Abstract
Mycobacterium tuberculosis causes active tuberculosis in only a small percentage of infected persons. In most cases, the infection is clinically latent, although immunosuppression can cause reactivation of a latent M. tuberculosis infection. Surprisingly little is known about the biology of the bacterium or the host during latency, and experimental studies on latent tuberculosis suffer from a lack of appropriate animal models. The Cornell model is a historical murine model of latent tuberculosis, in which mice infected with M. tuberculosis are treated with antibiotics (isoniazid and pyrazinamide), resulting in no detectable bacilli by organ culture. Reactivation of infection during this culture-negative state occurred spontaneously and following immunosuppression. In the present study, three variants of the Cornell model were evaluated for their utility in studies of latent and reactivated tuberculosis. The antibiotic regimen, inoculating dose, and antibiotic-free rest period prior to immunosuppression were varied. A variety of immunosuppressive agents, based on immunologic factors known to be important to control of acute infection, were used in attempts to reactivate the infection. Although reactivation of latent infection was observed in all three variants, these models were associated with characteristics that limit their experimental utility, including spontaneous reactivation, difficulties in inducing reactivation, and the generation of altered bacilli. The results from these studies demonstrate that the outcome of Cornell model-based studies depends critically upon the parameters used to establish the model.
Current estimates are that one-third of the world’s population is infected with Mycobacterium tuberculosis (33). In most cases, the infected individual mounts an effective immune response that culminates in granuloma formation around the infective foci and subsequent arrest of disease progression. Clinical studies suggest that the bacilli within these granulomas are not killed but, instead, remain dormant (30, 31); this is termed a latent infection. Approximately 10% of latent infections reactivate, resulting in active, infectious tuberculosis months to years after the initial infection (31). The risk of reactivation increases to 5 to 15% annually in persons coinfected with human immunodeficiency virus (28). Thus, the large number of latently infected individuals presents a major impediment to reducing the incidence of tuberculosis and the rate of M. tuberculosis transmission.
Recent studies have provided significant insight into the immune responses that mediate control of acute M. tuberculosis infection in the murine model of tuberculosis. In particular, essential roles have been demonstrated for T cells (reviewed in reference 3), gamma interferon (IFN-γ) (6, 13), tumor necrosis factor alpha (TNF-α) (14), interleukin-12 (7), and reactive nitrogen intermediates (RNI) generated by the macrophage enzyme inducible nitric oxide synthase (NOS2) (2, 18). However, little is known about the basic mechanisms involved in maintaining a latent M. tuberculosis infection or the causes of reactivation. In large part, this is due to the difficulty in developing and manipulating animal models of latent tuberculosis. The design of an adequate animal model of latent M. tuberculosis infection is hampered by the lack of knowledge about the biological characteristics of both the tubercle bacilli and host immunity during human latent tuberculosis.
Variations on two murine models of latent M. tuberculosis infection have been described in the literature. Whether these models truly represent latent human tuberculosis remains controversial. Nevertheless, studies using these two models have yielded important information concerning the pathogenesis of tuberculosis (1, 15, 18, 25). In the first model (which will be referred to in this work as the low-dose model), mice were aerogenically infected with a low dose of M. tuberculosis (5 to 10 CFU), and within 3 months the pulmonic bacillary burden stabilized at ∼3 to 4 log10 (25). This clinically quiescent phase of the infection was maintained for 15 to 18 months, after which time the infection began to reactivate and the mice succumbed to tuberculosis. This low-dose model has the important advantage of mimicking natural latency in the sense that it relies solely on the host immune response for control of the infection, but it has the disadvantage of a high bacillary burden that is unlike that found in human latent M. tuberculosis infection. Using a modified low-dose model of murine latent tuberculosis, we have previously demonstrated that RNI play an important role in preventing reactivation of this infection (15).
The second model of M. tuberculosis latency has been referred to as the Cornell model and was first described in the 1950s (19, 20). In the original Cornell model (Table 1), mice were inoculated intravenously (i.v.) with 1 × 106 to 3 × 106 viable bacilli of the H37Rv strain of M. tuberculosis, and the resultant infection was treated for 12 weeks with the antimycobacterial drugs isoniazid (INH) and pyrazinamide (PZA) beginning within 20 min after infection. Mice so treated had been apparently sterilized, as they harbored no culturable tubercle bacilli at the time of completion of the antibiotic course. However, 90 days after cessation of antibiotics, approximately one-third of the mice yielded INH-sensitive tubercle bacilli upon organ culture (20). This drug-induced model of latent tuberculosis has the advantage of achieving very low or undetectable numbers of bacilli and maintaining those low levels for many weeks, analogous to latent infection in humans, but has the disadvantage of artificially inducing latency. Using this original Cornell model, McCune et al. have shown that administration of cortisone, a broad immunosuppressant, after chemotherapy reduced the time required for 50% of the mice to revert to a culture-positive state from 7 to 2.5 months (21). These early studies focused more on the ability of the mycobacteria to persist or replicate following antibiotic regimens and less on the role of host immunity in maintaining a latent infection. In fact, in the original Cornell model (Table 1), the antibiotic regimen was initiated 20 min postinfection, interfering with the induction of a natural immune response to the infection. Recently, we have used a modified Cornell model to show that reactivation occurs if the production of RNI is blocked by aminoguanidine, a nitric oxide synthase inhibitor with relative specificity for NOS2 (15).
TABLE 1.
Summary of Cornell model and variants
| Model | Inoculating dosed (CFU) | Infection perioda | Antibiotic (concn)b | Length of treatment | Postantibiotic rest period |
|---|---|---|---|---|---|
| Original Cornellc | 1 × 106, 3 × 106 | 20 min | INH (0.0125%) | 12 wk | Variable |
| PZA (2.0%) | |||||
| Variant A | 5 × 103 | 4 wk | INH (0.1 g/liter) | 4 wk | 2 wk |
| PZA (15 g/liter) | |||||
| Variant B | 5 × 103 | 4 wk | INH (0.1 g/liter) | 12 wk | 8 wk |
| PZA (15 g/liter) | |||||
| Variant C | 1 × 105 | 4 wk | INH (0.1 g/liter) | 12 wk | 11 wk |
| PZA (8 g/liter) |
Unfortunately, there is no standardized protocol for establishing latency with the Cornell model. In the present study, we examine several Cornell model variants for their applicability to immunologic studies of latent tuberculosis. Three variants of the original Cornell model were established by modulating the M. tuberculosis inoculum, the duration of antibiotic therapy, the antibiotic dosages, and the interval between cessation of antibiotics and immunologic intervention. The rate of (i) spontaneous reactivation following the antibiotic regimen and (ii) reactivation upon immunosuppression were evaluated for these variants. The immunosuppressive regimens included NOS2 inhibition, in vivo neutralization of IFN-γ, in vivo neutralization of TNF-α, and pharmacologic pan-immunosuppression using glucocorticoids. These regimens were chosen since each targets an immunologic component previously demonstrated to be important in controlling acute or latent tuberculosis. NOS2 inhibition has been shown to exacerbate acute murine tuberculosis and to accelerate disease progression in murine models of latent tuberculosis (2, 15, 18). IFN-γ plays a crucial role in controlling acute M. tuberculosis infections in mice (6, 13) and humans (reviewed in reference 26) and is crucial for inducing NOS2 expression (8, 13). In vivo neutralization of TNF-α, using a monoclonal antibody or an adenoviral vector expressing the p55 receptor for TNF-α, resulted in accelerated disease progression following acute M. tuberculosis challenge (1, 14) as well as in the low-dose, non-drug-induced model of latency (1). Glucocorticoids represent the best-known broad-spectrum immunosuppressants and are associated with enhanced susceptibility to M. tuberculosis infection, both clinically (reviewed in reference 5) and experimentally (18, 24).
We show that the outcome of the Cornell model is highly dependent upon the parameters used to establish latency and that each variant of the Cornell model has certain limitations, which results in either spontaneous reactivation, difficulties in inducing reactivation, or production of phenotypically altered mycobacteria. These studies provide essential information for investigators interested in using this model as a tool for studying latent tuberculosis and for immunologists who look to Cornell model-based reactivation studies for general immunologic principles operant in chronic infectious diseases. The results are significant in that an investigator must invest substantial resources to use latent tuberculosis animal models and must consider how the parameters used to establish the model may affect experimental outcomes.
MATERIALS AND METHODS
Animals.
Eight- to ten-week-old C57BL/6 female mice (The Jackson Laboratory, Bar Harbor, Maine, or Charles River, Rockland, Mass.) were maintained in biosafety-level-3, specific-pathogen-free animal facilities. Mice were routinely monitored for murine pathogens by comprehensive serological screens as well as histopathological studies of various organs, including the brain, liver, lungs, spleen, heart, kidneys, and gastrointestinal tract.
Mycobacteria.
The virulent Erdman strain of M. tuberculosis was passed through mice, grown in culture once, and frozen in aliquots. Prior to infection, an aliquot was thawed, diluted in phosphate-buffered saline (PBS) containing 0.05% Tween 80, and briefly sonicated.
Infection and antibiotic treatment of mice.
For variants of the Cornell model, mice were infected i.v. via the lateral tail vein with 5 × 103 to 1 × 105 viable bacilli, depending on the experiment (Table 1). After 4 weeks, the mice were treated with INH at 0.1 g/liter and PZA at either 8 or 15 g/liter delivered ad libitum in drinking water. The length of the antibiotic course was varied (Table 1). For testing the efficacy of MP6-XT22 or l-N6-(1-iminoethyl)lysine (L-NIL) in acute tuberculosis, mice were infected i.v. with 2 × 105 viable bacilli.
Chemicals and reagents.
Unless noted otherwise, all chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.). The 7H10 agar substrate was purchased from Difco (Detroit, Mich.). The NOS2 inhibitor L-NIL was synthesized according to a published protocol (23). α-t-butoxycarbonyl–lysine was purchased from United States Biochemicals (Cleveland, Ohio), ethyl acetimidate HCl was purchased from Aldrich Chemical Co. (Milwaukee, Wis.), and pyridine and p-dioxane were purchased from J. T. Baker (Philipsburg, N.J.). The XMG-6 rat anti-murine IFN-γ monoclonal antibody (MAb) hybridoma was developed by Cherwinski et al. (4, 12). The MP6-XT22 rat anti-murine TNF-α MAb hybridoma (DNAX Research Institute of Molecular and Cellular Biology, Inc., Palo Alto, Calif.) was obtained through the American Type Culture Collection (Manassas, Va.). Both hybridomas were used to produce ascites by Harlan Bioproducts for Science (Indianapolis, Ind.) and the immunoglobulin G (IgG) from the resultant ascitic fluid was purified by sodium ammonium sulfate precipitation and dialyzed into PBS. The MAbs were tested for the ability to neutralize TNF-α or IFN-γ in vitro according to the protocols published in references 11 and 17, respectively. Normal rat IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa.).
Immunosuppressive regimens. (i) NOS2 inhibition.
Mice acutely infected with M. tuberculosis were given aminoguanidine (2.5%, wt/vol) or L-NIL (4 or 9 mM; pH 2.7) ad libitum in drinking water. Control mice were given plain water. Water with L-NIL was changed every 48 h; water with aminoguanidine was changed twice weekly. For reactivation experiments, 4 mM L-NIL was provided in drinking water, beginning 8 weeks after mice completed the INH-PZA course.
(ii) In vivo IFN-γ neutralization.
Beginning 2 weeks after M. tuberculosis-infected mice completed the antibiotic regimen, the mice received injections of 0.5 mg of XMG-6 intraperitoneally (i.p.) twice per week. Control mice received 0.5 mg of normal rat IgG on an identical schedule.
(iii) In vivo TNF-α neutralization.
To confirm the efficacy of MP6-XT22 in vivo, antibody treatment was begun 1 day prior to infection with M. tuberculosis. The antibody was delivered by weekly i.p. injections of 1 mg or by twice-weekly i.p. injections of 0.5 mg of MP6-XT22. Control animals received equivalent amounts of rat IgG. For reactivation experiments, 0.5 mg of MP6-XT22 was injected biweekly, beginning 8 (variant B) or 11 (variant C) weeks after completion of the antibiotic regimen.
(iv) Glucocorticoid treatment.
Dexamethasone was injected i.p. at a dosage of 0.08 mg/day (6 times/week). PBS was administered to the control group on the same schedule. Hydrocortisone acetate (HCA) was administered at 1.5 mg subcutaneously (s.c.) every third day for 88 days and then at 1.0 mg s.c. daily for an additional 38 days. Control animals received PBS only.
Quantitation of viable mycobacteria in organs.
At regular intervals, mice were sacrificed and their lungs and spleens were removed. As previously described (13, 14), one-quarter or one-half of each organ was homogenized in PBS containing 0.05% Tween 80 and serial dilutions of the homogenates were plated onto 7H10 agar. The plates were incubated at 37°C in 5% CO2 for 21 days, the colonies were counted, and the data were presented as CFU per organ. The acid fastness of colonies recovered from organs of mice after reactivation with MP6-XT22 (the TNF-α-specific neutralizing antibody) or dexamethasone was examined with Ziehl-Neelsen stain as described previously (15).
IS6110-specific PCR.
Species confirmation of colonies recovered from organs of mice upon reactivation by administration of MP6-XT22 or dexamethasone was carried out by the detection of IS6110, a mycobacterial insertional element specific for the M. tuberculosis complex (34). Briefly, a single colony was suspended in 150 μl of Tris-EDTA buffer (10 mM Tris, pH 8.0; 1 mM EDTA). The bacterial suspension was incubated at 100°C for 15 min. The heated bacterial suspension was vortexed vigorously after the addition of 100 μl of glass beads (106-μm diameter; Sigma). PCR was carried out for 35 cycles with the forward primer GCGTAGGCGTCGGTGACAAA and the reverse primer CGTGAGGGCATCGAGGTGGC.
In vitro RNI production.
The murine macrophage cell line J774 was grown in Dulbecco’s modified Eagle medium (Gibco, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (Gibco). Cells were plated in 96-well plates at 1.5 × 105 cells/well and primed overnight with recombinant murine IFN-γ (100 U/ml; Genentech, San Francisco, Calif.). When appropriate, cells were treated with NOS2 inhibitors for 4 h prior to activation with lipopolysaccharide (1 μg/ml). Plates were incubated at 37°C in 5% CO2 for 24 h, the supernatants were collected, and nitrite levels were determined by Griess assay as previously described (16).
Statistical analysis.
Statistical significance was tested by the Wilcoxon rank sum test or by Fisher’s exact test as noted. Differences with a P of ≤0.05 were considered significant.
RESULTS
Variant A of the Cornell model.
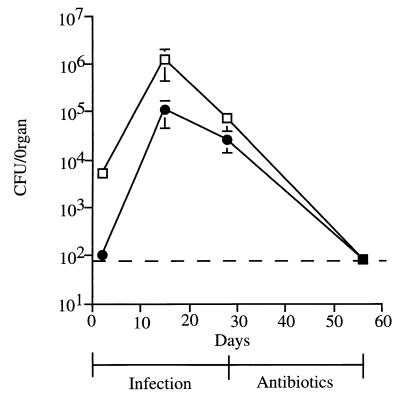
In variant A of the Cornell model, C57BL/6 mice were infected i.v. with a relatively low dose of M. tuberculosis Erdman (5 × 103 viable bacilli). One month later, mice were treated with INH and PZA at doses of 0.1 and 15 g/liter, respectively, administered in drinking water, for 4 weeks (Table 1). The numbers of viable bacilli in spleen and lungs were 104 to 105 at 4 weeks postinfection (Fig. 1). The 4-week course of INH-PZA resulted in low or undetectable numbers of viable bacilli in spleens and lungs (Fig. 1). Administration of immunosuppressive agents in attempts to reactivate the drug-induced latent infection began 2 weeks after completion of antibiotic treatment. We previously used this variation of the Cornell model to show that inhibition of NOS2 in latently infected mice induced reactivation of the infection (15).
FIG. 1.
Drug-induced model of latent tuberculosis (variant A of the Cornell model). Mice were infected i.v. with 5 × 103 CFU of M. tuberculosis for 4 weeks, and the infection was then treated with a 4-week course of the antimycobacterial drugs INH (0.1 g/liter) and PZA (15 g/liter) delivered in drinking water. After the treatment period, mice were maintained on plain water. Numbers of viable bacilli were determined at the indicated times by plating homogenates of spleen (squares) and lungs (circles) and counting colonies 21 days later. The dashed line denotes the limit of detection for the assay (80 CFU). Each point represents the mean for two to four mice; error bars show standard errors.
(i) IFN-γ neutralization.
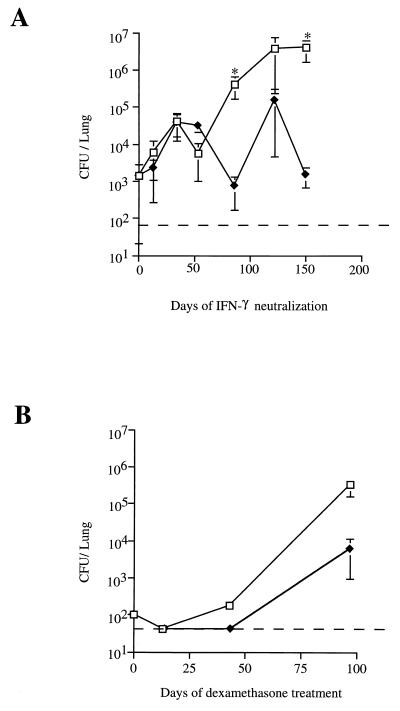
Neutralization of IFN-γ induced a slow but sustained increase in the number of mycobacteria in the lungs (Fig. 2A), resulting in a bacterial burden ∼750-fold higher than that in IgG-treated controls by 87 days after initiation of XMG-6 treatment (P = 0.02). The bacillary load in the lungs of XMG-6-treated animals continued to rise, reaching a level of ∼5 × 106 by 150 days of IFN-γ neutralization (P = 0.02). At some time points the bacterial burden in lungs of IgG-treated control mice increased above the level attained immediately following antibiotic treatment, but there was no significant upward trend (P = 0.39). Thus, in vivo neutralization of IFN-γ caused recrudescence of infection in variant A of the Cornell model.
FIG. 2.
Reactivation of infection in lungs of mice by immunosuppression in variant A of the Cornell model. Mice were infected with 5 × 103 CFU of M. tuberculosis for 4 weeks and then treated for 4 weeks with INH-PZA. (A) Following the antibiotic regimen, IFN-γ was neutralized in vivo by using monoclonal anti-murine IFN-γ (squares) while control mice (diamonds) received normal rat IgG1. (B) Following the antibiotic regimen, mice were treated with dexamethasone (squares) or with PBS (diamonds). For both panels, mice were sacrificed at various time points, and the number of viable bacilli in the lungs was determined by plating serial dilutions of lung homogenates onto 7H10 agar and counting colonies after 21 days at 37°C. The dashed line denotes the limit of detection for the assay (80 CFU). Each point represents the mean for three to four mice; error bars show standard errors. ∗, P ≤ 0.05 by Wilcoxon rank sum test.
(ii) Dexamethasone pan-immunosuppression.
In the low-dose (non-drug-induced) model of latent murine tuberculosis (described in reference 15), treatment with the glucocorticoid dexamethasone resulted in rapidly fatal reactivation with a mean survival time of 24 ± 4 days (data not shown). In variant A of the Cornell model, dexamethasone caused a marked increase in pulmonic bacillary burden. By 98 days of dexamethasone therapy, the bacterial load in the lungs was 1,000-fold greater than that prior to the start of immunosuppression (Fig. 2B). However, spontaneous reactivation resulted in an increased bacillary burden in the nonimmunosuppressed control group (Fig. 2B) and precluded a significant difference in tissue bacterial burden between the steroid-treated and control groups 98 days after the start of immunosuppression (P = 0.38).
It is noteworthy from these data, particularly those presented in Fig. 2A, that the antibiotic regimen of variant A of the Cornell model failed to render the infected animals consistently culture negative. Additionally, there was significant variability in the bacterial load among mice in the postantibiotic period. These variables may obfuscate conclusions derived from data obtained with this variant of the Cornell model. We therefore investigated the applicability of a more stringent variation of the Cornell model, one that consistently would result in culture-negative mice following chemotherapy.
Variant B of the Cornell model.
To reduce spontaneous reactivation during the postantibiotic treatment period, variant A of the Cornell model was modified in two ways (Table 1). First, the INH (0.1 g/liter) and PZA (15 g/liter) course was extended from 4 to 12 weeks. This 12-week regimen was used in the original Cornell model (Table 1) and was shown previously to be reliable in rendering the organs of M. tuberculosis-infected mice culture negative; this state was maintained in two-thirds of the mice for more than 3 months (19). Second, an 8-week drug-free rest period was introduced between completion of the antibiotic course and commencement of the immunologic intervention. McCune et al. (21), using the original Cornell model, showed that cortisone treatment was more effective in reactivating a drug-induced latent infection when the mice were rested for 8 to 12 weeks after antibiotic therapy. This may reflect a recovery period for the bacteria following drug treatment. Thus, attempts to induce reactivation were initiated 8 weeks following completion of the INH-PZA. Variant B of the Cornell model (Table 1) was tested with several immunosuppressive agents.
(i) Immunosuppression by glucocorticoid.
Despite a powerfully immunosuppressive course of the glucocorticoid HCA, all animals remained culture negative until the final time point (126 days), when three of four HCA-treated animals had culturable M. tuberculosis in the lungs, albeit in very low numbers (70 ± 44 CFU/lung; range, 0 to 200 CFU) (Table 2). No bacteria were recovered from the spleens of any HCA-treated mice during this period, and both the lungs and spleens of the control animals remained culture negative throughout the entire postantibiotic period.
TABLE 2.
Reactivation of latent M. tuberculosis infection in Cornell model variant Ba
| Agent | Action or result | Days of treatment with agent | No. of culture positive mice/total no. of mice |
|---|---|---|---|
| HCA | Immunosuppressant | 0 | 0/3 |
| 28 | 0/4 | ||
| 58 | 0/1 | ||
| 70 | 0/4 | ||
| 126 | 3/4 | ||
| L-NIL | Inhibits NOS2 | 0 | 0/3 |
| 35 | 0/3 | ||
| 84 | 0/4 | ||
| 126 | 0/3 | ||
| 300 | 0/5 | ||
| MP6-XT22 | Neutralizes TNF-α | 32 | 0/4 |
| 56 | 0/4 | ||
| 98 | 0/4 | ||
| 154 | 0/4 | ||
| None | Advanced age | 326 | 0/4 |
| 510 | 0/5 |
C57BL/6 mice were infected with 5 × 103 to 10 × 103 CFU of M. tuberculosis for 4 weeks and then treated with INH-PZA for 12 weeks. Eight weeks after completion of antibiotics, the indicated agent was administered as detailed in Materials and Methods. Control mice were culture negative at all postantibiotic time points in all experiments. The limit of detection was 80 CFU/organ.
(ii) Immunosuppression by NOS2 inhibition.
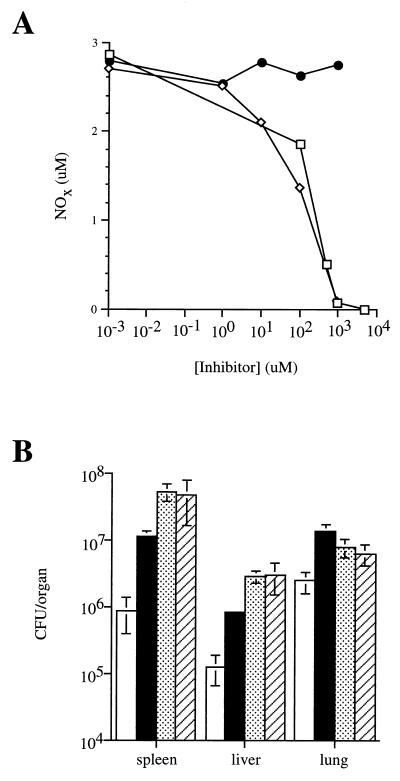
Given the important role of NOS2-generated RNI in maintaining M. tuberculosis latency (15, 18), L-NIL, a nitric oxide synthase inhibitor most specific for NOS2 (32), was used to attempt reactivation in variant B of the Cornell model. The efficacy of the L-NIL preparation was confirmed in vitro by inhibition of lipopolysaccharide (LPS)-induced RNI production by IFN-γ-primed J774 cells (Fig. 3A). The L-NIL preparation was also effective in vivo: both 4 and 9 mM L-NIL in drinking water equivalently exacerbated an acute M. tuberculosis infection in C57BL/6 mice (Fig. 3B), as previously reported by others (18). However, despite 210 days of L-NIL administration to mice (4 mM in drinking water) in which a latent M. tuberculosis infection was established in the variant B model, all mice remained culture negative (Table 2).
FIG. 3.
L-NIL inhibits nitric oxide production in vitro and exacerbates acute M. tuberculosis infection. (A) IFN-γ-primed J774 cells were treated with dilutions of L-NIL (diamonds), aminoguanidine (squares), or α-t-butoxycarbonyl–lysine, the precursor for L-NIL synthesis (circles), for 4 h prior to activation with lipopolysaccharide (1 μg/ml). Nitrite levels in the medium were determined 24 h later by Griess assay. (B) Mice were infected i.v. with 5.5 × 105 CFU of M. tuberculosis and then given 2.5% (wt/vol) aminoguanidine (solid bars), 4 mM L-NIL (stippled bars), or 9 mM L-NIL (hatched bars) ad libitum in drinking water or plain water (open bars). Mice were sacrificed 21 days later, and the numbers of viable bacilli were determined by plating serial dilutions of organ and counting colonies after 21 days at 37°C. Each bar represents the mean for three mice; error bars show standard errors.
(iii) Immunosuppression by neutralization of TNF-α.
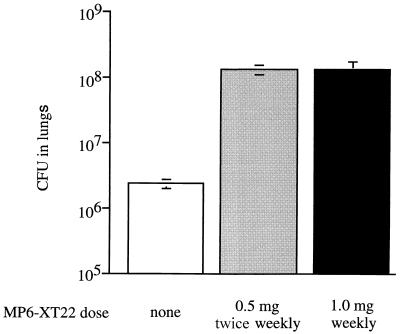
The variant B model was tested for reactivation following neutralization of TNF-α with the MAb MP6-XT22. The efficacy of the MP6-XT22 MAb was established in vivo by its ability to exacerbate an acute M. tuberculosis infection (Fig. 4). Mice treated with MP6-XT22 had 57-fold more mycobacteria in the lungs than did similarly infected control mice 17 days after infection (Fig. 4). However, when 0.5 mg of MP6-XT22 was administered twice weekly to mice in which a latent M. tuberculosis infection was established in the variant B model, no mice converted to a culture-positive state despite 154 days of antibody treatment (Table 2).
FIG. 4.
In vivo neutralization of TNF-α with MP6-XT22 exacerbates acute murine tuberculosis. Mice were infected i.v. with 2 × 105 CFU of M. tuberculosis 1 day after being injected i.p. with MP6-XT22 or normal rat IgG1. After 17 days of infection, numbers of viable bacteria were determined in lungs of mice that received 0.5 mg of MP6-XT22 i.p. twice weekly (shaded bar), 1.0 mg of MP6-XT22 i.p. weekly (solid bar), or 0.5 mg of normal rat IgG1 i.p. twice weekly (open bar). Each bar represents the mean for three to four mice; error bars show standard errors.
Remarkably, no spontaneous reactivation was observed in any control animals throughout the HCA, L-NIL, or MP6-XT22 treatment periods even though 32 of 49 animals were examined 100 days (range, 112 to 356 days) postchemotherapy (data not shown). Even when variant B model mice were monitored for 510 days post-INH-PZA treatment, none proved culture positive (Table 2) despite the advanced age of the mice (25 months). These data are in stark contrast to the collective data of 22 experiments performed by McCune et al. using the original Cornell model, in which >50% of mice had culturable bacilli 6 months postchemotherapy (21).
Variant C of the Cornell model.
The infrequent reactivation observed following immunocompromise of mice with a latent M. tuberculosis infection induced by variant B of the Cornell model suggested near-sterilization of the infection. This near-sterile state may have been due to the aggressive antibiotic regimen relative to the low inoculum, which was ∼200- to 330-fold lower than that used in the original Cornell model (Table 1). Accordingly, the model was further modified by adjusting both the M. tuberculosis inoculum and the dose of antibiotics used. In this third Cornell model variation, variant C, mice were infected i.v. with 105 CFU M. tuberculosis and the PZA concentration was reduced from 15 to 8 g/liter while the INH concentration remained the same. As in variant B, antibiotic treatment was started 4 weeks postinfection and maintained for 12 weeks. To test the applicability of variant C to studies on latent tuberculosis, mice were treated with MP6-XT22 (anti-TNF-α MAb) or normal rat IgG beginning 11 weeks after completion of antibiotic therapy. Control animals remained culture negative throughout the 126-day normal rat IgG treatment period (Table 3). In contrast, three of five MP6-XT22-treated mice had culturable bacilli in lungs and spleen after 98 days of antibody treatment, and by 126 days four of four MP6-XT22-treated mice (P ≤ 0.05 [Fisher’s exact test]) showed reactivation (Table 3). Interestingly, bacteria isolated from the lungs of one animal after 48 days of MP6-XT22 treatment formed atypical small, smooth colonies on 7H10 agar (Table 3). Acid-fast staining revealed that the bacilli that comprised these atypical colonies were negative for acid-fast bacilli and they did not grow in liquid 7H9 media upon subculturing. PCR amplification of IS6110 confirmed these bacilli to be M. tuberculosis.
TABLE 3.
Neutralization of TNF-α in vivo caused reactivation in variant C of the Cornell modela
| Days of antibody treatment | MP6-XT22
|
Normal rat IgG
|
||||
|---|---|---|---|---|---|---|
| Mouse no. | CFU in spleen | CFU in lung | Mouse no. | CFU in spleen | CFU in lung | |
| 48 | 1 | 0 | 0 | 4 | 0 | 0 |
| 2 | 0 | 0 | 5 | 0 | 0 | |
| 3 | 0 | 320b | 6 | 0 | 0 | |
| 98 | 7 | 0 | 0 | 12 | 0 | 0 |
| 8 | 0 | 0 | 13 | 0 | 0 | |
| 9 | 12,000c | 6,000c | 14 | 0 | 0 | |
| 10 | 12,000c | 6,000c | 15 | 0 | 0 | |
| 11 | 2,360 | 6,000c | 16 | 0 | 0 | |
| 126 | 17 | 480 | 0 | 21 | 0 | 0 |
| 18 | 0 | 28 | 22 | 0 | 0 | |
| 19 | 1,204 | 0 | 23 | 0 | 0 | |
| 20 | 16 | 0 | 24 | 0 | 0 | |
The numbers of viable M. tuberculosis bacilli in spleen and lungs of individual animals were determined by plating organ homogenates. The limit of detection was 10 CFU/organ.
Bacteria with altered growth and staining properties.
Plates were confluent at the time of counting, so the reported number of bacteria is approximate and likely is underestimated.
Interestingly, the numbers of bacteria in the spleens and lungs of the culture-positive MP6-XT22-treated mice varied widely (Table 3). Similar wide variation in bacterial burden was observed in Cornell model mice with spontaneous reactivation (21). While the mechanisms underlying this phenomenon remain unclear, variability inherent in experimental murine tuberculosis may be responsible, at least in part. For example, it is commonly observed that survival time varies widely among infected mice, even of the same genetic background.
The limited quantity of MP6-XT22 precluded further tracking of reactivation due to TNF-α neutralization in the variant C model. In an effort to gain more insight into the biology of variant C, dexamethasone was administered to 8 of the 15 control rat IgG-treated mice that remained from the experiment reported in Table 3. At that time, these mice had been infected for 43 weeks and were 27 weeks (189 days) postchemotherapy. Bacterial burdens were determined at 50 and 80 days of dexamethasone treatment (Table 4). Surprisingly, reactivation was noted in both control and dexamethasone-treated mice at the same rate beginning 239 days postchemotherapy. Furthermore, some lung isolates formed colonies with atypical morphology and exhibited the same staining and growth characteristics as some of the bacilli reactivated by the MP6-XT22 treatment described above (Table 4).
TABLE 4.
M. tuberculosis bacilli that reactivate late in the variant C model are phenotypically altered, and the rate of reactivation is not increased by dexamethasone treatmenta
| Day of treatment | Group | Mouse no. | CFU in:
|
AFBc | Growth in 7H9 | IS6110 PCR | |
|---|---|---|---|---|---|---|---|
| Spleen | Lung | ||||||
| 50 | DEX | 1 | 416 | 1,296 | − (lung) | − | + |
| 2 | 0 | 0 | NAb | NA | NA | ||
| 3 | 0 | 0 | NA | NA | NA | ||
| 4 | 0 | 0 | NA | NA | NA | ||
| Control | 1 | 0 | 0 | NA | NA | NA | |
| 2 | 0 | 0 | NA | NA | NA | ||
| 3 | 0 | 0 | NA | NA | NA | ||
| 4 | 0 | 0 | NA | NA | NA | ||
| 80 | DEX | 1 | 0 | 40 | + | + | + |
| 2 | 276 | 0 | + | + | + | ||
| 3 | 0 | 0 | NA | NA | NA | ||
| 4 | 0 | 0 | NA | NA | NA | ||
| Control | 1 | 3,176 | 0 | + | + | + | |
| 2 | 0 | 40 | − | − | + | ||
| 3 | 0 | 0 | NA | NA | NA | ||
Mice were infected with M. tuberculosis for 4 weeks, treated with INH-PZA for 12 weeks, rested for 11 weeks, and then treated with normal rat IgG for 16 weeks before treatment with dexamethasone (DEX) (0.08 mg/day; s.c.) or with PBS s.c. The limit of detection for CFU determination was 10 CFU/organ. −, negative; +, positive.
NA, not applicable.
AFB, acid-fast bacillus staining.
DISCUSSION
Human latent M. tuberculosis infection remains in large part an enigma. Although neither of the two murine models of latent tuberculosis currently available exactly mimics human latent tuberculosis, both have yielded important information concerning the pathogenesis of latent tuberculosis (15, 18, 25). The purpose of the present study was to evaluate three variants of the original Cornell model of latent tuberculosis (Table 1) for their utility in experimental studies of M. tuberculosis latency and reactivation. The results presented indicate that (i) the outcome of Cornell model-based experiments is largely dependent on experimental parameters, such as the size of the M. tuberculosis inoculum and the dose of the antibiotics used, and (ii) all three Cornell model variants are associated with limitations which must be considered when evaluating Cornell model-based studies.
The Cornell model is widely accepted as a suitable experimental model of latent tuberculosis, but paradoxically, the model lacks a standard protocol. This may be due, in part, to the myriad experimental parameters that comprise the Cornell model. Such parameters include (i) the strain of M. tuberculosis used, (ii) the methods of preparation and propagation of stock bacilli (animal-passaged bacteria such as those used in this study are, in general, more virulent); (iii) the M. tuberculosis inoculum; (iv) the route of infection; (v) the time interval between infection and initiation of antibiotic therapy; (vi) the dose and nature of the antimycobacterial agents employed; (vii) the length of the drug-free rest period after which reactivation regimens are implemented; and (viii) the genetic background, sex, and health conditions of the mice. Indeed, in the early Cornell model studies, male outbred Swiss Webster mice were used (19, 21, 29), while female inbred strains of different genetic constitutions, including C57BL/6 and BALB/c, have been used more recently (9, 10, 15). In addition, the H37Rv strain of M. tuberculosis was most commonly used in previous studies (9, 10, 19, 29) but under various culture conditions, which may contribute to differences in virulence. The inoculating dose used to establish infection has ranged from 5 × 103 CFU (15) to 7 × 106 CFU (29), and the elapsed time between infection and chemotherapy has ranged from 0 days (19, 21) to 60 days (29). Neither the dose of INH and PZA nor the length of treatment has been constant among studies. The murine pathogen status of mice in the older studies is unknown, and it is possible that concurrent infection with murine pathogens had an effect on reactivation of the M. tuberculosis infection. Finally, these lengthy and costly studies are intrinsically difficult to carry out. For example, even when identical conditions were used by the same group, relapse rates varied from 0% (9) to 70% (10). In these studies, the remarkable discrepancy in spontaneous revival of quiescent bacilli was attributed to the different sources from which the animals were obtained.
Thus, it is not surprising that the three variants of the original Cornell model (Table 1) described herein vary greatly in terms of the rate of spontaneous increase in bacillary load following chemotherapy. In the least stringent of the models (variant A) (Table 1), the postantibiotic culture-negative state was attained only occasionally, and spontaneous increases in tissue bacillary burden occurred in all three experiments performed (reference 15 and this study). Despite this shortcoming, mice immunosuppressed by in vivo neutralization of IFN-γ and by dexamethasone administration experienced enhanced reactivation in variant A of the Cornell model (Fig. 2). Interestingly, both regimens failed to induce notable reactivation until 45 days after the initiation of those regimens. These reactivation kinetics are remarkably similar to those achieved by NOS2 inhibition in variant A of the Cornell model (15). These data underscore the crucial role of NOS2-derived RNI in maintaining a latent M. tuberculosis infection since dexamethasone, a powerful glucocorticoid immunosuppressant, did not accelerate reactivation relative to NOS2 inhibition. Finally, it is interesting that in these experiments, neither NOS2 inhibition, dexamethasone treatment, nor IFN-γ neutralization led to rapidly fatal reactivation as seen in previous studies that involved the use of NOS2 inhibitors (15, 18) or dexamethasone in the low-dose, non-drug-induced latency model. This may be due to a decrease in efficacy of the agents over the treatment period, but it is also possible that a compensatory immune response that is capable of circumventing the loss of a single immunologic component develops. Alternatively, the M. tuberculosis bacilli in variant A of the Cornell model may have been altered by the long-term antibiotic treatment, as might have occurred also in variants B and C, and consequently may be less virulent than the original inoculum.
In the most-stringent Cornell model variant (variant B) (Table 1), the length of the antibiotic course was extended to 12 weeks, and a rest period of 8 weeks was introduced. The culture-negative state of the mice following chemotherapy was so stable that it was difficult to induce reactivation. L-NIL, a potent NOS2 inhibitor, accelerates disease progression in quiescent murine tuberculosis (18) and exacerbated an acute M. tuberculosis infection but did not induce reactivation in the variant B model. In vivo neutralization of TNF-α with MP6-XT22 was capable of exacerbating acute M. tuberculosis infection but also did not result in reactivation in this model. Only after an extended course of high-dose HCA was any reactivation observed, and the numbers of bacilli in the lungs of mice after prolonged HCA treatment were very low, even though HCA has been shown previously to exacerbate disease progression in both the acute and chronic phase of murine M. tuberculosis infections (18, 24). Even when variant B model mice were monitored for 510 postantibiotic days, none were observed to revert to a culture-positive state. Thus, a long course of antibiotic treatment, coupled with a relatively low infecting dose, established a latent infection that was very difficult to reactivate, except in response to severe, prolonged immunosuppression. This may closely mimic latency in the human, in which both aging (27) and iatrogenic immunosuppression (5) increase the risk of reactivation but neither causes reactivation in the majority of latent M. tuberculosis infections. Experimentally, however, the model has little utility for studies of reactivation of latent tuberculosis.
In variant C of the Cornell model, in vivo neutralization of TNF-α induced reactivation without concomitant spontaneous reactivation. In this variant, the inoculum was increased to 105 CFU and the concentration of PZA was reduced. Under these conditions, neutralization of TNF-α resulted in substantive reactivation after 48 days of antibody treatment. No difference in general health between the MP6-XT22-treated and the control mice that might account for their diametric reactivation rates was noted. In addition, mice in our biosafety-level-3 facilities routinely are monitored for murine pathogens (see Materials and Methods). The pattern of reactivation was similar to that induced by NOS2 inhibition, dexamethasone treatment, or IFN-γ neutralization in variant A of the Cornell model, but without spontaneous reactivation.
Although variant C yielded reactivation subsequent to TNF-α neutralization, it is curious that dexamethasone treatment initiated 27 weeks following chemotherapy did not result in reactivation at a higher frequency than that which occurred in control animals (i.e., spontaneous reactivation). The length of the postchemotherapy drug-free resting period has been shown to correlate well with the susceptibility to spontaneous reactivation (22). This phenomenon could explain, at least in part, the comparable dexamethasone-induced and spontaneous reactivation rates observed in variant C in the immunosuppressed and control mice, respectively. These results also imply that the utility of variant C for the study of latent and/or reactivated tuberculosis is limited beyond 23 to 27 weeks postchemotherapy, when spontaneous reactivation begins to occur.
Some of the isolates of reactivated M. tuberculosis that appeared in anti-TNF-α antibody-treated, glucocorticoid-treated, and control animals had altered morphology, growth, and staining characteristics. In some animals, altered bacilli were isolated from the lungs while phenotypically normal M. tuberculosis was isolated from the spleen. The aberrant staining characteristics of bacilli from atypical colonies suggests damage to the mycobacterial cell wall, possibly arising from the prolonged antibiotic regimen. Although isolated on solid medium, these altered bacilli were defective for growth in liquid culture. It is possible that these altered bacilli are more fastidious than the parental strain and that growth of these isolates is supported only on very rich media as occurs when undiluted organ homogenates are plated onto 7H10 agar. Altered bacilli recovered upon reactivation of latent tuberculosis in the Cornell model, albeit with phenotypic changes different from those observed in the present study, have been described previously (22). The recovery of reactivated bacilli with altered growth characteristics in variant C of the Cornell model suggests that the capacity to reactivate may be affected by deleterious changes to the bacteria induced by the model itself.
The influence of experimental parameters on the outcome of Cornell model-based studies has been recognized since the inception of this model (19–22). McCune et al. realized that the apparent sterility of the postantibiotic state actually had a gradation attached to it; for example, when treatment with INH and PZA was extended to 26 weeks, M. tuberculosis-infected mice were rendered virtually sterile (22). There was, however, little information concerning the suitability of specific experimental parameters when establishing the Cornell model for immunologic studies of latent tuberculosis. McCune et al. used the original Cornell model and its variant to study the ability of cortisone to convert M. tuberculosis-infected mice from a culture-negative to a culture-positive state (21, 22). In the present study, we have used the perturbation of a wide variety of host factors to test the experimental utility of three variants of the original Cornell model of latent murine tuberculosis. The results show that each variant is associated with characteristics that limit its usefulness for reactivation tuberculosis studies. Spontaneous reactivation occurred in variants A and C, reactivation was too difficult to induce in variant B, and in variants B and C there is a possibility that the reactivation outcome depends on the nature of the immunosuppressive regimens employed. Finally, some isolates of reactivating bacilli in variant C had altered growth and staining properties. The in vitro growth defect of these altered bacilli introduces yet another variable to the Cornell models: in the postchemotherapy culture-negative state, viable M. tuberculosis capable of replication only in enriched media may exist.
Collectively, our data serve as a cogent reminder for the investigator interested in using the Cornell model—a reminder that the parameters used to establish latency, such as the inoculating dose, the concentrations of INH and PZA, and the duration of chemotherapy, must be considered carefully. These parameters may also affect interpretation of the data obtained from these models. Clearly, the various models tested vary in their suitability for the characterization of immune mechanisms involved in latent and/or reactivated tuberculosis. Success is dependent upon achieving a low rate of spontaneous reactivation and upon retaining the ability to induce reactivation, and such goals may be difficult to attain. These factors, combined with the time and cost of using this model, are important considerations for the investigator.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Amy Myers Caruso. We thank Michael Cascio and Jordan Bennett for their invaluable assistance in synthesizing L-NIL.
This work was supported by National Institutes of Health grant AI36990 (J.L.F. and J.C.).
REFERENCES
- 1.Adams L B, Mason C M, Kolls J K, Scollard D, Krahenbuhl J L, Nelson S. Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J Infect Dis. 1995;171:400–405. doi: 10.1093/infdis/171.2.400. [DOI] [PubMed] [Google Scholar]
- 2.Chan J, Tanaka K, Carroll D, Flynn J L, Bloom B R. Effect of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Kaufmann S H E. Immune mechanisms of protection. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 389–415. [Google Scholar]
- 4.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cisneros J R, Murray K M. Corticosteroids in tuberculosis. Ann Pharmacother. 1996;30:1298–1303. doi: 10.1177/106002809603001115. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffen J P, Russell D G, Orme I M. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2248. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 9.de Wit D, Wooten M, Dhillon J, Mitchison D A. The bacterial DNA content of mouse organs in the Cornell model of dormant tuberculosis. Tubercle Lung Dis. 1995;76:555–562. doi: 10.1016/0962-8479(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon J, Mitchison D A. Effect of vaccines in a murine model of dormant tuberculosis. Tubercle Lung Dis. 1994;75:61–64. doi: 10.1016/0962-8479(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 11.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 12.Finkleman F D, Katona I M, Mosmann T R, Coffman R L. IFN-γ regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- 13.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against M. tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 15.Flynn J L, Scanga C A, Tanaka K E, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 16.Green L C, Wagner D A, Glogowske J A, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrite, nitrate, and [15N]-nitrate in biological fluids. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in graft-versus-host reaction. J Immunol. 1990;145:2167–2176. [PubMed] [Google Scholar]
- 18.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCune R M, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1957;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCune R M, Tompsett R, McDermott W. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1957;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCune R M, Feldmann F M, Lambert H P, McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCune R M, Feldman F M, McDermott W. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966;123:469–486. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore W M, Webber R K, Jerome G M, Tjoeng F S, Misko T P, Currie M G. l-N6-(1-Iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994;37:3886–3888. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- 24.North R J, Izzo A A. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–1733. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orme I M. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988;137:716–718. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- 26.Ottenhoff T H M, Kumararatne D, Casanova J-L. Novel human immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–498. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 27.Powell K E, Farer L S. The rising age of tuberculosis patients. J Infect Dis. 1980;142:946–948. doi: 10.1093/infdis/142.6.946. [DOI] [PubMed] [Google Scholar]
- 28.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a global epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 29.Rees R J W, Hart D. Analysis of the host-parasite equilibrium in chronic murine tuberculosis by total and viable bacillary counts. Br J Exp Pathol. 1961;42:83–88. [PMC free article] [PubMed] [Google Scholar]
- 30.Stead W W, Kerby G R, Schleuter D P, Jordahl C W. The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med. 1968;68:731–745. doi: 10.7326/0003-4819-68-4-731. [DOI] [PubMed] [Google Scholar]
- 31.Stead W W. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Respir Dis. 1967;95:729–745. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 32.Stenger S, Thuring H, Rollinghoff M, Manning P, Bogdan C. l-N6-(1-Iminoethyl)-lysine potently inhibits inducible nitric oxide synthase and is superior to Ng-monomethyl arginine in vitro and in vivo. Eur J Pharmacol. 1995;294:703–712. doi: 10.1016/0014-2999(95)00618-4. [DOI] [PubMed] [Google Scholar]
- 33.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 34.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D A. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]