Abstract
Introduction
Alcohol use disorder (AUD) is on the ascendancy in the US older adult population. The association between AUD and adverse brain outcomes remains inconclusive.
Method
In a retrospective cohort design using US insurance claim data (2007–2020), 129,182 individuals with AUD were matched with 129,182 controls by age, sex, race, and clinical characteristics. We investigated the association between AUD and adverse brain outcomes using Cox analysis, Kaplan–Meier analysis, and log‐rank test.
Results
After adjusting for covariates, AUD was associated with a higher risk of Alzheimer's disease (female adjusted hazard ratio [HR] = 1.78, 95% confidence interval [CI]: 1.68–1.90, p < 0.001; male adjusted HR = 1.80, 95% CI: 1.71–1.91, p < 0.001) and a higher risk of Parkinson's disease (female adjusted HR = 1.49, 95% CI: 1.32–1.68, p < 0.001; male adjusted HR = 1.42, 95% CI: 1.32–1.52, p < 0.001) in the overall sample. In separate analyses of Black, White, and Hispanic individuals, those with AUD had higher risk of Alzheimer's disease (adjusted HRs ≥1.58; Ps ≤ 0.001). A significantly elevated risk for Parkinson's disease was found only in the White subpopulation (female adjusted HR = 1.55, 95% CI: 1.36–1.77, p < 0.001; male adjusted HR = 1.45, 95% CI: 1.33–1.57, p < 0.001).
Conclusions
AUD is associated with Alzheimer's disease. AUD is associated with Parkinson's disease in White people. Cognitive screening and neurological examination among older adults with AUD hold the promise for early detection of Alzheimer's disease and Parkinson's disease.
HIGHLIGHTS
Alcohol use disorder is associated with Alzheimer's disease and dementia.
Alcohol use disorder is associated with Parkinson's disease in White people.
1. INTRODUCTION
Alcohol consumption is common among the United States (US) older adult population. US nation‐wide survey data from 2017 to 2019 has shown that: (1) over 40% of US older adults used alcohol and (2) over 13% of US older adults engaged in binge or excessive alcohol consumption. 1 , 2 The prevalence of alcohol use disorder (AUD) in the older adult population increased from 3.2% (95% confidence interval [CI]: 2.6%–4.0%) to 5.6% (95% CI: 4.8%–6.6%) from years 2001–2002 to 2012–2013, a 1.75‐fold increase (p < 0.05). 3
Despite the fact that excessive alcohol consumption is known to have neurotoxic consequences, 4 , 5 population‐based association studies investigating adverse brain outcomes for alcohol consumers and/or individuals with AUD have shown mixed results. 6 AUD was associated with increased risk of dementia including Alzheimer's disease (hazard ratio [HR] = 3.34, p < 0.001) in an analysis of 31.6 million French patients. 7 Based on the aforementioned study and literature reviews, excessive alcohol consumption (>21 units/week) was considered a new risk factor for dementia in the 2020 report of the Lancet Commission on Dementia Prevention, Intervention, and Care. 8 AUD was also associated with an increased risk of Parkinson's disease (HR = 1.38, p < 0.001) among 602,930 Swedish patients with an average of 13 years of follow‐up. 9 Additionally, an over 30‐year observation study in England concluded that both moderate and high alcohol consumption (i.e., 14–21 and > 21 units/week) increased the risk (odds ratios [ORs] > 3., Ps ≤ 0.007) for developing hippocampal atrophy. 10 On the other hand, some studies found that alcohol consumption and AUD had no association or were associated with decreased risk of degenerative brain diseases. For instance, a 3‐year observational study in Germany found that alcohol consumers had reduced risk of overall dementia (HR = 0.71, p = 0.028) and Alzheimer disease (HR = 0.58, p = 0.013). 11 A cohort study of middle‐aged and older US adults (N = 19,887) concluded a U‐shaped relationship between weekly alcohol consumption and cognitive function with better cognitive function at 10–14 drinks per week. 12 Another 13‐year longitudinal study of a large US cohort (N = 132,403) identified no association between alcohol consumption and risk of Parkinson disease. 13
Currently, AUD is on the ascendancy in the older adult population. 3 Thus, more real‐world evidence on adverse brain outcomes for individuals with AUD is warranted. 14 In this study, we investigated the association between AUD and Alzheimer dementia and Parkinson disease using a large‐scale US insurance claim dataset.
2. METHODS
2.1. Data
We used the OPTUM Clinformatics Data (2007Q1–2020Q4). The data are derived from commercially insured US individuals and US Medicare Advantage beneficiaries. The data included individual level demographics (e.g., sex, birth year, and “race”), monthly enrollment records, medical claims (e.g., emergency department [ED] visits, inpatient hospitalizations, and outpatient visits), procedure claims, pharmacy claims, laboratory results, and death records. Because the OPTUM data only contain a single variable designated “RACE” with five categories: “White”, “Hispanic”, “Black”, “Asian” and “unknown”, our analyses were limited to this single variable to cover the more complex dimensions of ethnicity and genetic background.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literatures using traditional sources. Alcohol use disorder (AUD) has been reported to have mixed associations with adverse brain outcomes (e.g., Alzheimer's disease, dementia, and Parkinson's disease).
Interpretation: Cognitive screening and neurological examination among older adults with severe problematic alcohol use holds the promise for early detection of Alzheimer's disease, dementia, and Parkinson's disease.
Future directions: A large scale longitudinal study with measurements of alcohol consumption and cognitive function is warranted to further validate the impact of alcohol use on brain health.
2.2. Data accessibility and Institutional Review Board
Data accessibility is granted by the license agreement between Indiana University and OPTUM. The OPTUM Clinformatics Data are de‐identified. The Indiana University Institutional Review Board (IRB) designated this study as exempt.
2.3. Phenotyping algorithm
We used ICD‐9 and ICD‐10 codes to identify phenotypes. Specifically, we used alcohol related diagnosis codes defined by the US Centers for Disease Control and Prevention (CDC) to identify alcohol use disorders (ICD‐9: 291*, 303*, 305.0*, 357.5, 425.5, 535.3, 571.0, 571.1, 571.2, 571.3; ICD‐10: F10*, G62.1, G31.2, G72.1, I42.6, K29.2, K70*, K85.2, K86.0, Q86.0). 15 We used algorithms from the published literature to identify Alzheimer's disease (ICD‐9 331.0; ICD‐10: F00*, G30*) 16 and Parkinson's disease (ICD‐9: 332*, 331.82, 333.0; ICD‐10: G20, G21*, G31.83, G31.85, G23.1, G23.2, G90.3). 17 We used the R package comorbidity 18 to identify dementia, heart disease (myocardial infarction and congestive heart failure), cerebrovascular disease, chronic pulmonary disease, liver disease, diabetes, renal disease, HIV infection, hemiplegia or paraplegia, and malignancy. We used the algorithm derived by Chen et al. 19 to identify fall and hypertension. We used the algorithm derived by Quan et al. 20 to identify depression. We used the algorithm defined in Anna Nordström and Peter Nordström to identify traumatic brain injury. 21
2.4. Study design
We used a cohort design to compare the AUD group and a matched control group with respect to adverse brain outcomes. The study design was illustrated in Figure 1. We included individuals that were: (1) ≥60 years on the first day of enrollment and (2) continuously enrolled for ≥3 years. We identified 251,321 individuals with AUD. Among these, we excluded 111,528 individuals with AUD that were: (1) diagnosed with AUD within 365 days of enrollment and (2) diagnosed with Alzheimer's disease, cancer, dementia, hemiplegia/paraplegia, HIV infection, and/or Parkinson's disease prior to the diagnosis of AUD. After exclusion, 139,793 were eligible to be matched. We matched individuals with AUD and controls on age, sex, “race” and comorbidities common among older adults and/or associated with AUD and adverse brain outcomes. 8 , 22 Specifically, we matched on the following conditions: cerebrovascular disease, chronic pulmonary disease, depression, diabetes, fall, heart disease (myocardial infarction and congestive heart failure), hypertension, liver disease, renal disease, and traumatic brain injury. For individuals with AUD, the index date was the diagnosis date for AUD. For each matched pair, the control index date was chosen to be the corresponding AUD individual's index date (Figure S1). To summarize, individuals within a matched pair: (1) were not diagnosed with Alzheimer's disease, cancer, dementia, hemiplegia/paraplegia, HIV infection, and/or Parkinson's disease prior to the index date and (2) had the same demographics (age, “race” and sex) and comorbidity status on the index date. 10,611 individuals with AUD were unable to be matched. The final data set included 129,182 individuals with AUD and 129,182 matched control individuals.
FIGURE 1.
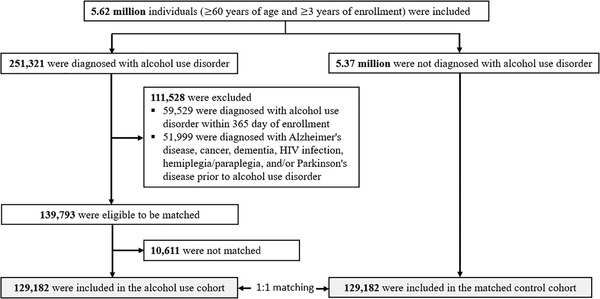
Study population and cohort matching process
2.5. Dependent variable
The primary outcomes were time from index date (i.e., day‐0) to the earliest diagnosis date of Alzheimer's disease or Parkinson's disease (Figure S1). The secondary outcome was time from index date to the earliest diagnosis of dementia. For all outcomes, the earliest diagnosis date is defined as the first diagnosis date after the index date. Individuals without any outcome were censored at the last month of enrollment or on 12/31/2020, whichever came first.
2.6. Statistical analysis
We used a Cox proportional hazard model to estimate covariate‐adjusted HRs, 95% confidence intervals (CIs), and p‐values. 23 We used the Kaplan–Meier estimator to estimate cumulative incidence curves for the individuals with AUD and the matched controls. 24 We used the log‐rank test to compare the incidence rates over time between the individuals with AUD and the matched controls. 25 The Cox proportional hazard models were adjusted for the following covariates: age (60–75 and 76–100), “race” (White, Hispanic, Black, Asian, and unknown), cerebrovascular disease (yes/no), chronic pulmonary disease (yes/no), depression (yes/no), diabetes (yes/no), fall (yes/no), heart disease (yes/no), liver disease (yes/no), renal disease (yes/no), and traumatic brain injury (yes/no). All analyses were stratified by sex because men and women have different incidence of AUD and adverse brain outcomes. 26 , 27
In addition to analyzing the whole dataset (N = 258,364), we did sensitivity analyses in: (1) White, Hispanic, Black, and Asian subpopulations, (2) individuals with alcohol misuse (i.e., ICD‐9/10: 305.0* and F10.1*) and the matched controls, and (3) used age as a continuous variable in Cox models. In all sensitivity analyses, we conducted Cox proportional hazard regression, computed Kaplan–Meier estimator, and conducted log‐rank test. In secondary analysis, we investigated the association between AUD and dementia, as well as the cumulative incidence of dementia for individuals with AUD and the matched controls after index date. All analyses were performed with R 4.0.2.
3. RESULTS
The whole study dataset included 258,364 individuals (129,182 individuals with AUD and 129,182 matched control individuals). Table 1 shows the demographic characteristics of the AUD group and the matched control group at baseline (i.e., the index dates). Demographics of subpopulations are presented in Supplementary Material Tables S1 and S4.
TABLE 1.
Demographics of the study population (N = 258,364)
| Female (N = 90,486) | Male (N = 167,878) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alcohol use disorder | Matched control | Alcohol use disorder | Matched control | |||||
| Age: [mean (SD)] | ||||||||
| 73.0 (6.5) | 73.0 (6.5) | 72.1 (6.1) | 72.1 (6.1) | |||||
| Age group: (N, %) | ||||||||
| 60‐75 | 30,511 | 67.4% | 30,511 | 67.4% | 61,190 | 72.9% | 61,190 | 72.9% |
| 76‐100 | 14,732 | 32.6% | 14,732 | 32.6% | 22,749 | 27.1% | 22,749 | 27.1% |
| Subpopulation: (N, %) | ||||||||
| White | 34,230 | 75.7% | 34,230 | 75.7% | 59,904 | 71.4% | 59,904 | 71.4% |
| Hispanic | 3605 | 8.0% | 3605 | 8.0% | 10,221 | 12.2% | 10,221 | 12.2% |
| Black | 4147 | 9.2% | 4147 | 9.2% | 7617 | 9.1% | 7617 | 9.1% |
| Asian | 676 | 1.5% | 676 | 1.5% | 1652 | 2.0% | 1652 | 2.0% |
| Unknown | 2585 | 5.7% | 2585 | 5.7% | 4545 | 5.40% | 4545 | 5.4% |
| Comorbidity: (N, %) | ||||||||
| Cerebrovascular disease | 9315 | 20.6% | 9315 | 20.6% | 16,294 | 19.4% | 16,294 | 19.4% |
| Depression | 16,655 | 36.8% | 16,655 | 36.8% | 14,792 | 17.6% | 14,792 | 17.6% |
| Diabetes | 11,697 | 25.9% | 11,697 | 25.9% | 27,258 | 32.5% | 27,258 | 32.5% |
| Fall | 8552 | 18.9% | 8552 | 18.9% | 8069 | 9.6% | 8069 | 9.6% |
| Heart disease | 7984 | 17.6% | 7984 | 17.6% | 18,848 | 22.5% | 18,848 | 22.5% |
| Hypertension | 35,028 | 77.4% | 35,028 | 77.4% | 65,923 | 78.5% | 65,923 | 78.5% |
| Liver disease | 7350 | 16.2% | 7350 | 16.2% | 11,258 | 13.4% | 11,258 | 13.4% |
| Chronic pulmonary disease | 18,136 | 40.1% | 18,136 | 40.1% | 29,046 | 34.6% | 29,046 | 34.6% |
| Renal disease | 7625 | 16.9% | 7625 | 16.9% | 14,770 | 17.6% | 14,770 | 17.6% |
| Traumatic brain injury | 695 | 1.5% | 695 | 1.5% | 629 | 0.7% | 629 | 0.7% |
Figure 2 shows the covariate adjusted HRs for individuals with AUD relative to matched controls. For Alzheimer's disease, both females and males with AUD were associated with higher risk in the whole study population (female adjusted HR = 1.78 [95% CI: 1.68–1.90; p < 0.001]; male adjusted HR = 1.80 [95% CI: 1.71–1.91; p < 0.001]). In subgroup analyses, females with AUD were associated with higher risk of Alzheimer's disease (adjusted HRs ≥ 1.58; Ps p < 0.001) in White, Hispanic, and Black subpopulations; males with AUD were associated with higher risk of Alzheimer's disease (adjusted HRs ≥ 1.59; Ps ≤ 0.02) in White, Hispanic, Black, and Asian subpopulations. White females with AUD had risks for Alzheimer's disease comparable to White males with AUD. Hispanic and Black females with AUD had higher risks for Alzheimer's disease than corresponding males with AUD. For Parkinson's disease, both females and males with AUD were associated with higher risk in the whole study population (female adjusted HR = 1.49 [95% CI: 1.32–1.68]; male adjusted HR = 1.42 [95% CI: 1.32–1.52]; Ps < 0.001). In subgroup analysis, only White individuals with AUD were associated with higher risk of Parkinson's disease (female adjusted HR = 1.55 [95% CI: 1.36–1.77]; male adjusted HR = 1.45 [95% CI: 1.33–1.57]; Ps < 0.001). White females with AUD had higher risk for Parkinson's disease relative to White males with AUD. Results from multivariate Cox analysis of Alzheimer's disease and Parkinson's disease are given in Tables S2 and S3.
FIGURE 2.
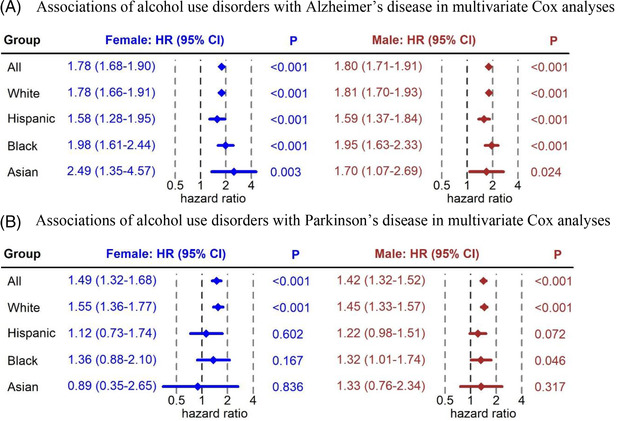
(A) Associations of alcohol use disorders with Alzheimer's disease in multivariate Cox analyses (N = 258,364). (B) Associations of alcohol use disorders with Parkinson's disease in multivariate Cox analyses (N = 258,364)
Figure 3 presents the sex‐specific cumulative incidence of Alzheimer's disease after diagnosis date or matched control index date. White, Hispanic, and Black females with AUD had significant higher cumulative incidence of Alzheimer's disease than matched controls. Black females with AUD had the highest 5‐year cumulative incidence (8.4%), followed by White females with AUD (8.0%), and then Hispanic females with AUD (7.4%). Males with AUD had a significantly higher cumulative incidence of Alzheimer's disease than matched controls in White, Hispanic, Black, and Asian subpopulations. Black males with AUD had the highest 5‐year cumulative incidence (6.2%), followed by White males with AUD (5.4%), then Hispanic males with AUD (5.0%), and last Asian males with AUD (4.0%).
FIGURE 3.
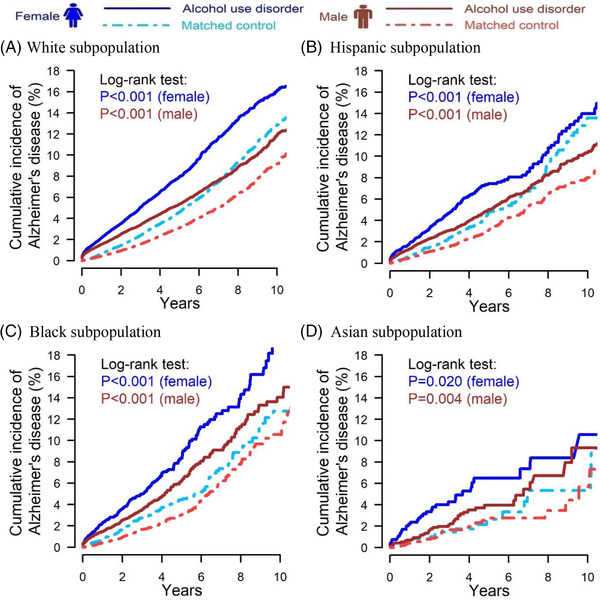
Cumulative incidence of Alzheimer's disease for individuals with alcohol use disorder and matched controls after index date; (A) White subpopulation (female N = 68,460, male N = 119,808); (B) Hispanic subpopulation (female N = 7210, male N = 20,442); (C) Black subpopulation (female N = 8294, male N = 15,234); (D) Asian subpopulation (female N = 1352, male N = 3304)
Figure 4 presents the sex‐specific cumulative incidence of Parkinson's disease after diagnosis date or matched control index date. Both White females and males with AUD had a significantly higher cumulative incidence of Parkinson's disease compared with matched controls. White females with AUD had a 2.1% 5‐year cumulative incidence, while matched controls had a 1.5% 5‐year cumulative incidence. White males with AUD had a 3.0% 5‐year cumulative incidence, while matched controls had a 2.1% 5‐year cumulative incidence.
FIGURE 4.
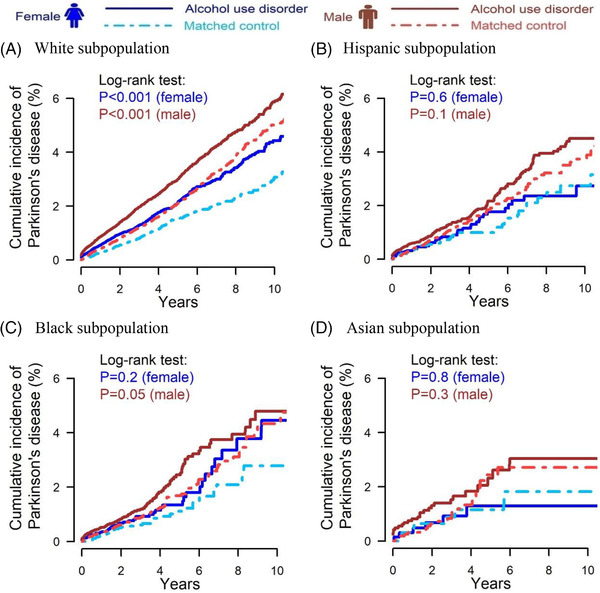
Cumulative incidence of Parkinson's disease for individuals with alcohol use disorder and matched controls after index date; (A) White subpopulation (female N = 68,460, male N = 119,808); (B) Hispanic subpopulation (female N = 7210, male N = 20,442); (C) Black subpopulation (female N = 8294, male N = 15,234); (D) Asian subpopulation (female N = 1352, male N = 3304)
Secondary and sensitivity analyses are presented in the supplementary figures and tables. AUD was associated with higher risks of dementia in the whole study population and all subpopulations (adjusted HRs≥1.74, Ps < 0.001 [Figures S2 and S3]). Alcohol misuse was associated with: (1) higher risk of Alzheimer's disease in the whole study population (female adjusted HR = 1.82 [95% CI: 1.70–1.99]; male adjusted HR = 1.80 [95% CI: 1.66–1.95]; Ps < 0.001 [Figures S4 and S6]); (2) higher risk of Parkinson's disease in the whole study population (female adjusted HR = 1.49 [95% CI: 1.25–1.77]; male adjusted HR = 1.39 [95% CI: 1.25–1.55]; Ps < 0.001 [Figures S5 and S6]); and (3) higher risk of dementia in the whole study population (female adjusted HR = 2.08 [95% CI: 1.96–2.02]; male adjusted HR = 2.16 [95% CI: 2.05–2.27]; Ps < 0.001 [Figures S7 and S8]). In sensitivity Cox analysis using age as a continuous variable (Figure S9), the association between AUD and adverse brain outcomes were consistent with the results of Cox analysis using dichotomized age groups.
4. DISCUSSION
This study highlights real‐world evidence on adverse brain outcomes following diagnosis of AUD. We found that AUD was associated with higher risks of Alzheimer's disease and dementia consistently in the whole study population and White, Hispanic, and Black subpopulations. We also found that AUD was associated with higher risks of Parkinson's disease in the whole study population and the White subpopulation.
The primary aim of the study was to investigate the associations between AUD and adverse brain outcomes. Our findings are in agreement with most of the literature. 7 , 9 Our analyses of subpopulations provide novel real‐world evidence on adverse brain outcomes following diagnosis of AUD in the (overly) broad categories of White, Black, Hispanic, and Asian subpopulations. Pre‐clinical and clinical studies have identified mechanisms of AUD‐induced neurodegeneration. 6 Alcohol consumption could induce oxidative stress in brain, hyperglutamatergic excitotoxicity, and neuroinflammation, all of which may subsequently trigger neuronal apoptosis. Thus, our findings together with alcohol related neurotoxicity suggest cognitive screening and neurological examination among older adults with AUD, and perhaps with excessive alcohol consumption holds the promise for early detection of adverse brain outcomes. 6
This study has several limitations. First, the analytical data set only includes commercially insured individuals and US Medicare Advantage beneficiaries. In 2020, Medicare Advantage beneficiaries reached 24 million (i.e., 40% of all Medicare beneficiaries), which represents a geographically and racially diverse older adult population. The study results could be generalized to Medicare Advantage beneficiaries. However, the results may not be generalizable to traditional Medicare beneficiaries, as most Medicare Advantage enrollees have access to additional benefits that not covered by traditional Medicare. 28
Second, the claims data only represent individuals diagnosed with AUD; in the analytical data set, the prevalence of AUD is 4.3%, which is likely to be underestimated based on epidemiologic studies. 3 This would tend to reduce the significance of the differences by potentially including some individuals with AUD among the controls, so the risks could be higher than calculated. Additionally, socioeconomic status and education level are not explicitly measured in the analytical data and these may have significant impact on the outcomes. Other possible confounders that we were unable to measure include (but are not limited to) neurological exams, cognitive tests, brain imaging studies, and genetic factors.
A third limitation, also associated with the constraints of administrative data, is that we did not have alcohol consumption records. We can assume that an AUD diagnosis is associated with high alcohol consumption prior to the diagnosis. In the US, 23% of older adults engaged in risky alcohol consumption, which is defined as consuming 15 or more standard drinks per week or 5 or more on an occasion. 29 The observed associations between AUD and adverse brain outcomes are likely to be induced by excessive alcohol consumption. It would be interesting to directly study the risks associated with different levels of consumption, but the available data did not allow this.
A fourth limitation was imposed by the categorization in the OPTUM database of genetic background and ethnicity into a single variable designated “RACE” with 5 broad categories: “White”, “Hispanic”, “Black”, “Asian” and “unknown.” There is much variability within these broad categories that could not be analyzed in the OPTUM data.
In summary, we found that AUD was associated with an increased risk of Alzheimer's disease and Parkinson's disease in the whole study population. AUD was associated with the highest risk of Alzheimer's disease in the Black subpopulation, followed by the White subpopulation, and the Hispanic subpopulation. AUD was associated with increased risk of Parkinson's disease in the White subpopulation. Females with AUD had higher or comparable risks of adverse brain outcomes relative to males with AUD.
Human subjects: Informed consent was not necessary.
CONFLICT OF INTEREST
All authors declare no conflict of interest. Author disclosures are available in the supporting information.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENT
The work was not supported by any funding.
Zhang P, Edenberg HJ, Nurnberger J, Lai D, Cheng F, Liu Y. Alcohol use disorder is associated with higher risks of Alzheimer's and Parkinson's diseases: A study of US insurance claims data. Alzheimer's Dement. 2022;14:e12370. 10.1002/dad2.12370
REFERENCES
- 1. Substance and Mental Health Services Sdministration (SAMHSA). 2017 National Survey of Drug Use and Health (NSDUH) Releases. 2017. Accessed 07/31, 2021. https://www.samhsa.gov/data/release/2017‐national‐survey‐drug‐use‐and‐health‐nsduh‐releases
- 2. Substance and Mental Health Services Sdministration (SAMHSA). 2019 National Survey of Drug Use and Health (NSDUH) Releases. 2019. Accessed 07/31, 2021. https://www.samhsa.gov/data/release/2019‐national‐survey‐drug‐use‐and‐health‐nsduh‐releases
- 3. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12‐month alcohol use, high‐risk drinking, and DSM‐IV alcohol use disorder in the United States, 2001‐2002 to 2012‐2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harper C. The neurotoxicity of alcohol. Hum Exp Toxicol. 2007;26(3):251‐257. [DOI] [PubMed] [Google Scholar]
- 5. de la Monte SM, Kril JJ. Human alcohol‐related neuropathology. Acta Neuropathol. 2014;127(1):71‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamal H, Tan GC, Ibrahim SF, et al. Alcohol use disorder, neurodegeneration, Alzheimer's and Parkinson's Disease: interplay between oxidative stress, neuroimmune response and excitotoxicity. Front Cell Neurosci. 2020;14:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J. QalyDays Study G. Contribution of alcohol use disorders to the burden of dementia in France 2008‐13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3(3):e124‐e132. [DOI] [PubMed] [Google Scholar]
- 8. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eriksson AK, Lofving S, Callaghan RC, Allebeck P. Alcohol use disorders and risk of Parkinson's disease: findings from a Swedish national cohort study 1972‐2008. BMC Neurol. 2013;13:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357:j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weyerer S, Schaufele M, Wiese B, et al. Current alcohol consumption and its relationship to incident dementia: results from a 3‐year follow‐up study among primary care attenders aged 75 years and older. Age Ageing. 2011;40(4):456‐463. [DOI] [PubMed] [Google Scholar]
- 12. Zhang R, Shen L, Miles T, et al. Association of low to moderate alcohol drinking with cognitive functions from middle to older age among US adults. JAMA Netw Open. 2020;3(6):e207922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palacios N, Gao X, O'Reilly E, et al. Alcohol and risk of Parkinson's disease in a large, prospective cohort of men and women. Mov Disord. 2012;27(8):980‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caputo F, Vignoli T, Leggio L, Addolorato G, Zoli G, Bernardi M. Alcohol use disorders in the elderly: a brief overview from epidemiology to treatment options. Exp Gerontol. 2012;47(6):411‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention (CDC) Alcohol‐Related ICD Codes. 2021. Accessed 07/31, 2021. https://www.cdc.gov/alcohol/ardi/alcohol‐related‐icd‐codes.html
- 16. Wilkinson T, Ly A, Schnier C, et al. Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement. 2018;14(8):1038‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang W, Hamilton JL, Kopil C, et al. Current and projected future economic burden of Parkinson's disease in the U.S. NPJ Parkinsons Dis. 2020;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gasparini A. R Package ‘comorbidity’. 2022; Accessed 03/07, 2022. https://cran.r‐project.org/web/packages/comorbidity/comorbidity.pdf
- 19. Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the U.S. Veterans Affairs Frailty Index: transitioning from ICD‐9 to ICD‐10. J Gerontol A Biol Sci Med Sci. 2021;76(7):1318‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676‐682. [DOI] [PubMed] [Google Scholar]
- 21. Nordstrom A, Nordstrom P. Traumatic brain injury and the risk of dementia diagnosis: a nationwide cohort study. PLoS Med. 2018;15(1):e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stark SL, Roe CM, Grant EA, et al. Preclinical Alzheimer disease and risk of falls. Neurology. 2013;81(5):437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cox D. Regression models and life‐tables. J Rl Stat Soc. 1972;34(2):187‐220. [Google Scholar]
- 24. Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958(53):471‐481. [Google Scholar]
- 25. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163‐170. [PubMed] [Google Scholar]
- 26.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327‐406. [DOI] [PubMed] [Google Scholar]
- 27. Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174(11):1094‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaiser Family Foundation (KKF). A Dozen Facts About Medicare Advantage in 2020. 2021. Accessed 08/07, 2021. https://www.kff.org/medicare/issue‐brief/a‐dozen‐facts‐about‐medicare‐advantage‐in‐2020
- 29. Friedmann PD. Alcohol use in adults. N Engl J Med. 2013;368(17):1655‐1656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION
Data Availability Statement
Data accessibility is granted by the license agreement between Indiana University and OPTUM. The OPTUM Clinformatics Data are de‐identified. The Indiana University Institutional Review Board (IRB) designated this study as exempt.


