Abstract
Helicobacter infection leads to chronic inflammation of the stomach. Although the infection persists in spite of an immune response, animal studies have shown that adjuvant-based oral vaccines can protect against infection and even eliminate established infection. These vaccines are thought to induce a Th2 immune response, counterbalancing the Th1 response seen with natural infections. As a prelude to using adenovirus vectors carrying cytokine genes to modulate the immune response to established Helicobacter felis infection, we first examined the effect of the replication-defective adenovirus (RDA) vector itself. C57BL/6 mice chronically infected with H. felis (8 to 10 weeks) received intramuscular injections of RDA. The effect of RDA on the severity of H. felis colonization and the degree of gastric inflammation was assessed 2 weeks later. RDA caused a significant decrease in H. felis colonization without significantly altering the associated inflammation. RDA did not alter the H. felis-specific immunoglobulin G1 (IgG1), IgG2a, and IgA responses in the serum but was associated with an increase in gamma interferon (IFN-γ)-producing CD8+ spleen cells. To determine if IFN-γ or Th1 cytokines were involved in the response to RDA, we examined RDA treatment of H. felis infection in mice lacking either IFN-γ or interleukin-12 (IL-12). RDA failed to alter H. felis colonization in either of these two mouse strains. Thus, viral infection of mice chronically infected with H. felis led to a significant decrease in H. felis colonization in an IFN-γ- and IL-12-dependent manner. These results demonstrate that Th1 responses associated with systemic viral infection can influence an established H. felis infection.
Helicobacter pylori causes gastritis, has a causal role in the development of peptic ulcers, and is considered a risk factor for the development of gastric cancer and mucosa-associated lymphoid tissue lymphomas (34). Once H. pylori infection is established, it persists in spite of the immune response that develops. Eradication of Helicobacter infection leads to cure of the ulcer and prevention of its recurrence (47). Studies of H. felis in mice suggest that oral vaccines given with a mucosal adjuvant such as cholera toxin can prevent Helicobacter infection and can even lead to elimination of established infection (6, 8, 12). Cholera toxin induces a shift in the helper T-cell response from a Th1- to a Th2-type cytokine response. Although it has been proposed that the shift from a Th1- to a Th2-type response is responsible for the induction of protection, cholera toxin can also induce mixed Th1 and Th2 responses (17, 43) and even Th1 responses (4). Therefore, the possibility remains that Th1 responses can influence Helicobacter infection.
The use of mucosal adjuvants like cholera toxin or the heat-labile enterotoxin of Escherichia coli in humans is potentially associated with toxicity, and preliminary data on the clinical effectiveness of these adjuvant-based H. pylori vaccines in humans is disappointing (36). Therefore, alternative means of modulating the immune response to Helicobacter infection need to be explored. We and others have shown that injection of replication-defective adenovirus (RDA) carrying cytokine genes can modulate the immune and inflammatory responses to a number of infectious agents and allergens (21, 33, 41, 44). The availability of recombinant RDA containing immunomodulatory molecules provides an alternate approach to the modulation of the immune response to established Helicobacter infections. The present study was designed to determine the effects of the RDA vector itself on H. felis colonization and on the inflammatory response to an established H. felis infection in the mouse model.
MATERIALS AND METHODS
Animals and bacteria.
Specific-pathogen-free female C57BL/6 and gamma interferon (IFN-γ)−/− mice (6 to 8 weeks old) were purchased from The Jackson Laboratory, Bar Harbor, Maine. The generation of interleukin-12 (IL-12) p40−/− mice (C57BL/6 background) has previously been described (24). These mice were bred in our central animal facility. All mice were housed in microisolator cages with free access to autoclaved chow and water. H. felis ATCC 49179 (CS1) was obtained from the Laboratory Center for Disease Control of Canada and stored at −70°C in 80% brain heart infusion broth plus 10% horse serum and 10% glycerol. The bacteria were cultured on chocolate agar plates (PML Microbiologicals, Mississauga, Ontario, Canada) under microaerophilic conditions. Before harvesting in sterile saline, bacterial cultures were examined by Gram staining to exclude possible contamination and by phase-contrast microscopy to ensure viability and motility.
Establishment of gastric H. felis infection.
Six- to 8-week-old mice were infected with three doses of 5 × 108 H. felis bacteria (in 300 μl of sterile saline) by oral gavage at 2-day intervals. Sham-treated mice were gavaged with a solution of sterile saline. The date of the first infection was counted as day 1. This study was approved by the McMaster University Animal Care Committee and conforms to the guidelines of the Canadian Council on Animal Care.
Preparation of H. felis whole-cell sonicate.
Whole-cell sonicate (WCS) was prepared from freshly harvested H. felis. Cell pellets of bacteria were resuspended in sterile distilled water and submitted to ultrasonication at 4°C in a Fisher sonic dismembrator (Artek Systems Corp., Farmingdale, N.Y.). The WCS was centrifuged for 10 min at 4°C to clear the cellular debris and then filtered through a 0.2-μm-pore-size Acrodics filter (Gelman Sciences, Ann Arbor, Mich.). The protein concentration of the filtrate was determined by Lowry assay (22), and aliquots were stored at −70°C until use. WCS was used to immunize mice to generate positive serum standards for an H. felis-specific antibody isotype enzyme-linked immunosorbent assay (ELISA).
Grading of gastric inflammation and infection.
Stomachs of mice were removed and fixed in 10% neutral buffered formalin and then embedded in paraffin. Sagittal sections at three different levels were stained with hematoxylin and eosin. Histological evaluation of inflammation and infection was carried out in a blinded manner as previously described (28, 30). The inflammation was graded on the basis of the intensity of inflammation in the longitudinal axis of the mucosa and the vertical extent of inflammation within the gastric glands. The intensity of inflammation was measured in the areas showing the most significant changes under ×10 magnification and scored on a scale of 0 to 4 (grades: 0, no inflammatory cells; 1, rare inflammatory cells; 2, multiple clusters of inflammatory cells; 3, diffuse inflammation with variable intensity; 4, diffuse and uniformly severe inflammation). The longitudinal extent of inflammation was scored on the basis of the percentage of the mucosal surface involved in the inflammation as assessed for the entire sagittal section examined at ×10 magnification (grades: 0, none; 1, <25%; 2, 25 to 50%; 3, 50 to 75%; 4, >75%). The vertical extent of inflammation was scored on the basis of the degree to which the inflammation extended to the different mucosal layers in the area with the greatest involvement (grades: 0, none; 1, only basal area involved by inflammation [i.e., not extending to the surface of the mucosa]; 2, transmural [i.e., full-thickness involvement of the mucosa]; 3, deep [i.e., involvement of both the mucosa and the submucosa]). These grading scales, i.e., intensity, longitudinal extent, and vertical extent, were combined, and the sum was used to represent the degree of inflammation. The extent of infection was estimated by determining first the number of H. felis-positive glands per 20 glands and then the maximum number of H. felis organisms per gland. These two numbers were averaged. The average extent of infection in the antrum was then combined with the average extent of infection in the fundus. The degree of infection represents the combined extent of infection in the antrum and fundus (28).
RDA.
The human type 5 RDA carries a deletion of the E1 gene and a partially crippled E3 gene in the adenoviral genome (adenovirus type 5 strain DL70-3) (46). RDA was harvested and purified by ultracentrifugation, and the titer was determined as previously described (2). The virus was diluted to a final concentration of 6 × 108 PFU/100 μl. Each mouse was injected twice with 6 × 108 PFU of RDA in the hind legs over a 5-day period (46). The mice receiving the RDA injection had had an established H. felis infection for at least 8 weeks.
Collection of serum and gut wash samples.
The serum used as a standard for the ELISA was obtained from C57BL/6 mice that had received four weekly immunizations with H. felis WCS antigen plus Freund’s incomplete adjuvant (Gibco, Grand Island, N.Y.). Negative control serum was collected from naive C57BL/6 mice. Serum was collected and stored at −20°C until use. To measure H. felis-specific antibody levels in gut washings, the small intestine of each mouse was removed, cut open longitudinally, and immersed in 3 ml of ice-cold phosphate-buffered saline (PBS) containing 1.0 mM phenylmethylsulfonyl fluoride, 0.005 M EDTA, and 0.005 U of aprotinin (Sigma, St. Louis, Mo.) per ml (40). After 30 min at 4°C, the solution was centrifuged at 200 × g at 4°C for 10 min and supernatants were separated and stored at −20°C.
H. felis-specific antibody ELISA.
H. felis-specific antibody levels were determined by an isotype-specific ELISA. Heat-killed H. felis bacteria were used to coat 96-well flat-bottom microtiter plates (F96 Maxisorp Nunc-Immuno plates; Nunc, Roskilde, Denmark). Antigen was diluted with 0.3% glyoxal buffer (2% glyoxal in 10% bicarbonate solution, pH 8.0) to give a final concentration of 107 bacteria/ml. An aliquot of 100 μl of the diluted antigen preparation was added to each well. After overnight incubation at 4°C, the wells were aspirated and refilled with 150 μl of serum diluent buffer (Tris-buffered saline containing 0.1% gelatin and 0.05% Tween 20, pH 7.2) and incubated for another 2 h at room temperature. Gelatin (BDH Inc.) was used to block nonspecific binding sites. Antigen-coated wells were then washed three times with Tris-buffered saline containing 0.05% Tween 20. Each test serum was diluted 1:20, 1:60, and 1:180 in serum diluent buffer. Aliquots (100 μl) of each diluted test serum were added to the wells in triplicate and incubated for 60 min at 37°C. Biotin-labeled goat anti-mouse immunoglobulin G1 (IgG1), IgG2a (1:2,000), and IgA (1:1,000) (Southern Biotechnology Associates Inc., Birmingham, Ala.), prepared in serum diluent buffer, were used as secondary antibodies. Aliquots (100 μl) of the diluted, biotin-labeled secondary antibodies were added to each well and incubated for 60 min at 37°C. Wells were subsequently washed, and 100 μl of ExtrAvidin-alkaline phosphatase (1:5,000; Sigma) prepared in serum diluent buffer was added, and the mixture was incubated for 1 h at room temperature. Wells were washed, and 100 μl of substrate solution (containing 1 mg of p-nitrophenylphosphate per ml in ethanolamine substrate buffer [Sigma]) was incubated for 10 min at room temperature. The optical density of the individual wells was read with an ELISA reader (TiterTek Multiscan Plus MK II) at a wavelength of 405 nm. A standard serum was used to calculate a relative antibody activity in each of the isotype-specific assays. Each standard serum was given an arbitrary value of 10,000 U/ml. Standard sera were diluted from 1:100 to 1:218,700 and the resulting optical density was plotted against units of antibody per milliliter. Optical density readings derived for dilutions of test sera were then interpolated from the standard curve if they fell within the linear range of the curve.
Flow cytometry analysis of intracellular cytokines.
Single-cell suspensions of spleen cells were isolated by forcing tissue through a fine wire mesh. Cells were resuspended in RPMI medium with 10% fetal calf serum and centrifuged at 150 × g for 5 min. The supernatant was discarded, and the cells were resuspended in 1 ml of ACK lysing buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA) for 2 min to lyse erythrocytes. After being rinsed in medium, 5 ml of cells (2 × 106/ml) was plated in six-well plates and incubated overnight at 37°C with H. felis WCS (1 μg/ml). The following day, cells were stimulated with phorbol myristate acetate (50 ng/ml; Sigma) and ionomycin (5 ng/ml; Sigma) in the presence of 10 μg of brefeldin A (Sigma) per ml for 4 h. After rinsing with PBS, surface staining was carried out with biotin-conjugated anti-CD8α or anti-CD4 (Pharmingen, Mississauga, Ontario, Canada) at 0.5 μg of antibody per 106 cells for 30 min at room temperature. This was followed by treatment with streptavidin-PerCP (10 μl/106 cells; Becton Dickinson) for another 30 min at room temperature. After washing, cells were resuspended in PBS and an equal volume of 4% paraformaldehyde for 15 min. Intracellular cytokine staining was carried out by incubating cells in 100 μl of fluorescein isothiocyanate-conjugated anti-IFN-γ (0.1 μg) or anti-IL-2 (0.1 μg) and phycoerythrin-conjugated anti-IL-4 (0.2 μg) (Pharmingen) diluted in permeabilization buffer (PBS containing 1% saponin, 1% fetal calf serum, and 0.1% NaN3) for 30 min. Finally, the cells were rinsed and fixed in 500 μl of 1% paraformaldehyde. Data on a minimum of 10,000 events was collected for each sample on a Becton Dickinson FACScan using Cellquest software. Mononuclear cells were identified by their forward-by-side-scatter properties and were gated for analysis using PC Lysis software.
Statistical analysis.
Data is presented as the mean value plus the standard error of the mean (SEM). Statistical significance was determined by the Student t test or, if the data was not normally distributed, the nonparametric Mann-Whitney U test. Differences between groups were considered statistically significant at P < 0.05.
RESULTS
Effect of RDA infection on established H. felis colonization of the mouse stomach.
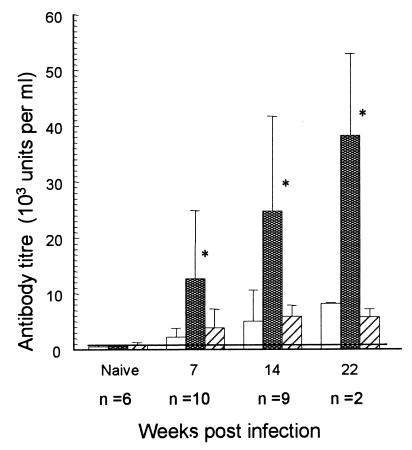
C57BL/6 mice infected with H. felis for 8 weeks or more received RDA or PBS injections in a hind limb. H. felis infection is well established at 8 weeks and is associated with chronic gastritis and a significant antibody response (Fig. 1) (30). Two weeks after RDA injection, the mice were sacrificed and the stomachs were removed for histological examination. Mice receiving RDA injections showed a significant decrease in H. felis colonization compared to mice receiving PBS alone (P < 0.005). The degree of gastric inflammation did not change significantly after RDA injection (Table 1).
FIG. 1.
Mean (+SEM) anti-H. felis antibody in serum of mice inoculated orally on days 1, 3, and 5 with 5 × 108 H. felis bacteria. Isotype (□, IgG1; , IgG2a; ▨, IgA) antibodies were measured by using a standard serum and calculation of units of antibody per milliliter as described in Materials and Methods. The horizontal line shows the limit of detection of the assays (approximately 800 U/ml). n, number of mice tested per time point. ∗ P < 0.05 compared to naive mice.
TABLE 1.
Effect of RDA on the severity of an established H. felis infection and the associated gastric inflammation in C57BL/6 mice
| Treatment | No. of experiments | Mean
degreea (±SEM) of:
|
|
|---|---|---|---|
| Infection | Inflammation | ||
| H. felis | 5 | 12.6 ± 1.3 | 6.6 ± 0.5 |
| H. felis + RDA | 4 | 3.2 ± 1.3b | 7.4 ± 0.8 |
Degrees of infection and inflammation were graded as described in Materials and Methods. Two to six mice were used per experiment.
Significantly different (P < 0.005) from the value for control H. felis-infected mice not treated with RDA as calculated by Student’s t test.
Effect of RDA infection on the immune response to established H. felis colonization.
In C57BL/6 mice, an increase in serum IgG2a H. felis-specific antibody was detected by 7 weeks after H. felis infection (Fig. 1). In contrast, minimal serum IgG1 or IgA anti-H. felis antibody responses were measured, even up to 22 weeks after infection. Previous studies indicate that Th1 responses favor IgG2a over IgG1 isotype responses (31, 32). Therefore, the increase in IgG2a is in keeping with the tendency of C57BL/6 mice to develop Th1-type responses to infections in general and is also in keeping with the predominant Th1-type responses demonstrated in helicobacter infections specifically (14, 29). We compared the H. felis-specific antibody responses in H. felis-infected mice 2 weeks after RDA injection (10 to 12 weeks post H. felis infection) with that of H. felis-infected mice given PBS. No significant difference was found in any of the three isotype responses between PBS- and RDA-treated H. felis-infected mice (data not shown).
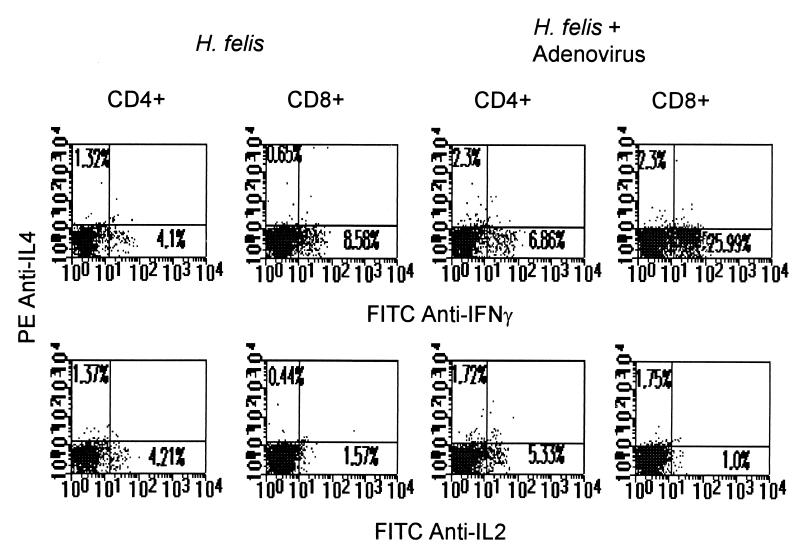
We also analyzed the effect of RDA infection on IFN-γ, IL-4, and IL-2 production by H. felis-stimulated spleen cells by using flow cytometry analysis of intracellular cytokine expression. In the absence of in vitro H. felis stimulation, there was no detectable cytokine (data not shown). After H. felis WCS stimulation of spleen cells from RDA-infected, H. felis-colonized mice, there was a significant increase in IFN-γ-producing CD8+ splenic T cells (6.5 to 25%) (Fig. 2). There was no significant change in the number of IL-4- or IL-2-producing spleen cells. RDA infection of naïve mice not colonized with H. felis failed to increase IFN-γ-producing spleen cells after in vitro stimulation with H. felis antigen (data not shown). Therefore, RDA infection of H. felis-colonized mice increased IFN-γ-producing H. felis antigen-specific cells.
FIG. 2.
Flow cytometry analysis of intracellular IL-4 and IFN-γ (top panels) or IL-4 and IL-2 (bottom panels) in CD4+ and CD8+ spleen cells isolated from mice at 10 weeks after infection with H. felis (left series of panels), 10 weeks after oral infection with 5 × 108 H. felis bacteria, and 2 weeks after infection of 0.6 × 109 PFU of RDA into each hind leg, twice over a 5-day period. The cell suspensions were incubated overnight with H. felis WCS antigen and then stimulated with phorbol myristate acetate (50 ng/ml; Sigma), ionomycin (5 ng/ml; Sigma), and 10 μg of brefeldin A (Sigma) per ml for 4 h. Three-color staining was performed as described in Materials and Methods. These results are representative of two experiments, each consisting of three mice. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Effect of RDA on H. felis infection in mice lacking the IFN-γ or IL-12 p40 gene.
IFN-γ and IL-12 are thought to contribute to the gastritis and mucosal damage that develop during Helicobacter infection in humans and mice (13, 29). In order to directly examine the role of these cytokines in the development of H. felis-associated gastritis and in regulating H. felis colonization, we infected mice lacking the IFN-γ or IL-12 p40 gene. Lack of the IFN-γ gene causes loss of IFN-γ production in vivo (9), and CD4+ T cells respond to antigens by differentiation to a Th2 response (42). IL-12-deficient mice are impaired in the ability to produce IFN-γ following endotoxin administration. IL-12 is an essential regulator of IFN-γ production and facilitates delayed-type hypersensitivity responses. However, cell-mediated immunity, i.e., IL-2 secretion, and cytotoxic T-cell generation are not compromised in IL-12-deficient mice (24). The severity of gastric inflammation and the degree of H. felis colonization were analyzed at 8 to 12 weeks postinfection. Both IFN-γ and IL-12 p40 knockout mice developed a chronic H. felis infection associated with gastric inflammation (Table 2).
TABLE 2.
Effect of RDA on the severity of H. felis infection and gastric inflammation in IFN-γ- and IL-12-deficient mice
| Treatment | Mouse strain | No. of mice | Mean
degreea (±SEM) of:
|
|
|---|---|---|---|---|
| Infection | Inflammation | |||
| H. felis | IFN-γ−/− | 9 | 9.1 ± 1.7 | 5.1 ± 0.4 |
| H. felis | IL-12 p40−/− | 4 | 10.5 ± 1.0 | 3.3 ± 0.3 |
| H. felis + RDA | IFN-γ−/− | 3 | 6.8 ± 3.1 | 3.8 ± 0.4 |
| H. felis + RDA | IL-12 p40−/− | 3 | 7.2 ± 2.0 | 3.2 ± 0.3 |
Degrees of infection and inflammation were graded as described in Materials and Methods.
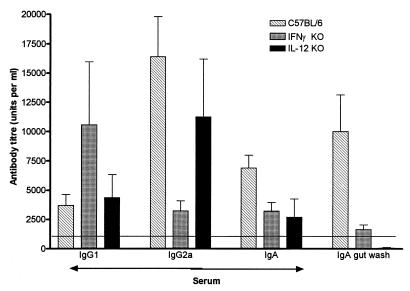
Given the increase in IFN-γ-producing spleen cells after RDA treatment of H. felis-infected mice, we examine the possibility that the effect of RDA on H. felis colonization was mediated via IFN-γ and IL-12 by injecting H. felis-infected IFN-γ and IL-12 knockout mice with RDA. RDA infection of both of these mouse strains failed to reduce H. felis colonization, suggesting that IFN-γ and IL-12 were required for the RDA effect on H. felis colonization (Table 2). The lack of an effect of RDA infection on H. felis colonization in IFN-γ- and IL-12-deficient mice may be related to an altered H. felis immune response. H. felis-specific IgG1, IgG2a, and IgA titers were measured in the sera and IgA titers were measured in gut washes of these mice after H. felis infection. H. felis-specific IgG2a was decreased fourfold in IFN-γ−/− mice compared to C57BL/6 mice, while the IgG1 response was increased twofold (Fig. 3). This is in keeping with the notion that Th1 cytokines such as IFN-γ favor IgG2a antibody responses (31). In spite of the increase in Th2-type antibody responses in IFN-γ−/− mice, the IgA response in the gut wash and, to a lesser degree, in the serum from IFN-γ−/− mice was significantly less than in C57BL/6 mice. This is in keeping with the previously described influence of IFN-γ on IgA responses (19). In IL-12 p40−/− mice, we failed to detect a significant decrease in serum H. felis-specific IgG2a but did see a decrease in serum IgG1 and IgA, as well as in IgA in gut wash (Fig. 3).
FIG. 3.
Mean (+SEM) anti-H. felis antibody isotype levels in serum and gut wash of: C57BL/6 mice (n = 6); IFN-γ knockout (KO) mice (n = 6); and IL-12 p40 knockout mice (n = 6). Samples were taken 8 to 10 weeks after oral infection with 5 × 108 H. felis bacteria. Serum antibody was measured as described in Materials and Methods.
DISCUSSION
RDA lacking functional E1 and E3 genes was constructed to serve as a vector capable of incorporating cytokine genes for in vivo delivery (2, 46). We have shown previously that RDA can influence the induction of the immune response to a protein allergen (41). In an effort to explore the utility of such vectors in modulating mucosal infections and inflammation, we examined the effect of RDA alone on chronic H. felis infection in the mouse. The results show that infection of mice with RDA led to a significant reduction of H. felis colonization in the stomachs of C57BL/6 mice but not in those of mice deficient in the IFN-γ or IL-12 cytokine gene. The effect of RDA on H. felis was independent of changes in the profile of antibody isotype responses but was associated with an increase in IFN-γ-producing splenic CD8+ T cells.
Previous work has documented that virus infection stimulates IFN-γ and IL-12 cytokine responses (7). Natural helicobacter infection is also associated with predominately Th1 responses characterized by increases in local IFN-γ (28). However, in the face of a vigorous immune response to helicobacter infection, this immune response is ineffective in eliminating the helicobacter infection. The reasons for this are not clear. Oral vaccines incorporating helicobacter antigens require mucosal adjuvants such as cholera toxin to effectively prevent or eliminate H. felis and H. pylori infections in the mouse (6, 25, 26, 28, 29, 37). These observations have led to the argument that cholera toxin shifts the immune response toward a Th2 response, leading to a more effective antibody response (37, 39). This argument is further supported by studies in which adoptive transfer of helicobacter-specific Th2 cell lines decreased helicobacter infection in the mouse (29). On the other hand, there is data that suggests that oral vaccines do not require antibody since they are effective in mice deficient in antibody production (3).
Our data further showed that the effect of RDA on H. felis infection in the mouse was dependent on IFN-γ and IL-12, i.e., Th1 cytokines. This seems to contradict the prevailing notion that Th1 cytokines do not effect protective immunity as opposed to Th2 responses stimulated by cholera toxin-based vaccines. As mentioned, cholera toxin can induce a mixed Th1 and Th2 response and even Th1 responses. Furthermore, while some studies have shown that H. pylori infection in humans was associated with a Th1 cytokine response (1, 10, 16), others have shown that gastritis due to H. pylori was associated with fewer IFN-γ-producing cells in the gastric antrum than are seen in gastritis not due to H. pylori (18). It remains possible therefore, that Th1 immune responses are involved in controlling helicobacter infection. In a recent study by Blanchard et al. (3), systemic vaccination with complete Freund’s adjuvant, a strong inducer of Th1 responses, protected against H. felis infection in mice. This supports a role for Th1 responses in controlling helicobacter infection. The means by which Th1 cytokines effect protective immunity or decrease colonization by H. felis is not clear. Our data suggests that in the absence of IFN-γ and IL-12, there is a decrease in serum and secretory IgA levels. Previous work has shown that IL-12 administered intranasally can increase serum and secretory IgA levels in response to tetanus toxin (5). Therefore, it is tempting to speculate that these cytokines influence the infection by altering IgA responses. On the other hand, antibody responses do not seem to be required for the effectiveness of oral vaccines. The possibility that cellular responses influenced by IFN-γ and IL-12 are important in the control of helicobacter infection remains to be explored.
Viral infections influence T-cell responses to bacteria (48), but few studies have directly examined the relationship between viral infections and helicobacter. Studies of hepatitis infection influencing helicobacter have focused primarily on hepatitis A as a surrogate marker of fecal-oral routes of transmission (23, 35). On the other hand, helicobacter infection was shown to influence viral infections in a study of vaccinia virus in the mouse. The decreased clearance of the vaccinia virus infection was mediated by a reduction in cytotoxic T-cell responses and Th1 cytokines associated with helicobacter infection (38). Helicobacter infection commonly occurs in childhood and remains chronic in spite of the development of an immune response (11, 15). Cohort and cross-sectional population studies indicate that the rate of spontaneous elimination of H. pylori infection is low (20, 27, 45); nonetheless, spontaneous eradication does occur. The mechanism leading to spontaneous clearance of helicobacter infection is not known. Furthermore, once helicobacter infection in humans is eradicated, the rate of reinfection is very low (27). One interpretation of this observation is that the immune response can become effective in protecting against helicobacter infection and may even participate in eliminating established infection. Our data raise the possibility that viral infection is one means of inducing an effective immune response.
ACKNOWLEDGMENTS
This work was supported by grants from the Chedoke-McMaster Hospital Foundation and The Medical Research Council of Canada. K.C. gratefully acknowledges the award of an Ontario Ministry of Health Career Scientist Award.
We are grateful to Pam Lyn for skilled technical assistance.
REFERENCES
- 1.Bamford K B, Fan X J, Crowe S E, Leary J F, Gourley W K, Luthra G K, Brooks E G, Graham D Y, Reyes V E, Ernst P B. Lymphocytes in the human gastric mucosa during Helicobacter pylorihave a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 2.Bett A J, Haddara W, Prevec L, Graham F L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard T G, Gottwein J M, Targoni O S, Eisenberg J C, Zagorksi B M, Trezza T P, Redline R, Nedrud J G, Tary-Lehman P V, Czinn S J. Systemic vaccination inducing either Th1 or Th2 immunity protects mice from challenge with H. pylori. Gastroenterology. 1999;116:A695. [Google Scholar]
- 4.Bourguin I, Chardes T, Bout D. Oral immunization with Toxoplasma gondiiantigens in association with cholera toxin induces enhanced protective and cell-mediated immunity in C57BL/6 mice. Infect Immun. 1993;61:2082–2088. doi: 10.1128/iai.61.5.2082-2088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyaka P N, Marinaro M, Jackson R J, Menon S, Kiyono H, Jirillo E, McGhee J R. IL-12 is an effective adjuvant for induction of mucosal immunity. J Immunol. 1999;162:122–128. [PubMed] [Google Scholar]
- 6.Corthésy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A-C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacterinfection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 7.Coutelier J P, Van Broeck J, Wolf S F. Interleukin-12 gene expression after viral infection in the mouse. J Virol. 1995;69:1955–1958. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca R, Blanchard T G, Czinn S J, Nedrud J G, Monath T P, Lee C K, Redline R W. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets [see comments] Gastroenterology. 1996;110:1770–1775. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 9.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.D’Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pyloriin the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 11.DeWitt D L, Meade E A. Serum and glucocorticoid regulation of gene transcription and expression of the prostaglandin H synthase-1 and prostaglandin H synthase-2 isozymes. Arch Biochem Biophys. 1993;306:94–102. doi: 10.1006/abbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- 12.Doidge C, Gust I, Lee A, Buck F, Hazell S, Manne U. Therapeutic immunization against helicobacter infection. Lancet. 1994;343:914–915. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 13.Ernst P B, Crowe S E, Reyes V E. How does Helicobacter pyloricause mucosal damage? The inflammatory response. Gastroenterology. 1997;113:S35–S42. doi: 10.1016/s0016-5085(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 14.Ernst P B, Jin Y, Reyes V E, Crowe S E. The role of the local immune response in the pathogenesis of peptic ulcer formation. Scand J Gastroenterol Suppl. 1994;205:22–28. doi: 10.3109/00365529409091405. [DOI] [PubMed] [Google Scholar]
- 15.Graham D Y, Malaty H M, Evan D G, Evans D J, Jr, Klein P D, Adam E. Epidemiology of Helicobacter pyloriin an asymptomatic population in the United States: effect of age, race and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 16.Haberle H, Kubin M, Trinchieri G, Luthra R, Gourley W K, Crowe S E, Reyes V E, Ernst P B. Activated Th cells are recruited to the gastric epithelium and lamina propria during H. pyloriinfection. Clin Immunol Immunopathol. 1995;76:S8. [Google Scholar]
- 17.Hörnquist E, Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol. 1993;23:2136–2143. doi: 10.1002/eji.1830230914. [DOI] [PubMed] [Google Scholar]
- 18.Karttunen R, Karttunen T, Ekre H P, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pyloripositive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjerrulf M, Grdic D, Ekman L, Schön K, Vajdy M, Lycke N Y, Schon K. Interferon-gamma receptor-deficient mice exhibit impaired gut mucosal immune responses but intact oral tolerance. Immunology. 1997;92:60–68. doi: 10.1046/j.1365-2567.1997.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumagai T, Malaty H M, Graham D Y, Hosogaya S, Misawa K, Furihata K, Ota H, Sei C, Tanaka E, Akamatsu T, Shimizu T, Kiyosawa K, Katsuyama T. Acquisition versus loss of Helicobacter pyloriinfection in Japan: results from an 8-year birth cohort study. J Infect Dis. 1998;178:717–721. doi: 10.1086/515376. [DOI] [PubMed] [Google Scholar]
- 21.Lei X F, Ohkawara Y, Stämpfli M R, Gauldie J, Croitoru K, Jordana M, Xing Z. Compartmentalized transgene expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in mouse lung enhances allergic airways inflammation. Clin Exp Immunol. 1998;113:157–165. doi: 10.1046/j.1365-2249.1998.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Luzza F, Imeneo M, Maletta M, Paluccio G, Giancotti A, Perticone F, Foca A, Pallone F. Seroepidemiology of Helicobacter pylori infection and hepatitis A in a rural area: evidence against a common mode of transmission. Gut. 1997;41:164–168. doi: 10.1136/gut.41.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 25.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pyloriinfection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 26.Michetti P, Corthésy-Theulaz I, Davin C, Haas R, Vaney A-C, Heitz M, Bille J, Kraehenbuhl J-P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pyloriurease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell H M, Hu P, Chi Y, Chen M H, Li Y Y, Hazell S L. A low rate of reinfection following effective therapy against Helicobacter pyloriin a developing nation (China) Gastroenterology. 1998;114:256–261. doi: 10.1016/s0016-5085(98)70475-5. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 29.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felisinfection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosmann T R, Coffman R L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–148. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 33.Papp Z, Middleton D M, Mittal S K, Babiuk L A, Baca-Estrada M E. Mucosal immunization with recombinant adenoviruses: induction of immunity and protection of cotton rats against respiratory bovine herpesvirus type 1 infection. J Gen Virol. 1997;78:2933–2943. doi: 10.1099/0022-1317-78-11-2933. [DOI] [PubMed] [Google Scholar]
- 34.Parsonnet J. Helicobacter pylori: the size of the problem. Gut. 1998;43:S6–S9. doi: 10.1136/gut.43.2008.s6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pretolani S, Stroffolini T, Rapicetta M, Bonvicini F, Baldini L, Megraud F, Ghironzi G C, Sampogna F, Villano U, Cecchetti F, Giulianelli G, Stefanelli M L, Armuzzi A, Miglio F, Gasbarrini G. Seroprevalence of hepatitis A virus and Helicobacter pylori infections in the general population of a developed European country (the San Marino study): evidence for similar pattern of spread. Eur J Gastroenterol Hepatol. 1997;9:1081–1084. doi: 10.1097/00042737-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Saldinger P F, Blum A L, Corthesy-Theulaz I E. Perspectives of anti-H. pylori vaccination. J Physiol Pharmacol. 1997;48(Suppl. 4):59–65. [PubMed] [Google Scholar]
- 37.Saldinger P F, Porta N, Launois P, Louis J A, Waanders G A, Bouzourène H, Michetti P, Blum A L, Corthésy-Theulaz I E. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacterinfection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 38.Shirai M, Arichi T, Nakazawa T, Berzofsky J A. Persistent infection by Helicobacter pylori down-modulates virus-specific CD8+cytotoxic T cell response and prolongs viral infection. J Infect Dis. 1998;177:72–80. doi: 10.1086/513827. [DOI] [PubMed] [Google Scholar]
- 39.Snider D P. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15:317–348. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 40.Snider D P, Underdown B J. Quantitative and temporal analyses of murine antibody response in serum and gut secretions to infection with Giardia muris. Infect Immun. 1986;52:271–278. doi: 10.1128/iai.52.1.271-278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stampfli M R, Ritz S A, Neigh G S, Sime P J, Lei X F, Xing Z, Croitoru K, Jordana M. Adenoviral infection inhibits allergic airways inflammation in mice. Clin Exp Allergy. 1998;28:1581–1590. doi: 10.1046/j.1365-2222.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z E, Reiner S L, Zheng S, Dalton D K, Locksley R M. CD4+effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson A D, Bailey M, Williams N A, Stokes C R. The in vitroproduction of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991;21:2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- 44.Wilson M E, Young B M, Davidson B L, Mente K A, McGowan S E. The importance of TGF-beta in murine visceral leishmaniasis. J Immunol. 1998;161:6148–6155. [PubMed] [Google Scholar]
- 45.Xia H X, Talley N J. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol. 1997;92:1780–1787. [PubMed] [Google Scholar]
- 46.Xing Z, Ohkawara Y, Jordana M, Graham F L, Gauldie J. Adenoviral vector-mediated interleukin-10 expression in vivo: intramuscular gene transfer inhibits cytokine responses in endotoxemia. Gene Ther. 1997;4:140–149. doi: 10.1038/sj.gt.3300371. [DOI] [PubMed] [Google Scholar]
- 47.Yamada T, Ahnen D J, Alpers D H, Greenberg H B, Gray L, Joscelyn K B, Kauffman G, Podolsky D K, Ray W A, Schaberg D, Silverstein F E, Sivak M V, Williams A L B, Yolken R. Helicobacter pyloriin peptic ulcer disease. JAMA. 1994;272:65–69. [Google Scholar]
- 48.Zhang W J, Sarawar S, Nguyen P, Daly K, Rehg J E, Doherty P C, Woodland D L, Blackman M A. Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice. J Immunol. 1996;157:5049–5060. [PubMed] [Google Scholar]