Abstract
Based on the findings from the National Lung Screening Trial, the U.S. Preventive Services Task Force recommends annual low dose computed tomography (LDCT) lung cancer screening (LCS) among high-risk adults. Approximately 54% of individuals seeking LCS report current cigarette smoking. Effective smoking cessation interventions, offered at the time of LCS, enhances the health benefits of screening that are attributable to reductions in lung cancer overall and tobacco-related mortality. Considering these data, the Centers for Medicare & Medicaid Services’ (CMS) 2015 decision to cover LCS with LDCT required that radiology imaging facilities make tobacco cessation interventions available for people who smoke. In February 2022, CMS reversed their 2015 coverage requirement for delivering tobacco use treatment at the time of LDCT; CMS retained the requirement for counseling during the shared decision-making visit prior to the exam. The policy change does not diminish the importance of offering high-quality tobacco cessation services in conjunction with routine LDCT for LCS. However, LCS programs face a range of barriers to implementing tobacco use treatment in their settings. As a result, implementation has lagged. Closing the “evidence to practice” gap is the focus of implementation science, a field that offers a set of rigorous methods and a systematic approach to identifying and overcoming contextual barriers to implementing evidence-based guidelines in a range of clinical settings. In this paper, we describe how implementation science frameworks and methods can be used to help guide LCS programs in their efforts to integrate tobacco use treatment and discuss policy changes needed to further facilitate the delivery of TUT as an essential component of the LCS process.
Keywords: Lung cancer screening, Tobacco cessation, Tobacco use treatment, Implementation science
Integrating tobacco use treatment at the time of lung cancer screening will save lives.
Implications.
Practice: Applying an Implementation science lens through which to plan, implement and evaluate TUT integration can facilitate the design of a feasible, sustainable screening and clinical pathway that aligns with organizational infrastructure and resources and increases the reach of cessation services in the context of LCS.
Policy: Policymakers who support reducing cancer inequities should strengthen the current CMS coverage mandates by further specifying a minimum set of cessation services that includes screening all patients for tobacco use, strongly advising tobacco users to quit and offering an active referral to a treatment program like a state Quitline or the national smokefree.gov text message program.
Research: Future research is needed to further guide program decisions about the most feasible and effective strategies for implementing tobacco use treatment (TUT) across the wide range of LCS programs and to facilitate evidence-based policymaking.
PROBLEM
Lung cancer is the leading cause of cancer death in the United States (U.S.), accounting for over one fifth of cancer mortality [1]. In 2011, results from the U.S. National Lung Screening Trial demonstrated that early diagnosis using low dose computed tomography (LDCT) lung cancer screening (LCS) can lower mortality rates by 20% compared to chest x-ray [2, 3]. Based on these findings, the U.S. Preventive Services Task Force (USPSTF) recommended annual screening among eligible adults. In 2021, the eligibility guidelines were expanded to include adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years [4]. Approximately 54% of individuals seeking LCS report current cigarette smoking [5]. With the expansion of screening eligibility criteria, the total number of individuals who smoke who are referred for LCS will grow substantially [6].
The Centers for Medicare & Medicaid Services’ (CMS) 2015 decision to cover LCS with LDCT required that prior to screening, tobacco users receive counseling on the importance of cessation as part of a shared decision-making process, and that radiology imaging facilities make smoking cessation interventions available for people who smoke [7]. Unfortunately, as part of an effort to reduce potential barriers to uptake of LCS, in February 2022, CMS reversed the coverage mandate for delivery of cessation support at the time of LCS; CMS still requires counseling during the shared decision-making visit prior to referral for screening [8].
The 2015 CMS decision was based on trial data and simulation studies that demonstrate significant reductions in lung cancer–specific and overall tobacco-related mortality when LCS and smoking cessation interventions are combined compared with either screening or treatment alone [9–15]. Consistent with current clinical guidelines, these studies further suggest that more intensive, multimodal cessation interventions (e.g., combining pharmacotherapy with individual or telephone counseling) are the most effective approaches for achieving smoking abstinence in the context of LCS [12]. On the strength of these data, the change in coverage determination should not diminish the importance of offering high-quality tobacco cessation services in conjunction with LCS.
More than 2,500 LDCT facilities are registered with the Lung Cancer Screening Registry of the American College of Radiology; they vary from large academic health centers to freestanding, community-based imaging facilities [16]. These sites experience a range of barriers to implementing and sustaining evidence-based tobacco use treatment (TUT) (e.g., brief advice to quit, and FDA-approved pharmacotherapy). These may include inadequate staff training, a lack of reimbursement, and policies that limit options for integrating tobacco cessation support into the screening process [17]. Additionally, there is lack of guidance on how best to deliver effective smoking cessation treatment in the context of LCS [18]. As a result, even with the financial incentive, implementation has lagged. A recent study in the VA found high rates of screening among patients undergoing LCS. However, 82% of those identified as tobacco users received neither counseling nor pharmacotherapy, and only 1% of smokers receiving the recommended, most effective form of treatment: behavioral treatment plus pharmacotherapy [19]. Given the CMS policy change, it is now even more important to build commitment and capacity among LCS programs to implement TUT in their settings.
Closing the “evidence to practice” gap is the focus of implementation science (IS), a relatively new field that offers a set of rigorous methods and a systematic approach to identifying and overcoming contextual barriers to implementing evidence-based guidelines in a range of clinical settings [20–24]. In this paper, we describe how IS frameworks and methods can be used to help LCS programs optimize the delivery of TUT in their specific context and discuss the policy changes needed to further facilitate TUT as a core component of the LCS process.
PRACTICE
Overview of implementation science (IS) frameworks and strategies
Implementation science (IS) is the study of methods to promote the uptake of evidence-based interventions (EBIs) into routine practices in a range of clinical settings [20]. IS theories, models, and frameworks (hereafter referred to as frameworks) support this process by defining determinants of successful implementation and guiding the multistage iterative process of planning, implementing, evaluating, and sustaining improvements in health care delivery [22, 23]. For example, determinants frameworks, like the well-established Consolidated Framework for Implementation Research (CFIR), define a range of factors across several domains that may influence the effectiveness of efforts to implement TUT in an LCS program (e.g., CMS policies) that align with program priorities and organizational capacity [23].
Implementation outcomes frameworks capture outcomes that are proximal to measures of clinical effectiveness [24, 25]. These include program reach, or the degree to which a program is reaching the target population, how acceptable a policy or practice change is to program staff and patients, how well the program fits into current workflow (i.e., appropriateness), to what extent a new treatment model is implemented as intended (i.e., fidelity), the cost of implementing the new clinical practice, and the explicit consideration of equity in program delivery and outcomes [26]. Implementation outcomes can help organizations explain why new interventions or programs may or may not work.
Process frameworks describe the stages of implementation and guide a systematic process for planning and preparing, implementing, monitoring progress, and sustaining effective practices (Table 1) [27–30].
Table 1.
Designing, implementing, evaluating, and sustaining tobacco use treatment services
| Implementation stages | Questions |
|---|---|
|
Pre-implementation
1. Establish an implementation team, identify a team leader, and define program objectives |
• Who will lead the project? • Who are the decision makers? • Who are the implementers? (i.e., frontline staff) • Who will provide resources? • How will this plan promote equity and inclusion? • What are we trying to accomplish and how will we know we succeeded? |
| 2.Conduct a needs assessment | • What are the training and staffing needs? • What system changes are needed to facilitate consistent assessment and treatment? • How will this fit into current workflow? • Is there a need for technical assistance (TA)? • What additional funding, staffing, or other resources are needed? |
| 3.Define the core components of the TUT intervention model | • What TUT components will be delivered onsite vs offsite through referrals? • What are the referral resources? How often are patients screened for TUT? • Are there staff licensed to prescribe pharmacotherapy? |
| 4.Select and define implementation strategies | • What specific strategies are needed to address capacity gaps and barriers? (e.g., changes to electronic health record [EHR], training, practice facilitation/TA) |
| 5.Develop a detailed implementation plan (who, what, when, where) | • What is the timeline? • What is the new workflow? (e.g., who will screen for tobacco use?) • What are the specifications for system changes? |
| Evaluation and continuous quality improvement (CQI) | • How will you track that TUT was provided? What are your quality measures? • What is the frequency of assessing those measures? • Who will run reports? Who will review the reports? |
| Sustainability | • Has the program secured adequate funding, staffing, and leadership commitment? |
Finally, implementation strategies, or the “how to” of implementation efforts, are methods or techniques that are used to increase implementation of an EBI (i.e., TUT in the context of LCS) [31]. There is emerging evidence for effective strategies that increase implementation of TUT in clinical settings. These include capacity building (i.e., LCS staff training), system changes that facilitate referrals (e.g., to state tobacco quitlines), revised roles and workflows, and external practice facilitation for programs that need more hands-on support to build capacity for integrating TUT within the LCS clinical pathway [31–34].
Applying implementation science to improve integration of TUT in LCS programs
Drawing on current literature, we provide an overview of how IS methods can support the process of developing and executing a plan for effectively implementing TUT across a wide range of LCS settings. Table 1 outlines the stages of implementation, from pre-implementation to planning for sustainability, and provides a sample of practical questions that the implementation team can use to elicit contextual information that will inform the final plan.
Pre-implementation planning.
1. Establish an implementation team and define objectives
An effective team will include both decision makers (e.g., medical directors) and implementers (e.g., frontline clinical staff), who can facilitate the implementation process and plans for program sustainability. Depending on the type of LCS program (e.g., embedded in health care system, community program), the team may include site leadership (e.g., medical director, chief medical officer, or chief medical informatics officer), administrators and/or quality improvement leads (e.g., Director for Quality and Safety), imaging leadership, CT technicians, and information technology (IT) staff who can support system changes to facilitate clinical workflow and reporting. The team should be engaged early in the planning process to establish roles, responsibilities, and shared goals.
2. Conduct a needs assessment
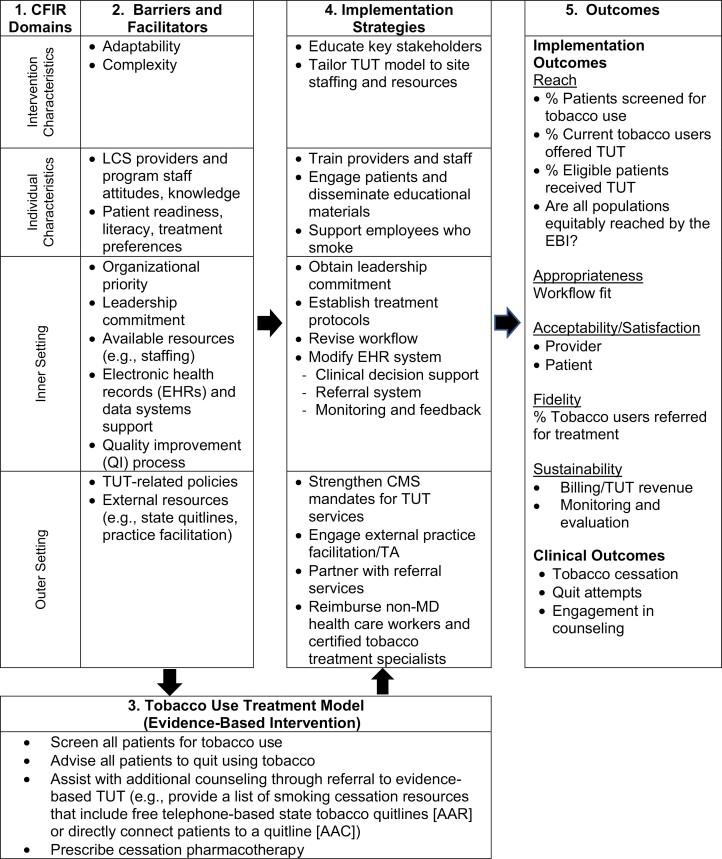
The needs assessment is essential to highlight assets (e.g., leadership support), to anticipate potential obstacles that may impede implementation efforts, and to facilitate the decision-making process. We use the CFIR (Fig. 1) to illustrate how a determinants framework can guide a pre-implementation needs assessment [23]. Here, CFIR is embedded in an implementation research logic model to further show the relationship between findings from the needs assessment and the selection of various elements of the program (i.e., components of the TUT model and implementation strategies) and expected outcomes [25, 34]. Fig. 1, Section #1, shows four CFIR domains, and column 2 outlines potential barriers and facilitators within those domains that have been shown to influence implementation of EBIs [23]. For example, the TUT intervention characteristics may create challenges because of perceived complexity and misalignment with the LCS program staffing and workflow.
Fig 1.
Logic model: Applying the Consolidated Framework for Implementation Research for integrating evidence-based tobacco use treatment (TUT) in lung cancer screening programs.
Individual healthcare provider and patient characteristics that create barriers if not addressed may include the staff’s lack of knowledge and skills, variation in patients’ readiness to quit, their willingness to engage in treatment, and language needs. Inner setting (i.e., organization or program characteristics) challenges may include gaps in electronic health record (EHR) functionality that is needed to support adoption of TUT, such as clinical decision support, automated referral systems, or the ability to generate performance reports. Policy or outer setting factors, like misaligned financial incentives, may impede TUT adoption. However, outer setting resources may also facilitate program implementation. These include telephone counseling offered through free state-funded quitlines available in all 50 states and the District of Columbia [35], the national smokefree.gov text message program [36], and state Medicaid policies that cover pharmacotherapy [37].
Developing a logic model fosters a shared understanding among stakeholders of the implementation process, including the rationale for the selection of a TUT service model (Fig. 1, Section #3), and the selection of implementation strategies (Fig. 1, Section #4) that are designed to enhance uptake of TUT by targeting barriers and leveraging program strengths [38]. Revisiting the needs assessment may be necessary, particularly when major health system or program shifts occur (e.g., changes in priorities or policies, departure of program champions).
3. Select and define the core components of a TUT model (Fig. 1, Section #3)
There is strong evidence for safe and effective TUT options such as health care worker-delivered advice to quit, behavioral support delivered in person or via proactive telephone support, and FDA-approved pharmacotherapy [39, 40]. Smoking abstinence rates are highest when counseling and pharmacotherapy are combined [17]. However, the TUT service delivery model, or care pathway that LCS programs select, may vary depending on resources, infrastructure, and other factors identified during the needs assessment. For example, the Ask, Advise, Refer (AAR), and Ask, Advise, Connect (AAC) models offer LCS programs an effective approach for engaging smokers in TUT while delegating more intensive counseling to state quitlines [41, 42]. The former involves a passive referral (e.g., providing a list of smoking cessation resources that include free telephone-based state tobacco quitlines and/or National Cancer Institute’s text-messaging quit smoking program). This is in contrast to AAC in which the program actively connects patients at the point of service to a resource like a state quitline. An important advantage of these two service delivery models is that they can be implemented by site coordinators or administrative staff in LCS programs, such as Independent Diagnostic Testing Facilities, where health care professionals are not readily available. With smoker quitlines available in every state, AAC and AAR are highly feasible population-based models for LCS programs to implement.
4. Select implementation strategies
The needs assessment will also guide the selection of implementation strategies that are designed to address specific barriers to integrating and sustaining TUT in the LCS setting. Figure 1 (Section #4) shows examples of implementation strategies, drawn from the literature, that target gaps in organizational readiness across CFIR domains [31–34]. These may include system changes such as creating a registry of patients who smoke to facilitate automated referrals to the quitline, and clinical decision support to facilitate adherence to TUT protocols. Onsite IT staff or state quitlines can offer technical assistance to help programs design a data exchange process to streamline referrals. Revising workflows and professional roles based on information from the needs assessment will facilitate routine delivery of TUT, and such revisions can be accomplished without compromising patient care or clinical revenue. Communication materials, such as print brochures, or digital messages sent via patient portals, can alert patients to a program’s TUT policy, and offer resources. Finally, training can be obtained through several existing resources. For example, sites may enroll staff in one of the 25 accredited tobacco treatment specialist training programs in the USA to increase program and system capacity to deliver more intensive multisession TUT [43]. Site coordinators and nurse navigators may be ideal patient-facing staff for additional training.
5. Develop a detailed implementation plan
The plan is the policy document that defines what services will be offered onsite (e.g., brief advice to quit) and/or through offsite cessation resources (e.g., quitline). The plan defines who is responsible for assessing and documenting current smoking and if pharmacotherapy is offered at the point of service. The program’s ability to monitor program fidelity requires this degree of specification. Like the TUT services, implementation strategies should be specified to ensure consistency of use and reproducibility in other sites, and to aid in evaluation [44]. This includes identifying a training resource, defining which staff are responsible for implementing revised workflows, who will be responsible for generating performance reports and how often, and how the findings will be used. Piloting the new workflow will identify any additional modifications needed prior to a full launch.
Evaluation and continuous quality improvement
Standard quality measures for TUT include: 1) the percentage of all individuals with a visit, within a defined time period, who are screened for tobacco use, and 2) the percentage of those identified as current smokers who are offered treatment [45]. Most EHRs have a location to document this information. However, sites may require assistance from their EHR vendor or from IT staff to generate real-time dashboards and standard reports on these measures. Integrating screening and TUT as part of the order for LCS and other LCS relational databases (e.g., LCS registry) can facilitate consistent monitoring and annual updates in smoking status at follow-up screening visits. Reports that are stratified by subgroups (e.g., race and ethnicity, age, health insurance status) can be used to identify (Fig. 1, Section #5) disparities regarding which patients receive treatment, and inform changes to the TUT process and workflows to achieve equity in service delivery.
Quality improvement (QI) models like Plan-Do-Study-Act cycles (PDSA) provide a pragmatic structure for an iterative evaluation of practice changes to ensure that the program is meeting its goals [46]. Effective QI methods such as PDSA cycles enable rapid information gathering that is then used to adapt and change clinical processes. If targets are not being reached, programs can move through another iterative cycle in which clinicians and staff revisit the needs assessment, discuss what is or is not working and create a plan to make modifications to improve program fit. Documenting each stage of the PDSA cycle is important to support local learning and efforts to scale TUT to other settings, if applicable.
Sustainability
Planning for sustaining TUT services begins during the needs assessment. Many of the predictors of effective implementation overlap with those associated with program sustainability. These include the presence of an internal champion to advocate for the program, and business policies that continue to align incentives with consistent delivery of TUT [47–49]. Other strategies for sustaining TUT programs include developing strategic internal partners (e.g., with complementary initiatives) and external collaborations (e.g., state quitlines, American Lung Association, GO2 Foundation), instituting routine monitoring of program outcomes, and adapting a web-based training or train-the-trainer program to address staff turnover. Finally, for LCS programs that exist in larger health care systems, there is an opportunity to align with the system’s QI goals and infrastructure to improve TUT outcomes and facilitate sustainability.
POLICY
Federal and local policies exert significant influence on decisions about if and how to adopt and implement TUT in the context of LCS. In 2015, the CMS coverage determination mandating offering smoking cessation in the context of lung cancer screening was seen as a forward-thinking breakthrough in reducing the burden of lung cancer [7]. The CMS decision to remove the requirement for radiology imaging facilities to offer even minimal cessation advice, quitting resources, and referral information threatens to diminish the overall benefit of LCS, and the opportunity to reduce tobacco-related disparities [8].
There is a strong rationale and evidence for offering cessation support in the context of LCS [9–12]. First, both the LDCT visit, and the moment when patients receive results, represent “teachable moments” when the health risk of tobacco use is most salient, and therefore smokers may be more willing to engage in tobacco use treatment [50, 51]. In two LCS trials, the majority of smokers who quit during the study period reported that screening was a catalyst for quitting [52, 53]. Second, tobacco dependence is a chronic, relapsing disorder that, like other chronic diseases, often requires repeated intervention and support [54]. The LDCT visit provides an additional interaction between a smoker and the healthcare system, and therefore, another occasion to reinforce the benefits of quitting and to link patents to cessation services. Third, there is overwhelming evidence that promoting the integration of smoking cessation support and treatment within the context of lung cancer screening saves lives [11, 12]. Additionally, there are cost-effective models for referring and connecting patients to evidence-based interventions that are highly feasible to implement across the full range of LCS settings, including Independent Diagnostic Testing Facilities in which health care professionals are not readily available [11].
Finally, the prevalence of smoking is increasingly concentrated in U.S. populations with poor access to and low utilization of primary care [40]. These include individuals with behavioral health conditions (i.e., mental health conditions, substance use disorders), persons of low socioeconomic status, and specific racial and ethnic minorities. Disparities in access to, and utilization of, TUT contribute to the growing disparities in tobacco-related health outcomes. The revised USPSTF recommendations will substantially increase the number of racial and ethnic minorities who are eligible for screening [6]. This change creates new opportunities for simultaneously increasing access to cessation treatment among these underserved populations, and further emphasizes the importance of requiring that patients be connected to treatment resources at every patient visit along the clinical pathway for lung cancer screening. This includes the shared decision-making visit, at the point of service for LDCT, at the time of disclosure of LDCT screening results, and at annual follow-up LDCT visits.
Absent a reimbursement mandate, there are policies that CMS can promote to improve uptake of cessation services in LCS programs. CMS can continue to strongly recommend support for tobacco cessation at the time of screening and specify the provision of behavioral and pharmacologic cessation treatment options that are consistent with current guidelines at both the point of referral and the LCS visit [40]. CMS can also expand billing compliance to provide incentives for LCS programs to optimize TUT delivery [51]. Billing policies that narrowly define which providers are eligible for TUT reimbursement create unwarranted constraints on how TUT is delivered. For example, CMS does not currently allow Certified Tobacco Treatment Specialists (CTTS) who are not licensed independent practitioners to bill for services. Some states have expanded the range of Medicaid providers that are eligible to bill for cessation services. However, for programs for which this option is not feasible, a reversal in Medicare (i.e., CMS polices) restricting reimbursement may incentivize programs to offer onsite treatment delivered by a CTTS or other staff, in addition to referring patients to quitlines or other community-based programs.
RESEARCH
Additional research is needed to determine the best possible strategies for implementing TUT in the context of LCS. The importance of these data is twofold: 1) to guide LCS programs’ decisions about what type of TUT model (e.g., AAC vs AAR) to select and what strategies they will use to effectively implement treatment services, and 2) to facilitate evidence-based policymaking, specifically as it relates to CMS policies that support integration of TUT in these settings. High-priority research questions include the following: Which TUT interventions are feasible and most effective in the context of LCS programs? What are the barriers to implementing cessation services in the LCS program? What are effective strategies to overcome these barriers? How do organizational characteristics of LCS programs impact reach and effectiveness of treatment options?
The National Cancer Institute’s (NCI) Smoking Cessation at Lung Examination (SCALE) Collaboration was designed to address these questions [18]. The SCALE Collaboration includes eight research trials that are testing the efficacy and effectiveness of a range of multicomponent smoking cessation interventions (e.g., referral to quitlines, pharmacotherapy, gain vs. loss message framing, web-based programs, and text messaging) and tobacco cessation outcomes. These studies are simultaneously exploring how to optimize the implementation of effective interventions in a range of LCS program settings. There are study limitations. For example, racial and ethnic groups will not be fully represented in the participant pool, head-to-head comparisons of pharmacotherapy interventions are limited, and findings may not be generalizable across the varied types screening programs [18]. However, the use of common data elements to identify what interventions are most effective for treating tobacco use among current smokers undergoing lung cancer screening, and how LCS programs can implement TUT into varying screening program contexts, will allow for additional hypotheses testing in secondary analyses and increase the generalizability of findings. CMS will benefit from these data for evidence-informed policymaking regarding TUT integration during LCS. While more research is needed, the current state of the science creates a compelling case to support LCS programs to implement the evidence for effective TUT interventions in the context of LCS, and begin to realize the full benefits of LCS.
Acknowledgments
The authors acknowledge the support of the National Cancer Institute’s SCALE Collaboration.
Contributor Information
Donna Shelley, NYU School of Global Public Health, New York, NY, USA.
Vivian Hsing-Chun Wang, NYU School of Global Public Health, New York, NY, USA.
Kathryn Taylor, Georgetown University, Washington, DC, USA.
Randi Williams, Georgetown University, Washington, DC, USA.
Benjamin Toll, Medical University of South Carolina, Charleston, SC, USA.
Alana Rojewski, Medical University of South Carolina, Charleston, SC, USA.
Kristie L Foley, Wake Forest University Health Sciences, Winston-Salem, NC, USA.
Nancy Rigotti, Massachusetts General Hospital, Boston, MA, USA.
Jamie S Ostroff, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Funding
Supported by NIH grants R01CA207078; (DS, JSO), R01CA207229 (BAT); R01CA207158 (KLF); R01CA207228 (KLT)
Compliance with Ethical Standards
Conflicts of Interest: Nancy Rigotti has received research grants and consulting fees from Achieve Life Sciences and loyalties from UpToDate. Benjamin Toll consulted on an Advisory Board about e-cigarettes with Pfizer and testifies on behalf of plaintiffs who have filed litigation against the tobacco industry. Kristie Foley received a grant from the National Cancer Institute that supported the work in a peer-reviewed publication. Jamie Ostroff, Alana Rojewski, Donna Shelley, Kathryn L. Taylor, Vivian Wang, and Randi Williams declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Transparency Statement: This article did not include any formal data collection or analyses, and as such there is no registration information, analytic code, or materials available for this manuscript.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2. Aberle DR, Black WC, Chiles C, et al. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019; 14(10):1732–1742. doi: 10.1016/j.jtho.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. United States Preventive Services Taskforce. Final Recommendation Statement—Lung Cancer: Screening; 2021. Rockville, MD: Author; 2021. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening. Accessibility verified February 24, 2021. [Google Scholar]
- 5. Richards TB, Soman A, Thomas CC, et al. Screening for lung cancer—10 states, 2017. MMWR Morb Mortal Wkly Rep. 2020; 69(8):201–206. doi: 10.15585/mmwr.mm6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meza R, Cao P, Jeon J, et al. Impact of joint lung cancer screening and cessation interventions under the new recommendations of the U.S. Preventive Services Task Force. J Thorac Oncol. 2022; 17(1):160–166. doi: 10.1016/j.jtho.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jensen TS, Chin J, Ashby L, et al. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). Baltimore, MD: CMS; 2015. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274&bc=CAAAAAAACAAA&. Accessibility verified February 11, 2022. [Google Scholar]
- 8. Jensen TS, Chin J, Baldwin J, et al. Reconsideration—Final National Coverage Determination for Lung Cancer Screening with Low Dose Computed Tomography (LDCT). Baltimore, MD: CMS; 2022. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304&=. Accessibility verified February 11, 2022. [Google Scholar]
- 9. Cadham CJ, Jayasekera JC, Advani SM, et al. Smoking cessation interventions for potential use in the lung cancer screening setting: a systematic review and meta-analysis. Lung Cancer. 2019; 135:205–216. doi: 10.1016/j.lungcan.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao P, Jeon J, Levy DT, et al. Potential impact of cessation interventions at the point of lung cancer screening on lung cancer and overall mortality in the United States. J Thorac Oncol. 2020; 15(7):1160–1169. doi: 10.1016/j.jtho.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cadham CJ, Cao P, Jayasekera J, et al. Cost-effectiveness of smoking cessation interventions in the lung cancer screening setting: a simulation study. J Natl Cancer Inst. 2021; 113(8):1065–1073. doi: 10.1093/jnci/djab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moldovanu D, de Koning HJ, van der Aalst CM.. Lung cancer screening and smoking cessation efforts. Transl Lung Cancer Res. 2021; 10(2):1099–1109. doi: 10.21037/tlcr-20-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brain K, Carter B, Lifford KJ, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK Lung Cancer Screening Trial. Thorax. 2017; 72(10):912–918. doi: 10.1136/thoraxjnl-2016-209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Aalst CM, van den Bergh KAM, Willemsen MC, de Koning HJ, van Klaveren RJ.. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax. 2010; 65(7):600–605. doi: 10.1136/thx.2009.133751. [DOI] [PubMed] [Google Scholar]
- 15. Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL.. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014; 106(6):dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American College of Radiology. Lung Cancer Screening Registry. Reston, VA: Author. Available from: https://www.acr.org/Practice-Management-Quality-Informatics/Registries/Lung-Cancer-Screening-Registry. Accessibility verified July 21, 2022. [Google Scholar]
- 17. Ostroff JS, Copeland A, Borderud SP, Li Y, Shelley DR, Henschke CI.. Readiness of lung cancer screening sites to deliver smoking cessation treatment: current practices, organizational priority, and perceived barriers. Nicotine Tob Res. 2016; 18(5):1067–1075. doi: 10.1093/ntr/ntv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joseph AM, Rothman AJ, Almirall D, et al. Lung cancer screening and smoking cessation clinical trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med. 2018; 197(2):172–182. doi: 10.1164/rccm.201705-0909CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heffner JL, Coggeshall S, Wheat CL, et al. Receipt of tobacco treatment and one-year smoking cessation rates following lung cancer screening in the veterans health administration. J Gen Intern Med. 2022; 37:1704–1712. doi: 10.1007/s11606-021-07011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM.. An introduction to implementation science for the non-specialist. BMC Psychol. 2015; 3:32. doi: 10.1186/s40359-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eccles MP, Mittman BS.. Welcome to implementation science. Implement Sci. 2006; 1(1):1. doi: 10.1186/1748-5908-1-1. [DOI] [Google Scholar]
- 22. Nilsen P. Making sense of implementation theories, models, and frameworks. In: Albers B, Shlonsky A, Mildon R, eds. Implementation Science 3.0. New York: Springer International Publishing; 2020:53–79. doi: 10.1007/978-3-030-03874-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC.. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009; 4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019; 7:64. doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011; 38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shelton RC, Chambers DA, Glasgow RE.. An extension of RE-AIM to enhance sustainability: addressing dynamic context and promoting health equity over time. Front Public Health. 2020; 8:134. doi: 10.3389/fpubh.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson KM, Brady TJ, Lesesne C; NCCDPHP Work Group on Translation. An organizing framework for translation in public health: the knowledge to action framework. Prev Chronic Dis. 2011; 8(2):A46. [PMC free article] [PubMed] [Google Scholar]
- 28. Chamberlain P, Brown CH, Saldana L.. Observational measure of implementation progress in community-based settings: the stages of implementation completion (SIC). Implement Sci. 2011; 6(1):116. doi: 10.1186/1748-5908-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandez ME, Ten Hoor GA, van Lieshout S, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. 2019; 7:158. doi: 10.3389/fpubh.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA.. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci. 2019; 14(1):1. doi: 10.1186/s13012-018-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015; 10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D’Angelo H, Ramsey AT, Rolland B, et al. Pragmatic application of the RE-AIM framework to evaluate the implementation of tobacco cessation programs within NCI-designated cancer centers. Front Public Health. 2020; 8:221. doi: 10.3389/fpubh.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A.. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010; 51(3):199–213. doi: 10.1016/j.ypmed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 34. Khanna N, Klyushnenkova E, Rao V, Siegel N, Wolfe S.. Electronic referrals to the tobacco quitline: implementation strategies in a large health system to optimize delivery of tobacco cessation to patients. Transl Behav Med. 2021; 11(5):1107–1114. doi: 10.1093/tbm/ibaa094. [DOI] [PubMed] [Google Scholar]
- 35. North American Quitline Consortium. 2020 Survey. Phoenix, AZ: Author. Available from: https://www.naquitline.org/page/2020survey. Accessibility verified November 24, 2021. [Google Scholar]
- 36. National Cancer Institute. SmokefreeTXT. Rockville, MD: U.S. Department of Health and Human Services. Available from: https://smokefree.gov/tools-tips/text-programs/quit-for-good/smokefreetxt. Accessibility verified November 24, 2021. [Google Scholar]
- 37. DiGiulio A, Jump Z, Babb S, et al. State Medicaid coverage for tobacco cessation treatments and barriers to accessing treatments—United States, 2008-2018. MMWR Morb Mortal Wkly Rep. 2020; 69(6):155–160. doi: 10.15585/mmwr.mm6906a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith JD, Li DH, Rafferty MR.. The Implementation Research Logic Model: a method for planning, executing, reporting, and synthesizing implementation projects. Implement Sci. 2020; 15(1):84. doi: 10.1186/s13012-020-01041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. West R, Raw M, McNeill A, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015; 110(9):1388–1403. doi: 10.1111/add.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. United States Public Health Service Office of the Surgeon General, National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services; 2020. Available from: http://www.ncbi.nlm.nih.gov/books/NBK555591/. Accessibility verified February 7, 2022. [Google Scholar]
- 41. Burris J, Borger T, Bernstein S, et al. Proposing a model of proactive outreach to advance clinical research and care delivery for patients who use tobacco. J Gen Intern Med. 2022; 37(10):2548–2522. doi: 10.1007/s11606-022-07553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piñeiro B, Vidrine DJ, Wetter DW, et al. Implementation of Ask-Advise-Connect in a safety net healthcare system: quitline treatment engagement and smoking cessation outcomes. Transl Behav Med. 2020; 10(1):163–167. doi: 10.1093/tbm/iby108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Council for Tobacco Treatment Training Programs. Accredited Programs. Madison, WI: Author. Available from: https://ctttp.org/accredited-programs/. Accessibility verified November 28, 2021. [Google Scholar]
- 44. Proctor EK, Powell BJ, McMillen JC.. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013; 8:139. doi: 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. National Committee for Quality Assurance. Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention. Washington, DC: National Quality Forum. Available from: https://www.qualityforum.org/QPS/0028. Accessibility verified November 28, 2021. [Google Scholar]
- 46. Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE.. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014; 23(4):290–298. doi: 10.1136/bmjqs-2013-001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whelan J, Love P, Pettman T, et al. Cochrane update: predicting sustainability of intervention effects in public health evidence: identifying key elements to provide guidance. J Public Health (Oxf). 2014; 36(2):347–351. doi: 10.1093/pubmed/fdu027. [DOI] [PubMed] [Google Scholar]
- 48. Birken SA, Bunger AC, Powell BJ, et al. Organizational theory for dissemination and implementation research. Implement Sci. 2017; 12(1):62. doi: 10.1186/s13012-017-0592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malone S, Prewitt K, Hackett R, et al. The Clinical Sustainability Assessment Tool: measuring organizational capacity to promote sustainability in healthcare. Implement Sci Commun. 2021; 2(1):77. doi: 10.1186/s43058-021-00181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pistelli F, Aquilini F, Falaschi F, et al. Smoking cessation in the ITALUNG lung cancer screening: what does “teachable moment” mean? Nicotine Tob Res. 2020; 22(9):1484–1491. doi: 10.1093/ntr/ntz148. [DOI] [PubMed] [Google Scholar]
- 51. Fucito LM, Czabafy S, Hendricks PS, Kotsen C, Richardson D, Toll BA.. Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Cancer. 2016; 122(8):1150–1159. doi: 10.1002/cncr.29926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ostroff JS, Buckshee N, Mancuso CA, Yankelevitz DF, Henschke CI.. Smoking cessation following CT screening for early detection of lung cancer. Prev Med. 2001; 33(6):613–621. doi: 10.1006/pmed.2001.0935. [DOI] [PubMed] [Google Scholar]
- 53. Balata H, Traverse-Healy L, Blandin-Knight S, et al. Attending community-based lung cancer screening influences smoking behaviour in deprived populations. Lung Cancer. 2020; 139:41–46. doi: 10.1016/j.lungcan.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 54. Steinberg MB, Schmelzer AC, Richardson DL, Foulds J.. The case for treating tobacco dependence as a chronic disease. Ann Intern Med. 2008; 148(7):554–556. doi: 10.7326/0003-4819-148-7-200804010-00012. [DOI] [PubMed] [Google Scholar]