within 20 days. On days 1, 3, 5, 7 and 10, photographs were taken, and the percentage of wound healing was calculated. Also, on the 10th day, the skin area was sampled for histopathology and on the 20th day, the skin was sampled for biomechanical and total protein assessments.
Results
The results of wound healing percentage showed that from day 3 onwards, there was a significant improvement between the group treated with 20% v/v hydroalcoholic extract of Cumin carvi L. and the negative control and basal gel groups (p < 0.05). According to histopathological and total protein content evaluations, the amount of collagen production and inflammation score in the Cumin carvi‐treated groups confirmed the healing process compared to other groups.
Conclusions
According to the results of this project, 20% v/v ethanolic extract of Cumin carvi L. has potential therapeutic effects in the treatment of skin wounds.
Wounds are one of the health concerns, and the economic burden of wound care and healing has continued to increase over the past years.
The healing effects of C. carvi. ethanolic extract on the wound were investigated.
The histopathological and macroscopical evaluations, as well as total protein content, were measured to investigate its wound healing properties.
Keywords: Carum carvi, histopathology, macroscopic scale, total protein, wound healing
1. INTRODUCTION
Skin, as a first‐line defence barrier, is essential for the fundamental structure and protection against microorganisms and harmful substances (Archer, 2010). However, this barrier is mostly effective when its surface is not damaged. The skin constantly regenerates itself through the shedding of dead cells and production of new cells made in the epidermis (the top layer and protective layer of the skin) (Rodrigues et al., 2019). When a wound occurs, this layer may become infected and allow microorganisms, some of which live on the surface of the skin, to enter the bloodstream (Ellis et al., 2018). Cells in the lower layer of the dermis, after skin damage, begin to make collagen and repair the epithelial cells (Qing, 2017). Accordingly, finding therapeutic agents for faster healing of the dermis and epidermis against skin damage is important.
There are different types of wounds including acute or chronic wounds, penetrating wounds, puncture wounds, surgical wounds and incisions, gunshot wounds, or other high‐velocity projectiles that can penetrate the body. Other forms of wounds are thermal, chemical or electric burns, bites and stings, blunt force trauma, abrasions, lacerations and skin tears.
Wound healing, as a natural physiological reaction, is classically divided into 4 stages: (1) haemostasis through vasoconstriction of blood vessels and platelet aggregation; (2) inflammation characterised by release of soluble mediators such as proinflammatory cytokines (including IL‐1, IL‐6, IL‐8 and TNF‐α), growth factors (such as PDGF, TGF‐β, TGF‐α, IGF‐1 and FGF) and small regulatory proteins which occur within the first 24 h after injury and can last for up to 2 weeks; (3) proliferation phase including replacement of the provisional fibrin matrix with a new matrix of collagen fibres, proteoglycans and fibronectin; and (4) remodelling, which happens when collagen is remodelled from type III to type I and the wound is fully closed (Fitridge & Thompson, 2011; Reinke and Sorg, 2012).
Impaired wound healing due to predisposing diseases such as diseases that weaken the immune system as well as diabetes ultimately slow down the wound healing process. The reasons for the delayed healing are the prolongation of the inflammatory phase, the presence of infection in the area and the formation of abnormal tissues in the area (Mangoni et al., 2016; Wilkinson & Hardman, 2020). In detail, the excessive inflammatory response leads to delay in wound closure and improper wound healing which results in scar formation. Proinflammatory cytokines such as interleukin 1 beta (IL‐1β), IL‐6, tumour necrosis factor‐alpha (TNF‐α) and prostaglandin E2 (PGE2) are released by macrophages and involved in the upregulation of inflammatory reactions, while wound healing is accelerated by appropriate temporal downregulation of proinflammatory cytokines levels (Nguyen et al., 2017).
Therefore, therapeutic interventions should be performed to prevent impaired wound healing. Plants are one of important natural sources with unique medicinal properties that have long been used to treat different diseases (Hajialyani et al., 2018). The therapeutic potencies of many traditional medicines are conferred by natural ingredients produced within the plant. Carum carvi L. (Caraway), belonging to the family Apaiaceae, are one of the earliest cultivated herbs in Asia, Africa and Europe. Caraway seeds from C. carvi have remained popular as culinary spices and have also been overwhelmingly used in folklore therapy since antiquity in diverse geographical areas (Johri, 2011). Photochemical analysis of C. carvi indicated a different class of compounds including monoterpenoids, flavonoids, glucosides, nucleosides and aromatic compounds. The essential oils of C. carvi main parts include carvone and limonene. C. carvi exhibited a wide range of biological and pharmacological potencies such as anticarcinogenic, antimutagenic, antidiabetic, antiulcer, antiosteoporotic and immunomodulatory activities (Agrahari & Singh, 2014; Johri, 2011).
Several investigations have demonstrated a potential antimicrobial activity of C. carvi against a range of pathogenic Gram‐positive and Gram‐negative bacterial strains like Klebsiella pneumonia, Streptococcus mutans and Streptococcus pyogenes. Noteworthy, the antifungal activity of this plant was observed in numerous human pathogens, including dermatophytes, Vibrio spp., yeasts, aflatoxins and mycotoxin producers (Boyraz & Özcan, 2005; Derakhshan et al., 2008; Derakhshan et al., 2010; Johri, 2011; Shayegh et al., 2008). Also, caraway is known as a powerful antioxidant due to the high content of phenolic and flavonoid compounds with anti‐inflammatory activities through inhibiting nitric oxide (NO) release in lipopolysaccharide (LPS) (Bourgou et al., 2020; Fang et al., 2010; Keshavarz et al., 2013).
In the present study, for the first time, the healing effects of C. carvi ethanolic extract, as a natural source of powerful antioxidants with antimicrobial and anti‐inflammatory activities on the wound, were investigated to determine the efficacy and potency of treatment. Also, the histopathological and macroscopical evaluations, as well as total protein content, were measured to investigate its wound healing properties.
2. MATERIALS AND METHODS
2.1. Ethical considerations
The Animal Care Committee approved the study. The authors followed all institutional and international guidelines for animal care and use during this study. The Animal Research Reporting in vivo Experiments guidelines (ARRIVE) were also followed. At the end of the study, the rats were euthanised with the rapid and humane method using a 70% volume displacement rate of CO2 increase above 100 % in the induction chamber.
2.2. Plant materials and preparation of hydroalcoholic extracts of C. carvi
The C. carvi seeds were obtained and its species was endorsed by taxonomists with the herbarium number of 2617. One hundred gram of dried C. carvi was powdered and percolated (3 times) with 70% ethanol, at room temperature, and the extracts were filtered and evaporated under reduced pressure to obtain 12.7 g of dried C. carvi seed (CCS) extract (Mardani et al., 2016).
2.3. Preparation of carboxymethylcellulose (CMC) extract
To prepare a gel‐forming agent, we added 5% glycerol to 2% sodium carboxymethyl cellulose (CMC) in deionised water (NaCMC) and continuously stirred it with a mixer at 500 RPM. Next, the stock solution of a caraway extract with 1 mg/ml concentration in 20% ethanol with a total volume of 50 ml was prepared. Next, 20 ml of this solution was added to 80 ml of 2% CMC solution; as a result, 20% volume /volume was prepared. In the case of 10% v/v solution, 10 ml of this solution was added to 90 ml of 2% CMC solution (volume final = 100 ml). The mixtures were gradually stirred for 1 h and a uniform gel was obtained, collected in an aluminium tube and stored in the refrigerator (Alizade Naini et al., 2021; Naini et al., 2021). In each treatment, 0.5 ml of gel was applied daily to the wound site using a dropper to fully cover the wound site.
2.4. Cytotoxicity assessments of hydroalcoholic extracts of C. carvi
The effect of hydroalcoholic extracts of C. carvi on the proliferation rate of MCF‐7 was studied by 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay (a tetrazolium salt; Sigma‐Aldrich, St. Louis, MO, USA), which determines the cell number as a function of mitochondrial activity. MCF‐7 cells were obtained from the Iranian Biological Resource Center, Tehran, Iran. The cells were grown in RPMI1640 medium supplemented with foetal bovine serum (FBS, 10%) and maintained at 37°C in the presence of 5% CO2. Cells were plated in 96‐well culture plates (5000 cells/200 μl) and after 24 h, the medium was changed with the solutions of C. carvi extract at different concentrations. Cells were further incubated with the extract at 37°C for 72 h. At the end of the exposure time, 20 μl of 0.5 mg/ml of (MTT solution) was added to each well and incubated for 4 h at 37°C. The medium was removed gently and to dissolve the formazan crystals, and 200 μl/well of DMSO (Merck, Germany) was added. Cell viability was evaluated by assessing the optical density at 570 nm on a microplate reader (FLUOstar Omega, BMG LABTECH, Germany) (Barzegar et al., 2021; Tanideh et al., 2020).
2.5. Evaluation of hydroalcoholic extracts of C. carvi release
The release profile of the extract was evaluated in deionised water at room temperature and calculated spectrophotometrically at a wavelength of 263 nm. 0.5 ml of gel 20% v/v was placed in a cellulose ester dialysis tube (12 kDa), soaked in 9 ml of deionised water and then incubated at 37°C. 30 μl of the suspension which was diluted in 3 ml of deionised water was sampled while it was in contact with dialysis tubes. Finally, the absorbance was monitored, and the relationship between the extract concentration plus the absorbance value was obtained by the calibration diagram (Allafchian et al., 2020; Kayani et al., 2018; Peptu et al., 2021).
2.6. Skin wound model of the animals
This research was performed with relevant guidelines and regulations of animal studies approved. Fifty male Sprague–Dawley rats aged 10–12 weeks and weight of 200 ± 25 g were housed in rat's cage and all rats had free access to food and water. The cage was kept at a temperature of 24 ± 1°C and 12 h light/dark control condition. After 7 days, all rats were anaesthesia with ketamin‐xylazine (200 mg/kg ketamine and 10 mg/kg xylazine, IM). The hair of the wound area of all animals was shaved and then one wide circular wound with a diameter of 2 × 2 cm was created with surgical curved scissors. Then, all animals were randomly divided into five groups.
Group 1: The negative control group – wounds were created but did not receive any treatment.
Group 2: The basal gel group – wound was created and received the CMC base gel.
Groups 3: The standard group – wound was created and treated with 3% tetracycline ointment.
Group 4: The treatment 10% v/v group – wound was created and treated with a gel. In this group, 0.5 ml of gel containing 50 μg of the extract was applied to the wound site.
Group 5: The treatment 20% v/v group – wound was created and treated with a gel. In this group, 0.5 ml of gel containing 100 μg of the extract was applied to the wound site.
2.7. Macroscopic evaluation
After the wound was created (day 0), photos were taken from the wound area with a smartphone camera. Also, photos were taken on days 3, 5, 7 and 10. The area of the wound was measured with digitiser software (MedCalc Software Ltd, Belgium), and the percentage of healing was measured using the following formula:
Wound healing ratio (%) = 100 × (specific day wound size/initial wound size)
All images used in the manuscript are not edited or altered using software and the brightness of image were adjusted using PowerPoint software.
2.8. Histopathological evaluation
On day 10, the rats were euthanised, and tissue samples were taken from the healing area using scissors and a scalpel. The samples were placed in 10% formalin buffer for fixation. The samples were then dewatered and prepared by tissue processing and moulded in paraffin. Paraffin blocks were cut transversely with a diameter of 5 μm. The prepared slides were stained with haematoxylin and eosin and examined by a light microscope (Olympus, Japan). Magnifications 10 and 40 were used to take the picture. Magnification 10 was used to evaluate the overall wound, arteries and epithelial formation, and magnification 40 was used to evaluate the cell type and collagen fibres. Histopathology examinations were performed blindly by a pathologist. In histopathological examination, the index of connective tissue, the amount of collagen, granulation tissue, re‐epithelisation, angiogenesis and inflammation were scored qualitatively (Table 1). For final scoring, all index scores were added and one number from 0 to 6 was obtained. Scores 0–2 mean no healing or start healing, 2–4 moderate healing and 4–6 successful wound healed (Tanideh et al., 2021).
TABLE 1.
Criteria for histologic sections scoring
| Score | Parameter | Criteria |
|---|---|---|
| 0 | Collagen deposition | None or few |
| Angiogenesis | Fewer than 5 vessels, unmatured | |
| Epithelialization | None to very minimal | |
| Cellular content | Mostly acute and chronic inflammatory cells | |
| Granulation tissue | None to the sparse amount at wound edges | |
| Connective tissue | Few and irregular | |
| 1 | Collagen deposition | Oriented collagen fibers |
| Angiogenesis | More than 5 vessels, matured | |
| Epithelialization | Complete and thick epithelium | |
| Cellular content | More fibroblast. Moderate inflammatory cells | |
| Granulation tissue | Uniformly thick and mature | |
| Connective tissue | multiple layers of fibrous connective tissue |
2.9. Biomechanical evaluation
After 20 days of treatment, a thin specimen from the wound skin was obtained (8 × 20 mm, width × length, respectively), wrapped with the foil and stored in –20°C. The specimens were defrosted before the biomechanical examination. The sections were subjected to a tensile strength test. The device moved at a speed of 60 mm per minute (BIOPAC MP‐30 Acq system (Santa Barbara, Goleta, CA).
2.10. Measuring the amount of total protein
The specimen was homogenised in 1.15% potassium chloride. 5 ml of protein reagent was added and mixed into 0.2 ml of homogenate. The absorbance was read at 595 nm using a spectrometer (Bradford, 1976). A standard curve was prepared using 0, 20, 40, 60, 80 and 100 μg/ml of BSA in normal saline‐treated similarly.
2.11. Statistical analyses
All statistical analyses were performed using Graph pad prism (v7.0a, GraphPad Software, Inc., San Diego, CA, USA). The results were reported as mean ± standard error of the mean (mean ± SEM). Quantitative data were evaluated by one‐way ANOVA and post hoc Tukey's test. Histopathological data were evaluated with the nonparametric Mann‐Whitney U test. The data were considered statistically significant at p ≤ 0.05.
3. RESULTS
3.1. Cytotoxic evaluation of hydroalcoholic extracts of C. carvi
Hydroalcoholic extracts of C. carvi were evaluated for cytotoxicity using a cells proliferation assay. The extract showed an IC50 value of 125.89 ± 5.41 μg/ml. Interesting results were obtained in the concentration ranging from 25 ng/ml to 1 μg/ml, so the proliferation of the cells was improved and this range of concentration was an ideal dose for cell division and proliferation.
3.2. Hydroalcoholic extracts of C. carvi release test
The release profiles of 20% gel extract were evaluated in deionised water at room temperature. As can be seen in Figure 1, the release profile of the extract began with a sharp increase over 15 min followed by a gradual increase over time and then reached the plateau in 25 min.
FIGURE 1.
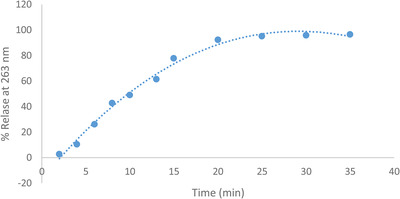
Release diagram of hydroalcoholic extracts of C. carvi of CMC gel
3.3. Biomechanical evaluation
Biomechanical examination of the tissues after 20 days of treatment showed that in the group treated with C. carvi extract, more force was needed for tissue rupture. There was a significant difference between the group treated with C. carvi extract and the negative control groups, standard treatment and base gel with p values of 0.005, 0.006 and 0.0052, respectively (Figure 2). There was no statistically significant difference between the negative control groups, standard treatment and treatment with the base gel at the level of p < 0.05.
FIGURE 2.
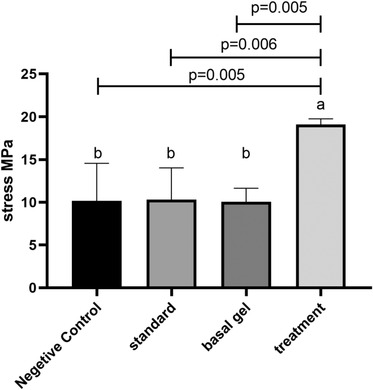
Tensile strength of skin in different groups. According to the post hoc test, groups with the same superscripted letters were not significantly different at α = 0.05 (p ≥ 0.05). However, dissimilar letters indicate a significant difference (p < 0.05)
3.4. Macroscopical evaluation
According to Figures 3 and 4, which shows the average percentage of wound healing in different groups, no significant difference was observed on day 3 in different groups. Noteworthy, a significant difference was observed in the percentage of wound healing on day 5 between the negative control groups and 10% and 20% CCS extract‐treated group (p = 0.04 and p = 0.02, respectively). A significant difference was observed in the percentage of repair on the 7th day between the negative control group and the 20% CCS extract‐treated group (p = 0.03). Improvement in wound healing was seen on the 10th day in the group which received 10% and 20% CCS extract compared with the negative control group at the level of p = 0.01 and 0.005 on. A significant difference was seen between the negative control group and the tetracycline‐treated group (p = 0.04). It was clear that 10% and 20% CCS extract treatment could better improve the wound healing profile compared to the tetracycline as a positive control.
FIGURE 3.
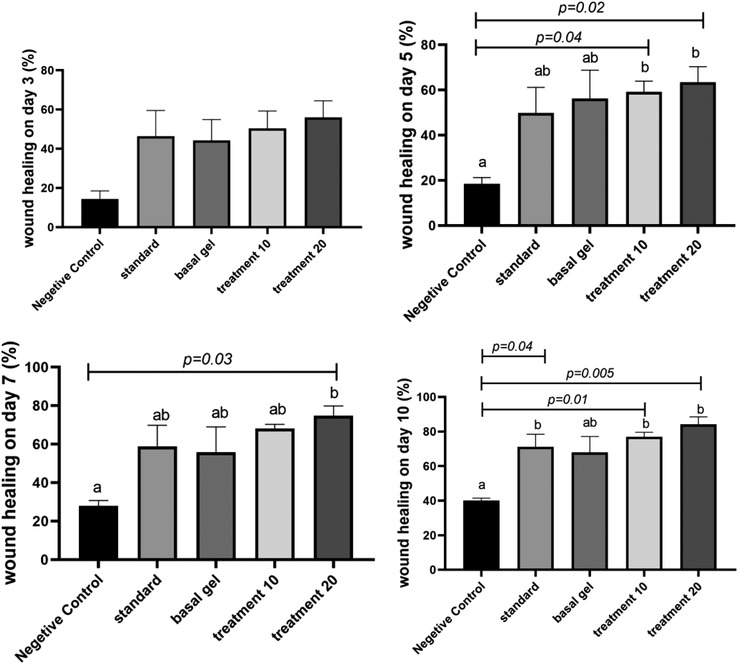
Wound healing percentage on days 3, 5, 7 and 10. According to the post hoc test, groups with the same superscripted letters were not significantly different at α = 0.05 (p ≥ 0.05). However, dissimilar letters indicate a significant difference (p < 0.05)
FIGURE 4.
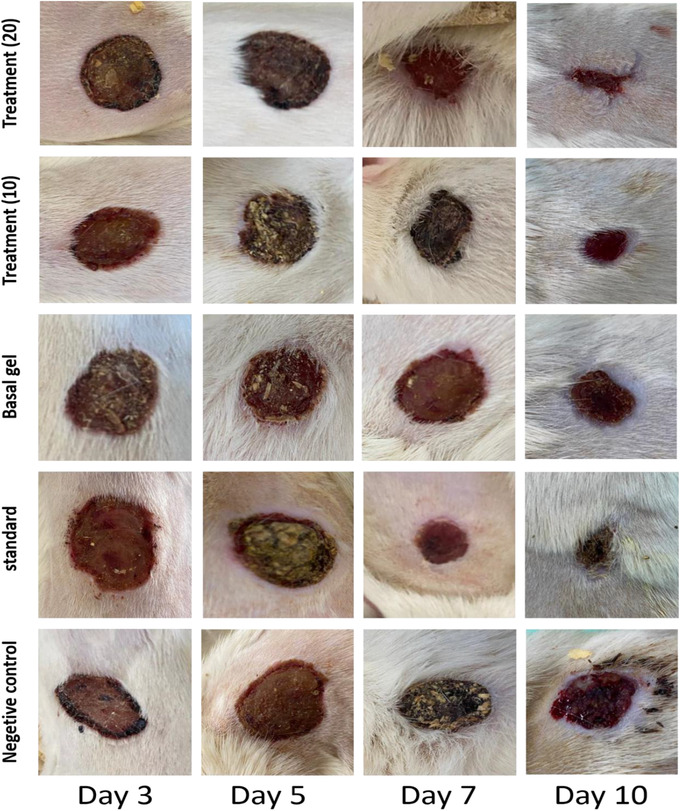
Image of wound healing after 3, 5, 7 and 10 days. After 10 days, wound was not completely closed in all groups, but in treatment groups and standard group, wound area was smaller than other groups. Negative control = no treatment, standard = tetracycline ointment, basal gel = CMC, treatment (10) = 10% Carum carvi extract and treatment (20) = 20% Carum carvi extract.
3.5. Biochemical evaluation
The total protein content of the wound tissue on day 20 was significantly higher in the extract treated groups compared to both negative control and basal gel groups (p < 0.05). Total protein in the group treated with CCS extract 20% and 10% was significantly higher than negative control group (p = 0.003 and p = 0.008, respectively) and basal gel group (p = 0.008, p = 0.02, respectively). However, there was no significant difference between treatment groups and standard control groups (Figure 5).
FIGURE 5.
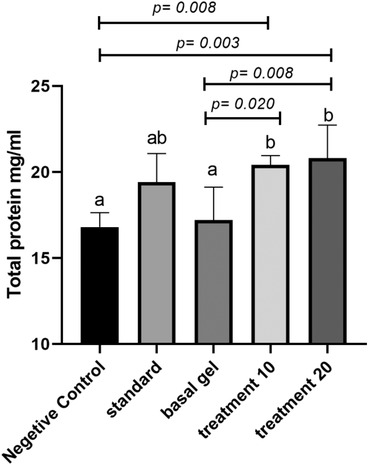
Total protein concentration in different groups after 20 days of wound creation. According to the post hoc test, groups with the same superscripted letters were not significantly different at α = 0.05 (p ≥ 0.05). However, dissimilar letters indicate a significant difference (p < 0.05)
3.6. Histopathological evaluation
In histopathological evaluation, re‐epithelialisation (in terms of complete or incomplete), tissue granulation (in terms of maturity or immaturity), connective tissue density, collagen fibre content, angiogenesis and inflammation were examined. Also, the final histopathology score was calculated and statistically evaluated. According to Figure 6, the mean of the re‐epithelialisation index, which indicates complete or incomplete epithelium, was reported to be statistically significant in different groups. A significant increase was detected in the animals which received 20% CCS extract compared to the control negative control group and base gel (p = 0.01); however, the statistical analysis did not show any significant difference between the other groups.
FIGURE 6.
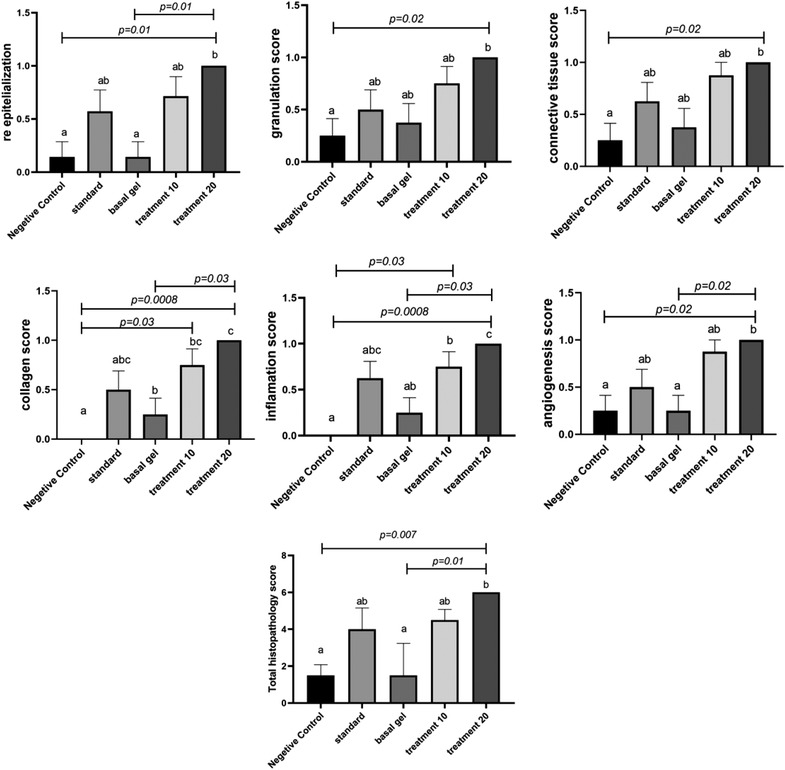
Histopathology criteria and final histopathology score in different groups
The mean granular tissue index was also examined. A significant difference was observed between the 20% CCS extract‐treated group and the negative control group (p = 0.04).
The mean of connective tissue index revealed a significant difference between the 20% CCS extract‐treated and the negative control group (p = 0.02). No significant difference was observed between the other groups at the level of p < 0.05. It was found that the collagen index, which shows the amount and dispersion of collagen fibres, was improved in 20% CCS extract‐treated rats compared with the negative control groups and the base gel (p = 0.03).
Angiogenesis index, which indicates the amount and maturity of the blood vessels, was assessed. According to Figure 6, the rate of angiogenesis in the 20% CCS extract group compared to the base and negative control group was significant with a p value of 0.02. The 10% CCS extract‐treated group showed improvement in Angiogenesis index although the differences were not significant compared to other groups. The inflammation index, which indicates the amount and type of inflammatory cells, was studied in different groups. Inflammation in the 20% CCS extract‐treated group was better than the group without treatment and the base gel with a p value of 0.008 and 0.02, respectively. Also, a significant difference was observed between the 10% CCS extract‐treated group and the negative control group (p = 0.03). According to the final score of histopathology, 20% CCS extract‐treated group demonstrated better results in comparison with the rest of the groups. A clear improvement was observed in the 20% CCS extract‐treated group with p = 0.007 and p = 0.01, compared to the negative control group and the base gel group, respectively (Figures 6 and 7).
FIGURE 7.
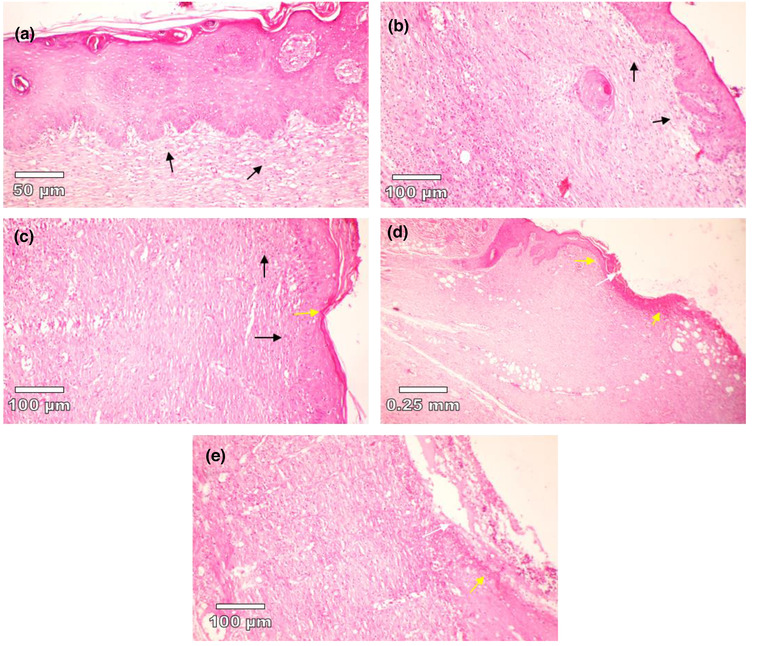
Histopathology of skin at days 10 stained with H&E. (a) 20% v/v CCS treated group, (b) 10% v/v CCS treated group, (c) tetracycline‐treated group, (d) basal gel‐treated group, (e) negative control (without treatment). White arrows show ulceration that is visible in d and e images. Yellow arrows show early epithelisation. 20% and 10% CCS‐treated groups showed a large amount of granulation tissue (black arrow). 20% treated group showing healed skin structure with near to normal epidermis; also fibrosis and collagen tissue is notable in the dermis. No quality image enhancement software was used.
4. DISCUSSION
Skin wounds include damage or any change in the structure or function of any part of the skin. Acute wound healing is seen after 8–12 weeks if the human is healthy and has no background problems. Chronic wounds, however, take longer (several months) to heal completely. Also, any complications or disease might delay and impair wound healing in any wound healing stages (Guo & DiPietro, 2010).
The wound healing process involves haemostasis, appropriate inflammation, differentiation, proliferation and migration of mesenchymal cells to the wound site, angiogenesis, re‐epithelialisation and finally, proper synthesis, cross‐linking and alignment of collagen to the tissue. However, some factors might impair the wound healing process including infections, reactive oxygen and nitrogen species (ROS and RNS), acute inflammation and decreased host immunity. Noteworthy, the prolonged inflammatory phase delays the formation of granulation tissue and its maturation, interrupting the conversion of proinflammatory factor M1 to anti‐inflammatory factor M2, which causes more secretion of TNF‐α and reactive radicals. TNF‐α activity prevented proliferating and caused the wound to become chronic (Hesketh et al., 2017; Zhao et al., 2016).
It was shown that the natural therapies were rich in a wide range of phytochemicals possessing potent anti‐inflammatory, antioxidant and antimicrobial properties, synergistically playing a vital role in wound healing. In these cases, traditional Iranian medicine (also known as Persian medicine) had promising wound healing effects (Hassanpour et al., 2019). In one study, wound healing effect of Pistacia atlantica (Persian turpentine tree) was evaluated and showed improved collagen production and organisation (Hamidi et al., 2017). However, more research on the plants to obtain information about the wound healing properties of herbal compounds is needed (Rex et al., 2018). C. carvi has exerted antioxidant, antimicrobial and anti‐inflammatory activities, which promote wound healing by reducing the complications of the microbial contamination, chronic inflammation and overoxidation process (Hosseini et al., 2020; Ibrahim et al., 2018; Johri, 2011). Compounds that have antioxidant activity in C. carvi including monoterpene alcohol, linalool, carvacrol, anethole and estragol, flavonoids and polyphenolic compounds have been reported to improve the speed of wound healing (Johri, 2011).
In a study conducted by Patil et al. (2009), the wound healing effect of ethyl acetate and petroleum extract of Cuminum cyminum seed on rats was evaluated. According to Patil et al.’s (2009), the ethyl acetate and its petroleum ether fraction showed promoted wound healing activity on excision, incision and granuloma wound models. Carvacrol is one of the major compounds in C. carvi which has also shown to produce elastin in favour of wound healing process (Johri, 2011). In another study, Mardani et al. (2016) displayed the healing effect of C. carvi on oral mucositis in golden hamsters because of containing γ‑terpinene as a hydroxyl radical's quencher which produces antioxidant properties.
The anti‐inflammatory activity of C. carvi extract stops the secretion of inflammatory factors such as TNF‐α, IL‐1 and IL‐6 and thus accelerates wound healing and the transition from the inflammatory stage to the proliferative stage (Seddighfar et al., 2020; Bourgou et al., 2020). Keshavarz et al. (2013) used cumin essential oil and cumin extract to treat colitis as the anti‐inflammatory agents. The results of the study showed that terpenoids, flavonoids, fatty acids, triglycerides, polysaccharides, lignin and polystyrene in C. carvi extract reduced inflammatory factors in colitis similar to the mechanism of glucocorticoid drugs. It seems that this plant reduced the production of prostaglandin E2 and increased the production of leukotriene B4 in polymorphonuclear leukocytes (Keshavarz et al., 2013). Carvone in C. carvi also inhibited the activity of 5‐lipoxygenase and cyclooxygenase and thus reduced the activity of leukotrienes and prostaglandins (Keshavarz et al., 2013). In a previous study, the amount of granular tissue in terms of maturity and immaturity was also significant in the group treated with C. carvi extract (De Filippo et al., 2013; Hesketh et al., 2017).
Antibacterial and antifungal agents are also effective in speeding wound healing (Pachuau, 2015). Cumin and its major compounds including carvone, linalool, limonene have strong antibacterial compounds that affect a wide range of Gram‐positive and Gram‐negative bacteria (Ghazi et al., 2019; Johri, 2011; Yakoubi et al., 2020). The antifungal effects of this plant on the soil, water, animal and human food such as dermatophyte, Vibrio, yeast, aflatoxin and mycotoxin have also been studied (Johri, 2011).
Wright et al. reported that Iberogast®, 10% of which is CCS, partially improved the histopathology features of 5‐FU‐induced jejunal mucositis (Bourgou et al., 2020). Since no specific research has been published on the wound healing and collagen‐producing effects of C. carvi, in this study, 10% and 20% (v/v) ethanolic extract of C. carvi was used to treat skin wounds in rats, and the amount of tensile strength and collagen score was also evaluated. Due to the lack of reports on the toxicity and allergic effects of this extract, the 10% and 20% v/v concentrations of CCS extract were chosen.
According to the biomechanical results, the amount of elasticity in the group treated with 20% CCS extract was higher than in other groups, showing an increase in collagen and elastin fibres. In the collagen index of histopathological evaluation, the amount of collagen in 10% and 20% (W/W) ethanolic extract treatment was higher than the negative control and the base gel groups. As a result, the increase in tissue elasticity was due to the increase in collagen and elastin in the groups treated with the extract. The histopathology evaluation showed that this plant had potential collagen production activity. Given that protein and amino acid play a significant role in wound healing such as adhesion of regenerating tissue, transporting trace elements, formation of extracellular matrix and inhibiting microbial migration (Soliman et al., 2018; Volksdorf et al., 2017), high amounts of total protein in the CCS groups were beneficial in this context. Also, collagen index in histopathology assessments supported this result and showed that CCS could enhance protein synthesis and increase the collagen content.
In the evaluation related to wound closure, in all groups, there was not any significant difference in the wound healing percentage on day 3, but from day 3 onwards, the percentage of wound healing in the group treated with 20% extract was better than the other groups. Also, in histopathological slides in the group that was treated with 20% CCS extract, the re‐epithelialisation index yielded a better score than other groups.
Given angiogenesis in histopathological evaluation, in the group treated with 20% CCS, angiogenesis was better than the control and base gel groups, so that the treated group had mature vessels with skin appendages. According to our histopathological evaluation and inflammatory score, the anti‐inflammatory effect of C. carvi extract was approved; this is consistent with the results of other articles mentioned above.
The main limitation of the current study was that the exact mechanisms that explain the mechanism of wound healing of C. carvi are lacking to correctly interpret the results of animal models into clinical practice extract. Also, more research is needed on the collagen production activity of C. carvi. In the current study, two dosages of C. carvi (10% and 20% v/v) were used. Assessments on the different dosages of C. carvi might affect the effectiveness of treatments. In the next step, further clinical trial study can be conducted to ensure that medical intervention and treatment are safe and effective.
5. CONCLUSION
In this study, the healing effects of C. carvi ethanolic extract, as a powerful antioxidant with antimicrobial and anti‐inflammatory potencies on the wound, were investigated to determine the efficacy and potency of treatment. Current findings indicated that the administration of 20% v/v ethanolic extract of C. carvi seed, as a potential herbal medicine on experimental wounds in rats, can significantly improve the total protein content and biomechanical factors as compared to the control and other treated groups. This plant increased collagen production and improved the angiogenesis and connective tissue index. Also, a reduction in inflammatory factors was observed in the 20% v/v ethanolic extract of C. carvi‐treated group, thus speeding up the wound healing process. These data could contribute to the formulation of therapeutic products for wound healing purposes that can be used on human. Further phytochemical analyses are needed to identify the chemical constituents and elucidate the structural compounds of the extract to find the lead compound.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
FUNDING
The authors wish to thank the support of Shiraz University. This study was part of the Ph.D. thesis of Mojtaba Salari Rafsanjani (IR.Shiraz1399.11.9564195).
AUTHOR CONTRIBUTIONS
MSR: methodology, validation, investigation. ATN: conceptualisation methodology, validation, supervision. AMP: conceptualisation, methodology, validation, formal analysis. FN: formal analysis, validation, writing. MMW: software, formal analysis, writing. AI: conceptualisation, methodology, writing – review & editing. all authors have read and approved the manuscript.
ETHICAL STATEMENT
Ethical approval was confirmed by the Animal Care Committee. The authors followed up all institutional and international guidelines for animal care and use during this study. The Animal Research Reporting in Vivo Experiments guidelines (ARRIVE) were also followed up (ethical number: 11011/46/19). Experimental research on the plant was under international legislation and guidelines of the Pharmacognosy Department of Shiraz University of Medical Sciences, Shiraz, Iran. At the end of the study, rats were euthanised with the rapid and humane method using a 70 % volume displacement rate of CO2 increased to around 100 % in the induction chamber.
ANIMAL WELFARE
This research is performed with relevant guidelines and regulations of animal studies approved. Fifty male Sprague–Dawley rats with the age of 10–12 weeks and weight of 200 ± 25 g were housed in rat's cages and all rats have free access to food and water. The cage was kept at a temperature of 24 ± 1°C and 12 h light/dark control condition.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.961.
Salari Rafsanjani, M. , Tabatabaei Naeini, A. , Meimandi‐Parizi, A. , Nowzari, F. , Mujtaba Wani, M. , & Iraji, A. (2022). Wound healing effect of Carum carvi L. on the incised skin wound in male rats: Histopathology, total protein and biomechanical evaluations. Veterinary Medicine and Science, 8, 2726–2737. 10.1002/vms3.961
Contributor Information
Aboutorab Tabatabaei Naeini, Email: tabatabaei_a@yahoo.com.
Aida Iraji, Email: iraji@sums.ac.ir, Email: aida.iraji@gmail.com.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are published in the literature.
REFERENCES
- Archer, C. B. (2010). Functions of the skin. Rook's Textbook of Dermatology, 1, 1–11. [Google Scholar]
- Rodrigues, M. , Kosaric, N. , Bonham, C. A. , & Gurtner, G. C. (2019). Wound healing: A cellular perspective. Physiological Reviews, 99(1), 665–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, S. , Lin, E. J. , & Tartar, D. (2018). Immunology of wound healing. Curr Dermatol Rep, 7(4), 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing, C. (2017). The molecular biology in wound healing & non‐healing wound. Chinese Journal of Traumatology = Chung‐Hua Chuang Shang Tsa Chih, 20(4), 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitridge, R. , & Thompson, M. (2011). Mechanisms of vascular disease: a reference book for vascular specialists, University of Adelaide Press. [PubMed] [Google Scholar]
- Reinke, J. M. , & Sorg, H. (2012). Wound repair and regeneration. European Surgical Research, 49(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Mangoni, M. L. , McDermott, A. M. , & Zasloff, M. (2016). Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Experimental Dermatology, 25(3), 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, H. N. , & Hardman, M. J. (2020). Wound healing: Cellular mechanisms and pathological outcomes. 10(9) 200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, V. ‐L. , Truong, C. ‐T. , Nguyen, B. C. Q. , Vo, T. ‐N. V. , Dao, T. ‐T. , Nguyen, V. ‐D. , Trinh, D. ‐T. T. , Huynh, H. K. , & Bui, C.‐B. (2017). Anti‐inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS One., 12(10), e0185674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajialyani, M. , Tewari, D. , Sobarzo‐Sánchez, E. , Nabavi, S. M. , Farzaei, M. H. , & Abdollahi, M. (2018). Natural product‐based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomedicine, 13, 5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri, R. (2011). Cuminum cyminum and Carum carvi: An update. Pharmacognosy Reviews, 5(9), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri, R. (2011). Cuminum cyminum and Carum carvi: An update. Pharmacognosy Reviews, 5(9), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahari, P. , & Singh, D. K. (2014). A review on the pharmacological aspects of Carum carvi . Journal of Biology and Earth Sciences, 4(1), M1–M13. [Google Scholar]
- Derakhshan, S. , Sattari, M. , & Bigdeli, M. (2008). Effect of subinhibitory concentrations of cumin (Cuminum cyminum L.) seed essential oil and alcoholic extract on the morphology, capsule expression and urease activity of Klebsiella pneumoniae . International Journal of Antimicrobial Agents, 32(5), 432–436. [DOI] [PubMed] [Google Scholar]
- Derakhshan, S. , Sattari, M. , & Bigdeli, M. (2010). Effect of cumin (Cuminum cyminum) seed essential oil on biofilm formation and plasmid integrity of Klebsiella pneumoniae . Pharmacognosy Magazine, 6(21), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegh, S. , Rasooli, I. , Taghizadeh, M. , & Alipoor Astaneh, S. D. (2008). Phytotherapeutic inhibition of supragingival dental plaque. Natural Product Research, 22(5), 428–439. [DOI] [PubMed] [Google Scholar]
- Boyraz, N. , & Özcan, M. (2005). Antifungal effect of some spice hydrosols. Fitoterapia, 76(7–8), 661–665. [DOI] [PubMed] [Google Scholar]
- Fang, R. , Jiang, C. H. , Wang, X. Y. , Zhang, H. M. , Liu, Z. L. , Zhou, L. , Du, S. S. , & Deng, Z. W. (2010). Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules (Basel, Switzerland), 15(12), 9391–9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz, A. , Minaiyan, M. , Ghannadi, A. , & Mahzouni, P. (2013). Effects of Carum carvi L. (Caraway) extract and essential oil on TNBS‐induced colitis in rats. Research in Pharmaceutical Sciences, 8(1), 1. [PMC free article] [PubMed] [Google Scholar]
- Bourgou, S. , Bettaieb Rebey, I. , Dakhlaoui, S. , Msaada, K. , Saidani Tounsi, M. , Ksouri, R. , Fauconnier, M. ‐L. , & Hamrouni‐Sellami, I. (2020). Green extraction of oil from Carum carvi seeds using bio‐based solvent and supercritical fluid: Evaluation of its antioxidant and anti‐inflammatory activities. Phytochemical Analysis, 31(1), 37–45. [DOI] [PubMed] [Google Scholar]
- Mardani, M. , Afra, S. , Tanideh, N. , Andisheh Tadbir, A. , Modarresi, F. , Koohi‐Hosseinabadi, O. , Iraji, A. , & Sepehrimanesh, M. (2016). Hydroalcoholic extract of Carum carvi L. in oral mucositis: A clinical trial in male golden hamsters. Oral Diseases, 22(1), 39–45. [DOI] [PubMed] [Google Scholar]
- Alizade Naini, M. , Mehrvarzi, S. , Zargari‐Samadnejadi, A. , Tanideh, N. , Ghorbani, M. , Dehghanian, A. , Hasanzarrini, M. , Banaee, F. , Koohi‐Hosseinabadi, O. , Irajie, C. , & Iraji, A. (2021). The antioxidant and anti‐inflammatory effects of Quercus brantii extract on TNBS‐induced ulcerative colitis in rats. Evidence‐Based Complementary and Alternative Medicine, 2021, p. 3075973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naini, M. A. , Zargari‐Samadnejad, A. , Mehrvarz, S. , Tanideh, R. , Ghorbani, M. , Dehghanian, A. , Hasanzarrini, M. , Banaee, F. , Koohi‐Hosseinabadi, O. , Tanideh, N. , & Iraji, A. (2021). Anti‐inflammatory, antioxidant, and healing‐promoting effects of Aloe vera extract in the experimental colitis in rats. Evidence‐Based Complementary and Alternative Medicine, 2021, 9945244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar, S. , Zare, M. R. , Shojaei, F. , Zareshahrabadi, Z. , Koohi‐Hosseinabadi, O. , Saharkhiz, M. J. , Iraji, A. , Zomorodian, K. , & Khorram, M. (2021). Core‐shell chitosan/PVA‐based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. International Journal of Pharmaceutics, 597, 120288. [DOI] [PubMed] [Google Scholar]
- Tanideh, N. , Azarpira, N. , Sarafraz, N. , Zare, S. , Rowshanghiyas, A. , Farshidfar, N. , Iraji, A. , Zarei, M. , & El Fray, M. (2020). Poly(3‐Hydroxybutyrate)‐multiwalled carbon nanotubes electrospun scaffolds modified with curcumin. Polymers, 12(11), 2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allafchian, A. , Hosseini, H. , & Ghoreishi, S. M. (2020). Electrospinning of PVA‐carboxymethyl cellulose nanofibers for flufenamic acid drug delivery. International Journal of Biological Macromolecules, 163, 1780–1786. [DOI] [PubMed] [Google Scholar]
- Peptu, C. A. , Băcăiță, E. S. , Savin, C. ‐ L. , Luțcanu, M. , & Agop, M. (2021). Hydrogels based on alginates and carboxymethyl cellulose with modulated drug release – An experimental and theoretical study. Polymers, 13(24), 4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayani, Z. , Firuzi, O. , & Bordbar, A. ‐K. (2018). Doughnut‐shaped bovine serum albumin nanoparticles loaded with doxorubicin for overcoming multidrug‐resistant in cancer cells. International Journal of Biological Macromolecules, 107, 1835–1843. [DOI] [PubMed] [Google Scholar]
- Tanideh, R. , Delavari, S. , Farshad, O. , Irajie, C. , Javad Yavari Barhaghtalab, M. , Koohpeyma, F. , Koohi‐Hosseinabadi, O. , Jamshidzadeh, A. , Tanideh, N. , & Iraji, A. (2021). Effect of flaxseed oil on biochemical parameters, hormonal indexes and stereological changes in ovariectomized rats. Veterinary Medicine and Science, 7(2), 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem., 72(1‐2), 248–254. [DOI] [PubMed] [Google Scholar]
- Guo, S. A. , & DiPietro, L. A. (2010). Factors affecting wound healing. Journal of Dental Research, 89(3), 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R. , Liang, H. , Clarke, E. , Jackson, C. , & Xue, M. (2016). Inflammation in chronic wounds. International Journal of Molecular Sciences, 17(12), 2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh, M. , Sahin, K. B. , West, Z. E. , & Murray, R. Z. (2017). Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci., 18(7), 1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour, I. , Naeini, A. T. , Aminlari, M. , & Nazifi, S. (2019). Effect of punicalagin nanofibrous‐dressing on tissue total antioxidant capacity index through wound healing in adult Wistar rats. Zahedan Journal of Research in Medical Sciences, 21(2), . [Google Scholar]
- Hamidi, S. A. , Tabatabaei Naeini, A. , Oryan, A. , Tabandeh, M. R. , Tanideh, N. , & Nazifi, S. (2017). Cutaneous wound healing after topical application of Pistacia atlantica gel formulation in rats. Turkish Journal of Pharmaceutical Sciences, 14(1), 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex, J. , Muthukumar, N. , & Selvakumar, P. (2018). Phytochemicals as a potential source for anti‐microbial, anti‐oxidant and wound healing – A review. MOJ Bioorganic & Organic Chemistry, 2(2), 61–70. [Google Scholar]
- Hosseini, S. , Ramezan, Y. , & Arab, S. (2020). A comparative study on physicochemical characteristics and antioxidant activity of sumac (Rhus coriaria L.), cumin (Cuminum cyminum), and caraway (Carum carvil) oils. Journal of Food Measurement and Characterization, 14(6), 3175–3183. [Google Scholar]
- Ibrahim, N. I. , Wong, S. K. , Mohamed, I. N. , Mohamed, N. , Chin, K. ‐Y. , Ima‐Nirwana, S. , & Shuid, A. N. (2018). Wound healing properties of selected natural products. Int J Environ Res Public Health., 15(11), 2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, D. , Kulkarni, A. , Shahapurkar, A. , & Hatappakki, B. C. (2009). Natural cumin seeds for wound healing activity in albino rats. 3(4), 148–152. [Google Scholar]
- Bourgou, S. , Bettaieb Rebey, I. , Dakhlaoui, S. , Msaada, K. , Saidani Tounsi, M. , Ksouri, R. , Fauconnier, M. L. , & Hamrouni‐Sellami, I. (2020). Green extraction of oil from Carum carvi seeds using bio‐based solvent and supercritical fluid: Evaluation of its antioxidant and anti‐inflammatory activities. Phytochemical Analysis, 31(1), 37–45. [DOI] [PubMed] [Google Scholar]
- Seddighfar, M. , Mirghazanfari, S. M. , & Dadpay, M. (2020). Analgesic and anti‐inflammatory properties of hydroalcoholic extracts of Malva sylvestris, Carum carvi or Medicago sativa, and their combination in a rat model. Journal of Integrative Medicine, 18(2), 181–188. [DOI] [PubMed] [Google Scholar]
- Keshavarz, A. , Minaiyan, M. , Ghannadi, A. , & Mahzouni, P. (2013). Effects of Carum carvi L.(Caraway) extract and essential oil on TNBS‐induced colitis in rats. Res Pharm Sci, 8(1), 1. [PMC free article] [PubMed] [Google Scholar]
- De Filippo, K. , Dudeck, A. , Hasenberg, M. , Nye, E. , van Rooijen, N. , Hartmann, K. , Gunzer, M. , Roers, A. , & Hogg, N. J. B. (2013). Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. The Journal of the American Society of Hematology, 121(24), 4930–4937. [DOI] [PubMed] [Google Scholar]
- Pachuau, L. (2015). Recent developments in novel drug delivery systems for wound healing. Expert Opin Drug Deliv, 12(12), 1895–1909. [DOI] [PubMed] [Google Scholar]
- Yakoubi, S. , Bourgou, S. , Mahfoudhi, N. , Hammami, M. , Khammassi, S. , Horchani‐Naifer, K. , Msaada, K. , & Tounsi, M. S. (2020). Oil‐in‐water emulsion formulation of cumin/carvi essential oils combination with enhanced antioxidant and antibacterial potentials. 32(6), 536–544. [Google Scholar]
- Ghazi, M. , Goudarzi, H. , Ardekani, H. A. Z. , Habibi, M. , Azargashb, E. , Hajikhani, B. , & Goudarzi, M. (2019). Investigation of antibacterial effect of Cuminum cyminum and Carum carvi against Streptococcus mutans and Streptococcus pyogenes . Novelty in Biomedicine., 7(1), 30–34. [Google Scholar]
- Volksdorf, T. , Heilmann, J. , Eming, S. A. , Schawjinski, K. , Zorn‐Kruppa, M. , Ueck, C. , Vidal‐y‐Sy, S. , Windhorst, S. , Jücker, M. , Moll, I. , & Brandner, J. M. (2017). Tight junction proteins claudin‐1 and occludin are important for cutaneous wound healing. American Journal of Pathology, 187(6), 1301–1312. [DOI] [PubMed] [Google Scholar]
- Soliman, A. M. , Lin, T. S. , Ghafar, N. A. , & Das, S. (2018). Virgin coconut oil and diabetic wound healing: Histopathological and biochemical analysis. 22, 135–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are published in the literature.


