Abstract
Background
Coagulopathy following cardiac surgery is associated with considerable blood product transfusion and high morbidity and mortality. The treatment of coagulopathy following cardiac surgery is challenging, with the replacement of clotting factors being based on transfusion of fresh frozen plasma (FFP). Prothrombin complex concentrate (PCCs) is an alternative method to replace clotting factors and warrants evaluation. PCCs are also an alternative method to treat refractory ongoing bleeding post‐cardiac surgery compared to recombinant factor VIIa (rFVIIa) and also warrants evaluation.
Objectives
Assess the benefits and harms of PCCs in people undergoing cardiac surgery who have coagulopathic non‐surgical bleeding.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE, Embase and Conference Proceedings Citation Index‐Science (CPCI‐S) on the Web of Science on 20 April 2021. We searched Clinicaltrials.gov (www.clinicaltrials.gov), and the World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/), for ongoing or unpublished trials. We checked the reference lists for additional references. We did not limit the searches by language or publication status.
Selection criteria
We included randomised controlled trials (RCTs) and non‐randomised trials (NRSs).
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
Eighteen studies were included (4993 participants). Two were RCTs (151 participants) and 16 were NRSs. Both RCTs had low risk of bias (RoB) in almost all domains. Of the 16 NRSs, 14 were retrospective cohort analyses with one prospective study and one case report. The nine studies used in quantitative analysis were judged to have critical RoB, three serious and three moderate.
1. PCC versus standard treatment
Evidence from RCTs showed PCCs are likely to reduce the number of units transfused compared to standard care (MD ‐0.89, 95% CI ‐1.78 to 0.00; participants = 151; studies = 2; moderate‐quality evidence). Evidence from NRSs agreed with this, showing that PCCs may reduce the mean number of units transfused compared to standard care but the evidence is uncertain (MD ‐1.87 units, 95% CI ‐2.53 to ‐1.20; participants = 551; studies = 2; very low‐quality evidence).
There was no evidence from RCTs showing a difference in the incidence of red blood cell (RBC) transfusion compared to standard care (OR 0.53, 95% CI 0.20 to 1.40; participants = 101; studies = 1; low‐quality evidence). Evidence from NRSs disagreed with this, showing that PCCs may reduce the mean number of units transfused compared to standard care but the evidence is uncertain (OR 0.54, 95% CI 0.30 to 0.98; participants = 1046; studies = 4; low‐quality evidence).
There was no evidence from RCTs showing a difference in the number of thrombotic events with PCC compared to standard care (OR 0.68 95% CI 0.20 to 2.31; participants = 152; studies = 2; moderate‐quality evidence). This is supported by NRSs, showing that PCCs may have no effect on the number of thrombotic events compared to standard care but the evidence is very uncertain (OR 1.32, 95% CI 0.87 to 1.99; participants = 1359; studies = 7; very low‐quality evidence).
There was no evidence from RCTs showing a difference in mortality with PCC compared to standard care (OR 0.53, 95% CI 0.12 to 2.35; participants = 149; studies = 2; moderate‐quality evidence). This is supported by evidence from NRSs, showing that PCCs may have little to no effect on mortality compared to standard care but the evidence is very uncertain (OR 1.02, 95% CI 0.69 to 1.51; participants = 1334; studies = 6; very low‐quality evidence).
Evidence from RCTs indicated that there was little to no difference in postoperative bleeding (MD ‐107.05 mLs, 95% CI ‐278.92 to 64.83; participants = 151, studies = 2; low‐quality evidence).
PCCs may have little to no effect on intensive care length of stay (RCT evidence: MD ‐0.35 hours, 95% CI ‐19.26 to 18.57; participants = 151; studies = 2; moderate‐quality evidence) (NRS evidence: MD ‐18.00, 95% CI ‐43.14 to 7.14; participants = 225; studies = 1; very low‐quality evidence) or incidence of renal replacement therapy (RCT evidence: OR 0.72, 95% CI 0.14 to 3.59; participants = 50; studies = 1; low‐quality evidence) (NRS evidence: OR 1.46, 95% CI 0.71 to 2.98; participants = 684; studies = 2; very low‐quality evidence).
No studies reported on additional adverse outcomes.
2. PCC versus rFVIIa
For this comparison, all evidence was provided from NRSs.
PCC likely results in a large reduction of RBCs transfused intra‐operatively in comparison to rFVIIa (MD‐4.98 units, 95% CI ‐6.37 to ‐3.59; participants = 256; studies = 2; moderate‐quality evidence).
PCC may have little to no effect on the incidence of RBC units transfused comparative to rFVIIa; evidence is very uncertain (OR 0.16, 95% CI 0.02 to 1.56; participants = 150; studies = 1; very low‐quality evidence).
PCC may have little to no effect on the number of thrombotic events comparative to rFVIIa; evidence is very uncertain (OR 0.51, 95% CI 0.23 to 1.16; participants = 407; studies = 4; very low‐quality evidence).
PCC may have little to no effect on the incidence of mortality (OR 1.07, 95% CI 0.38 to 3.03; participants = 278; studies = 3; very low‐quality evidence) or intensive care length of stay comparative to rFVIIa (MD ‐40 hours, 95% CI ‐110.41 to 30.41; participants = 106; studies = 1; very low‐quality evidence); evidence is very uncertain .
PCC may reduce bleeding (MD ‐674.34 mLs, 95% CI ‐906.04 to ‐442.64; participants = 150; studies = 1; very low‐quality evidence) and incidence of renal replacement therapy (OR 0.29, 95% CI 0.12 to 0.71; participants = 106; studies = 1; very low‐quality evidence) comparative to rFVIIa; evidence is very uncertain.
No studies reported on other adverse events.
Authors' conclusions
PCCs could potentially be used as an alternative to standard therapy for coagulopathic bleeding post‐cardiac surgery compared to FFP as shown by moderate‐quality evidence and it may be an alternative to rFVIIa in refractory non‐surgical bleeding but this is based on moderate to very low quality of evidence.
Keywords: Humans, Cardiac Surgical Procedures, Cardiac Surgical Procedures/adverse effects, Erythrocyte Transfusion, Hemorrhage, Hemorrhage/etiology, Hemorrhage/therapy
Plain language summary
Prothrombin complex concentrate in the treatment of bleeding that occurs with heart surgery
This purpose of this review was to assess the current evidence on whether prothrombin complex concentrates are safe to use to prevent bleeding following heart surgery. We also assessed its ability to reduce death and other serious complications when compared to other therapies.
Background
Bleeding following complex heart surgery can be challenging to manage. The blood clotting pathway is complex, and when the patient is placed on the heart bypass machine, there is a reduction of certain components from blood. Clotting factors can be significantly reduced depending on the duration of bypass. Fresh frozen plasma and prothrombin complex concentrates are the only recognised methods of replacing these clotting factors. Fresh frozen plasma is presented in 250 to 300 mL volume bags which can increase the total blood volume but may place extra strain on the heart. Prothrombin complex concentrates are presented in a powder that is reconstituted and delivered in a smaller volume. This product works faster as the factors are concentrated and given quickly compared to the slow infusion of dilute fresh frozen plasma. Recombinant factor VIIa (rFVIIa) is another blood clotting factor made in the lab, but not from humans. It is used when the bleeding is so bad than no blood products can fix it. We compared how effective prothrombin complex concentrates are to rFVIIa.
Study characteristics
The evidence is up‐to‐date to 20 April 2021. We included 18 studies with a total of 4993 participants who were undergoing heart surgery. From these 18 studies, two were pilot randomised control trials (RCTs) and 16 were non randomised studies (NRSs). Thirteen of these NRS studies were in adults and three were in children. The two types of prothrombin complex concentrates used were 3‐factor (contains three clotting factors) and 4‐factor (contains four clotting factors). These prothrombin complex concentrates were compared to standard therapy in eleven studies and rFVIIa in five studies, with the remaining two studies having no comparator.
The clotting products were given in the operating room in nine studies, intensive care and operating room in three studies and were not described in the remaining six studies. We excluded any study that used the clotting products to reverse the actions caused by blood thinning medications that the patient was already taking.
Key results
Prothrombin complex concentrates compared to standard therapy
Prothrombin complex concentrates had an overall reduction in red blood cell (RBC) transfusion (both units of RBC transfusion and incidence of RBC transfusion) when compared to fresh frozen plasma. There was potentially no reduction in chest drain output (bleeding) in the RCTs. There was no difference in the reported outcomes of blood clots, death, intensive care stay and the requirement of dialysis in both RCTs and NRSs. The RCTs had moderate to low quality of evidence and the NRS had very low to low quality of evidence.
Prothrombin complex concentrates compared to rFVIIa
Prothrombin complex concentrates had a large reduction in red blood cell transfusion when compared to rFVIIa. The quality of this evidence was moderate. For the remaining outcomes that we reviewed, there were only two studies that could be analysed. These studies found that there was no difference in blood clots, death, bleeding into drains, intensive care stay and the requirement for dialysis. This lack of difference could result from the lack of ability of low participant numbers to find these rarer outcomes. The quality of the evidence for these outcomes was very low.
Quality of evidence
The RCTs had a low risk of bias, but the overall quality of the evidence was graded as moderate for the majority of outcomes, rather than high due to low sample numbers. For the remaining outcomes, the evidence was graded as low as there was only one RCT that contributed to those outcomes.
The quality of evidence for the NRSs was low to very low. Many of the retrospective studies had significant confounding that may have influenced the final outcome.
Conclusion
Prothrombin complex concentrates may reduce RBC transfusion rates (both the quantity of RBC transfusion and the incidence of RBC transfusion) in patients with bleeding issues following heart surgery when compared with standard care. We didn't identify a difference in any of the other outcomes but the total number of participants in the studies was likely insufficient to detect an outcome difference.
Summary of findings
Summary of findings 1. PCC compared to standard treatment for cardiac surgery for the treatment of non‐surgical bleeding.
| PCC compared to standard treatment for cardiac surgery for the treatment of non‐surgical bleeding | ||||||
| Patient or population: cardiac surgery for the treatment of non‐surgical bleeding Setting: hospital (intraoperative and postoperative) Intervention: PCC Comparison: standard treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard treatment | Risk with PCC | |||||
| Blood products transfused (RBC) in units | The mean RCT: Blood products transfused (RBC) in units was 3 | MD 0.89 lower (1.78 lower to 0) |
‐ | 151 (2 RCTs) |
⊕⊕⊕⊝ MODERATE1 | Two pilot RCTs Green 2021; reported blood components in units at 24 hours and Karkouti 2021 reported units within 24 hours of start of surgery. |
| The mean NRS: blood products transfused (RBC) in units was 5 | MD 1.87 lower (2.53 lower to 1.20 lower) | ‐ | 551 (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 | Two observational studies described intraoperative red cell transfusion (Biancari 2019; Cappabianca 2016). They described this in mLs of red cells transfused and converted to units by assuming an average of 250 mL of blood per unit. | |
| Blood products transfused (RBC) % of patients | 830 per 1000 | 721 per 1000
(494 to 872) |
OR 0.53 (0.20 to 1.40) | 101 (1 RCT) | ⊕⊕⊝⊝ LOW 4 5 | One RCT Karkouti 2021 reported on incidence of RBC transfusion. |
| 889 per 1000 |
812 per 1000
(706 to 887) |
OR 0.54 (0.30 to 0.98) | 1046 (4 observational studies) | ⊕⊕⊝⊝ LOW 2 | Three observational studies described the incidence of a red cell transfusion (Biancari 2019; Cappabianca 2016; Zweng 2019). Fitzgerald 2018 reported avoidance of red cell transfusion. Zweng 2019 had a greater number of the PCC group receiving red cell transfusion but this was not significant. | |
| Thrombotic events | 95 per 1000 | 64 per 1000
(20 to 194) |
OR 0.68 (0.20 to 2.31) | 152 (2 RCTs) | ⊕⊕⊕⊝ MODERATE4 | Two pilot RCTs. Green 2021 reported on many thrombotic events; we only included those of stroke as the other outcomes e.g. mesenteric artery thrombosis and spinal cord ischaemia are more likely related to other complex factors. Karkouti 2021 reported on stroke/TIA, atrial and vascular thrombosis. |
| 68 per 1000 |
87 per 1000
(60 to 126) |
OR 1.32 (0.87 to 1.99) | 1359 (7 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | Biancari 2019 reported on acute cerebral infarcts only. Cappabianca 2016 reported on postoperative myocardial infarction and cerebral infarcts. Fitzgerald 2018 reported on cerebral infarcts and venous thromboembolism (deep vein thrombosis and pulmonary embolism). Arachchillage 2016 and Harris 2020a did not define how they measured thrombosis. Zweng 2019 measured both arterial and venous thrombosis. One study (Giorni 2013) reported 0 events for both PCC and standard care groups and therefore did not contribute to the meta‐analysis. | |
| Mortality (30‐day) | 70 per 1000 | 39 per 1000
(9 to 151) |
OR 0.53 (0.12 to 2.35) | 149 (2 RCTs) | ⊕⊕⊕⊝ MODERATE4 | Two pilot RCTs reported on this (Karkouti 2021; Green 2021); lost 4 to follow‐up at 30 days. |
| 83 per 1000 |
84 per 1000
(59 to 120) |
OR 1.02 (0.69 to 1.51) | 1334 (6 observational studies) | ⊕⊝⊝⊝ VERY LOW 23 4 | Arachchillage 2016; Biancari 2019; Zweng 2019 described 30‐day mortality. Cappabianca 2016; Fitzgerald 2018; Harris 2020a reported on in‐hospital mortality with no time frame given. | |
| Intensive care length of stay in hours | The mean RCT intensive care length of stay in hours was 84 | MD 0.35 lower (19.26 lower to 18.57 higher) |
‐ | 151 (2 RCTs) |
⊕⊕⊕⊝ MODERATE1 | Two pilot RCTs with Green 2021 reporting on ICU or HDU stay in days and Karkouti 2021 median ICU stay in days. |
| The mean NRS intensive care length of stay in hours was 128 | MD 18.00 lower (43.14 lower to 7.14 higher) | ‐ | 450 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 2 3 5 | Cappabianca 2016 reported on ICU length of stay with a mean of 110 hours (+/‐ 118) in the PCC group and a mean of 128 hours (+/‐ 152) in the standard treatment group. | |
| Incidence of renal impairment | 160 per 1000 | 120 per 1000
(30 to 482) |
OR 0.72 (0.14 to 3.59) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 4 5 | One pilot RCT (Green 2021) reported on number of patients requiring haemodialysis. Karkouti 2021 combined both haemodialysis and acute kidney injury with a 2‐fold increase in creatinine. |
| 41 per 1000 |
59 per 1000
(29 to 113) |
OR 1.46 (0.71 to 2.98) | 684 (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | Cappabianca 2016 and Fitzgerald 2018 reported on renal impairment postoperatively. Overall incidence was low with 20 patients in the PCC group and 14 in the standard treatment group. Cappabianca 2016 used the RIFLE criteria to define acute kidney injury. Fitzgerald 2018 used serum creatinine measured from before surgery to the highest creatinine concentration on postoperative days 1 or 2. | |
| Bleeding (chest drain output) in mLs for the first 12 hours | The mean RCT: Bleeding (chest drain output) in mLs for the first 12 hours was 552 |
MD 107.05 lower (278.92 lower to 64.83 higher) |
‐ | 151 (2 RCTs) |
⊕⊕⊝⊝ LOW 1 6 | |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | None of the studies measured this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level for imprecision as number of participants < 400
2Downgraded two levels for risk of bias associated with lack of randomisation
3Downgraded one level for risk of bias
4Downgraded one level for imprecision as number of events were < 400
5Downgraded one level for indirectness as only one study which did not represent all potential participants
6Downgraded one level for inconsistency
Summary of findings 2. PCC compared to FVIIa for cardiac surgery for the treatment of non‐surgical bleeding.
| PCC compared to FVIIa for cardiac surgery for the treatment of non‐surgical bleeding | ||||||
| Patient or population: cardiac surgery for the treatment of non‐surgical bleeding Setting: hospital (intraoperative and postoperative) Intervention: PCC Comparison: FVIIa | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with FVIIa | Risk with PCC | |||||
| Blood products transfused (RBC) in units ‐ intraoperative | The mean blood products transfused (RBC) in units ‐ intraoperative was 12 | MD 4.98 lower (6.37 lower to 3.59 lower) | ‐ | 256 (2 observational studies) | ⊕⊕⊕⊝ MODERATE 1 2 3 4 | Harper 2018 and Tanaka 2013 reported on intraoperative red cell transfusion. Harper 2018 used mLs of red cells transfused and we converted to units by assuming 250 mL per unit of red cells. Overall effect was clinically significant with a 5 unit of red cells difference in the PCC group. However, in both Harper 2018 and Tanaka 2013, the rFVIIa group may include higher risk cardiac surgical patients. |
| Blood products transfused (RBC) % of patients | 990 per 1000 |
941 per 1000
(664 to 994) |
OR 0.16 (0.02 to 1.56) | 150 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 2 5 6 | Only Tanaka 2013 reported on incidence of red cell transfusion in patients. |
| Thrombotic events | 90 per 1000 |
48 per 1000
(22 to 103) |
OR 0.51 (0.23 to 1.16) | 407 (4 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 5 | Three studies reported on incidence of postoperative thrombosis; Audley 2019; Harper 2018; Mehringer 2018. Audley 2019 reported this as all thromboembolic events but this was not defined. Harper 2018 defined thromboembolism as new cerebral vascular events, deep vein thrombosis, pulmonary embolism, myocardial infarction or new intracardiac thrombus. In Mehringer 2018, thromboembolic events defined as venous thromboembolism, arterial thromboembolism or pulmonary embolism that occurred at any time postoperatively. |
| Mortality (30‐day) | 135 per 1000 |
143 per 1000
(56 to 320) |
OR 1.07 (0.38 to 3.03) | 278 (3 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 5 | Harper 2018 and Tanaka 2013 reported on 30‐day mortality. Audley 2019 reported on in‐hospital mortality. |
| Bleeding (chest drain output) in mLs for the first 12 hours | The mean bleeding (chest drain output) in mLs for the first 12 hours was 1398 | MD 674.34 lower (906.04 lower to 442.64 lower) | ‐ | 150 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 3 6 | Tanaka 2013 was the only study that reported on 12‐hour chest drain output with a mean of 723.33 mL (+/‐ 442.78) in the PCC group and mean of 1397.67 mL (+/‐ 1002.69) in the rFVIIa group. |
| Intensive care length of stay in hours | The mean intensive care length of stay in hours was 196 | MD 40 lower (110.41 lower to 30.41 higher) | ‐ | 106 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 3 6 | Harper 2018 was the only study that reported on ICU length of stay with a mean of 156 hours (+/‐ 155.46) in the PCC group and a mean of 196 hours (+/‐ 210.32) in the rFVIIa group. |
| Incidence of renal impairment | 415 per 1000 |
171 per 1000
(78 to 335) |
OR 0.29 (0.12 to 0.71) | 106 (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 5 6 | Harper 2018 reported on new acute kidney injury by the incidence of patients requiring postoperative dialysis. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | None of the studies measured this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded two levels for risk of bias associated with lack of randomisation
2Downgraded one level for risk of bias
3Downgraded one level for precision as number of participants < 400
4Upgraded two levels due to very large effect size
5Downgraded one level for precision as number of events were < 400
6Downgraded one level for indirectness as only one study which did not represent all potential participants
Background
Description of the condition
Cardiac surgery is known to be associated with high blood product transfusion requirements and, in turn, allogeneic blood transfusion is associated with higher rates of morbidity and mortality (Arias‐Morales 2017; Kilic 2014). In 2016, it was estimated that one million people throughout the world undergo cardiac surgery each year (Veluz 2017). This number is only likely to increase with our ageing population. In the UK, there are 30,000 cardiac procedures performed each year and it is estimated that 30% of these require plasma transfusion for bleeding and management of coagulopathy (Bortolussi 2019; Green 2019).
There is considerable risk of postoperative bleeding due to contact activation within the extracorporeal circulation system, factor degradation, platelet dysfunction and activation, fibrinogen consumption, reduced liver production of factors, foreign graft material, multiple suture lines, and raw open vascular surfaces (Achneck 2010).
Platelets may become activated but injury to these platelets may occur due to the shear forces of the cardiopulmonary bypass circuit and pump leading to impaired function. At the surgical site, blood is exposed to air and tissue factor, further activating the coagulation cascade. These processes will ultimately result in consumption of coagulation factors, platelets, and fibrinogen, as well as increased fibrinolysis (O'Carroll‐Kuehn 2007). A non‐systematic search and review of the coagulation changes post‐cardiopulmonary bypass showed that plasma fibrinogen concentration decreases during cardiopulmonary bypass with a median reduction of 36% with platelet count decreasing by 44% (Höfer 2016). They also showed that coagulation factors had an overall decrease in activity during cardiopulmonary bypass with factors II, V, VII, X, XI, and XIII all strongly decreased by an average of 47.0%, 39.9%, 23.5%, 40.3%, 35.6%, and 33.6%, respectively (Höfer 2016). An animal study looking at swine, showed that, following cardiopulmonary bypass for two hours at 25 degrees Celsius, there was a fall of coagulation factors II, VII, IX and X up to 48% (Kaspereit 2010). It is hypothesised that these factors exponentially decrease the longer the patient is on cardiopulmonary bypass and the lower the temperature.
Description of the intervention
Prothrombin complex concentrate (PCC) is fractionated and includes both 4‐factor concentrates (coagulation factors II, VII, IX, X) and 3‐factor concentrates (coagulation factors II, IX, X). In Europe and Canada, 4‐factor concentrates are predominantly used, for example, Beriplex and Octaplex (Octapharma), whereas in Australia and New Zealand the only preparation available is the 3‐factor concentrate e.g. Prothrombinex (CSL Behring) (Sørensen 2011).
In the UK, factor concentrates became popular for treatment of haemophilia in the 1990s due to infectious risks associated with fresh frozen plasma (FFP) and cryoprecipitate (Köhler 1999). On a global scale, in 2017, the EACTS/EACTA (European Association for Cardio‐Thoracic Surgery/European Association of Cardiothoracic Anaesthesiology) taskforce published a comprehensive patient blood management guideline. The authors have recommended the use of PCC for coagulation factor deficiency in the treatment of microvascular bleeding but do not give a recommended dose or timing (Pagano 2018). Following on from this, in 2019, the American Society of Cardiovascular Anaesthesiologists have now included the use of low‐dose PCC in their perioperative blood management guideline as an alternative to FFP; however, this treatment is recommended with caution as there is still uncertainty about dosing and side effects (Raphael 2019).
PCC comes with a potential prothrombic risk, given it is a low‐volume, high‐concentration of coagulation factors infused directly into the systemic circulation (Song 2014). Three‐factor concentrates do not have protein S and C, therefore, they can add to the potential risk of thrombosis. Thrombosis has been described as cerebrovascular events, myocardial infarction, pulmonary embolism and deep vein thrombosis (Franchini 2010). In patients with a prior history of venous thromboembolism given 3‐factor PCC for reversal of warfarin in the setting of intracerebral haemorrhage, there was a 4.5 times increased risk of developing a venous thromboembolism within 30 days (Felton 2016). There has been one documented case study of massive thrombosis following PCC administration, of the superior vena cava to the pulmonary artery requiring reinstitution of cardiopulmonary bypass and thrombectomy (Koster 2014).
The true risk of acute kidney injury with PCC is unknown. Cappabiancca and colleagues showed an increased risk incidence of acute kidney injury and dialysis with PCC when compared to FFP (Cappabianca 2016). Subsequently, contrasting studies showed no increased risk of acute kidney injury (Fitzgerald 2018; Harper 2018). Further unknowns include the use of PCC for bleeding in patients with mechanical support such as left ventricular assist devices and extracorporeal membrane oxygenation.
PCC has a low transfusion volume (a 500‐unit vial is reconstituted in 20 mL). The patient receives less overall fluid volume, which will potentially avoid volume overload of the right ventricle and reduce incidence of lung oedema. PCC is also not associated with transfusion‐related acute lung injury (TRALI). The advised rate of transfusion is 3 mL to 6 mL per minute or as tolerated by the patient (Behring; Pabinger 2010). PCC has a shelf life of six months at room temperature and will allow for immediate availability for factor replacement. Unlike other clotting factors, PCC does not require blood group specificity and has an improved safety profile (Tanaka 2010).
PCC is a sterile freeze‐powder containing purified human coagulation factors. The concentrate is produced by ion‐exchange chromatography from the cryoprecipitate of large plasma pools after removal of factor IX and antithrombin (Franchini 2010).
The 3‐factor PCC (e.g. Prothrombinex‐VF) is presented in 500 IU vials that contain 500 IU of factors II, IX and X, 25 IU of antithrombin 3, 192 IU of heparin and electrolyte buffers.
The 4‐factor PCC (e.g. Beriplex) is presented in 500 IU vials that contain 380 to 800 IU of factor II, 200 to 500 IU of factor VII, 500 IU of factor IX, 500 to 1020 IU of factor X, 420 to 820 IU of protein C and 240 to 680 IU of protein S.
Intravenous administration means that the preparation is available immediately, and bioavailability is 100%. Patients who received a 50 IU/kg intravenous dose, showed that peak plasma concentrations of the coagulation factors occur within five minutes of infusion (Ostermann 2007).
PCC is distributed and metabolised in the same way as endogenous coagulation factors (Franchini 2010).
PCC administration is contraindicated in patients with known allergy to heparin or history of heparin‐induced thrombocytopenia and with active thrombosis or disseminated intravascular coagulopathy. Heparin‐induced thrombocytopenia is related to the low level of porcine heparin in some types of PCCs e.g. Prothrombinex. There were no documented heparin‐induced thrombocytopenias secondary to PCCs in a pharmacovigilance study of Beriplex from 1996 to 2012 (Hanke 2013). There are no known drug interactions with PCCs.
Elimination half‐life of the coagulation factors is: factor II, 60 hours; factor VII, 4.2 hours; factor IX, 17 hours; and factor X, 31 hours (Franchini 2010).
How the intervention might work
The treatment of bleeding diathesis following cardiac surgery is a considerable challenge and has been traditionally based on transfusion of allogeneic blood products (Kilic 2014). Typically, a volume of 20 mL/kg of FFP is required to produce a 30% increase in factor levels with a subsequent risk of transfusion‐associated fluid overload (TACO) (Nascimento 2010). Substantial volumes of FFP are required to ensure adequate factor replacement and, as a consequence, there can be dilution of other clotting constituents, including platelets, fibrinogen and red blood cells (Ishikura 2017; Nascimento 2010). PCC is currently used in the treatment and perioperative prophylaxis of acquired deficiency of prothrombin complex factors and bleeding in patients with congenital deficiency of individual coagulation factors when specific products are not available (Estrada 2016; Siddon 2016; Van Veen 2007). It is also used in the treatment of warfarin reversal prior to urgent or emergency surgery (Bordeleau 2015; Unold 2015; Van Veen 2007). Studies of PCC for warfarin reversal show that there is reversal of anticoagulation within 10 minutes following administration (Riess 2007), in comparison to FFP, which takes hours, and with which INR (International Normalised Ratio) correction can also be incomplete (Cartmill 2000). Furthermore, FFP correction is also delayed due to prescription, cross‐matching and administration time (Bordeleau 2015), and it is unable to correct an INR to less than 1.6 (Yazer 2010).
FFP contains all the coagulation factors except platelets. It is the plasma portion of a unit of whole blood that is frozen. FFP contains all coagulation factors and other plasma proteins (albumin), including fibrinogen (400 to 900 mg/unit), physiological anticoagulants (protein C and S, antithrombin and tissue factor pathway inhibitor). Following thawing of FFP, factors V and VIII have a gradual decline requiring re‐administration if there is ongoing bleeding (Nascimento 2010).
In comparison, following administration of PCC, there is correction of vitamin K‐dependent coagulation factors II, VII, IX and X and antithrombotic proteins C and S (in 4‐factor PCC). The 3‐factor PCC contains only factors II, IX and X with generally small amounts of factor VII, antithrombin and small amounts of heparin. Following increase of these substrate coagulation proteins, there is enhanced thrombin generation, which illustrates the ability of PCC to support the enzyme complexes that convert factor II to IIa (Ghadimi 2016).
Factor VII is converted to VIIa and binds to tissue factor, which then activates factor IX and the primary coagulation pathway. Factor IX in the presence of VIIIa activates factor X. Factor X is activated to convert prothrombin to thrombin in the presence of phospholipids and calcium ions. Factor II is converted to thrombin by the presence of activated factor X. Thrombin converts fibrinogen to fibrin which is the substance of the clot, and activates factors VIII, V and XI to continue the coagulation pathway. Protein C is activated by thrombin to then exert an antithrombotic effect, whereas protein S exists in a free form as a cofactor for activated protein C (Ghadimi 2016).
FFP has the advantage of containing all the required factors but in a dilute form, and large volumes are required for relatively small increments in factor levels. Conversely, PCC increases the key factors to a much larger extent. PCC is the ideal reversal agent of warfarin as the depleted factors are the vitamin K‐dependent ones.
Why it is important to do this review
Internationally, there is a growing collection of hospital‐based coagulation algorithms utilising PCC as factor replacement and as rescue therapy for the correction of coagulopathy post‐cardiac surgery. These centres mentioned are using PCC with point‐of‐care testing with thromboelastography such as rotational thromboelastometry (ROTEM) and thromboelastography (TEG). Montreal Heart Institute published their coagulation algorithm utilising PCC with ROTEM guidance with a dose of 10‐15 units/kg (Denault 2014). Duke University Hospital have recently published their algorithm (Hashmi 2019). Prince Charles hospital in Brisbane have also published an algorithm in association with National Blood Authority Australia (NBAA).
There are currently no randomised controlled trials (RCTs) in this area. There are, however, two trials listed on clinicaltrials.gov. One is a pilot in a single centre in London comparing FFP to PCC for patients who are bleeding during cardiac surgery (Green 2019). The other is a Mayo Clinic trial based in Rochester, sponsored by CSL Behring, looking at PCC compared to FFP for post‐cardiopulmonary bypass coagulopathy and bleeding (Roman 2019). This second study utilises laboratory testing and not point‐of‐care coagulation testing.
One publication, published in 2019, has stated that it is a “systematic review and meta‐analysis”, and it identified four studies to analyse with a total of 861 adult participants, none of which were randomised. The four studies that the authors included were all retrospective cohorts. The authors concluded that PCC appeared to be more effective than FFP in reducing perioperative blood transfusions and with no additional risk of thromboembolic events (Roman 2019). The studies identified only included the one comparator, which was FFP, and it was limited to adults. In this growing area of research, there are additional studies that need to be included.
This review would be the first step in summarising the entire literature in order to perform a comprehensive study that will assist in coagulation management and lead on to creating an international guideline. We believe that, with the introduction of PCC, there should be robust literature to support its use. PCC is potentially a very effective treatment option, which may reduce incidence of organ dysfunction, reduce blood transfusion and postoperative bleeding. It is cost‐effective but its safety and side effects need to be established before this becomes standard treatment worldwide.
Objectives
To assess the benefits and harms of prothrombin complex concentrate in people undergoing cardiac surgery who have coagulopathic non‐surgical bleeding
Methods
Criteria for considering studies for this review
Types of studies
We included individual randomised controlled trials (RCTs) with both blinded and unblinded assessment of outcome. We did not include cluster‐ and cross‐over RCTs. Due to the low incidence of patients that could potentially benefit, this treatment is more likely to be studied in non‐randomised studies.
In conjunction with these studies, we included non‐randomised trials: cohort trials, both prospective and retrospective in design; case‐control studies, as this is a reasonable study design to use, due to the rarity of the patients undergoing this procedure; and before‐and‐after studies, as the research may have occurred when hospitals changed their guidelines or policies. As this treatment is already in practice, it is important to summarise the current available evidence. Studies must analyse our described intervention and, if possible, compare with FFP or recombinant factor VIIa, or both.
For the non‐randomised studies, we chose the most robust designs that we believe will be able to answer the question of interest with minimal risk of bias. We did not exclude studies on the basis of language of publication or publication status. We excluded animal studies and non‐clinical trials (in vitro, ex vivo, in vivo and in silico).
We included case reports, which included outcomes on adverse events.
Types of participants
We included studies with participants of all age groups undergoing cardiac surgery who had coagulopathic bleeding (coagulopathy post‐cardiopulmonary bypass).
We excluded studies that used PCC for reversal of warfarin or vitamin K antagonists, and preoperative haemorrhagic diathesis (for example, haemophilia A and B, myelodysplastic syndrome, von Willebrand disease, immune thrombocytopenic purpura).
We believe that part of the resultant coagulopathy is a consequence of cardiopulmonary bypass, consequently, we will exclude any off‐pump cardiac surgery.
If we cannot separate participants from cardiac surgery and other forms of surgery, then we will write to the study authors to obtain data. As long as 80% were cardiac patients, data from these studies would be included.
Types of interventions
We included studies where PCC was prescribed for the intended purpose of factor replacement, as first‐line or rescue therapy (last‐resort therapy for refractory bleeding), or both, to reduce coagulopathic bleeding. There are many forms of PCC available internationally and we will review both 3‐ and 4‐factor products (Appendix 1). We excluded single‐factor concentrates labelled 'prothrombin complex concentrates'.
Comparators included standard therapy (current institutional protocol for bleeding diathesis), FFP and recombinant factor VIIa.
We included studies that used PCC as monotherapy and also PCC in combination with FFP (delivery separately or together) for the same intended therapeutic effect (reduction in coagulopathic bleeding). We included studies with any described doses providing that they gave our intervention and comparators intravenously. We excluded studies using or comparing activated PCC because it contains an activated form of factor VII and this would cause confounding since recombinant factor VIIa is one of our direct comparators.
The possibility that patients that receive PCC are likely to be higher risk of perioperative mortality and coagulopathy is a confounding factor. Factors that define high risk are defined further in the analysis section.
Types of outcome measures
Primary outcomes
Blood products transfused: defined as all products (whole blood, red blood cells, FFP, cryoprecipitate, platelets and fibrinogen concentrate) transfused in theatre and postoperatively, before and after the intervention or comparator, within 24 hours (mLs)
Thrombotic events: defined as new venous and arterial thromboses within 30 days
Mortality: defined as all‐cause mortality following cardiac surgery within 30 days
Secondary outcomes
Bleeding: reviewed by postoperative drain output in the intensive care unit. We will not use intraoperative blood loss as a primary outcome for bleeding because it is poorly mentioned in the literature following cardiac surgery. Drain output is defined as total blood loss from the mediastinal and pleural drains in the first 12 hours (mLs).
Intensive care unit length of stay: defined as the total stay in intensive care following surgery (hours)
Incidence of renal impairment: defined as new or acute renal impairment requiring temporary continuous renal replacement therapy or sustained low‐efficiency daily diafiltration within 30 days
Ventilator hours: the duration of intubation while in the intensive care unit
Adverse events: any other adverse event reported within the primary studies not included in the above outcomes
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify relevant studies on 20 April 2021:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 3 of 12, 2021);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 19 April 2021);
Embase (Ovid, 1980 to 2021 week 15);
Conference Proceedings Citation Index‐Science (CPCI‐S) on the Web of Science (Clarivate Analytics, 1990 to 19 April 2021).
We searched Clinicaltrials.gov (www.clinicaltrials.gov), and the World Health Organisation (WHO), International Clinical Trials Registry platform (ICTRP; apps.who.int/trialsearch/), for ongoing or unpublished trials on 10/02/2022 using the terms 'prothrombin complex concentrate' and 'cardiac surgery'.
The preliminary search strategy for MEDLINE was adapted for the other databases (Appendix 2). There was production and use of PCC prior to 2000, however, these PCCs are known to have different constituents and posed an increased thrombosis risk (Köhler 1999), therefore, we have chosen to start the literature search from 2000. This is in relation to the European Medicines Authority (EMA) gaining regulatory approval in 2005 (European Medicine Authority).
We imposed no restriction on language of publication or publication status. We did not perform a separate search for adverse effects of interventions used for the treatment of coagulopathy post‐cardiopulmonary bypass. We considered adverse effects described in included studies only.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also contacted principal investigators of identified studies to ascertain if they were aware of any other relevant published or unpublished matching clinical studies.
Data collection and analysis
Selection of studies
Two review authors (KH and CF) independently screened titles and abstracts for inclusion of all the potential studies we identified as a result of the search using Covidence and coded them as 'yes include' (eligible or potentially eligible/unclear), 'do not include', or 'maybe' if full text was required to clarify (Covidence). We resolved any disagreements about abstract suitability by discussion and consensus or a third party decision (VJ). We retrieved the full‐text study reports or publication and two review authors (KH and CF) independently screened the full text and identified studies for inclusion; we also identified and recorded reasons for exclusion of the ineligible studies. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of included studies' table (Liberati 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data, which had been piloted on at least one study in the review. Two review authors (KH and MF) extracted study characteristics from included studies separately and then compared and resolved conflicts. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, and date of study. For cohort and case control studies, we collected information about where the control group was sourced. For the cohorts, we determined whether they were retrospective or prospective in design.
Participants: number randomised, number lost to follow‐up or withdrawn, number analysed, mean age, age range, gender, inclusion criteria, and exclusion criteria. Cardiac‐specific data that we collected includes: type of cardiac surgery, duration of cardiopulmonary bypass, deep hypothermic arrest required, emergency surgery, pre‐operative anticoagulants, redo surgery and, when available, laboratory coagulation test results and point‐of‐care test results.
Interventions: interventions and comparisons; we also planned to include any information regarding co‐interventions, though we did not expect any co‐interventions at this stage.
Outcomes: primary and secondary outcomes specified and collected, and time points reported. We also collected both adjusted and unadjusted data. When collecting the adjusted data, we noted what variables that the data had been adjusted for.
Notes: funding for trial, and notable conflicts of interest of study authors.
Two review authors (KH, MF) independently extracted outcome data from included studies. We resolved disagreements by consensus. One review author (KH) transferred data into the Review Manager 5 (Review Manager 2014) file. We then double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data extraction form.
Assessment of risk of bias in included studies
Two review authors (KH and MF) independently assessed risk of bias, in both RCTs and NRSs, for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
For RCTs, we assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the risk of bias table for RCTs and in a supplementary table for non‐RCTs. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a study author, we noted this in the risk of bias table.
For non‐RCTs, we used the ROBINS‐I tool (version 19 September 2016) for assessing the bias (Sterne 2016). This tool shows substantial overlap with the risk of bias ratings in RCTs, but additionally includes two domains at the pre‐intervention level (bias due to confounding, bias in selection of participants into the study), and one domain at the intervention level (bias in classification of interventions). This tool uses a five‐point scale (low/moderate/serious/critical/unclear risk) for the assessment of bias in non‐randomised studies of interventions (NRSI). The ROBINS‐I tool was used to asses the effect of the assignment. The primary analysis included all studies regardless of their risk of bias. Please see Sensitivity analysis for how studies with serious and critical risk of bias were dealt with.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome. Risk of bias in NRSs was assessed for all outcomes which had included studies; please see Additional tables.
The most important confounding domains were those factors that increased the risk of bleeding. The factors affecting this are:
age over 75 years
active endocarditis
redo surgery
more than one cardiac surgical procedure
All of these factors are covered in the EuroSCORE II (Nashef 2012). However, in addition to these EuroSCORE II factors, there are also these factors:
Use of deep hypothermic cardiopulmonary arrest
CBP more than 180 minutes
BMI less than 25
urgent/emergent
pre‐operative anticoagulants
aortic surgical work
pre‐operative anaemia
aortic valve disease (regurgitation/stenosis/both)
history of thrombosis or coagulation defect
Measures of treatment effect
For data supplied by a randomised controlled trial, we analysed continuous data as mean difference (MD) with 95% CI. We entered data presented as a scale with a consistent direction of effect. We measured dichotomous data with odds ratios (ORs). If data were presented as medians, we used the Bland Method to estimate means and standard deviations (Wan 2014).
For non randomised studies, where possible, we chose adjusted over unadjusted estimates. We collected adjusted odds ratios (ORs) by preference, with 95% confidence intervals (CIs) from the NRSIs and, if adjusted data were not available, we collected unadjusted ORs with 95% CIs. If adjusted data were supplied by the NRSIs, we analysed these using generic inverse variance by using log ORs and standard errors. We noted adjustments made by the individual studies within the footnote section of the forest plot.
We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
Cross‐over studies and cluster‐RCTs were not included. For the outcomes where information was described as overall measures, that is, number of units of blood transfused and hours of hospital stay, we extracted these as mean numbers per person to avoid unit of analysis issues.
For multiple time points, we used the outcome that was closest to the prespecified outcome measure. For NRSIs, when multiple adjusted estimates were reported, we chose the one that was judged to minimise the risk of bias due to confounding.
Dealing with missing data
We contacted study authors or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data, where possible (e.g. when we identified a study that was only available as an abstract). We used the Review Manager 5 (Review Manager 2014) calculator to calculate missing standard deviations using other data from the study, such as confidence intervals, based on methods outlined in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). When this was not possible, and we thought that the missing data would introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. We addressed the potential impact of the missing data in our discussion.
Assessment of heterogeneity
Any variability among the studies in a systematic review may be caused by clinical, methodological or statistical heterogeneity. Any variability in the participants, interventions and outcomes studied were described as clinical diversity and any variability in the study design and risk of bias were described as methodological diversity (Deeks 2019). Variability from the intervention effects studied is known as statistical heterogeneity (referred to simply as heterogeneity) and can be a consequence of clinical or methodological diversity, or both. This may result in the observed intervention effects being more different from each other than one would expect due to random error alone (Deeks 2019).
With the known lack of RCTs on the topic to be reviewed, we expected to see heterogeneity due to both clinical and methodological diversity.
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We used the I² statistic (Higgins 2003), to measure heterogeneity among the studies in each analysis but, acknowledging that there is substantial uncertainty in the value of the I² statistic when there is only a small number of studies, we also considered the P value from the Chi² test.
When we identified substantial heterogeneity greater than 50%, we reported it and explored possible causes by prespecified subgroup analysis (Deeks 2019).
Assessment of reporting biases
If we were able to pool more than 10 studies, we created and examined a funnel plot to explore possible small study biases for the primary outcomes (Page 2019).
Data synthesis
We carried out statistical analysis using Review Manager 5 (Review Manager 2014). We undertook meta‐analyses only where this was meaningful, that is, the treatments, participants and the underlying clinical questions were similar enough for pooling to make sense. We used a random‐effects model as we anticipated heterogeneity in the participant or intervention characteristics. We carried out separate meta‐analysis for RCTs and NRSIs.
We analysed separately NRSIs with different study features.
If data were unavailable to be pooled, we presented them in a narrative summary with tables, if appropriate.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
High risk for coagulopathy versus low risk for coagulopathy. For the definition of high risk versus low risk we relied on the definition of the primary studies.
Use of PCC for rescue treatment in refractory bleeding versus recombinant factor VIIa
Adults (> 18 years) versus children (0‐18 years)
Four‐factor PCC versus three‐factor PCC
We used the formal test for subgroup differences in Review Manager 5 (Review Manager 2014), and based our interpretation on this.
Sensitivity analysis
We carried out the following sensitivity analyses, to test whether key methodological factors or decisions had affected the main result.
For RCTs, we only included studies with a low risk of bias for selection bias and attrition. For the NRSIs, we undertook a sensitivity analysis looking at the studies deemed to be at an overall low to moderate risk of bias by the ROBINS‐I tool excluding those judged as serious and critical.
We intended to carry out a sensitivity analysis on the inclusion of unadjusted data versus adjusted data but as no included studies supplied adjusted data, we did this for matched vs unmatched.
We carried out a sensitivity analysis on the impact of missing data, excluding studies judged at high risk for missing data.
We carried out a sensitivity analysis for NRSIs, looking at different study design features (if pooled).
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes.
Blood products transfused
Thrombotic events
Mortality
Bleeding
Intensive care unit length of stay
Incidence of renal impairment
Adverse events
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019a) using GRADEpro software (GRADEpro GDT). We created different summary of findings tables for RCTs and NRSIs.
We compared both of the two comparators, FFP and recombinant factor VIIa, to PCC. We developed a separate summary of findings table for each comparison and we analysed each comparison separately. We justified all decisions to downgrade the quality of studies using footnotes and made comments to aid readers' understanding of the review, where necessary.
Two review authors (KH and CF) worked independently to judge evidence quality, and resolved any disagreements by discussion or by involving a third review author, VJ. We justified and documented our judgements and incorporated them into reporting of results for each outcome. We made our judgements in accordance with recommendations on how the ROBINS‐I tool should integrate with GRADE. Evidence started at high quality and was downgraded according to the five domains that can lower certainty (Schünemann 2019b).
We extracted study data, formatted our comparisons in data tables and prepared a summary of findings table before writing the results and conclusions of our review.
Results
Description of studies
We provide descriptions of previously mentioned studies in the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
The search was performed on the 20 April 2021. The searches resulted in 1112 citations and an additional three papers were obtained from ongoing trials. None were identified from reviewing reference lists of included studies.
From the 1112 citations, 1055 were irrelevant. The remaining 57 studies underwent full‐text screening, of which 18 were included. Amongst the 33 excluded studies, 20 of these had incorrect study design, 10 wrong participant population, two wrong intervention, one wrong comparator and three studies are still ongoing (Figure 1).
1.
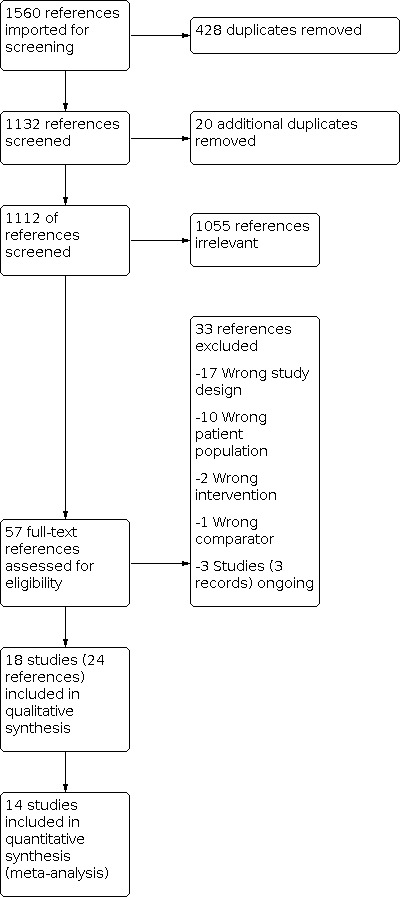
Study flow diagram
Of the 18 included studies, one of these was later excluded as no data could be obtained during the 10‐year period during which the study was conducted.
Included studies
Design
Two pilot RCTs were included (Green 2021; Karkouti 2021) with nine months duration.
Fourteen non‐randomised (NRS) retrospective cohort analyses were included (Arachchillage 2016; Arnekian 2012; Audley 2019; Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Fraser 2006; Harper 2018; Harris 2020a; Hashmi 2019; Mehringer 2018; Rybka 2015; Tanaka 2013; Zweng 2019). One prospective study (Giorni 2013) and one case report of an adverse event (Koster 2014) were included. The study duration ranged from seven months to nine years with two years median study duration.
Sample
The pilot RCTs had a total of 151 participants consisting of 50 Green 2021 and 101 Karkouti 2021. The NRS had a total of 4842 participants with varying sample sizes, the largest pre‐matching being Fitzgerald 2018 (1355 patients) and the smallest being Giorni 2013 (25 patients). Cappabianca 2016 analysed the largest series of post‐propensity matched patients with 450. There were no single‐sex studies and age distribution was an adequate representation of those undergoing cardiac surgery. There were 1986 post‐propensity matched studies that included 1312 (66%) men and 674 (34%) women.
Location
Most studies had single‐centre design except one that was conducted in multiple countries. Six studies were conducted in the USA (Audley 2019, Harper 2018, Harris 2020a, Hashmi 2019, Mehringer 2018, Tanaka 2013), and two were conducted in Italy (Cappabianca 2016, Giorni 2013), Australia (Fraser 2006, Zweng 2019), Canada (Fitzgerald 2018, Karkouti 2021) and the United Kingdom (Arachchillage 2016, Green 2021). One study each was included: from France (Arnekian 2012), Germany (Koster 2014), and Russia (Rybka 2015). One multicentre study (Biancari 2019) included patients across nine centres in Finland, France, Italy, Germany, Sweden and United Kingdom.
Participants
Age and gender
Three studies reviewed paediatric patients (Giorni 2013, Harris 2020a, Rybka 2015), with Giorni 2013 having mean age of 13 days and 17 days in the intervention and comparison group, respectively, and Harris 2020a a mean age of 164 days and 139 days in the intervention and comparison group, respectively. The remaining 15 studies (Arachchillage 2016; Arnekian 2012; Audley 2019; Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Green 2021; Fraser 2006; Harper 2018; Hashmi 2019; Karkouti 2021; Koster 2014; Mehringer 2018; Tanaka 2013; Zweng 2019) reviewed adult patients, with a mean age in the intervention group of 63 years and in the comparison group 64 years. The gener proportion of the population was 69% male and 31% female.
Comparison
Eleven studies compared PCC to standard therapy. This included PCC compared to FFP alone, PCC and FFP compared to FFP alone, and PCC compared to an institutional transfusion therapy protocol.
Two RCTs compared PCCs to FFP. Green 2021 randomised only one dose of PCCs, whereas Karkouti 2021 had up to two doses of PCCs; both studies then instituted standard practice with FFP following the intervention if further factor replacement was required.
Of the NRSs, two studies compared PCCs to FFP alone (Arachchillage 2016 and Arnekian 2012), five studies compared PCCs and FFP to FFP alone (Arnekian 2012, Biancari 2019, Cappabianca 2016, Fitzgerald 2018 and Zweng 2019) and two studies compared PCCs to standard blood product transfusion therapy (Giorni 2013; Harris 2020a).
We identified five studies that compared PCCs to rFVIIa (Audley 2019, Harper 2018, Mehringer 2018, Rybka 2015, Tanaka 2013).
The remaining two studies reviewed PCCs alone without a comparator (Fraser 2006; Hashmi 2019).
Intervention
All of our included studies used either 3‐factor or 4‐factor PCCs. Nine studies used 4‐factor PCCs (Arnekian 2012; Audley 2019; Fitzgerald 2018; Giorni 2013; Green 2021; Karkouti 2021; Koster 2014; Mehringer 2018; Rybka 2015) and seven studies used 3‐factor PCCs (Cappabianca 2016; Fraser 2006; Harper 2018; Harris 2020a; Hashmi 2019; Tanaka 2013; Zweng 2019).
One study evaluated both 3‐ and 4‐factor PCCs (Biancari 2019). One study did not provide the type of PCCs they reviewed (Arachchillage 2016).
Excluded studies
See Characteristics of excluded studies.
We excluded 33 studies. Of these, 30 were excluded: 17 had incorrect study design, 10 wrong participant population, two wrong intervention and one wrong comparator. Three were ongoing studies.
During our analysis, we had to exclude a further study (Ranucci 2017) as no data were obtainable due to a 10‐year study design. The study included a bundle of care which consisted of multiple interventions introduced over a 10‐year period. Data were not provided on the effect of the individual interventions.
Ongoing Studies
The three ongoing studies are NCT02557672; NCT04434001; and NCT04244981.
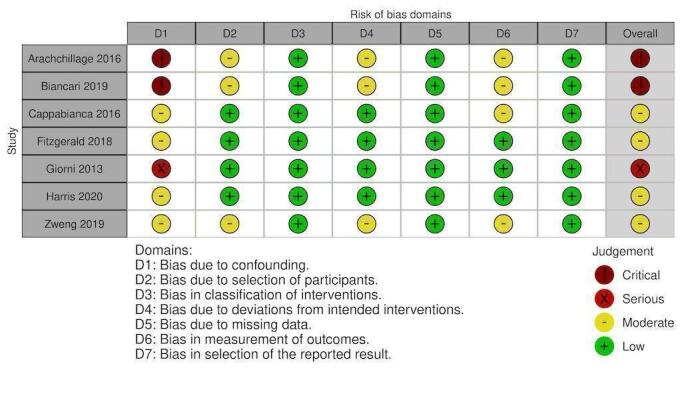
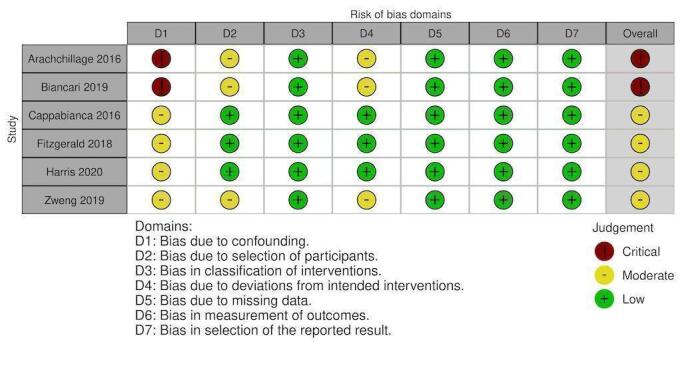
Risk of bias in included studies
Randomised controlled studies:
The two RCTs had an overall low risk of bias; please see figures (Figure 2; Figure 3).
2.
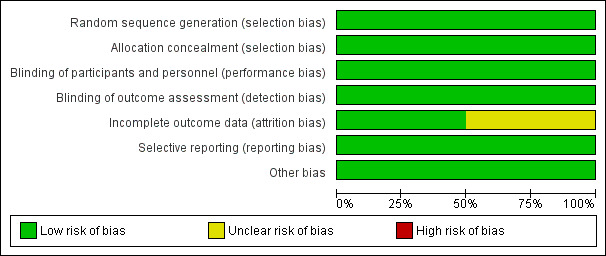
Risk of bias graph for RCT studies: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
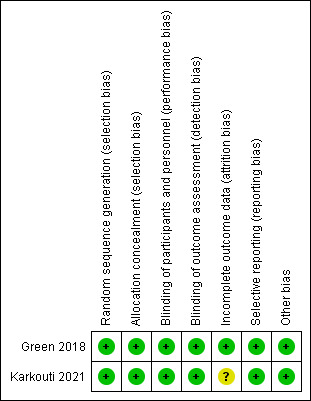
Risk of bias summary for RCT studies: review authors' judgements about each risk of bias item for each included study
Allocation
Both Green 2021 and Karkouti 2021 had low risk of bias due to appropriate random allocation and concealment of allocation of their participants into each treatment group.
Blinding
The intervention could not be blinded due to the different physical properties of the two products; both RCTs had a low risk of performance bias as outcomes were objective and judged to be at low risk of being influenced even with the knowledge of treatment allocation. In addition, Karkouti 2021 minimised this by the first set of products being released in weight‐matched, tamper‐sealed containers that were opened immediately before initiating treatment.
For detection bias, both included RCTs were judged to be at low risk of bias. In Green 2021, although clinicians collecting the data were not blinded to the interventions, these objective outcomes could not be manipulated as a result of having this knowledge. In Karkouti 2021, the clinicians were not involved in product administration, so they remained blinded to group assignment.
Incomplete outcome data
Green 2021 was judged at low risk of bias due to complete follow‐up and intention‐to‐treat analysis. Karkouti 2021 was judged as being at unclear risk of bias with up to 18% in the PCC group and 36% of in the FFP group not receiving their intervention despite being randomised. These patients were not included in the analysis. It did not appear to alter the baseline characteristics between the two groups, however, bias could have been introduced due to the low risk of bleeding patients being excluded as they stopped bleeding by the time the product arrived in the operating room.
Selective reporting
Both were at low risk of bias, with all outcomes from their protocol being measured.
Other potential sources of bias
No other bias was detected in either study.
Non‐randomised studies:
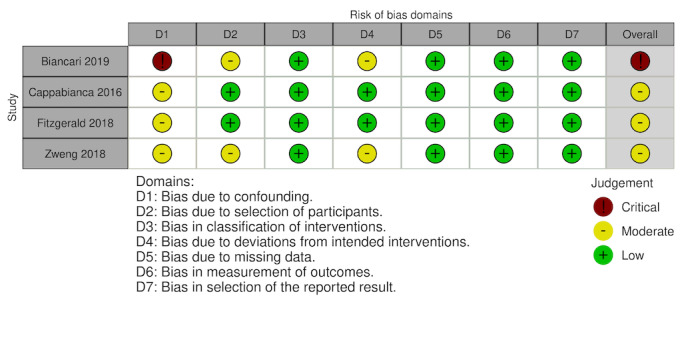
Overall, 10 studies had overall critical risk of bias (Arachchillage 2016; Arnekian 2012; Audley 2019; Biancari 2019; Fraser 2006; Hashmi 2019; Koster 2014; Mehringer 2018; Rybka 2015; Tanaka 2013). Three studies had an overall serious risk of bias (Cappabianca 2016; Giorni 2013; Harper 2018). Three had a overall moderate risk of bias (Fitzgerald 2018; Harris 2020a; Zweng 2019). Risk of bias was judged separately for studies contributing to each outcome and these judgements are documented in the following figures (Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12; Figure 13; Figure 14). Bias due to missing data and selection of reported results was judged low risk for the majority of studies as there were very few patients lost to follow‐up and most of the studies reported outcomes using appropriate methods. Bias due to confounding was the domain most likely to result in bias due to a lack of adequate control groups or appropriate analysis. For more information, please refer to the final paragraph in the Effects of interventions section for each outcome.
4.
NRS: Blood products transfused (RBC) % of patients
5.
NRS: PCCs thrombotic events
6.
NRS: PCCs mortality
7.
NRS: PCCs ICU length of stay
8.
NRS: PCCs renal replacement therapy
9.
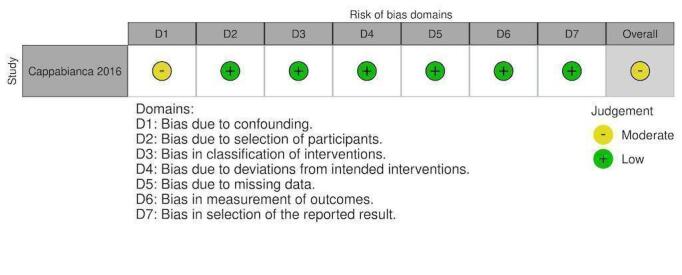
NRS: PCCS ventilator duration
10.
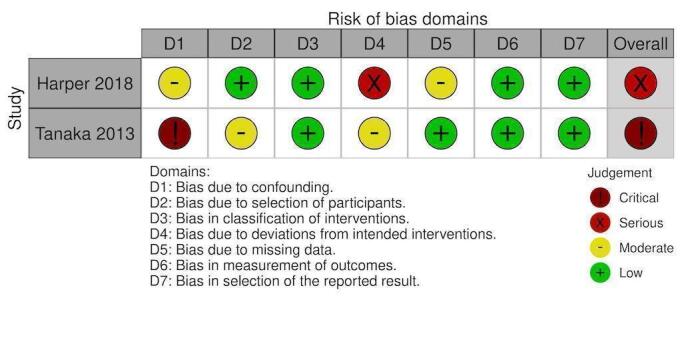
NRS: Factor VIIa blood products transfused (RBC) % patients
11.
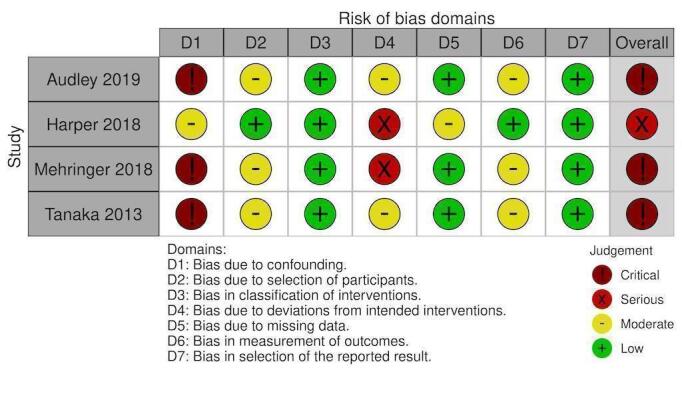
NRS: Factor VIIa thrombotic events
12.
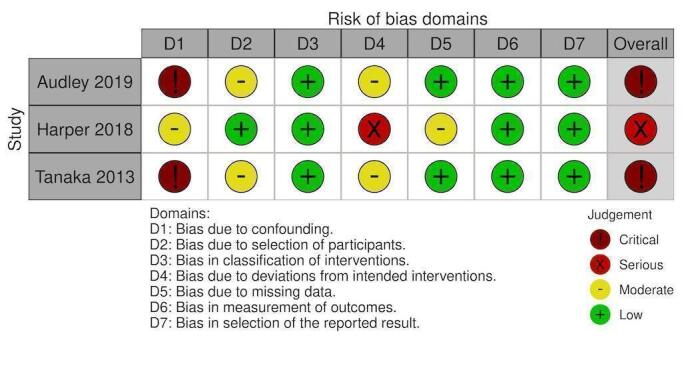
NRS: Factor VIIa mortality
13.
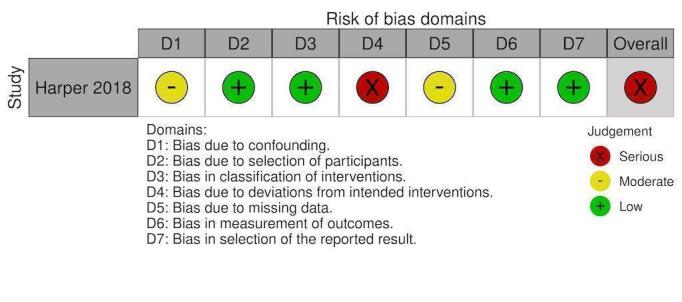
NRS: Factor VIIa ICU length of stay
14.
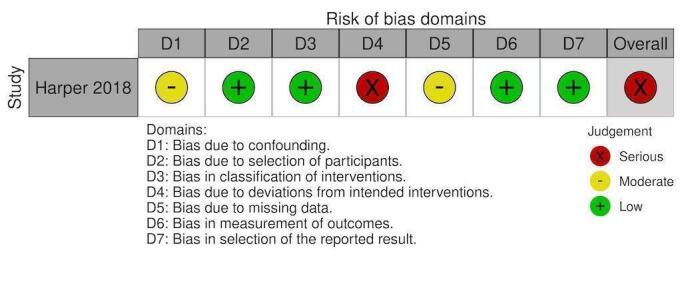
NRS: Factor VIIa renal replacement therapy
Effects of interventions
For detailed analysis, see the summary of findings table for PCC compared to standard treatment (Table 1) and for PCC compared to rFVIIa (Table 2).
1. PCC versus standard treatment
Blood products transfused
Two RCTs reported on red cell transfusion (Green 2021; Karkouti 2021). PCCs were likely to reduce the number of units transfused compared to standard care (MD ‐0.89, 95% CI ‐1.78 to 0.00; participants = 151; studies = 2; moderate‐quality evidence, I2 = 0%; Analysis 1.1).
1.1. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 1: RCT: Blood products transfused (RBC) in units
Two NRS studies reported on red cell transfusion (Biancari 2019; Cappabianca 2016). PCCs may reduce the mean number of units transfused compared to standard care but the evidence is uncertain (MD ‐1.87, 95% CI ‐2.53 to ‐1.20; participants = 551; studies = 2; very low‐quality evidence; I2 = 0%; Analysis 1.2). Sensitivity and subgroup analysis were unable to be performed as a result of the low number of studies.
1.2. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 2: NRS: Blood products transfused (RBC) in units
The risk of bias of these two studies were critical and moderate due to the inclusion of patients not undergoing cardiac surgery on cardiopulmonary bypass and multiple analysis methods (Figure 4, Table 3).
1. ROBINS‐I PCC versus standard treatment; assessments for blood products (RBC) transfused.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Biancari 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Critical risk |
| The intention of study was to review PCC vs FFP in patients undergoing cardiac surgery for CPB but they have included off‐CPB patients (FFP group 27% and PCC group 24%). ‐ Propensity‐matching occurred (101 patients each group). |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC +/‐ FFP or FFP alone. | ‐ Standardised heparin, protamine and TXA protocol ‐ Clinically appropriate dose of PCC and FFP ‐ No agreed coagulation algorithm amongst the hospitals ‐ There was no decision‐making process around when to give intervention or comparison. |
Collected data from e‐CABG registry |
Retrospective; assessors aware of intervention group |
No multiple subgroup analysis. Reported on what their methods stated | Critical bias in domain 1 | |
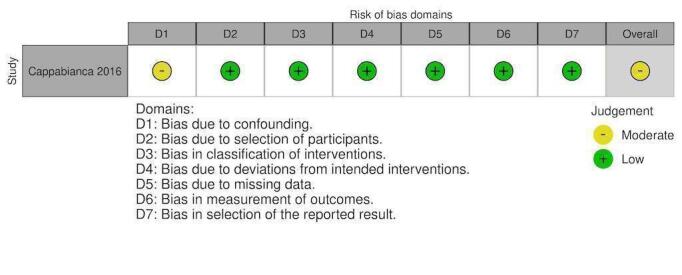
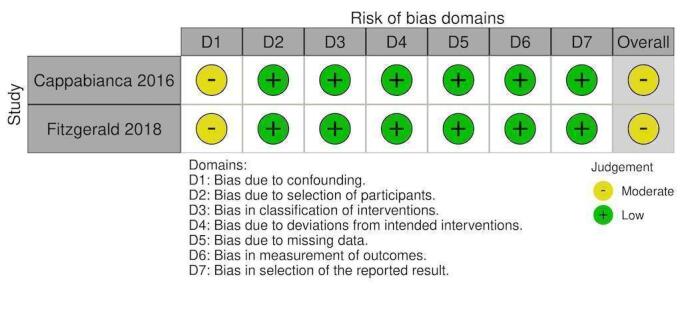
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Attempted to control with propensity‐matching ‐ Important domains were measured and accounted for ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not a deviation from standard practice. ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Use of extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | Retrospective; assessors aware of intervention group |
Two types of analyses were conducted with differing results. For RBC transfused they used: propensity score‐matched pairs and propensity score‐adjusted. |
‐ Unclear if 57 patients who received both interventions were included in either group |
|
| Fitzgerald 2018 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ There were matching patients in each intervention group ‐ 69 patients in PCC group could not be matched due to being a higher‐risk population (emergency surgery, longer CPB time and complex surgery). ‐ Two variables were not able to be matched with a SMD < 10% (CPB time and diabetes). |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs were all given intraoperatively and not in ICU |
Defined two groups from the beginning and stated that PCC group were able to receive FFP | ‐ Standardised care mentioned ‐ Both groups received rFVIIa with no statistical significance between the groups. ‐ POC testing and lab tests to guide transfusion requirements ‐ PCCs were given according to a protocol. |
‐ 18 patients excluded due to missing data (8%) ‐ Data were obtained from institutional databases. |
‐ Retrospective; assessors aware of intervention group |
Reported on what their methods stated | ‐ Unfortunate to not have 69 patients of higher complexity included in the study. We believe these patients would have provided objective evidence for the higher‐risk population group. |
|
| Zweng 2019 | Moderate risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Propensity‐matched ‐ No cross‐over occurred. ‐ 18 patients unmatched (25%) from the PCC group because of more complex surgical procedure ‐ Clear selection bias in choosing which patients received PCCs making it impossible to ascertain whether the complications observed were truly from the PCCs or the surgical complexity |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. |
Intervention groups were clearly defined. The groups received either PCC or clotting factor‐based therapy. | Unsure when intervention was given No mention of POC or lab tests No protocol The decision to give PCC was left up to the surgeon, anaesthetist, or intensivist Median dose of PCC 1500 units |
No reports of missing data. Collection of data ‐ from Austin hospital blood bank and cross‐referenced with the Australian Society of Cardiothoracic Surgeons database |
Assessors were aware of the intervention group. | ‐ Propensity‐matched pairs were evaluated by a multivariate logistic regression model adjusted for age, sex, total units of RBC, cryoprecipitate, FFP and platelets combined. |
‐ Unclear as to timing of interventions |
AKI: Acute Kidney Injury CPB: Cardiopulmonary Bypass e‐CABG: electronic Coronary Artery Bypass Graft FFP: Fresh Frozen Plasma ICU: Intensive Care Unit OR: Operating Room PCC: Prothrombin Complex Concentrate POC: Point Of Care SMD: Standard Mean Difference TXA: Tranexamic Acid
One RCT reported on incidence of red cell transfusion (Karkouti 2021). There was no evidence from this study showing a difference in the incidence of RBC transfusion compared to standard care (OR 0.53, 95% CI 0.2 to 1.4; participants = 101; studies = 1; low‐quality evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 3: RCT: Blood products transfused (RBC) % of patients
Four studies (Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Zweng 2019) also reported the incidence of red cell transfusion. The evidence suggests PCC reduces the incidence of red cell transfusion compared to standard care but the evidence is uncertain (OR 0.54, 95% CI 0.30 to 0.98; participants = 1046; studies = 4; low‐quality evidence; I2 = 63%; Analysis 1.4).
1.4. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 4: NRS: Blood products transfused (RBC) % of patients
Sensitivity analysis was conducted on the incidence of red cell transfusion, by removing studies judged to be of serious and critical risk of bias. Fitzgerald 2018 and Zweng 2019 showed no difference between PCC and standard treatment (OR 0.71; 95% CI 0.41 to 1.22, participants = 394; studies = 2).
Removing studies that contained unadjusted data did not alter the outcome.
The risk of bias for these four studies was critical for one and moderate for three; this is due to the inclusion of non‐cardiopulmonary bypass patients, a high proportion of the PCC groups being higher risk, and not able to be matched and therefore excluded in the matched data (Figure 4, Table 3).
Thrombotic events
Two RCTs reported on thrombotic events (Green 2021; Karkouti 2021). There is no evidence from RCTs showing a difference in the number of thrombotic events with PCC compared to standard care (OR 0.68, 95% CI 0.2 to 2.31; participants = 151; studies = 2; moderate‐quality evidence; I2 = 0%; Analysis 1.5).
1.5. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 5: RCT: Thrombotic events
Seven NRSs (Arachchillage 2016; Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Giorni 2013, Harris 2020a; Zweng 2019) reported on thrombotic events with a total of 1359 participants. One study (Giorni 2013) did not report any events in either PCC or standard care group and so has not contributed to the meta‐analysis. PCC may have no effect on the number of thrombotic events compared to standard care but the evidence is very uncertain (OR 1.32, 95% CI 0.87 to 1.99; participants = 1359; studies = 7; very low‐quality evidence; I2 = 0%; Analysis 1.6) .
1.6. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 6: NRS: Thrombotic events
Six NRSs presented propensity matched data (Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Giorni 2013; Harris 2020a; Zweng 2019) with an OR 1.33 (95% CI 0.87 to 2.01; participants = 1189; studies = 6; I2 = 0%; low‐quality evidence).
A sensitivity analysis reviewing thrombotic events in only trials considered to be of moderate risk of bias (Fitzgerald 2018; Harris 2020a; Zweng 2019) provided an OR of 1.41 (95% CI 0.76 to 2.63, participants = 512; studies = 3).
Subgroup analysis looking at 3‐factor versus 4‐factor PPC was conducted. Four trials specified whether they were using 3‐ or 4‐factor PCC (Cappabianca 2016; Fitzgerald 2018; Harris 2020a; Zweng 2019). We found that the different type of PCC may have no effect on thrombotic outcomes with the test for subgroup differences showing no difference between these groups (test for subgroup differences: Chi² = 0.74, df = 1 (P = 0.39), I² = 0%); Analysis 1.8).
1.8. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 8: NRS: Thrombotic events (3‐factor vs 4‐factor)
The risk of bias for the seven NRSs were critical for two, serious for one and moderate for four studies; this is due to unmatched data, inclusion of non‐cardiopulmonary bypass participants, multiple analysis, inadequate matching, small sample sizes, and exclusion of high‐risk patients due to no appropriate match (Figure 5, Table 4).
2. ROBINS‐I PCC versus standard treatment; assessments for thrombotic events.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Arachchillage 2016 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ No matching occurred. ‐ The PCC group had higher‐complexity surgery, duration and individual participant risk. |
Unknown whether PCCs given in OR or ICU, follow‐up may have started before intervention | Intervention groups were clearly defined. The groups received either PCC or FFP. | ‐ No mention of dose given ‐ No mention of co‐interventions ‐ No mention of usual clinical practice |
Assumed this was low because the authors have not mentioned any missing data. No mention of how authors collected their data. | ‐ Retrospective; assessors aware of intervention group ‐ Objective outcomes ‐ Did not mention how they detected thrombosis and thrombotic events (i.e. clinically or radiological assessment) |
No multiple subgroup analysis Reported on what their methods stated | PCC patients were in a higher‐risk group No mention of doses, clinical practice and standard care |
|
| Biancari 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ The intention of study was to review PCC vs FFP in patients undergoing cardiac surgery on CPB but they have included off‐CPB patients (FFP group 27% and PCC group 24%) ‐ Propensity matching occurred (101 patients each group) |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC +/‐ FFP or FFP alone. | ‐ Standardised heparin, protamine and TXA protocol ‐ Clinically appropriate dose of PCC and FFP ‐ No agreed coagulation algorithm amongst the hospitals ‐ There was no decision‐making process around when to give intervention or comparison. |
‐ Three patients had missing data on the location of surgical bleeding ‐ Collected data from e‐CABG registry |
‐ Retrospective, assessors aware of intervention group ‐ Objective outcomes ‐ Discussed incidence of stroke but no mention how this was diagnosed (i.e. clinical or radiological) |
No multiple subgroup analysis Reported on what their methods stated | ‐ Critical bias in domain 1 | |
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Moderate risk |
| ‐ Attempted to control with propensity matching ‐ Important domains were measured and accounted for ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not a deviation from standard practice. ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | ‐ Retrospective; assessors aware of intervention group ‐ Objective outcomes ‐ Discussed incidence of stroke but no mention how this was diagnosed (i.e. clinical or radiological) |
Two types of analyses were conducted: propensity‐score adjusted and propensity score‐matched |
‐ Unclear if 57 patients who received both interventions were included in either group |
|
| Fitzgerald 2018 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ There was matching of patients in each intervention group ‐ 69 patients in PCC group could not be matched due to being a higher‐risk population (emergency surgery, longer CPB time and complex surgery) ‐ Two variables were not able to be matched with a SMD < 10% (CPB time and diabetes) |
Retrospective collection with intervention and follow‐up coinciding for all participants. PCCs all given intraoperatively |
Defined two groups from the beginning and stated that PCC group participants were able to receive FFP | ‐ Standardised care mentioned ‐ Both groups received rFVIIa with no statistical significance between the groups. ‐ POC testing and lab tests to guide transfusion requirements ‐ PCCs were given according to a protocol. |
‐ 18 patients excluded due to missing data (8%) ‐ Data were obtained from institutional databases. |
‐ Retrospective; assessors aware of intervention group ‐ Objective outcomes Defined how they diagnosed stroke (clinically) and DVT/PE (radiological confirmation) |
Reported on all outcome measures | ‐ Unfortunate to not have 69 patients of higher complexity included in the study. We believe these patients would have provided objective evidence for the higher risk population group. | |
| Giorni 2013 | Serious risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| ‐ Not propensity‐matched ‐ The control group was a retrospectively‐matched cohort for baseline variables: "the treated and non‐treated patients were adequately matched for all the baseline variables" |
Retrospective collection in the non‐PCC group and follow‐up coincided for all participants. PCCs all given in the OR. FFP usage similar between the two groups |
Defined two groups from the beginning and stated that PCC group participants were able to receive FFP | Protocol for use of Confidex mentioned in methods Protocol stated how/when they received the standard treatment (FFP and cryo) vs PCCs. Used a clinically appropriate dose of PCC 25 u/kg |
‐ No mention of missing data ‐ Prospective enrolment of the PCC group and data on excel spreadsheet. ‐ Unclear how they retrospectively obtained data for the control group |
Daily echo monitoring performed on all enrolled patients to exclude intracardiac thrombi. They did not do this for the retrospective control group. Not comparable assessment. Unclear if this resulted in significant differences because the PCC group had no incidence of intracardiac thrombi |
Reported on all outcome measures | ‐ Due to serious bias in confounding ‐ Small sample size (14 patients in PCC group vs. 11 patients in control group) |
|
| Harris 2020a | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Matching occurred with a control based on demographic and surgical characteristics; 6/65 patients could not be matched |
Retrospective collection with intervention and follow‐up coincided for all participants. All PCCs and their 1:1:1 blood products given in OR |
Defined intervention group and comparison group | ‐ Unclear dose of protamine/antifibrinolytics ‐ If bleeding they used a 1:1:1: transfusion as per protocol ‐ Intervention used if ongoing bleeding despite transfusion; this was based on clinician judgement ‐ Control group got standard therapy, intervention group got PCCs if they continued to bleed (potentially a higher‐risk population) ‐ Unclear if they used POC testing or lab bloods |
Collection of data ‐ retrospective data base analysis | ‐ Retrospective, assessors aware of intervention group ‐ Objective outcomes Thromboembolic events were analysed for clinical signs of acute thrombosis in the medical charts and confirmed by radiographic evidence ‐ Database analysis |
Reported on all outcome measures | |
|
| Zweng 2019 | Moderate risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk |
| ‐ Propensity matched ‐ No cross‐over occurred. ‐ 18 patients unmatched (25%) from the PCC group because of more complex surgical procedure ‐ Clear selection bias in choosing which patients received PCCs making it impossible to ascertain whether the complications observed were truly from the PCCs or the surgical complexity |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. |
Intervention groups were clearly defined. The groups received either PCC or clotting factor based therapy. | Unsure when intervention was given No mention of POC or lab tests No protocol The decision to give PCC was left up to the surgeon, anaesthetist, or intensivist Normal standard dose of PCC was 1500 units |
No reports of missing data. Collection of data ‐ from Austin hospital blood bank and cross‐referenced with the Australian Society of Cardiothoracic Surgeons database |
‐ No mention of how they diagnosed thrombosis in patients ‐ Clinical or radiological assessment |
Reported on absolute numbers and on matched pairs |
|
AKI: Acute Kidney Injury CPB: Cardiopulmonary Bypass DVT: Deep Vein Thrombosis e‐CABG: electronic Coronary Artery Bypass Graft FFP: Fresh Frozen Plasma ICU: Intensive Care Unit OR: Operating Room PCC: Prothrombin Complex Concentrate PE: Pulmonary Embolism POC: Point Of Care rFVIIa: Recombinant Factor VIIa SMD: Standardised Mean Difference TXA: Tranexamic Acid vs: Versus
Mortality
Two RCTs reported on mortality (Green 2021; Karkouti 2021). There is no evidence from RCTs showing a difference in mortality with PCC compared to standard care (OR 0.53, 95% CI 0.12 to 2.35; participants = 151; studies = 2; moderate‐quality evidence; I2 = 0%; Analysis 1.9).
1.9. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 9: RCT: Mortality (30‐day)
Six NRS (Arachchillage 2016; Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Harris 2020a; Zweng 2019) reported on mortality data in a total of 1334 patients. PCC may have little to no effect on mortality compared to standard care but the evidence is very uncertain (OR 1.02, 95% CI 0.69 to 1.51; participants = 1334; studies = 6; very low‐quality evidence; I2 = 0%; Analysis 1.10). Five studies presented propensity‐matched data (Biancari 2019; Cappabianca 2016; Fitzgerald 2018; Harris 2020a; Zweng 2019) with an OR 1.02 (95% CI 0.68 to 1.53; participants = 1164; studies = 5; I2 = 0%; very low‐quality evidence).
1.10. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 10: NRS: Mortality (30‐day)
A sensitivity analysis that included Fitzgerald 2018; Harris 2020a; Zweng 2019, provided an OR of 0.96 (95% CI 0.55 to 1.69, participants = 512; studies = 3). Sensitivity analysis looking at the inclusion of trials with matched and unmatched data showed no difference between both the matched and unmatched data finding that PCCs result in little to no difference in mortality.
The risk of bias for the six NRSs were critical for two, serious for one and moderate for three studies; this is due to unmatched data, inclusion of non‐cardiopulmonary bypass patients, multiple analysis, not all patients able to be matched and exclusion of high risk patients due to no appropriate match (Figure 6, Table 5).
3. ROBINS‐I PCC versus standard treatment; assessments for mortality.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Arachchillage 2016 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Critical risk |
| ‐ No matching occurred ‐ The PCC group had higher‐complexity surgery, duration and individual participant risk. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention | Intervention groups are clearly defined. The groups received either PCC or FFP. | ‐ No mention of dose given ‐ No mention of co‐interventions ‐ No mention of usual clinical practice |
Assumed this was low because the authors have not mentioned any missing data. No mention on how authors collected their data | Retrospective; assessors aware of intervention group. |
No multiple subgroup analysis. Reported on what their methods stated. | No matching of groups PCC patients were of a higher‐risk group. |
|
| Biancari 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Critical risk |
| ‐ The intention of study was to review PCC vs FFP in patients undergoing cardiac surgery on CPB but they have included off‐CPB patients (FFP group 27% and PCC group 24%) ‐ Propensity matching occurred (101 patients each group) |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention | Intervention groups were clearly defined. The groups received either PCC +/‐ FFP or FFP alone. | ‐ Standardised heparin, protamine and TXA protocol ‐ Clinically appropriate dose of PCC and FFP ‐ No agreed coagulation algorithm amongst the hospitals ‐ There was no decision‐making process around when to give intervention or comparison. |
‐ Three patients had missing data on location of surgical bleeding ‐ Collected data from e‐CABG registry |
Retrospective; assessors aware of intervention group. |
No multiple subgroup analysis. Reported on what their methods stated. | ||
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Attempted to control with propensity matching ‐ Important domains were measured and accounted for ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coinciding for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not a deviation from standard practice. ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Use of extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | Retrospective; assessors aware of intervention group. |
Two types of analyses were conducted. ‐ Propensity score‐adjusted and propensity score‐matched |
|
|
| Fitzgerald 2018 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ There was matching of patients in each intervention group. ‐ 69 patients in PCC group could not be matched due to being a higher‐risk population (emergency surgery, longer CPB time and complex surgery) ‐ Two variables were not able to be matched with a SMD < 10% (CPB time and diabetes) |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs all given intraoperatively |
Defined two groups from the beginning and stated that PCC group were able to receive FFP | ‐ Standardised care mentioned ‐ Both groups received rFVIIa with no statistical significance between the groups. ‐ POC testing and lab tests to guide transfusion requirements ‐ PCCs were given according to a protocol. |
‐ 18 patients excluded due to missing data (8%) ‐ Data was obtained from institutional databases. |
‐ Retrospective; assessors aware of intervention group. |
Reported on all outcome measures | 69 patients of higher complexity were not included in the study. We believe these patients would have provided objective evidence for the higher‐risk population group. | |
| Harris 2020a | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Matching occurred with a control based on demographic and surgical characteristics. ‐ 6/65 patients could not be matched. |
Retrospective collection with intervention and follow‐up coincided for all participants. All PCCs and their 1:1:1 blood products given in OR |
Defined intervention group and comparison group | ‐ Unclear dose of protamine/antifibrinolytics ‐ If bleeding, they used a 1: 1:1: transfusion as per protocol. ‐ Intervention used if ongoing bleeding despite transfusion; this was based on clinician judgement ‐ Control group got standard therapy; intervention group got PCCs if they continued to bleed (potentially a higher‐risk population) ‐ Unclear if they used POC testing or lab bloods |
Collection of data ‐ retrospective data base analysis | Retrospective; assessors aware of intervention group. |
Reported on all outcome measures | |
|
| Zweng 2019 | Moderate risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Propensity matched ‐ No cross‐over occurred. ‐ 18 patients unmatched (25%) from the PCC group because of more complex surgical procedure ‐ Clear selection bias in choosing which patients received PCCs making it impossible to ascertain whether the complications observed were truly from the PCCs or the surgical complexity. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. |
Intervention groups were clearly defined. The groups received either PCC or clotting factor‐based therapy. | Unsure when intervention ws given. No mention of POC or lab tests No protocol The decision to give PCC was left up to the surgeon, anaesthetist, or intensivist. Normal standard dose of PCC was 1500 units |
No reports of missing data. Collection of data ‐ from Austin hospital blood bank and cross‐referenced with the Australian Society of Cardiothoracic Surgeons database |
Reported on absolute numbers and matched pairs |
AKI: Acute Kidney Injury CPB: Cardiopulmonary Bypass e‐CABG: electronic Coronary Artery Bypass Graft FFP: Fresh Frozen Plasma ICU: Intensive Care Unit OR: Operating Room PCC: Prothrombin Complex Concentrate POC: Point Of Care rFVIIa: Recombinant Factor VIIa SMD: Standardised Mean Difference TXA: Tranexamic Acid
Bleeding
Two RCTs reported on chest drain output; Green 2021 recorded 24‐hour chest drainage while Karkouti 2021 recorded both 12 and 24 hours of chest drainage. PCCs may result in little to no difference in chest drain output (MD = ‐107; 95% CI ‐78 to 65; participants = 151; studies = 2; low‐quality evidence; I2 = 66%; Analysis 1.12).
1.12. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 12: RCT: Bleeding (chest drain output) in mLs for the first 12 hours
No NRS studies reported chest drain output in the first 12 hours.
Intensive care unit length of stay
Two RCTs reported on ICU length of stay (Green 2021; Karkouti 2021). PCC may have little to no effect on this outcome compared to standard care (MD = ‐0.35; 95% CI ‐19.26 to 18.57; participants = 151; studies = 2; moderate‐quality evidence; I2 = 0%; Analysis 1.13).
1.13. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 13: RCT: Intensive care length of stay in hours
One NRS (Cappabianca 2016) reported on this outcome with a total of 450 patients. PCCs may have little to no effect on intensive care length of stay comparative to standard care but the evidence is very uncertain (MD = ‐18.00; 95% CI ‐43.14 to 7.14; participants = 450; studies = 1; very low‐quality evidence; Analysis 1.13).
The risk of bias for the one NRS was moderate; this is due to multiple analysis (Figure 7, Table 6).
4. ROBINS‐I PCC versus standard treatment; assessments for ICU length of stay.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Attempted to control with propensity matching ‐ Important domains were measured and accounted for ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Use of extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | Retrospective; assessors aware of intervention group. |
Two types of analysis: Propensity score‐adjusted and propensity score‐matched |
|
AKI: Acute Kidney Injury FFP: Fresh Frozen Plasma PCC: Prothrombin Complex Concentrate
Incidence of renal replacement therapy
One RCT reported on renal replacement therapy (Green 2021) which showed that PCCs may have little to no effect on this outcome (OR 0.72, 95% CI 0.14 to 3.59; participants = 50; studies = 1; low‐quality evidence; Analysis 1.15)
1.15. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 15: RCT: Incidence of renal replacement therapy
Two NRSs (Cappabianca 2016; Fitzgerald 2018) reported on this outcome with a total of 684 patients. PCC may have little to no effect on the incidence of renal replacement therapy comparative to standard care but the evidence is very uncertain (OR 1.46, 95% CI 0.71 to 2.98; participants = 684; studies = 2; very low‐quality evidence; I2 = 0%; Analysis 1.16).
1.16. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 16: NRS: Incidence of renal replacement therapy
The risk of bias for the two NRSs was moderate; this is due to multiple analysis and not all patients were able to be matched leading to exclusion of high‐risk patients (Figure 8, Table 7).
5. ROBINS‐I PCC versus standard treatment; assessments for renal replacement therapy.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Attempted to control with propensity matching ‐ Important domains were measured and accounted for ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not a deviation from standard practice. ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Use of extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | Retrospective; assessors aware of intervention group. |
Two types of analysis: propensity score‐adjusted and propensity score‐matched | ||
| Fitzgerald 2018 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ There was matching for patients in each intervention group. ‐ 69 patients in PCC group could not be matched due to being a higher‐risk population (emergency surgery, longer CPB time and complex surgery). ‐ Two variables were not able to be matched with a SMD <10% (CPB time and diabetes). |
Retrospective collection with intervention and follow‐up coincided for all participants. PCCs all given intraoperatively |
Defined two groups from the beginning and stated that PCC group were able to receive FFP | ‐ Standardised care mentioned ‐ Both groups received rFVIIa with no statistical significance between the groups. ‐ POC testing and lab tests to guide transfusion requirements ‐ PCCs were given according to a protocol. |
‐ 18 patients excluded due to missing data (8%) ‐ Data were obtained from institutional databases. |
Retrospective; assessors aware of intervention group. |
Reported on all outcome measures | 69 patients of higher complexity were not included in the study. These patients would have provided objective evidence for the higher‐risk population group. |
AKI: Acute Kidney Injury CPB: Cardiopulmonary Bypass PCC: Prothombin Complex Concentrate POC: Point of Care Testing rFVIIa: Recombinant Factor VIIa SMD: Standardisd Mean Difference
Ventilator hours
One RCT reported on this outcome (Karkouti 2021) which showed that PCCs may have little to no effect on this outcome compared to standard treatment (MD ‐0.8; 95% CI ‐4.49 to 2.89; participants = 101; studies = 1; low‐quality evidence; Analysis 1.17).
1.17. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 17: RCT: Ventilator hours
One NRS reported on this outcome (Cappabianca 2016) with a total of 450 patients. PCC may have little to no effect on the number of hours spent on a ventilator comparative to standard care but the evidence is very uncertain (MD ‐5.20; 95% CI ‐23.10 to 12.70; participants = 450; studies = 1; very low‐quality evidence; Analysis 1.18).
1.18. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 18: NRS: Ventilator hours
The risk of bias for the one NRS was moderate; this is due to multiple analysis (Figure 9, Table 8).
6. ROBINS‐I PCC versus standard treatment; assessments for ventilator hours.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Attempted to control with propensity matching ‐ Important domains were measured and accounted for. ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coinciding for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not a deviation from standard practice. ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Use of extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | Retrospective; assessors aware of intervention group. |
Propensity score‐adjusted and propensity score‐matched pairs |
AKI: Acute Kidney Injury FFP: Fresh Frozen Plasma PCC: Prothrombin Complex Concentrate POC: Point of Care
Adverse events
One RCT Green 2021 reported on other adverse events. Adverse events were reported for both PCC and FFP groups; most of these are likely to be related to the surgery and postoperative course, such as chest pain, malaise, pleuritic pain and pulseless electrical activity. Both groups had similar numbers of adverse events.
No NRS reported on additional adverse outcomes. One study reported on fluid overload (Arachchillage 2016); however, the authors did not define how they diagnosed fluid overload and so this is not included as a true adverse event.
2. PCC versus rFVIIa
There were no RCTs evaluating the use of this comparison.
Blood products transfused
We identified two NRS studies that reported on red cell transfusion (Harper 2018; Tanaka 2013). PCC likely results in a large reduction in the number of RBCs transfused intraoperatively in comparison to rFVIIa (MD ‐4.98, 95% CI ‐6.37 to ‐3.59; participants = 256; studies = 2; moderate‐quality evidence; I2 = 0%; Analysis 2.1). Harper 2018 also provided postoperative red cell transfusion. PCC may have little to no effect on the number of blood units transfused postoperatively comparative to rFVIIa but the evidence is very uncertain (MD ‐1.06; 95% CI ‐2.48 to 0.36; participants = 106; studies = 1; very low‐quality evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 1: Blood products transfused (RBC) in units
One study mentioned incidence of red cell transfusions (Tanaka 2013). PCC may have little to no effect on the incidence of RBCs transfused compared to rFVIIa but the evidence is very uncertain (OR 0.16; 95% CI 0.02 to 1.56; participants = 150; studies = 1; very low‐quality evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 2: Blood products transfused (RBC) % of patients
We were unable to conduct a sensitivity analysis on either of these outcomes due to having no studies with moderate or lower risk of bias. We were unable to conduct subgroup analysis on 3‐ versus 4‐factors and adult versus child due to the lack of studies.
The risk of bias for the two NRSs was serious for one and critical for one study; this is due to a disproportionately high dose of rFVIIa and not being adequately matched (Figure 10, Table 9).
7. ROBINS‐I PCC versus rFVIIa; assessments for blood products (RBC) transfused.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Harper 2018 | Moderate risk | Low risk | Low risk | Serious risk | Moderate | Low risk | Low risk | Serious risk |
| ‐ Propensity matching occurred for all baseline characteristics except EGFR and procedure type. ‐ Procedure type appeared to be more complex in the PCC group. ‐ 5 of the remaining 58 were then excluded due to inability to be matched with rFVIIa group. |
Retrospective collection with intervention and follow‐up coincided for all participants. Both PCCs and FVIIa were given in OR. |
Defined two groups from the beginning and stated that they analysed patients receiving PCCs or rFVIIa. No definition of what constituted refractory bleeding and initiation of interventions | ‐ rFVIIa group received 90 mcg/kg and PCC group received 27 IU/kg (median). ‐ The rFVIIa dose exceeded recognised and accepted doses for rFVIIa. ‐ No definition of when patients received intervention ‐ This was a safety study. |
‐ 14/72 patients had incomplete data therefore were excluded. Data were collected via an electronic medical record database that was retrospectively queried. |
Reported as described in their methods | Reported on all outcome measures | High rFVIIa dose exceeding common recommended practice ‐ Uncertainty around increased cell salvage and product administration in the rFVIIa group. This could be result of clinician bias or a higher risk population. |
|
| Tanaka 2013 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Critical risk |
| ‐ Matched on age, sex and CPB duration ‐ Other factors unmatched |
‐ 40% of patients received rFVIIa in ICU. ‐ 100% of patients received PCCs in theatre. Follow‐up may have started before intervention. |
Intervention groups were clearly defined. | ‐ Followed institutional protocol ‐ Cross‐over of patients in PCC group (24% patients in the PCC group also received rVIIa). |
No mention of missing data Collection of data not mentioned |
‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes |
Reported on all outcome measures | ‐ Editorial, not a published paper ‐ Comparable doses ‐ Not propensity matched ‐ Issues with initiation of intervention (theatre vs ICU) |
CPB: Cardiopulmonary Bypass EGFR: Estimated Glomerular Filtration Rate ICU: Intensive Care Unit OR: Operating Room PCC: Prothrombin Complex Concentrate rFVIIa: Recombinant Factor VIIa
Thrombotic events
Four studies (Audley 2019; Harper 2018; Mehringer 2018; Tanaka 2013) reported on the incidence of thrombosis. Tanaka 2013 reported zero events in both study arms, therefore, did not contribute to the meta‐analysis leaving a total of 257 patients. PCC may have little to no effect on the number of thrombotic events comparative to rFVIIa but the evidence is very uncertain (OR 0.51, 95% CI 0.23 to 1.16; participants = 257; studies = 3; very low‐quality evidence; I2 = 0%; Analysis 2.3).
2.3. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 3: Thrombotic events
Sensitivity analysis looking at the inclusion of trials with matched and unmatched data showed no difference, finding that PCC results in little to no difference in thrombotic events. One study presented matched data (Harper 2018), with an OR of 0.50 (95% CI 0.19 to 1.30; participants = 106; studies = 1; very low‐quality evidence).
One study (Tanaka 2013) did not have any thrombotic events in either group. We were unable to perform a sensitivity analysis due to having no studies with moderate or low risk of bias. We were unable to do subgroup analysis on 3‐ and 4‐factors versus rFVIIa and on adults versus children due to a lack of studies.
The risk of bias for the three NRSs were critical for two and serious for one study. This is due to no matching and a disproportionately high dose of rFVIIa (Figure 11, Table 10).
8. ROBINS‐I PCC versus rFVIIa; assessments for thrombotic events.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Audley 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ Only 22 patients ‐ No matching occurred ‐ Patients received either PCC or rFVIIa |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC or rFVIIa. | ‐ Unclear decision‐making process of clinicians treating patients with intervention No mention of POC or Lab tests ‐ No mention of use of tranexamic acid and protamine ‐ Increased FFP usage in the PCC group ‐ Mild dose of rFVIIa (32 mcg/kg) compared with PCC (20 IU/kg) |
No mention of missing data. Chart review from paper and electronic documentation | ‐ Retrospective; assessors aware of intervention group. ‐ Did not mention how they detected thrombosis and thrombotic events (i.e. clinically or radiological assessment) |
Reported on what their methods stated | ‐ No matching ‐ Small sample size |
|
| Harper 2018 | Moderate risk | Low risk | Low risk | Serious risk | Moderate | Low risk | Low risk | Serious risk |
| ‐ Propensity matching occurred for all baseline characteristics except EGFR and procedure type. ‐ Procedure type appeared to be more complex in the PCC group. ‐ 5 of the remaining 58 were then excluded due to inability to be matched with rFVIIa group. |
Retrospective collection with intervention and follow‐up coincided for all participants. Both PCCs and FVIIa were given in OR. |
Defined two groups from the beginning and stated that they analysed patients receiving PCCs or rFVIIa. No definition of what constituted refractory bleeding and initiation of interventions | ‐ rFVIIa group received 90mcg/kg and PCC group received 27 IU/kg (median). ‐ The rFVIIa dose exceeded recognised and accepted doses for rFVIIa. ‐ No definition of when patients received intervention ‐ This was a safety study. |
‐ 14/72 patients had incomplete data therefore were excluded. Data were collected via an electronic medical record database that was retrospectively queried. |
‐ CVA defined as new postoperative stroke being documented by a neurologist in the electronic medical record or on autopsy ‐ DVT defined as postoperative ultrasound diagnosing acute DVT ‐ PE defined as CT or autopsy demonstrating acute PE ‐ MI defined as new native coronary artery or coronary artery bypass graft occlusion demonstrated on postoperative cardiac catheterisation or wall motion abnormalities seen on echocardiogram that resolved with PCI or CABG ‐ All new intracardiac thrombus as noted on postoperative echo or autopsy Electronic medical record retrospectively queried |
Reported on all outcome measures | High rFVIIa dose exceeding common recommended practice Matched for most of baseline variables ‐ Uncertainty around increased cell salvage and product administration in the rFVIIa group. This could be result of clinician bias or a higher‐risk population. |
|
| Mehringer 2018 | Critical risk | Moderate risk | Low risk | Serious risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ No propensity matching occurred. ‐ Two groups had baseline differences in haemoglobin concentration and haematocrit. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. Unclear in the study when given |
Intervention groups were clearly defined. The groups received either PCC or rFVIIa alone. | ‐ No mention of coagulation studies or coagulation algorithm ‐ Decision to give intervention was based on clinical judgement. ‐ High rFVIIa dose ‐ Intervention not comparable due to differences in doses |
No mention of missing data Collection of data ‐ electronic health record system |
‐ Retrospective; assessors aware of intervention group. ‐ No mention of how they diagnosed thrombosis in patients: clinical or radiological assessment Collected data from electronic medical record system |
Reported on all outcome measures |
‐ Not matched ‐ High dose of rFVIIa dose (decided by surgeons) that was higher than most institutions standard practice ‐ 66 mcg/kg |
|
| Tanaka 2013 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ Matched on age, sex and CPB duration ‐ Other factors unmatched |
‐ 40% of patients received rFVIIa in ICU. ‐ 100% of patients received PCCs in theatre. ‐ Follow‐up may have started before intervention. |
Intervention groups were clearly defined. | ‐ Followed institutional protocol ‐ Cross‐over of patients in PCC group (24% patients in the PCC group also received rVIIa). |
No mention of missing data Collection of data not mentioned |
‐ Retrospective; assessors aware of intervention group. No mention of how they diagnosed thrombosis in patients: clinical or radiological assessment |
Reported on all outcome measures | ‐ Comparable doses ‐ Not matched ‐ Issues with initiation of intervention (theatre vs ICU) |
CABG: Coronary Artery Bypass Graft Surgery CPB: Cardiopulmonary Bypass CT: Computed Tomography CVA: Cerebrovascular Accident DVT: Deept Vein Thrombosis EGFR: Estimated Glomerular Filtration Rate FFP: Fresh Frozen Plasma ICU: Intensive Care Unit MI: Myocardial Infarction OR: Operating Room PCC: Prothrombin Complex Concentrate PCI: Percutaneous Coronary Intervention PE: Pulmonary Embolism POC: Point Of Care rFVIIa: Recombinant Factor VIIa
Mortality
We included three studies (Audley 2019; Harper 2018; Tanaka 2013) that reported on mortality.
PCC may have little to no effect on the incidence of mortality comparative to rFVIIa but the evidence is very uncertain (OR 1.07, 95% CI 0.38 to 3.03; participants = 278; studies = 3; very low‐quality evidence; I2 = 40%; Analysis 2.4).
2.4. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 4: Mortality (30‐day)
Sensitivity analysis looking at the inclusion of trials with matched and unmatched data showed no difference between groups finding that PCCs have little to no difference on mortality. One study presented matched data (Harper 2018) with an OR of 0.84 (95% CI 0.26 to 2.69; participants =106; studies = 1; very low‐quality evidence).
We were unable to perform a sensitivity analysis as no studies had moderate or low risk of bias.
The risk of bias for the three NRSs were critical for two and serious for one study. This is due to no matching and a disproportionately high dose of rFVIIa (Figure 12, Table 11).
9. ROBINS‐I PCC versus rFVIIa; assessments for mortality.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Audley 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Critical risk |
| ‐ Only 22 patients ‐ No matching occurred. ‐ Patients received either PCC or rFVIIa. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC or rFVIIa. | ‐ Unclear decision‐making process of clinicians treating patients with intervention No mention of POC or Lab tests ‐ No mention of use of tranexamic acid and protamine ‐ Increased FFP usage in the PCC group ‐ Mild dose of rFVIIa (32 mcg/kg) compared with PCC (20 IU/kg) |
No mention of missing data Chart review from paper and electronic documentation |
Retrospective; assessors aware of intervention group. |
Reported on what their methods stated | ‐ No matching of groups ‐ Small sample size may have contributed to analysing outcomes |
|
| Harper 2018 | Moderate risk | Low risk | Low risk | Serious risk | Moderate | Low risk | Low risk | Serious risk |
| ‐ Propensity matching occurred for all baseline characteristics except EGFR and procedure type. ‐ Procedure type appeared to be more complex in the PCC group. ‐ 5 of the remaining 58 were then excluded due to inability to be matched with rFVIIa group. |
Retrospective collection with intervention and follow‐up coinciding for all participants. Both PCCs and FVIIa were given in OR. |
Defined two groups from the beginning and stated that they analysed patients receiving PCCs or rFVIIa. No definition of what constituted refractory bleeding and initiation of interventions | ‐ rFVIIa group received 90 mcg/kg and PCC group received 27 IU/kg (median). ‐ The rFVIIa dose exceeded recognised and accepted doses for rFVIIa. ‐ No definition of when patients received intervention ‐ This was a safety study. |
‐ 14/72 patients had incomplete data, therefore were excluded. Data were collected via an electronic medical record database that was retrospectively queried. |
Retrospective; assessors aware of intervention group. | Reported on all outcome measures | High rFVIIa dose exceeding common recommended practice Matched for most of baseline variables Uncertainty around increased cell salvage and product administration in the rFVIIa group This could be a higher‐risk population. |
|
| Tanaka 2013 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Critical risk |
| ‐ Matched on age, sex and CPB duration ‐ Other factors unmatched |
‐ 40% of patients received rFVIIa in ICU. ‐ 100% of patients received PCCs in theatre. Follow‐up may have started before intervention. |
Intervention groups were clearly defined. | ‐ Followed institutional protocol ‐ Cross‐over of patients in PCC group (24% patients in the PCC group also received rVIIa). |
No mention of missing data Collection of data not mentioned |
Retrospective; assessors aware of intervention group. |
Reported on all outcome measures | Comparable doses Not matched |
CPB: Cardiopulmonary Bypass EGFR: Estimated Glomerular Filtration Rate FFP: Fresh Frozen Plasma ICU: Intensive Care Unit OR: Operating Room PCC: Prothrombin Complex Concentrate POC: Point Of Care rFVIIa: Recombinant Factor VIIa
Bleeding
One study (Tanaka 2013) reported on 12‐hour chest drain output with a total of 150 patients. PCC may reduce bleeding comparative to rFVIIa but the evidence is very uncertain (MD ‐674.34; 95% CI ‐906.04 to ‐442.64; participants = 150; studies = 1; very low‐quality evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 5: Bleeding (chest drain output) in mLs for the first 12 hours
We were unable to conduct any sensitivity or subgroup analysis due to a lack of studies.
The risk of bias for the one NRS was critical. This was due to inadequate matching.
Intensive care unit length of stay
One study (Harper 2018) reported on this outcome with a total of 106 patients. PCC may have little to no effect on intensive care length of stay comparative to rFVIIa but the evidence is very uncertain (MD ‐40.00; 95% CI ‐110.41 to 30.41; participants = 106; studies = 1; very low‐quality evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 6: Intensive care length of stay in hours
We were unable to conduct any sensitivity or subgroup analysis due to a lack of studies.
The risk of bias for the one NRS was serious. This was due to a disproportionately high dose of rFVIIa (Figure 13, Table 12).
10. ROBINS‐I PCC versus rFVIIa; assessments for ICU length of stay.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Harper 2018 | Moderate risk | Low risk | Low risk | Serious risk | Moderate risk | Low risk | Low risk | Serious risk |
| ‐ Propensity matching occurred for all baseline characteristics except EGFR and procedure type ‐ Procedure type appeared to be more complex in the PCC group ‐ 5 of the remaining 58 were then excluded due to inability to be matched with rFVIIa group. |
Retrospective collection with intervention and follow‐up coincided for all participants. Both PCCs and FVIIa were given in OR. |
Defined two groups from the beginning and stated that they analysed patients receiving PCCs or rFVIIa. No definition of what constituted refractory bleeding and initiation of interventions | ‐ rFVIIa group received 90 mcg/kg and PCC group received 27 IU/kg (median). ‐ The rFVIIa dose exceeded recognised and accepted doses for rFVIIa. ‐ No definition of when patients received intervention ‐ This was a safety study. |
‐ 14/72 patients had incomplete data, therefore were excluded. Data were collected via an electronic medical record database that was retrospectively queried. |
Reported on all outcome measures | High rFVIIa dose exceeding common recommended practice Matched for most of baseline variables Increased cell salvage and product administration in the rFVIIa group This could be a higher‐risk population. |
EGFR: Estimated Glomerular Filtration Rate OR: Operating Room PCC: Prothrombin Complex Concentrate rFVIIa: Recombinant Factor VIIa
Incidence of renal impairment
One study (Harper 2018) reported on acute renal impairment with a total of 106 patients. PCC may reduce the incidence of renal impairment comparative to rFVIIa but the evidence is very uncertain (OR 0.29; 95% CI 0.12 to 0.71; participants = 106; studies = 1; very low‐quality evidence; Analysis 2.7).
2.7. Analysis.

Comparison 2: PCC versus rFVIIa, Outcome 7: Incidence of renal replacement therapy
The risk of bias for the one NRS was serious. This was due to a disproportionately high dose of rFVIIa (Figure 14, Table 13).
11. ROBINS‐I PCC versus rFVIIa; renal replacement therapy.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Harper 2018 | Moderate risk | Low risk | Low risk | Serious risk | Moderate risk | Low risk | Low risk | Serious risk |
| ‐ Propensity matching occurred for all baseline characteristics except EGFR and procedure type. ‐ Procedure type appeared to be more complex in the PCC group. ‐ 5 of the remaining 58 were then excluded due to inability to be matched with rFVIIa group. |
Retrospective collection with intervention and follow‐up coinciding for all participants. Both PCCs and FVIIa were given in OR. |
Defined two groups from the beginning and stated that they analysed patients receiving PCCs or rFVIIa. No definition of what constituted refractory bleeding and initiation of interventions | ‐ rFVIIa group received 90 mcg/kg and PCC group received 27 IU/kg (median). ‐ The rFVIIa dose exceeded recognised and accepted doses for rFVIIa. ‐ No definition of when patients received intervention ‐ This was a safety study. |
‐ 14/72 patients had incomplete data, therefore were excluded. Data was collected via an electronic medical record database that was retrospectively queried. |
Reported on all outcome measures | ‐ High rFVIIa dose exceeding common recommended practice ‐ Authors matched for most of baseline variables Increased cell salvage and product administration in the rFVIIa group |
EGFR: Estimated Glomerular Filtration Rate OR: Operating Room PCC: Prothrombin Complex Concentrate rFVIIa: Recombinant Factor VIIa
Ventilator hours
No studies reported on time spent on a ventilator postoperatively in the intensive care unit.
Adverse events
No studies reported on other adverse events.
Paediatrics
In the three paediatric studies (Giorni 2013; Harris 2020a; Rybka 2015), doses ranged from 25 units/kg to 57 units/kg. Further analysis could not be performed due to lack of paediatric studies. The paediatric studies all underwent high‐risk complex congenital surgery and none showed an increased risk of thrombosis.
Harris 2020a used a large dose of PCC 50 u/kg and Giorni 2013 used a more standard dose of 25 u/kg. Giorni 2013 was the only study to do prospective screening of thrombotic events with daily bedside echocardiography to check for intra‐cardiac thrombosis. The study showed no difference in thrombotic events.
Harris 2020a had 59 participants, and the outcomes also showed similar death and thrombosis risk, with decreased hospital length of stay and blood products used. These paediatric patients were highly representative of the paediatric cardiac surgical population; although these results are very encouraging with respect to the safe use of PCC, we need studies with a larger population.
Case Study
We identified only one catastrophic thrombotic event related to PCC administration in a case report (Koster 2014). This should be interpreted with caution as the 22 year old congenital heart disease patient experiencing the event appeared to have underlying prothrombotic diathesis; this participant developed near complete thrombotic obstruction in their 12 year old tricuspid and pulmonic mechanical valves, despite been compliant with warfarin with a therapeutic INR at the time. Following cardiac surgery to correct these thrombotic obstructions, the participant was given PCC; during the infusion of 22 units/kg, a large thrombus formed.
Discussion
Summary of main results
Overall, we had a total of 18 studies (2 RCTs, 16 NRSs) that included 4993 patients. There were 4842 patients studied in the NRSs with 1986 patients having matched data.
PCC vs standard treatment
We found that PCCs were likely to reduce units of RBC transfusion in patients receiving PCCs. This was supported by moderate‐quality evidence from RCTs and by very low‐quality evidence from NRSs. We found that PCCs may reduce the incidence of RBC transfusion in patients receiving PCCs. This was supported by low‐quality evidence from an RCT and by low‐quality evidence from NRSs.
PCCs may result in little to no difference in the incidence of postoperative bleeding, thrombotic events, mortality, intensive care length of stay, and incidence of renal replacement therapy when compared to standard therapy. This is supported by moderate‐ and low‐quality evidence from the RCTs and low‐quality evidence from the NRSs.
PCCs vs rFVIIa
The evidence suggests that PCCs results in a large reduction in total RBC units transfused when compared to rFVIIa. Intraoperative RBC unit transfusion may result in a reduction of up to five units. The quality of this evidence was considered to be moderate.
PCCs may have little to no effect on thrombotic events, mortality, postoperative chest drain output, ICU length of stay and incidence of renal replacement therapy when compared to rFVIIa. We determined that the quality of evidence for these outcomes was very low.
Overall completeness and applicability of evidence
Our review method identified 18 studies (2 RCTs, 16 NRSs) with 4993 total participants. All the studies were patients undergoing cardiac surgery. Fifteen studies (including the two RCTs) were in adult patients and three were in paediatric patients.
Target study population
All but one study described the type of cardiac surgery analysed. The participants were representative of the general cardiac surgical population, however, not all the patients included in these studies were categorised as requiring high‐risk cardiac surgery which we believe is the target population.
Our major concern was that high‐risk cardiac surgery was poorly represented in the published studies comparing PCCs to standard therapy. The group of patients that are likely to benefit from PCCs are those undergoing high‐risk cardiac surgery. These are the patients that become factor deficient, need early and rapid correction of their coagulopathy and can often not tolerate the volume associated with adequate FFP factor replacement. We appreciate there are many other patients' risk factors to take into account when addressing coagulopathy, however, we were using high‐risk surgery as a major risk factor for coagulopathy.
Of the two RCTs, Green 2021 excluded first‐time isolated coronary artery bypass grafts and first‐time isolated aortic valve replacement (excluding active endocarditis) which helps to exclude the low risk of coagulopathy population. However, Biancari 2019 included 30% of patients who underwent a coronary artery bypass (CABG) procedure and were not on cardiopulmonary bypass (CPB). All other studies had cardiac surgical patients who underwent surgery on CPB.
Paediatrics
The paediatric study participants all underwent high‐risk complex congenital surgery and so were representative of the paediatric cardiac surgical population. We found that the dosing in the paediatric studies was higher than in the adult studies, with no increase in thrombotic events.
Timing of intervention
The timing of the intervention varied from either within the operating theatre (9 trials), intensive care and operating theatre (3 trials) or not described (6 trials). We didn't explore any outcome differences between these trials. Although this is what occurs clinically, the authors feel that factor deficiency should initially be replaced in theatre with guidance from point‐of‐care viscoelastic testing. However, ongoing refractory bleeding could occur in both the operating room or the ICU, therefore, in these studies it is appropriate to have the intervention starting in either location.
Quality of the evidence
We used GRADE to assess the quality of our evidence. Two pilot RCTs and 16 NRSs were included in our analysis. Please refer to our summary of finding tables (Table 1; Table 2).
PCC vs standard treatment
The RCTs had moderate quality of evidence, however, where there was only one study looking at incidence of blood products transfused and incidence of renal impairment, the quality of evidence was low due to overall low participant numbers.
The NRSs had very low quality of evidence, except for incidence of RBC transfusion which was graded as low due to a high number of patients and a well‐defined and measured outcome. The key contributor to the low quality of evidence was the risk of bias. The review authors, using the Robins‐I tool (Sterne 2016), determined that two of the NRSs had critical risk of bias, one had serious, and five had moderate and they were downgraded accordingly.
The low event rate may indicate that this review does not have enough power to detect a difference. The secondary outcomes had even lower event rates and sample sizes and, as a consequence, had large confidence intervals.
PCC Vs rFVIIa
In our PCC vs rFVIIa cohort, we found that there were even lower numbers of patients in all our outcomes, with similar downgrades due to risk of bias. Our primary outcome of RBC transfusion had a moderate quality of evidence as a result of a large effect size which allowed the evidence level to be upgraded despite having only two studies. The remaining primary and secondary outcomes had very low quality of evidence due to a low event rate and high risk of bias.
There were only two studies that compared RBC transfusion (Harper 2018, Tanaka 2013). Harper had a comparatively much larger dose of rFVIIa, and a large difference in the amount of cell saver blood collected between the two groups. This could suggest that the rFVIIa group was a higher‐risk population group. Tanaka had equivalent doses of the intervention, however, there was a difference in whether they received the treatment in the operating room or in the ICU. These factors could affect the observed outcome difference.
We have included studies with a critical risk of bias as we believe the totality of the evidence should be included as this clinical question has limited studies and evidence.
Potential biases in the review process
We conducted a thorough search and reduced potential bias by having two review authors assess study eligibility, data extraction, analysis and assessment of risk of bias in the included studies. We included all studies that compared PCCs to either standard therapy or rFVIIa. We contacted all the authors of any study where we could not extract relevant data for our outcomes. This was to reduce potential bias and also strengthen the overall outcome.
Agreements and disagreements with other studies or reviews
There are two other published systematic reviews by Roman 2019 and Van den Brink 2020. Roman 2019 reviewed the evidence of PCCs compared to FFP in cardiac surgery and Van den Brink 2020 reviewed the evidence of PCCs in three main areas of bleeding including trauma, cardiac surgery and liver surgery.
The main differences between this review and Roman 2019 is that we included studies that not only compared PCCs (with or without FFP) to FFP alone, but also PCC and rFVIIa. This is due to PCC being used in two ways clinically and in the literature, one as factor replacement instead of FFP and the other in refractory bleeding instead of the commonly used rFVIIa. We also included paediatric cardiac studies and conference abstracts and posters, both of which were excluded by Roman 2019. Roman 2019 included Ortmann 2015 which we did not include in our analysis. The review authors determined that the patients selected by Ortmann 2015 had a higher than normal incidence of preoperative anticoagulation and a resultant significantly higher INR compared to the normal population. We had specifically excluded studies with preoperative anticoagulation as this is a significant confounder.
Roman 2019 also included Arnekian 2012 in their systematic review which we did include but could not meta‐analyse. Arnekian 2012 had a discrepancy in the results between the text and the tables and, despite trying to contact these authors, we were unable to determine which data were correct. The paper also included three groups of PCC, FFP + PCC and FFP alone; however, 50% of the FFP alone group also received PCCs, therefore, there was large cross‐over and confounding.
The outcomes were similar between the two reviews for our comparator group of PCC versus standard treatment. Roman 2019 included 24‐hour chest drain output, re‐exploration for bleeding and stroke in their secondary outcomes. We expanded our outcomes to include all thrombotic events described and only the first 12 hours of chest drain output. We also reviewed additional outcomes of days in intensive care and total ventilator hours.
Our results showed similarities following meta‐analysis. The RBC transfused (units and % of patients transfused), mortality outcomes, and incidence of renal replacement therapy showed no difference between the two comparators. The Roman 2019 stroke outcome was similar to our incidence of thrombosis.
Van den Brink 2020 did a generalised systematic review looking at three areas of bleeding and PCC use. Their meta‐analysis of PCC use in cardiac surgery included the same studies as in this Cochrane Review, however, we excluded Ortmann 2015 and Bradford 2015 due to a high proportion of preoperative anticoagulation, specifically warfarin, which was an exclusion of this review. Like this review, they also excluded Arnekian 2012. However, their review combined both comparators of PCCs, FFP and rFVIIa. They included both Harper 2018 and Tanaka 2013 that exclusively looked at rFVIIa use compared to PCCs and added this to their meta‐analysis. So their meta‐analysis combined both interventions, PCCs and rFVIIa, but did not explain or highlight this.
Our results also showed similar outcomes in the reduction in RBC utilisation and no significant difference in thromboembolic events and mortality. They also discussed the credibility of these results due to the large heterogeneity of the studies.
The most recent publication is a European consensus statement on the use of 4‐factor PCC for cardiac and non‐cardiac surgical patients (Erdoes 2021). The authors concluded that, for cardiac surgery, the patients were at higher risk of thromboembolic events, therefore, an initial lower half dose of 12.5 IU/kg should be used, followed by a second dose, if microvascular bleeding persisted. They believed this to be a rational risk‐adjusted strategy. This recommendation was based on the systematic review by Roman 2019 and the retrospective cohort analysis done by Biancari 2019. The authors of this European consensus statement are members of the Scientific Subcommittee of Haemostasis and Transfusion in the European Association of Cardiothoracic Anaesthesiology.
These most recent publications show just how topical and important this question is in cardiac surgery as many centres have adopted PCC use worldwide, but we are lacking answers to guide our safe and effective use.
Authors' conclusions
Implications for practice.
PCCs versus standard treatment (FFP)
This review has found that PCCs could be used as an alternative to standard therapy for coagulopathic bleeding post‐cardiac surgery with moderate quality of evidence from RCTs which is in agreement with low‐quality evidence NRSs. There is a reduction in RBC transfusion and there may be a reduction in the incidence of RBC transfusion when PCCs are used as factor replacement in coagulopathic bleeding.
There is uncertainty around the optimal but safe dosing of PCCs. Adult doses in the included studies ranged from 12 units/kg to 28 units/kg. Some studies only reported a total dose, which ranged from 545 units to a maximum dose of 2000 to 3000 units, but without the weight range, the doses could not be compared.
In the three paediatric studies, doses ranged from 25 units/kg to 57 units/kg. Further analysis was unable to be performed due to the lack of paediatric studies. The paediatric studies all underwent high‐risk complex congenital surgery and are highly representative of the paediatric cardiac surgical population but, despite the high doses, did not show an increased risk of thrombosis.
There is a theoretical risk of increased thrombosis with 3‐factor PCC due to a lack of protein C and S. Our subgroup analysis looking at 3‐factor versus 4‐factor PCC showed there was no difference in the thrombotic events between these two types.
PCCs versus rFVIIa
PCCs may result in a large reduction in the number of RBCs transfused with moderate quality of evidence in refractory coagulopathic bleeding post‐cardiac surgery, but we are unsure of their effects on all other outcomes because the quality of evidence was very low.
Implications for research.
The current research published are two pilot RCTs and 16 NRSs. To increase the certainty of these conclusions, we propose that well‐constructed and powered multicentred trials would be required. These studies should use a well‐defined bleeding algorithm, set an appropriate dose of PCCs and its comparator, and study only the high‐risk cardiac surgical population. We also propose that future research clearly define outcomes such as thrombosis and how it was diagnosed.
A standardised PCC dose and FFP dose of equal factor replacement should be given and compared in future studies. An acceptable initial dose of PCCs is 10 to 20 units/kg. An equal dose of FFP for factor replacement is about 10‐20 mL/kg. This is general guidance based on equivalent doses of factor IX in each of the products, however, it must be noted that levels of factors in donor plasma will differ.
A standardised coagulation algorithm should also be used within institutions with the use of point‐of‐care viscoelastic testing. This ensures that a logical stepwise process in treating coagulopathy is completed, addressing deficiencies in protamine post‐bypass, followed by replacement of fibrinogen and platelets and finally, if point‐of‐care testing shows a factor deficiency, PCCs are given.
The second type of use of PCCs is in refractory coagulopathic bleeding instead of rFVIIa. This is where all coagulation constituents are optimised with adequate fibrinogen, platelet, coagulation factors and normalised physiological parameters, however, there is ongoing diffuse microvascular bleeding. Historically, rFVIIa has been used in this situation. Future studies need to ensure that the basics of coagulation are optimised first with a logical algorithm followed and PCCs only used in refractory bleeding.
We believe there is little benefit of PCCs in the low‐risk cardiac surgical population as these patients rarely become factor deficient and can tolerate the volume of FFP. Risk factors for developing factor deficiency during cardiac surgery include prolonged CPB time, low target temperature on bypass (hypothermia impairing liver production of coagulation factors), and foreign graft material such as aortic replacements. Higher‐risk patients often can not tolerate large volume transfusions of FFP due to poor right heart function or lung disease. These are the patients most likely to benefit from PCCs and should be studied in future. We are aware that some countries do not have access to FFP and all their factor replacement transfusions are done with PCCs alone. This study is therefore only relevant to countries that still use FFP as standard therapy in cardiac surgery.
Outcome measures should look at the incidence of acute renal impairment with a standardised internationally recognised acute kidney injury score such as the AKIN criteria. There is uncertainty whether PCCs may be causing renal microemboli causing acute kidney injury or via another unknown mechanism. There is also uncertainty whether the large volumes of FFP required for factor replacement is causing interstitial oedema within the kidneys and impairing glomerular blood flow and therefore contributing to acute kidney injury. We only looked at incidence of renal replacement therapy due to the varied and lack of consistent ways in which studies reported on kidney injury.
Only one study prospectively screened for embolic events with daily echocardiography and daily ultrasound (USS) of the legs. We can only assume that most studies made a clinical diagnosis of stroke or DVT and did not actively screen for them. This may be the most clinically relevant way to report on thromboembolic events, however, in future research, this should be prospectively screened for as clinical notes can miss important events.
Both moderate and low‐quality evidence suggests there is a benefit of PCCs in the cardiac surgical population, which justifies undertaking future high‐quality trials to confirm the place of PCCs.
History
Protocol first published: Issue 3, 2020
Acknowledgements
Cochrane Heart supported the authors in the development of this systematic review.
The following people conducted the editorial process for this review.
• Co‐ordinating Editor/Sign‐off Editor (final editorial decision): Professor Rui Providencia, Cochrane Heart, University College London.
• Managing Editors (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the review): Ghazaleh Aali, Cochrane Heart, University College London. Nicole Martin, Cochrane Heart, University College London.
• Copy Editor (copy‐editing and production): Anne Lethaby.
• Information Specialist: Dr Farhad Shokraneh, Cochrane Heart, University College London. Charlene Bridges, Cochrane Heart, University College London.
• Peer‐reviewers (provided comments and recommended editorial decisions): Authors would like to acknowledge the following people for their contribution to this review: Olaf Wendler (Contact Editor, Cochrane Heart,), Dr. Jerrold H Levy, Dr Giovanni Mariscalco, Kamrouz Ghadimi, Dr Gabor Erdoes (clinical reviewers). The authors also would like to thank Fiona Stewart, Cinzia Del Giovane, and Silvia Minozzi (Systematic Review/ method Specialist).
We would also like to acknowledge Dr Richard Charlewood, transfusion medicine specialist, for the information provided in equivalent doses of PCCs and FFP.
We would like to thank Dr Laura Young for her input in the protocol for this review and for expert haematological advice.
The background and methods section of this review is based on a standard template provided by Cochrane Heart.
Appendices
Appendix 1. Summary of constituents of prothrombin complex concentrate
| Name | FII | FVII | FIX | FX | Protein C/S | Additive |
| Beriplexa | 111 | 57 | 100 | 150 | Yes | Heparin, AT |
| Octaplexa | 98 | 66 | 100 | 96 | Yes | Heparin |
| Bebulina | 120 | 13 | 100 | 139 | No | Heparin |
| Profilninea | 148 | 11 | 100 | 64 | No | No heparin |
| Cofacta | 106 | 48 | 100 | 103 | Yes | No heparin |
| Prothrombinexb | 100 | ‐ | 100 | 100 | No | Heparin, AT |
| AT: antithrombin; FII: coagulation factor two; FVII: coagulation factor seven; FIX: coagulation factor nine; FX: coagulation factor ten. | ||||||
Appendix 2. Search strategies
CENTRAL ‐ Results 143
#1 MeSH descriptor: [Factor IX] this term only
#2 prothrombin complex concentrate
#3 pcc*
#4 factor IX
#5 beriplex
#6 octaplex
#7 bebulin
#8 profilnine
#9 cofact
#10 prothrombinex
#11 kcentra
#12 confidex
#13 kaskadil
#14 kedcom
#15 ocplex
#16 pronativ
#17 prothromplex
#18 PPSB
#19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 MeSH descriptor: [Thoracic Surgery] this term only
#21 MeSH descriptor: [Cardiovascular Surgical Procedures] explode all trees
#22 ((cardio* or cardiac* or heart) NEAR/3 surg*)
#23 ((cardio* or cardiac* or heart or coronary) NEAR/3 surg*)
#24 (non‐surgical NEAR/3 bleed*)
#25 Coagulopathy
#26 #20 or #21 or #22 or #23 or #24 or #25
#27 #19 and #26 with Publication Year from 2000 to 2021, in Trials
MEDLINE Ovid Results 507
1 Factor IX/
2 prothrombin complex concentrate.tw.
3 pcc*.tw.
4 factor IX.tw.
5 beriplex.tw.
6 octaplex.tw.
7 bebulin.tw.
8 profilnine.tw.
9 cofact.tw.
10 prothrombinex.tw.
11 kcentra.tw.
12 confidex.tw.
13 kaskadil.tw.
14 kedcom.tw.
15 ocplex.tw.
16 pronativ.tw.
17 prothromplex.tw.
18 PPSB.tw.
19 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20 Thoracic Surgery/
21 exp Cardiovascular Surgical Procedures/
22 ((cardio* or cardiac* or heart) adj3 surg*).tw.
23 ((cardio* or cardiac* or heart or coronary) adj3 surg*).tw.
24 (non‐surgical adj3 bleed*).tw.
25 Coagulopathy.tw.
26 20 or 21 or 22 or 23 or 24 or 25
27 19 and 26
28 limit 27 to yr="2000‐current"
Embase Ovid Results 829
1 blood clotting factor 9/
2 prothrombin complex concentrate.tw.
3 pcc*.tw.
4 factor IX.tw.
5 beriplex.tw.
6 octaplex.tw.
7 bebulin.tw.
8 profilnine.tw.
9 cofact.tw.
10 prothrombinex.tw.
11 kcentra.tw.
12 confidex.tw.
13 kaskadil.tw.
14 kedcom.tw.
15 ocplex.tw.
16 pronativ.tw.
17 prothromplex.tw.
18 PPSB.tw.
19 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20 thorax surgery/
21 exp cardiovascular surgery/
22 ((cardio* or cardiac* or heart) adj3 surg*).tw.
23 ((cardio* or cardiac* or heart or coronary) adj3 surg*).tw.
24 (non‐surgical adj3 bleed*).tw.
25 Coagulopathy.tw.
26 20 or 21 or 22 or 23 or 24 or 25
27 19 and 26
28 limit 27 to yr="2000‐current"
29 limit 28 to embase
CPCI‐S Results 81
# 24 #23 AND #18 Timespan=2000‐2021
# 23 #22 OR #21 OR #20 OR #19
# 22 TS=Coagulopathy
# 21 TS=(non‐surgical NEAR/3 bleed*)
# 20 TS=((cardio* or cardiac* or heart or coronary) NEAR/3 surg*)
# 19 TS=((cardio* or cardiac* or heart) NEAR/3 surg*)
# 18 #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
# 17 TS=PPSB
# 16 TS=prothromplex
# 15 TS=pronativ
# 14 TS=ocplex
# 13 TS=kedcom
# 12 TS=kaskadil
# 11 TS=confidex
# 10 TS=kcentra
# 9 TS=prothrombinex
# 8 TS=cofact
# 7 TS=profilnine
# 6 TS=bebulin
# 5 TS=octaplex
# 4 TS=beriplex
# 3 TS=factor IX
# 2 TS=pcc*
# 1 TS=prothrombin complex concentrate
Appendix 3. Risk of bias tables for non‐randomised studies
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Arachchillage 2016 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ No matching occurred ‐ The PCC group had higher‐complexity surgery, duration and individual patient risk. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC or FFP. | ‐ No mention of dose given ‐ No mention of co‐interventions ‐ No mention of usual clinical practice |
Assumed this was low because the authors have not mentioned any missing data. No mention of how authors collected their data. | ‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ Did not mention how they detected thrombosis and thrombotic events (i.e. clinically or radiological assessment) |
No multiple subgroup analysis Reported on what their methods stated | Critical bias in domain 1 No matching of groups PCC patients were from a higher‐risk group No mention of doses, clinical practice and standard care |
|
| Arnekian 2012 | Critical risk | Moderate risk | Low risk | Critical risk | Low risk | Low risk | Low risk | Critical risk |
| No matching occurred. Patients received both intervention and control. Administration of tranexamic acid and PCCs were left to the discretion of the attending physician. | Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. |
Intervention defined but not dose and timing which were left to the discretion of the attending physician. | Appropriate dosing of PCCs. Deviations from the intended intervention. Patients did not stay within their intended intervention group with 50% of the FFP group receiving PCCs. | Assumed low as nothing mentioned. | ‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐Well defined measurement outcomes of thrombotic events. Paper chart review |
No multiple analyses Reported on everything they said they would However, text reporting contradicted the table reporting. |
The groups were not as they reported. There was a large amount of cross‐over of intervention and control group. We could not use the data as patients did not stay within their intervention groups. | |
| Audley 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ Only 22 patients ‐ No matching occurred. ‐ Patients received either PCC or rFVIIa. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC or rFVIIa. | ‐ Unclear decision‐making process of clinicians treating patients with intervention. No mention of POC or Lab tests ‐ No mention of use of tranexamic acid and protamine ‐ Increased FFP usage in the PCC group ‐ Mild rFVIIa dose (32 mcg/kg) compared with PCC (20 IU/kg) |
No mention of missing data. Chart review from paper and electronic documentation | ‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ Did not mention how they detected thrombosis and thrombotic events (i.e. clinically or radiological assessment) |
No multiple subgroup analysis Reported on what their methods stated | ‐ Critical bias in Domain 1 ‐ No matching of groups |
|
| Biancari 2019 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ The intention of study was to review PCC vs FFP on patients undergoing cardiac surgery on CPB but they have included off‐CPB patients (FFP group 27% and PCC group 24%). ‐ Propensity matching occurred (101 patients each group). |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. | Intervention groups were clearly defined. The groups received either PCC +/‐ FFP or FFP alone. | ‐ Standardised heparin, protamine and TXA protocol ‐ Clinically appropriate dose of PCC and FFP ‐ No agreed coagulation algorithm amongst the hospitals ‐ There was no decision‐making process around when to give intervention or comparison. |
‐ Three patients had missing data on location of surgical bleeding. ‐ Collected data from e‐CABG registry |
‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ Discussed incidence of stroke but no mention how this was diagnosed (i.e. clinical or radiological) |
No multiple subgroup analysis Reported on what their methods stated | ‐ Critical bias in Domain 1 | |
| Cappabianca 2016 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Moderate risk | Serious risk | Serious risk |
| ‐ Attempted to control with propensity matching ‐ Important domains were measured and accounted for. ‐ Propensity one‐to‐one score and propensity score‐adjusted multivariate analysis was used (what score used changed AKI results) |
Retrospective collection with intervention and follow‐up coinciding for all participants. PCCs were all given intraoperatively. |
Intervention groups were clearly defined. The groups received either PCC or FFP alone. They did not mention a PCC + FFP group. | ‐ This was not a deviation from standard practice. ‐ This was not defined in their methods for analysing the two groups. ‐ Product usage was guided by POC testing, blood labs. ‐ Use of extra products was at the discretion of the treating clinician. |
No mention of missing data. Data were prospectively collected and recorded in computerised database registries that remained consistent during the study period. | ‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ Discussed incidence of stroke but no mention how this was diagnosed (i.e. clinical or radiological) |
Many types of analyses were conducted with differing results. ‐ Unadjusted univariate analysis (unmatched group) ‐ Multivariate analysis (unmatched group) ‐ One‐to‐one propensity‐matched analysis (matched group) ‐ Propensity score‐adjusted multivariate analysis (matched group) ‐ Group comparison in most recent years (2009 to 2013) in separate supplementary files |
‐ Amongst all the different ways of analysing data there was consistency of results except in AKI and RRT. ‐ This was discussed by the authors but no mention as to why this occurred. ‐ Unclear if 57 patients who received both interventions were included in either group |
|
| Fitzgerald 2018 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ There were matching patients in each intervention group. ‐ 69 patients in PCC group could not be matched due to being a higher‐risk population (emergency surgery, longer CPB time and complex surgery). ‐ Two variables were not able to be matched with a SMD < 10% (CPB time and diabetes). |
Retrospective collection with intervention and follow‐up coinciding for all participants. PCCs all given intraoperatively |
Defined two groups from the beginning and stated that PCC group was able to receive FFP | ‐ Standardised care mentioned ‐ Both groups received rFVIIa with no statistical significance between the groups. ‐ POC testing and lab tests to guide transfusion requirements ‐ PCCs were given according to a protocol. |
‐ 18 patients excluded due to missing data (8%) ‐ Data were obtained from institutional databases. |
‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes Defined how they diagnosed stroke (clinically) and DVT/PE (radiological confirmation) |
Reported on all outcome measures | ‐ Unfortunate to not have 69 patients of higher complexity included in the study. We believe these patients would have provided objective evidence for the higher‐risk population group. ‐ Outcome measures were difficult to interpret (avoidance of red cell, platelet, fibrinogen transfusion). |
|
| Fraser 2006 | Critical risk | Critical risk | Low risk | Low risk | Low risk | Moderate risk | Serious risk | Critical risk |
| ‐ Retrospective study ‐ Only one group of patients that received intervention and compared outcomes before and after the intervention ‐ They only analysed those 22/60 patients that received PCCs in ICU, therefore there could be potential selection bias of patients with ongoing/refractory bleeding in the ICU |
N/A ‐ No comparator group | Intervention group defined at start of study | ‐ Protocol for blood product administration provided ‐ All patients analysed received intervention. |
‐ They only analysed the 22 patients that received the PCCs in ICU, therefore, technically no missing data. ‐ Authors did not comment on the perioperative baseline characteristics of the missing/included patients. Collection of data ‐ retrospective chart review |
‐ Unclear definition of outcome measures: “we sought evidence from discharge summaries and chart review” | ‐ Theatre subgroup was not analysed/discussed for postoperative laboratory results and bleeding; these were excluded from analysis. Only 22/60 patients were analysed who received PCCs in ICU ‐ 3 deaths were included the 60 patients |
‐ Missing data accounted for 64% of patients that received PCCs. ‐ Authors did not comment on these patients' postoperative results except to say they had 3 deaths, 1 with CVA and 2 with superficial thrombophlebitis. |
|
| Giorni 2013 | Serious risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| ‐ Not propensity matched ‐ The control group was a retrospectively matched cohort on baseline variables: "the treated and non‐treated patients were adequately matched for all the baseline variables". |
Retrospective collection in the non‐PCC group and follow‐up coincided for all participants. PCCs all given in the OR. FFP usage similar between the two groups |
Defined two groups from the beginning and stated that PCC group was able to receive FFP | Protocol for use of Confidex mentioned in methods Protocol stated how/when they received the standard treatment (FFP and cryo) vs PCCs. Used a clinically appropriate dose of PCC (25 u/kg) |
No mention of missing data. Prospective enrolment of the PCC group and data on excel spreadsheet. Unclear how they retrospectively obtained data for the control group | Daily echo monitoring performed on all enrolled patients to exclude intracardiac thrombi. They did not do this for the retrospective control group. Not comparable assessment. Unclear if this resulted in significant differences because the PCC group had no incidence of intracardiac thrombi |
Reported on all outcome measures | ‐ Due to serious bias in confounding ‐ Small sample size (14 patients in PCC group vs. 11 patients in control group) ‐ Performed both prospective enrolment and retrospective collection of controls |
|
| Harper 2018 | Moderate risk | Low risk | Low risk | Serious risk | Moderate | Low risk | Low risk | Serious risk |
| ‐ Propensity matching occurred for all baseline characteristics except EGFR and procedure type ‐ Procedure type appeared to be more complex in the PCC group. ‐ 5 of the remaining 58 were then excluded due to inability to be matched with rFVIIa group. |
Retrospective collection with intervention and follow‐up coinciding for all participants. Both PCCs and FVIIa were given in OR. |
Defined two groups from the beginning and stated that they analysed patients receiving PCCs or rFVIIa. No definition of what constituted refractory bleeding and initiation of interventions | ‐ rFVIIa group received 90 mcg/kg and PCC group received 27 IU/kg (median). ‐ The rFVIIa dose exceeded recognised and accepted doses for rFVIIa. ‐ No definition of when patients received intervention ‐This was a safety study. |
‐ 14/72 patients had incomplete data, therefore, were excluded. Data were collected via an electronic medical record database that was retrospectively queried. |
‐ CVA defined as new postoperative stroke being documented by a neurologist in the electronic medical record or on autopsy ‐ DVT defined as postoperative ultrasound diagnosing acute DVT ‐ PE defined as CT or autopsy demonstrating acute PE ‐ MI defined as new native coronary artery or coronary artery bypass graft occlusion demonstrated on postopeative cardiac catheterisation or wall motion abnormalities seen on echocardiogram that resolved with PCI or CABG ‐ All new intracardiac thrombus as noted on postoperative echo or autopsy ‐ Patients did not routinely get a postoperative echocardiogram or cardiac catheter. Electronic medical record retrospectively queried |
Reported on all outcome measures | ‐ Serious bias in domain 4 (due to high rFVIIa dose exceeding common recommended practice) ‐ Authors matched for most of baseline variables except EGFR and surgical type (however, there was low SD between the two groups) ‐ Uncertainty around increased cell salvage and product administration in the rFVIIa group This could be the result of clinician bias or a higher‐risk population. |
|
| Harris 2020a | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| ‐ Matching occurred with a control based on demographic and surgical characteristics; 6/65 patients could not be matched. |
Retrospective collection with intervention and follow‐up coinciding for all participants. All PCCs and their 1:1:1 blood products given in OR |
Defined intervention group and comparison group | ‐ Unclear dose of protamine/antifibrinolytics ‐ If bleeding, they used a 1:1:1: transfusion as per protocol. ‐ Intervention used if ongoing bleeding despite transfusion; this was based on clinician judgement. ‐ Control group got standard therapy; intervention group got PCCs if they continued to bleed (potentially a higher‐risk population). ‐ Unclear if they used POC testing or lab bloods |
Collection of data ‐ retrospective database analysis | ‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ Thromboembolic events were analysed for clinical signs of acute thrombosis in the medical charts and confirmed by radiographic evidence. Database analysis |
Reported on all outcome measures | ‐ Well matched group ‐ Most data available for patients |
|
| Hashmi 2019 | Serious risk | Critical risk | Low risk | Low risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ Only one group; single arm only reviewing PCC efficacy | Not applicable as a single arm intervention of only PCCs | One group that was defined at the start of the study | ‐ Followed institutional protocol ‐ 52% of patients received rFVIIa, however, it may not lead to greater thrombotic complications; those that had complications were extremely high‐risk surgery. |
No mention of missing data Collection of data ‐ retrospective chart review with postoperative variables extracted from electronic records |
‐ Retrospective; assessors aware of intervention group ‐ Objective outcomes ‐ Unclear from paper how they defined thrombotic complications ‐ Appeared that they have reviewed the notes for clinical and radiological confirmation of thrombosis ‐ Retrospective chart review from electronic record |
Reported on all outcome measures | ‐ Only one group analysed ‐ Followed institutional protocol but 52% received rFVIIa |
|
| Koster 2014 | Serious risk | Critical risk | Critical risk | Serious risk | Low risk | Low risk | Low risk | Critical risk |
| ‐ Case report (single patient) ‐ Significant selection bias |
‐ Case report (single patient) |
‐ Case report (single patient) |
‐ 50 IU/kg dose about to be given | All data present for the one patient | ‐ Case report (single patient) |
‐ Case report (single patient) |
‐ Single patient case report of complication ‐ Unclear whether any PCCs were given preoperatively ‐ INR changed from 8 to 12 with hospital transfer. |
|
| Mehringer 2018 | Critical risk | Moderate risk | Low risk | Serious risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ No propensity matching occurred. ‐ Two groups had baseline differences in haemoglobin concentration and haematocrit. |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. Unclear in the study when given |
Intervention groups were clearly defined. The groups received either PCC or rFVIIa alone. | ‐ No mention of coagulation studies or coagulation algorithm ‐ Decision to give intervention was based on clinical judgement. ‐ High rFVIIa dose ‐ Intervention not comparable due to differences in doses |
No mention of missing data Collection of data ‐ electronic health record system |
‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ No mention of how they diagnosed thrombosis in patients: clinical or radiological assessment Collected data from electronic medical record system |
Reported on all outcome measures |
‐ Not propensity‐matched ‐ No mention of how they diagnosed coagulopathy or whether they used a coagulation algorithm ‐ High dose of rFVIIa dose (decided by surgeons) that was higher than most institutions standard practice ‐ 66 mcg/kg |
|
| Rybka 2015 | Critical risk | Moderate risk | Moderate risk | Serious risk | Low risk | Moderate risk | Low risk | Critical risk |
| No matching occurred. Physicians discretion whether to use intervention or comparison | Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. |
Both groups received predefined doses. There was no justification of when and why they gave the dose of PCC or rFVIIa. | Very high rFVIIa dose (100 mcg/kg) compared to high dose (PCC 42 u/kg) No protocol to aid decision‐making of when to give intervention or control This was a lab study; appeared that POC and lab tests were not used to guide treatment. No mention of co‐interventions or cross‐over Appeared the participants all stayed within their groups and adhered to the dosing protocol |
Not mentioned | No mention of how they collected their data. No mention of how they measured their outcomes of thrombosis | No multiple analyses Didn’t discuss what outcome measures were but they measured all the appropriate laboratory outcomes |
||
| Tanaka 2013 | Critical risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Critical risk |
| ‐ Matched on age, sex and CPB duration ‐ Other factors unmatched |
‐ 40% of patients received rFVIIa in ICU. ‐ 100% of patients received PCCs in theatre. Follow‐up may have started before intervention. |
Intervention groups were clearly defined. | ‐ Followed institutional protocol ‐ Cross‐over of patients in PCC group (24% patients in the PCC group also received rVIIa) |
No mention of missing data Collection of data not mentioned |
‐ Retrospective; assessors aware of intervention group. ‐ Objective outcomes ‐ No mention of how they diagnosed thrombosis in patients: clinical or radiological assessment |
Reported on all outcome measures | ‐ Editorial, not a published paper ‐ Comparable doses ‐ Not propensity‐matched ‐ Issues with initiation of intervention (theatre vs ICU) |
|
| Zweng 2019 | Moderate risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk | Low risk | Moderate risk |
| ‐ Propensity matched ‐ No cross‐over occurred. ‐ 18 patients unmatched (25%) from the PCC group because of more complex surgical procedure ‐ Clear selection bias in choosing which patients received PCCs making it impossible to ascertain whether the complications observed were truly from the PCCs or the surgical complexity |
Unknown whether PCCs given in OR or ICU; follow‐up may have started before intervention. |
Intervention groups were clearly defined. The groups received either PCC or clotting factor‐based therapy. | Unsure when intervention was given No mention of POC or lab tests No protocol The decision to give PCC was left up to the surgeon, anaesthetist, or intensivist. Normal standard dose of PCC was 1500 units |
No reports of missing data Collection of data ‐ from Austin hospital blood bank and cross‐referenced with the Australian Society of Cardiothoracic Surgeons database |
‐ No mention of how they diagnosed thrombosis in patients: clinical or radiological assessment | ‐ Propensity‐matched pairs were used for a multivariate logistic regression model adjusted for age, sex, total units of RBC, cryoprecipitate, FFP and platelets combined. |
‐ Selection bias ‐ Multiple statistical tools used to report data ‐ Unclear as to timing of interventions |
Data and analyses
Comparison 1. PCC versus standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 RCT: Blood products transfused (RBC) in units | 2 | 151 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.78, 0.00] |
| 1.2 NRS: Blood products transfused (RBC) in units | 2 | 551 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.53, ‐1.20] |
| 1.3 RCT: Blood products transfused (RBC) % of patients | 1 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.20, 1.40] |
| 1.4 NRS: Blood products transfused (RBC) % of patients | 4 | 1046 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.98] |
| 1.5 RCT: Thrombotic events | 2 | 152 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.31] |
| 1.6 NRS: Thrombotic events | 7 | 1359 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.87, 1.99] |
| 1.7 NRS: Thrombotic events matched data | 6 | 1189 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.87, 2.01] |
| 1.7.1 Matched data | 6 | 1189 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.87, 2.01] |
| 1.8 NRS: Thrombotic events (3‐factor vs 4‐factor) | 4 | 962 | Odds Ratio (M‐H, Random, 95% CI) | 1.30 [0.84, 2.00] |
| 1.8.1 3‐factor PCCs | 3 | 728 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.88, 2.31] |
| 1.8.2 4‐factor PCCs | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.33, 2.37] |
| 1.9 RCT: Mortality (30‐day) | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.12, 2.35] |
| 1.10 NRS: Mortality (30‐day) | 6 | 1334 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.51] |
| 1.11 NRS: Mortality (30‐day) | 5 | 1164 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.53] |
| 1.11.1 Matched data | 5 | 1164 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.53] |
| 1.12 RCT: Bleeding (chest drain output) in mLs for the first 12 hours | 2 | 151 | Mean Difference (IV, Random, 95% CI) | ‐107.05 [‐278.92, 64.83] |
| 1.13 RCT: Intensive care length of stay in hours | 2 | 151 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐19.26, 18.57] |
| 1.14 NRS: Intensive care length of stay in hours | 1 | 450 | Mean Difference (IV, Random, 95% CI) | ‐18.00 [‐43.14, 7.14] |
| 1.15 RCT: Incidence of renal replacement therapy | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.14, 3.59] |
| 1.16 NRS: Incidence of renal replacement therapy | 2 | 684 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.71, 2.98] |
| 1.17 RCT: Ventilator hours | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐4.49, 2.89] |
| 1.18 NRS: Ventilator hours | 1 | 450 | Mean Difference (IV, Random, 95% CI) | ‐5.20 [‐23.10, 12.70] |
1.7. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 7: NRS: Thrombotic events matched data
1.11. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 11: NRS: Mortality (30‐day)
1.14. Analysis.

Comparison 1: PCC versus standard treatment, Outcome 14: NRS: Intensive care length of stay in hours
Comparison 2. PCC versus rFVIIa.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Blood products transfused (RBC) in units | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Intraoperative | 2 | 256 | Mean Difference (IV, Random, 95% CI) | ‐4.98 [‐6.37, ‐3.59] |
| 2.1.2 Postoperative | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.48, 0.36] |
| 2.2 Blood products transfused (RBC) % of patients | 1 | 150 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.56] |
| 2.3 Thrombotic events | 4 | 407 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.16] |
| 2.4 Mortality (30‐day) | 3 | 278 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.38, 3.03] |
| 2.5 Bleeding (chest drain output) in mLs for the first 12 hours | 1 | 150 | Mean Difference (IV, Random, 95% CI) | ‐674.34 [‐906.04, ‐442.64] |
| 2.6 Intensive care length of stay in hours | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐40.00 [‐110.41, 30.41] |
| 2.7 Incidence of renal replacement therapy | 1 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arachchillage 2016.
| Study characteristics | ||
| Methods | Study design ‐ retrospective chart review Study duration ‐ 1 year (January 2015‐December 2015) Centres ‐ Royal Brompton and Harefield |
|
| Participants | 170 patients Mean age ‐ 56 yrs in the intervention group and 58 yrs in the comparison group Gender ‐ 111 men (65%) and 59 (35%) women overall Inclusion criteria ‐ Patients who underwent major cardiac surgery that received intervention or comparison Exclusion criteria ‐ Those who received both intervention and comparison |
|
| Interventions | Intervention group ‐ PCC (unknown type and dose) given IV Comparison group ‐ FFP (unknown dose) given IV No patients received both intervention and comparison. |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (RBC and platelet transfusion) ‐ Thrombotic events (VTE/arterial) ‐ Mortality (30‐day) Secondary outcomes ‐ Drain output (12‐hr and 24‐hr output with 95% CI) ‐ Incidence of new renal impairment ‐ Cardiac overload failure (incidence) |
|
| Notes | Conflicts of Interest ‐ None Funding ‐ M Laffan (CSL travel support and Octapharma Speakers Bureau) |
|
Arnekian 2012.
| Study characteristics | ||
| Methods | Study design ‐ retrospective chart review Study duration ‐ 2 years (January 2009‐December 2010) Centres ‐ Centre Chirurgical Marie Lannelongue, Le Plessis Robinson (France) |
|
| Participants | 77 patients Mean age ‐ intervention group 64 yrs +/‐ 13, comparison group (FFP) 72 yrs +/‐14 and comparison group (FFP + PCC) 73 yrs +/‐ 10 Gender ‐ 51 men (66%) and 26 women (34%) overall. Men in intervention group 18 patients (75%), comparison group (FFP) 18 patients (69%) and comparison group (FFP + PCC) 15 patients (55%) Inclusion criteria ‐ Patients who underwent cardiac surgery under CPB that received PCC and/or FFP Exclusion criteria ‐ not mentioned |
|
| Interventions | Intervention group ‐ PCC (Octaplex) given IV Comparison group ‐ No distinct comparison group |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (RBC, FFP and platelet transfusion in ICU) ‐ Thrombotic events (CVA/PE/other significant) ‐ Mortality (30‐day) Secondary outcomes ‐ ICU stay (days and range) ‐ Hospital stay (days and range) ‐ Ventilator hours (hours and range) ‐ Re‐exploration for bleeding (incidence) ‐ Pericardial effusion (incidence) ‐ Pulmonary oedema (incidence) ‐ ARDS (incidence) ‐ Mediastinitis (incidence) ‐ Other infections (incidence) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Audley 2019.
| Study characteristics | ||
| Methods | Study design ‐ retrospective cohort study Study duration ‐ 2 years (June 2016‐July 2018) Centres ‐ Christiana Care Health System (USA) |
|
| Participants | 22 patients Mean age ‐ intervention group 61 yrs +/‐ 13.5 and comparison group (rFVIIa) 61.7 yrs +/‐19.1 Gender ‐ 18 men (82%) and 4 women (18%) overall. Men in intervention group 4 patients (100%) and comparison group (rFVIIa) 14 patients (77.8%) Inclusion criteria ‐ >= 18 years of age, cardiac patients who received PCC or rVIIa perioperatively and measure of chest tube output Exclusion criteria ‐ Time of PCC and rFVIIa administered not documented, participant administered both PCC and rFVIIa and PCC or rFVIIa ordered for an indication other than surgical bleeding |
|
| Interventions | Intervention group ‐ PCC (4‐factor) with median/mean doses Comparison group ‐ rFVIIa with median/mean doses |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (RBC units and FFP units transfused) ‐ Thrombotic events ‐ Mortality Secondary outcomes ‐ Drain output (1 hr and 24 hrs in mL) ‐ ICU stay (days) ‐ Need for re‐exploration (incidence) ‐ Estimated blood loss (mL) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Biancari 2019.
| Study characteristics | ||
| Methods | Study design ‐ retrospective cohort study Study duration ‐ 2 years (January 2015‐December 2016) Centres ‐ 9 centres total in Europe, Finland, France, Italy, Germany, Sweden, UK |
|
| Participants | 535 patients in total with 202 (101 in each group) post‐propensity matched Mean age (matched) ‐ intervention group (PCC) 65.3yrs +/‐ 9.3 and comparison group (FFP) 65.9yrs +/‐ 9.7 Gender (matched) ‐ intervention group (PCC) 14 women (13.9%) and comparison group (FFP) 11 women (11%) Inclusion criteria ‐ Only coronary artery surgery including emergency, redo, and off pump Exclusion criteria ‐ Not mentioned |
|
| Interventions | Intervention group ‐ PCC (3 and 4‐factor) with initial doses and maximum dose range Comparison group ‐ FFP +/‐ PCC |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (RBC transfusion, RBC units transfused, patients receiving FFP, patients receiving platelet transfusion, patients receiving cryoprecipitate, patients receiving fibrinogen concentrate) ‐ Thrombotic events (strokes) ‐ Mortality (30‐day mortality) Secondary outcomes ‐ Drain output (over 12 h in mL) ‐ UDPB bleeding grades ‐ E‐CABG bleeding grades ‐ Surgical site bleeding (incidence) ‐ ICU stay (days) ‐ Hospital stay (days) ‐ New AF (incidence) ‐ Incidence of renal impairment via KDIGO AKI Score (incidence) ‐ Prolonged inotropes (incidence) ‐ Mediastinitis (incidence) ‐ IABP/ECMO (incidence) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Cappabianca 2016.
| Study characteristics | ||
| Methods | Study design ‐ retrospective observational study Study duration ‐ 9 years (January 2005‐December 2013) Centres ‐ Varese University Hospital (Italy) |
|
| Participants | 914 in total with 450 (225 in each group) post‐propensity matching Mean age ‐ Intervention group (PCC) 69.2 +/‐ 11.6 yrs and comparison group (no PCC) 69.7 +/‐ 10.6 yrs Female Gender ‐ Intervention group (PCC) 91 (40.4%) and comparison group (no PCC) 91 (40.4%) Inclusion criteria ‐ All patients having elective, urgent or emergency surgery ‐ CABG, valve surgery, proximal aortic procedures. Exclusion criteria ‐ Off‐pump CABG, other cardiac surgery (cardiac tumour, ACHD, post‐MI VSD, free wall rupture repairs). Patients who died intraoperatively without blood product administration |
|
| Interventions | Intervention group ‐ PCC (3‐factor Uman Complex DI) with median doses and IQR given IV Comparison group ‐ FFP with median doses and IQR given IV |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (incidence of RBC transfusion, RBC units transfused and incidence of patients receiving platelets) ‐ Thrombotic events (stroke/TIA) ‐ Mortality (in hospital) Secondary outcomes ‐ Drain output (24 hrs in mL) ‐ ICU stay (hours) ‐ Hospital stay (days) ‐ Incidence of renal impairment (AKI and RRT) determined by RIFLE criteria ‐ Ventilator hours (hours) ‐ Need for re‐exploration (incidence) ‐ Postoperative IABP (incidence) ‐ Perioperative MI (incidence) ‐ Postoperative AF (incidence) ‐ Blood loss (mL) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Fitzgerald 2018.
| Study characteristics | ||
| Methods | Study design ‐ retrospective observational study Study duration ‐ 5 years (January 2012‐December 2016) Centres ‐ Toronto General Hospital (Canada) |
|
| Participants | 1355 patients total with 234 (117 patients in each group) post‐propensity matching Median age ‐ Intervention group (PCC) 60 (50, 69) and comparison group (FFP) 61 (46, 70) Gender ‐ Intervention group (PCC) 77 men (65.8%), 40 women (34.2%) and comparison group (FFP) 72 men (61.5%), 45 women (38.5%) Inclusion criteria ‐ All patients having cardiac surgery under CPB (for patients who had multiple operations requiring CPB during study period only the data from the first surgery was used) Exclusion criteria ‐ Patients with transfusion data missing |
|
| Interventions | Intervention group ‐ PCC (Octaplex) with median doses and IQR given IV Comparison group ‐ FFP with median doses and IQR given IV |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (avoidance of RBC transfusion, massive transfusion, avoidance of platelet transfusion, avoidance of fibrinogen and use of rFVIIa) ‐ Thrombotic events (stroke, DVT/PE) ‐ Mortality (in hospital) Secondary outcomes ‐ Incidence of renal impairment (class I, II, III) ‐ Need for re‐exploration (incidence) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Fraser 2006.
| Study characteristics | ||
| Methods | Study design ‐ retrospective chart review Study duration ‐ 7 months (February 2003‐August 2003) Centres ‐ Geelong Hospital (Australia) |
|
| Participants | 60 patients total Median age ‐ Intervention group (PCC) 71 yrs (42‐81) Gender ‐ Intervention group (PCC) ‐ men 45 (75%) women 15 (25%) Inclusion criteria ‐ All patients that underwent cardiothoracic surgery and received PCCs Exclusion criteria ‐ Not described |
|
| Interventions | Intervention group ‐ PCC (Prothrombin‐VT) with mean doses and given IV Comparison group ‐ none |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (before‐and‐after: average RBC transfusion, average FFP transfusion and average platelet transfusion) ‐ Thrombotic events (stroke) ‐ Mortality (in hospital) Secondary outcomes ‐ Drain output (average bleeding for two hours before and two hours after PCCs) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Giorni 2013.
| Study characteristics | ||
| Methods | Study design ‐ prospective observational cohort study Study duration ‐ 16 months (November 2010‐February 2012) Centres ‐ Bambino Gesu`Children’s Hospital (Italy) |
|
| Participants | 25 patients total Median age ‐ Intervention group (PCC) median and IQR (25‐75) ‐ 13 days (8‐67) and comparison group (no PCCs) median and IQR (25‐75) ‐ 17 days (13‐25) Gender ‐ Not described Inclusion criteria ‐ Infants younger than 1 year of age who underwent cardiac surgery with cross clamp > 60 min or CPB > 120 min and had non‐surgical bleeding Exclusion criteria ‐ Not described |
|
| Interventions | Intervention group ‐ PCC (4‐factor Confidex) with mean doses and given IV Comparison group ‐ Standard therapy |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (RBC and FFP transfused) ‐ Thrombotic events (intracardiac thrombus) Secondary outcomes ‐ Drain output (for first 24 hrs mL/kg/hr) ‐ Intensive care stay (days) ‐ Ventilator (days) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Green 2021.
| Study characteristics | ||
| Methods | Study design ‐ pragmatic pilot open‐label phase II randomised controlled trial Intervention model ‐ parallel assignment Centres ‐ St Bartholomew's Hospital (UK) |
|
| Participants | Estimated enrolment ‐ 50 patients Inclusion criteria ‐ Age ≥ 18 years ‐ Able to give consent ‐ Any cardiovascular surgeries excluding procedures under exclusion criteria Exclusion criteria ‐ Unable to consent ‐ Patients refusing blood transfusion for any reason ‐ First‐time isolated coronary artery bypass grafts (CABG) ‐ First‐time isolated aortic valve replacement (excluding active endocarditis) ‐ Thoraco‐abdominal surgeries ‐ Minor surgeries that do not involve cardiopulmonary bypass ‐ Use of warfarin within four days ‐ Use of direct oral anticoagulants (i.e. dabigatran, rivaroxaban, apixaban or edoxaban) within 48 hrs (or 72 hours if participant has renal impairment ‐ i.e. estimated glomerular filtration rate of < 30mL/min) ‐ Inherited bleeding disorder (i.e. any inherited clotting factor deficiencies, or platelet disorders) ‐ Pregnancy ‐ Known or suspected allergy to FFP or PCC ‐ Known or suspected allergy to heparin, sodium citrate dihydrate, sodium dihydrogenphosphate dihydrate and glycine ‐ History of heparin‐induced thrombocytopenia ‐ Individuals who have immunoglobulin A (IgA) deficiency with known antibodies against IgA ‐ Documented venous thromboembolism in the last three months ‐ Documented antiphospholipid syndrome ‐ Severe protein S deficiency ‐ Participation in another clinical trial, where the patient has received investigational medicinal product in the last 3 months |
|
| Interventions | Intervention group ‐ Prothrombin Complex Concentrate (PCC) PCC 500 IU if the participant’s weight was < 60 kg; 1000 IU if 61–90 kg; and 1500 IU if > 90 kg. If bleeding continued after administration of the first PCC dose, the participant received standard care with FFP; therefore, no further PCC was administered to any participant. Comparison group ‐ Fresh Frozen Plasma (FFP) 15 mL/kg and based on the average volume of one FFP unit being 270 mL, dose was rounded up to reduce wastage to 3 units if the participant’s weight was ≤ 60 kg, 4 units if 61–90 kg and 5 units if > 90 kg. |
|
| Outcomes | Primary outcome: 1. The proportion of eligible patients who consented and received the intervention within 24 h of surgery Secondary outcomes: 1. Proportion of patients where there was protocol adherence and protocol violation 2. Difference in haemostatic capacity ‐ to assess this, blood samples were taken at three time points: before the intervention and during bleeding; within 1 h of the intervention being completed; and 24 h after the intervention. 3. Time to administration of study drug 4. Safety up to 90 days after surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled participants were randomly allocated by the transfusion laboratory to receive either PCC or FFP. Randomisation was by allocating participants in a 1:1 ratio to receive PCC or FFP using block randomisation, with block size varied randomly to ensure balance of treatments. |
| Allocation concealment (selection bias) | Low risk | The algorithm was written by the study statistician using the ralloc command in Stata. Randomisation occurred via a web‐based electronic database for the first 5 months of the trial and was switched to manual randomisation envelopes for the next 3 months. Laboratory staff found paper randomisation easier and simpler to use during an emergency. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The clinician giving the intervention could not be blinded due to the different physical properties of the two products. Very unlikely that the outcome measures could have been affected by the clinician knowing the treatment allocation. e.g. death, infection, haemodialysis, hospital stay |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All samples were analysed by a biomedical scientist who was blinded to the participant group. Unsure who collected the clinical outcome measures but very unlikely that outcome measurement could have been affected by clinician knowledge |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 25 subjects allocated to PCC and 25 allocated to FFP, no one withdrew from the trial but four were lost to follow‐up at 90 days. Reasons for loss to follow‐up were: patients returned to their homes abroad and were not able to be contacted (n = 2), and patients were not reachable by telephone following several attempts (n = 2). |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoints in the study protocol were assessed in the RCT. |
| Other bias | Low risk | No other bias detected in the study |
Harper 2018.
| Study characteristics | ||
| Methods | Study design ‐ retrospective cohort study Study duration ‐ 13 years and 4 months (January 2003‐April 2015) Centres ‐ Mayo Clinic, Rochester (USA) |
|
| Participants | 335 patients in total with 106 patients (53 in each group) post‐propensity matching Mean age ‐ Intervention group (PCC) mean +/‐ SD = 60.9 (17.4) and comparison group (rFVIIa) mean +/‐ SD = 58.5 (19.7) Gender ‐ Intervention group (PCC) = 36 (68%) men and 17 (32%) women and comparison group (rFVIIa) = 35 (66%) men and 18 (34%) women Inclusion criteria ‐ Patients who received PCC or rFVIIa intraoperatively during cardiac surgery requiring CPB Exclusion criteria ‐ Patients who declined to participate in research, those with haemophilia and undergoing cardiac surgery without CPB |
|
| Interventions | Intervention group ‐ PCC (3‐factor Bebulin) with mean/median doses and given IV Comparison group ‐ rFVIIa with mean/median doses and given IV |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (intraoperative/postoperative transfusions of RBC, FFP, platelets, cryoprecipitate, fresh whole blood) ‐ Thrombotic events (CVA/DVT/PE/MI/intracardiac thrombus) ‐ Mortality (30 days) Secondary outcomes ‐ Drain output (for first 24 hrs in mL) ‐ Intensive care stay (days) ‐ Hospital stay (days) ‐ Additional procedures within 24 hrs ‐ Incidence of renal impairment |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Harris 2020a.
| Study characteristics | ||
| Methods | Study design ‐ retrospective cohort study Study duration ‐ 6 years and 8 months (January 2009‐August 2015) Centres ‐ Health Science Center at Houston (USA) |
|
| Participants | 246 patients in total with 118 (59 patients in each group) post‐propensity matching Mean age ‐ Intervention group (PCC) mean + SD ‐ 0.45 yrs +/‐ 0.84 neonates ‐ 33 (56%) premature ‐ 18 (31%) and comparison group (all controls) mean + SD ‐ 0.38 yrs +/‐ 0.78 neonates ‐ 34 (58%) premature ‐ 15 (25%) Gender ‐ Intervention group (PCC) ‐ male 35 (59%) and comparison group (all controls) ‐ male 35 (59%) Inclusion criteria ‐ This was a retrospective database analysis of paediatric patients < 18 years of age undergoing cardiac surgery on CPB at a single institution Exclusion criteria ‐ Patients with known preexisting coagulopathy requiring prophylactic factor concentrates |
|
| Interventions | Intervention group ‐ PCC (3‐factor Bebulin) with mean doses and given IV Comparison group ‐ Standard therapy |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (transfusions of RBC, FFP, platelets, cryoprecipitate in mL/kg before and after PCCs) ‐ Thrombotic events (DVTs/PE) ‐ Mortality (in hospital) Secondary outcomes ‐ ECMO requirement (incidence) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Hashmi 2019.
| Study characteristics | ||
| Methods | Study design ‐ retrospective chart review Study duration ‐ 1 years and 4 months (February 2014‐June 2015) Centres ‐ Duke University Medical Centre (USA) |
|
| Participants | 114 patients in total Mean age ‐ Intervention group (PCC) 58.2 yrs (18‐92) Gender ‐ Intervention group (PCC) female 39 (36.4%) and male 68 (63.6%) Inclusion criteria ‐ Adults (age 18 years or older), that had received profilnine for refractory bleeding in the operating room or within the first 12 h of postoperative ICU admission, were included. Exclusion criteria ‐ Patients under the age of 18 and parturients were excluded, as were patients with a history of disseminated intravascular coagulation (DIC) and those that received both 3F and 4F‐PCC during the perioperative period.. |
|
| Interventions | Intervention group ‐ PCC (3‐factor profiline) with mean doses and given IV Comparison group ‐ None |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (transfusions of RBC, FFP, platelets, cryoprecipitate in mL/kg before and after PCCs) ‐ Thrombotic events (CVA/DVT/PE) Secondary outcomes ‐ Drain output (1st and 2nd hrs in mL and up to 11 hrs as B + W graph) ‐ Factor 2 levels (% of normal) |
|
| Notes | Funding of trial ‐ KG: grant support from NIH (T32GM008600); Consultant for UpToDate; NH, AS, YL, RR, JG, AR, YB: None declared. TLO: Research funding from Instrumentation Laboratory and Siemens, honoraria from BMS‐Squibb, and the UpToDate Board or Advisory Committee. JHL: receives fees for serving on advisory committees for CSL Behring, Boehringer Ingelheim, Instrumentation Laboratories, Octapharma, and Merck. IJW: grant support from NIH (R01HL121232‐01), CSL Behring and Biomet Biologics. Consultant for UpToDate. Conflicts of interest ‐ nothing to declare |
|
Karkouti 2021.
| Study characteristics | ||
| Methods | Study design ‐ randomised pilot trial Intervention model ‐ parallel assignment Centres ‐ Sunnybrook Health Science Centre and University Health Network, Toronto (Canada) |
|
| Participants | There were 169 screened patients: 131 were randomised, and 101 were treated (54 with PCC and 47 with FFP), provided consent, and were included in the analysis. Inclusion criteria: Adult patients undergoing cardiac surgery for whom coagulation factor replacement with FFP or PCC was ordered during surgery for management of bleeding. Exclusion criteria
|
|
| Interventions | Intervention group ‐ Prothrombin Complex Concentrate (PCC) Octaplex. For the first and second orders up to 24 hours after randomisation, patients assigned to the PCC group received Octaplex, 1500 IU if the participant weighed 60 kg or less or 2000 IU if the participant weighed more than 60 kg. Comparison group ‐ Fresh Frozen Plasma (FFP) received 3 units FFP if the participant weighed 60 kg or less or 4 units FFP if the participant weighed more than 60 kg for each order. For any additional orders, FFP was administered to both groups. These calculations resulted in a proportionally higher factor replacement dose in the PCC group compared to the FFP group with the median dose being 26 IU/kg compared to 12.5 mL/kg respectively. |
|
| Outcomes | The primary measures of haemostatic effects were: 1. Treatment response, based on receipt of any haemostatic therapies from 60 minutes to 4 and 24 hours after initiation of the intervention; 2. Cumulative and individual allogeneic blood component units (red blood cells, platelets, and FFP) administered within 24 hours after start of surgery; 3. Avoidance of red cell transfusion within 24 hours after start of surgery. Other measures of haemostatic effects included: 1. Cumulative and individual allogeneic blood component units administered within 24 hours and 7 days after cardiopulmonary bypass and within 24 hours after start of intervention; 2. Blood loss, as measured by chest tube drainage at 12 and 24 hours after surgery; 3. Number of patients receiving haemostatic factor concentrates; 4. Bleeding severity as measured by the universal definition of perioperative bleeding score. The measures for assessing feasibility of study procedures were successful randomisation, treatment according to group allocation, and attainment of informed consent after surgery. To assess the suitability of PCC as a substitute for FFP, the number of patients in the PCC group who ultimately required FFP was recorded. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (1:1 ratio) to study groups using a pseudo‐random number generator (PROC PLAN procedure in SAS) in randomly permuted blocks of 4, stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Allocation was blinded; the randomisation schedule was kept at the blood banks in sequentially numbered opaque sealed envelopes (prepared by Ergomed GmbH), which were opened when the order for PCC or FP was received. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Given that the products have different physical properties, it was not possible to blind treating clinicians to group assignment. To minimise bias, the first set of products was released in weight‐matched, tamper‐sealed containers that were opened immediately before initiating treatment, thereby ensuring that clinicians remained blinded to group allocation until after the decision was made to administer the investigational product. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians not involved in product administration, patients, family members, and all study personnel remained blinded to group assignment. Medical record labels for both products stated “FARES Study Product 1 U.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No patients in the analysis set had missing data on transfusions or adverse events. However once randomised, there were 18% in the PCC group and 36% of patients in the FFP group that did not receive their intervention due to cessation of bleeding prior to the intervention arriving from the laboratory. They were then not included in the analysis. Although clinically this can happen, this could have introduced bias into the results such as the low risk of bleeding patients being excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | Reported on all outcome measures they stated they would in the protocol, even the avoidance of red cell transfusion within 24 hours after start of surgery which was not in the tables, but in the text |
| Other bias | Low risk | N/A |
Koster 2014.
| Study characteristics | ||
| Methods | Study design ‐ case report Study duration ‐ N/A Centres ‐ Heart and Diabetes Center, North Rhine‐Westphalis (Germany) |
|
| Participants | 1 participant ‐ 22 yrs old | |
| Interventions | PCC | |
| Outcomes | Intracardiac thrombus | |
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Mehringer 2018.
| Study characteristics | ||
| Methods | Study design ‐ retrospective chart review Study duration ‐ 1 years and 8 months (April 2015‐December 2016) Centres ‐ TriStar Centennial Medical Centre (USA) |
|
| Participants | 129 patients in total Mean age ‐ Intervention group (PCC) 68 yrs and comparison group (rFVIIa) 64 yrs Gender ‐ Intervention group (PCC) = 34 men (68%) and 22 women (32%) and comparison group (rFVIIa) = 44 men (60%) and 29 women (40%) Inclusion criteria ‐ Patients were eligible for enrolment if they were at least 18 years of age and had any type of cardiothoracic surgery with bleeding requiring intervention. Exclusion criteria ‐ Patients were excluded if they were pregnant, if they received 4‐factor PCC or rFVIIa for indications other than bleeding associated with cardiac surgery, or if they received both 4‐factor PCC and rFVIIa. We did not intentionally exclude patients with underlying prothrombotic or antithrombotic disorders, such as haemophilia or factor V Leiden thrombophilia, but none of the patients included in our study had underlying coagulation disorders of any kind that were documented in the medical record. |
|
| Interventions | Intervention group ‐ PCC (4‐factor) with mean doses and given IV Comparison group ‐ rFVIIa with mean doses and given IV |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (transfusions of FFP) ‐ Thrombotic events (all VTE/arterial thrombosis and PE) Secondary outcomes ‐ Drain output (24 hr and average output in mL) ‐ Hospital stay (days) ‐ Incidence of re‐exploration |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
Rybka 2015.
| Study characteristics | ||
| Methods | Study design ‐ prospective analysis Study duration ‐ not mentioned in study Centres ‐ not mentioned in study |
|
| Participants | 56 patients in total Mean age ‐ Intervention group (PCC) 9 months to 3 years and comparison group (rFVIIa) 7 days to 5.5 years Gender ‐ not mentioned Inclusion criteria ‐ High‐risk congenital cardiac surgery with abnormal coagulation factors post‐operatively Exclusion criteria ‐ Not mentioned |
|
| Interventions | Intervention group ‐ PCC (4‐factor Prothromblex‐600) with mean dose and range and given IV Comparison group ‐ rFVIIa with mean dose and range and given IV |
|
| Outcomes | Primary outcomes ‐ Thrombotic events Secondary outcomes ‐ Blood tests before and after intervention ‐ TEG parameters before and after intervention ‐ Coagulation factor plasma concentrations before and after intervention ‐ Incidence of re‐exploration |
|
| Notes | Funding of trial ‐ not mentioned Conflicts of interest ‐ not mentioned |
|
Tanaka 2013.
| Study characteristics | ||
| Methods | Study design ‐ retrospective analysis Study duration ‐ 3 years and 6 months (December 2008‐May 2012) Centres ‐ University of Pittsburgh Medical Center (USA) |
|
| Participants | 150 patients in total Mean age ‐ Intervention group (PCC) mean + SD = 55.5 +/‐ 16.6 and comparison group (rFVIIa) mean + SD = 57.8 +/‐ 12.6 Gender ‐ Intervention group (PCC) = 35 men (70%) and 15 women (30%) and comparison group (rFVIIa) = 70 (70%) and 30 (30%) women Inclusion criteria ‐ Patients receiving PCC and rFVIIa for persistent non‐surgical bleeding despite 4 FFP, 2 of platelets and 20 units of cryoprecipitate Exclusion criteria ‐ Not mentioned |
|
| Interventions | Intervention group ‐ PCC (3‐factor bebulin and profiline) with median doses and given IV Comparison group ‐ rFVIIa with median doses and given IV |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (transfusions of RBC, platelets, FFP and cryoprecipitate) ‐ Thrombotic events (VTE/arterial) ‐ Mortality (30 day) Secondary outcomes ‐ Drain output (12 hr median output in mL) ‐ Cost ($) |
|
| Notes | Funding of trial ‐ KT has previously given lectures on coagulation (unrelated to prothrombin complex concentrates) and received speaking fees from Baxter and Grifols. Conflicts of interest ‐ nothing to declare |
|
Zweng 2019.
| Study characteristics | ||
| Methods | Study design ‐ retrospective analysis Study duration ‐ 2 years and 6 months (January 2011‐July 2013) Centres ‐ Austin Health (Australia) |
|
| Participants | 592 patients in total with 160 (80 in each group) post‐propensity matching Mean age ‐ Intervention group (PCC) = 66.9 +/‐12.18 and comparison Group (no PCC) = 69.4 +/‐10.5 Gender ‐ Intervention group (PCC) = male 55 (69%), female 25 (31%) and comparison group (no PCC) male 46 (58%), female 34 (42%) Inclusion criteria ‐ All patients admitted to Austin Hospital for cardiac surgery and subsequently received resuscitation with blood products Exclusion criteria ‐ Not mentioned |
|
| Interventions | Intervention group ‐ PCC (3‐factor Prothrombinex VT) with median/mean doses and given IV Comparison group ‐ Standard treatment |
|
| Outcomes | Primary outcomes ‐ Blood products transfused (transfusions of platelets and FFP) ‐ Thrombotic events (VTE/arterial/stroke/TIA/coronary thrombosis) ‐ Mortality (30 day) Secondary outcomes ‐ ICU readmission (within 30 days) |
|
| Notes | Funding of trial ‐ none Conflicts of interest ‐ nothing to declare |
|
ACHD: Adult Congenital Heart Disease AF: Atrial Fibrilation AKI: Acute Kidney Injury B + W: Box and Whiskers CABG: Coronary Artery Bypass Graft CI: Cardiac Index CPB: Cardiopulmonary Bypass CVA: Cerebrovascular Accident DIC: Disseminated Intravascular Coagulation DOAC: Direct Oral Anticoagulants DVT: Deep Vein Thrombosis E‐CABG: Electronic Coronary Artery Bypass Graft ECMO: Extra Corporeal Membrane Oxygenator FC: Fibrinogen Concentrate FFP: Fresh Frozen Plasma FP: Frozen Plasma IABP: Intra Aortic Balloon Pump IgA: Immunoglobulin A IQR: InterQuartile Range IV: Intravenous KDIGO: Kidney Disease Improving Global Outcomes MI: Myocardial Infarction N/A: Not Applicable PCC: Prothrombin Complex Concentrate PE: Pulmonary Embolism RBC: Red Blood Cell rFVIIA: Recombinant Factor VIIa RIFLE: Risk, Injury, Failure, Loss of Kidney Function, End‐Stage Kidney Disease RRT: Renal Replacement Therapy TEG: Thromboelastography TIA: Transient Ischaemic Attack UDPB: Universal Definition of Perioperative Bleeding VSD: Ventricular Septal Defect VT: Ventricular Tachycardia VTE: Venous Thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashikhmina 2017 | Wrong study design |
| Bhatt 2018 | Wrong study design |
| Bobbitt 2011 | Wrong patient population |
| Boswell 2021 | Wrong patient population |
| Bradford 2015 | Wrong patient population |
| Bruce 2008 | Wrong study design |
| Chowdary 2018 | Wrong study design |
| Demeyere 2006 | Wrong study design |
| Ghadmi 2015 | Wrong study design |
| Green 2019 | Ongoing study |
| Harris 2020 | Wrong comparator |
| Jooste 2016 | Wrong intervention |
| Katz 2021 | Wrong patient population |
| Lee 2016 | Wrong study design |
| Lee 2018 | Wrong study design |
| Levy 2014 | Wrong study design |
| Lin 2013 | Wrong study design |
| Maynard 2020 | Wrong study design |
| Mo 2016 | Wrong study design |
| Ortmann 2013 | Wrong patient population |
| Ortmann 2015 | Wrong patient population |
| Phillips 2019 | Wrong intervention |
| Pollock 2017 | Wrong patient population |
| Ranucci 2017 | Wrong study design as was a multifactorial intervention |
| Rao 2016 | Wrong study design |
| Robblee 2012 | Wrong study design |
| Roman 2019 | Wrong study design |
| Santibanez 2018 | Wrong patient population |
| Sarode 2009 | Wrong patient population |
| Stuklis 2001 | Wrong patient population |
| Urbanowicz 2013 | Wrong study design |
| Van den 2020 | Wrong study design |
Characteristics of ongoing studies [ordered by study ID]
NCT02557672.
| Study name | Prothrombin Complex Concentrate (PCC) compared to Fresh Frozen Plasma (FFP) for post‐cardiopulmonary bypass coagulopathy and bleeding, a prospective randomized trial at large US medical centre |
| Methods | Study design ‐ randomised Intervention model ‐ parallel assignment Centres ‐ Mayo Clinic (USA) |
| Participants | Estimated enrolment ‐ 100 patients Inclusion criteria
Exclusion criteria
|
| Interventions | After cardiopulmonary bypass, patients will receive protamine at dose 0.01 mg/unit of heparin given with target activated clotting time (ACT) within 10% of baseline value. After protamine administration, the ACT, complete blood count (CBC), prothrombin time (PT)/international normalised ratio (INR), activated partial thromboplastin time (APTT), and fibrinogen, will be collected via preexisting arterial access. If ACT > 10% baseline, additional protamine will be given at the anaesthesiologist's discretion. Evaluation and determination of excessive microvascular bleeding in the surgical field will occur 10 minutes after return of ACT to within 10% of baseline. Intervention ‐ Patients with clinical evidence of excessive microvascular bleeding in the surgical field as determined by the surgical team, along with a PT > 16.6 sec/INR > 1.6 sec will receive prothrombin complex concentrate (human) 15 units/kg Comparison ‐ Patients with clinical evidence of excessive microvascular bleeding in the surgical field as determined by the surgical team, along with a PT > 16.6 sec/INR > 1.6 sec will receive fresh frozen plasma as this is standard therapy per our institutional algorithm at a dose of 10‐15 mL/kg rounded up to the nearest unit. |
| Outcomes | Primary outcomes 1. Blood loss [time frame: 24 hours] blood loss as collected by chest tube drains 24 hours after surgery. 2. Blood product transfusion [time frame: 24 hours], total quantity of all blood products transfused 24 hours after surgery |
| Starting date | August 2016 |
| Contact information | Gregory Nuttall, MD |
| Notes |
NCT04244981.
| Study name | Efficacy of Prothrombin Complex Concentrate reducing perioperative blood loss in cardiac surgery, compared with Fresh Frozen Plasma: study protocol for a non‐inferiority, randomized controlled trial |
| Methods | Study Design ‐ randomised Intervention model ‐ parallel assignment Centres ‐ Chinese Academy of Medical Sciences, Fuwai Hospital and Peking Union Medical College Hospital (China) |
| Participants | Estimated enrolment ‐ 560 patients Inclusion criteria
Exclusion criteria
|
| Interventions | Intervention group ‐ When APTT is prolonged (> 1.5 times normal), patients will be given a 4‐factor PCC based on the patients' body weight and INR (INR 2‐4, PCC 25 IU/kg; INR 4‐6, PCC 35 IU/kg; INR > 6, PCC 50 IU/kg) Comparison group ‐ When APTT is prolonged (> 1.5 times normal), patients will be given a dose of 10‐15 mL/kg FFP |
| Outcomes | Primary outcomes Volume of blood loss during and within 24 hours after surgery [time frame: during the intraoperative and postoperative period up to 24 hours after surgery], the volume of blood loss during and within 24 hours after surgery Secondary outcomes 1. Total units of allogeneic RBCs transfused during and within 7 days after surgery [time frame: during the intraoperative and postoperative period up to 7 days after surgery], the total units of allogeneic RBC transfused during the intraoperative and postoperative period up to 7 days after surgery 2. Re‐exploration due to postoperative bleeding [time frame: within 7 days after surgery], re‐exploration due to postoperative bleeding within 7 days after surgery |
| Starting date | January 1, 2021 |
| Contact information | Shi Jia, M.D 86 10 88322467 shijia@fuwai.com |
| Notes |
NCT04434001.
| Study name | ZEPLAST‐PED: ZEro_PLASma Trial in small infants undergoing cardiac surgery (ZEPLAST‐PED) |
| Methods | Parallel RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Drug: Prothrombin Complex Concentrate Treatment of acquired postoperatively thrombin generation deficiency as assessed by ROTEM EXTEM test. Other Name: Confidex Control: Fresh Frozen Plasma Treatment of acquired postoperative coagulopathy as assessed by ROTEM FIBTEM and INTEM tests |
| Outcomes | Primary outcome measures Transfusion of Fresh Frozen Plasma (FFP) [time frame: first 48 hours after surgery], number of patients transfused with FFP Secondary outcome measures: Postoperative bleeding [time frame: First 12, 24 and 48 hours after surgery], amount of blood collected by chest drainage Severe bleeding [time frame: First 12 hours after surgery], number of patients who experienced severe bleeding (higher than 30 mL/kg in the first 12 hours after surgery) Surgical re‐exploration for bleeding [time frame: First 12, 24 and 48 hours after surgery], number of patients requiring surgical re‐exploration due to bleeding (bleeding with no coagulopathies detected or refractory to pharmacological treatment) |
| Starting date | February 27, 2020 |
| Contact information | |
| Notes |
ACT: Activated Clotting Time APTT: Activated Partial Thromboplastin Clotting Time CABG: Coronary Artery Bypass Graft Surgery CBC: Complete Blood Count FFP: Fresh Frozen Plasma INR: International Normalised Ratio PCC: Prothrombin Complex Concentrate PT: Prothrombin Time RBC: Red Blood Cell
Differences between protocol and review
We changed the types of interventions that we would compare.
Originally, we stated that we would include all studies that used PCC as monotherapy but not those that used PCC in combination with either of the comparators. On review of the articles and what standard practice is amongst cardiac surgical institutions, we changed the protocol to compare PCCs as monotherapy alone or in combination with FFP, to FFP alone. In many institutions, it is common to use PCC as replacement for factors, and also FFP as a source of volume and fibrinogen in coagulopathy post‐CPB. We would compare this to FFP alone.
We changed the types of participants we would exclude to be more specific.
Originally, we stated we would exclude studies that used PCC for reversal of warfarin or vitamin K antagonists. However, to be more specific, we excluded studies that had a high proportion of patients on preoperative warfarin with abnormal INRs.
We changed the methods from excluding studies with a critical risk of bias. The primary analysis now includes all studies, regardless of their risk of bias.
We originally intended to do sensitivity analyses on adjusted data. Included studies, instead of adjusting, used matching to account for confounders and so we have performed sensitivity analysis on matching instead.
Contributions of authors
KH ‐ Was involved in screening, data extraction, data analysis, writing of the results, grading of the evidence, discussion and conclusion
MF ‐ Was involved in screening, data extraction, data analysis, writing of the results, grading of the evidence, discussion and conclusion
VJ ‐ Was involved in screening, data extraction, data analysis, writing of the results, grading of the evidence, discussion and conclusion
Sources of support
Internal sources
-
Internal sources of support, Other
There were no other sources of support used.
External sources
-
NIHR, UK
This project was supported by the NIHR via Cochrane Infrastructure funding to Cochrane Heart. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
KH ‐ none known.
MF ‐ none known.
VJ ‐ none known.
New
References
References to studies included in this review
Arachchillage 2016 {published data only}
- Arachchillage DJ, Deplano S, Dunnett E, Owen S, Tillyer L, Laffan M. Efficacy and safety of prothrombin complex concentrate in patients undergoing major cardiac surgery. Blood 2016;128(22):6. [Google Scholar]
Arnekian 2012 {published data only}
- Arnekian V, Camous J, Fattal S, Rezaiguia-Delclaux S, Nottin R, Stephan F. Use of prothrombin complex concentrate for excessive bleeding after cardiac surgery. Interactive Cardiovascular and Thoracic Surgery 2012;15(3):382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Audley 2019 {published data only}
- Audley K, Rosini J, Tully A, Zizza L. Use of four-factor prothrombin complex concentrate versus activated factor seven in cardiac surgery. Critical Care Medicine 2019;47:1. [Google Scholar]
Biancari 2019 {published data only}
- Biancari F, Ruggieri VG, Perrotti A, Gherli R, Demal T, Franzese I, et al. Comparative analysis of prothrombin complex concentrate and fresh frozen plasma in coronary surgery. Heart, Lung and Circulation 2019;28(12):1881-7. [DOI] [PubMed] [Google Scholar]
Cappabianca 2016 {published data only}
- Cappabianca G, Mariscalco G, Biancari F, Maselli D, Papesso F, Cottini M, et al. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Critical Care 2016;20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2018 {published data only}
- Fitzgerald J, Lenihan M, Callum J, McCluskey SA, Srinivas C, Van Rensburg A, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. British Journal of Anaesthesia 2018;120(5):928-34. [DOI] [PubMed] [Google Scholar]
- Lenihan M, Fitzgerald J, Callum J, McCluskey S, Srinivas C, Karkouti K. Use of prothrombin complex concentrate versus frozen plasma for bleeding after cardiac surgery. Canadian Journal of Anaesthesia 2018;65(Suppl 1):S15-S16. [DOI] [PubMed] [Google Scholar]
Fraser 2006 {published data only}
- Fraser TA, Corke CF, Mohajeri M, Stevenson L, Campbell PJ. A retrospective audit of the use of Prothrombinex-HT for refractory bleeding following adult cardiac surgery. Critical Care and Resuscitation 2006;8(2):141-5. [PubMed] [Google Scholar]
Giorni 2013 {published data only}
- Giorni C, Ricci Z, Iodice F, Garisto C, Favia I, Polito A, et al. Use of confidex to control perioperative bleeding in pediatric heart surgery: prospective cohort study. Paediatric Cardiology 2013;35(2):208-14. [DOI] [PubMed] [Google Scholar]
Green 2021 {published data only}
- Green L, Roberts N, Cooper J, Agarwal S, Brunskill SJ, Chang I, et al. Prothrombin complex concentrate vs. fresh frozen plasma in adult patients undergoing heart surgery - a pilot randomised controlled trial (PROPHESY trial). Anaesthesia 2021;76(7):892-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Roberts N, Cooper J, Field J, Gill R, Klein A, et al. A pragmatic pilot phase II randomised controlled trial of prothrombin complex concentrates (PCC) versus fresh frozen plasma (FFP) in adult patients who are undergoing heart surgery (PROPHESY). Trials 2019;20(1):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Roberts N, Platton S, O'Brien B, Agarwal S, Gill R, et al. Impact of prothrombin complex concentrate and fresh frozen plasma on correction of haemostatic abnormalities in bleeding patients undergoing cardiac surgery (PROPHESY trial results). Anaesthesia 2021;76(7):997-1000. [DOI] [PubMed] [Google Scholar]
Harper 2018 {published data only}
- Harper PC, Smith MM, Brinkman NJ, Passe MA, Schroeder DR, Said SM, et al. Outcomes following three-factor inactive prothrombin complex concentrate versus recombinant activated Factor VII administration during cardiac surgery. Journal of Cardiothoracic and Vascular Anaesthesia 2018;32(1):151-7. [DOI] [PubMed] [Google Scholar]
Harris 2020a {published data only}
- Harris AD, Hubbard RM, Sam RM, Zhang X, Salazar J, Gautam NK. A retrospective analysis of the use of 3-factor prothrombin complex concentrates for refractory bleeding after cardiopulmonary bypass in children undergoing heart surgery: a matched case-control study. Seminars in Cardiothoracic and Vascular Anaesthesia 2020;24(3):227-31. [DOI] [PubMed] [Google Scholar]
Hashmi 2019 {published data only}
- Hashmi NK, Ghadimi K, Schroder J, Levy J, Welsby IJ. Three factor prothrombin complex concentrates in cardiac surgery: a novel algorithmic approach to perioperative bleeding. Blood 2016;128(22):5035. [Google Scholar]
- Hashmi NK, Ghadimi K, Srinivasan AJ, Li YJ, Raiff RD, Gaca JG, et al. Three-factor prothrombin complex concentrates for refractory bleeding after cardiovascular surgery within an algorithmic approach to haemostasis. Vox Sanguinis 2019;114(4):374-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karkouti 2021 {published data only}
- Bartoszko J, Callum J, Karkouti K. The association of prothrombin complex concentrates with transfusion requirement and postoperative outcomes in cardiac surgery: a post-hoc analysis of the fibres randomized controlled trial. Transfusion 2020;60:252A-3A. [Google Scholar]
- Karkouti K, Bartoszko J, Grewal D, Bingley C, Armali C, Carroll J, et al. Comparison of 4-factor prothrombin complex concentrate with frozen plasma for management of hemorrhage during and after cardiac surgery: a randomized pilot trial. JAMA 2021;4(4):e213936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkouti K, Callum J. The fares study: a multicenter, randomized, active-control, pragmatic, phase 2 pilot study comparing prothrombin complex concentrate versus frozen plasma in bleeding adult cardiac surgical patients. Research and Practice in Thrombosis and Haemostasis 2020;4(Suppl 1):378. [Google Scholar]
Koster 2014 {published data only}
- Koster A, Meyer-Jark T, Schirmer U, Sandica E. Fulminant intraoperative right heart and pulmonary artery thrombosis following prothrombin complex concentrate infusion after complex open heart surgery with cardiopulmonary bypass. A and A Case Reports 2014;2(8):89-91. [DOI] [PubMed] [Google Scholar]
Mehringer 2018 {published data only}
- Mehringer SL, Klick Z, Bain J, McNeely EB, Subramanian S, Pass LJ, et al. Activated factor 7 versus 4-factor prothrombin complex concentrate for critical bleeding post-cardiac surgery. Annals of Pharmacotherapy 2018;52(6):533-7. [DOI] [PubMed] [Google Scholar]
Rybka 2015 {published data only}
- Rybka MM, Samsonova NN, Klimovich LG, Rogalskaya EA, Khichagov DY, Tataryan FE. Correction of hemostasis with blood products in the surgical treatment of congenital heart disease in infants and young children. Anesteziologiia i Reanimatologiia 2015;60(5):42-6. [PubMed] [Google Scholar]
Tanaka 2013 {published data only}
- Tanaka KA, Mazzeffi MA, Grube M, Ogawa S, Chen EP. Three-factor prothrombin complex concentrate and hemostasis after high-risk cardiovascular surgery. Transfusion 2013;53(4):920-1. [DOI] [PubMed] [Google Scholar]
Zweng 2019 {published data only}
- Zweng I, Galvin S, Robbins R, Bellomo R, Hart GK, Seevanayagam S, et al. Initial experience of the use of 3-factor prothrombin complex concentrate and thromboembolic complications after cardiac surgery. Heart, Lung and Circulation 2019;28(11):1706-13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ashikhmina 2017 {published data only}
- Ashikhmina E, Said S, Smith MM, Rodriguez V, Oliver WC Jr, Nuttall GA, et al. Prothrombin complex concentrates in pediatric cardiac surgery: the current state and the future. Annals of Thoracic Surgery 2017;104(4):1423-31. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bhatt 2018 {published data only}
- Bhatt HV, Subramaniam K. PRO: prothrombin complex concentrate should be used in preference to fresh frozen plasma for hemostasis in cardiac surgical patients. Journal of Cardiothoracic and Vascular Anesthesia 2018;32(2):1062-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bobbitt 2011 {published data only}
- Bobbitt L, Merriman E, Raynes J, Henderson R, Blacklock H, Chunilal S. PROTHROMBINEX( R)-VF (PTX-VF) usage for reversal of coagulopathy: prospective evaluation of thrombogenic risk. Thrombosis Research 2011;128(6):577-82. [DOI: ] [DOI] [PubMed] [Google Scholar]
Boswell 2021 {published data only}
- Boswell MR, Stulak JM, Tchantchaleishvili V, Weber MP, Warner MA, Smith BB, et al. Intraoperative prothrombin complex concentrate administration and outcomes in patients undergoing left ventricular assist device implantation. Artificial Organs 2021;45(8):E223-E303. [DOI] [PubMed] [Google Scholar]
Bradford 2015 {published data only}
- Bradford CD, Stahovich MJ, Dembitsky WP, Adamson RM, Engelbert JJ, Perreiter A. Safety of prothombin complex concentrate to control excess bleeding during continuous flow LVAD insertion. ASAIO Journal 2015;61(5):509-13. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bruce 2008 {published data only}
- Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Critical Care 2008;12(4):R105. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chowdary 2018 {published data only}
- Chowdary P, Tang A, Watson D, Besser M, Collins P, Creagh MD, et al. Retrospective review of a prothrombin complex concentrate (Beriplex P/N) for the management of perioperative bleeding unrelated to oral anticoagulation. Clinical and Applied Thrombosis/Hemostasis 2018;24(7):1159-69. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demeyere 2006 {published data only}
- Demeyere R, Arnout J, Strengers P, Gasthuisberg UZ. Prothrombin complex concentrate versus fresh frozen plasma in patients on oral anticoagulant therapy undergoing cardiac surgery: a randomized study. Critical Care 2006;10(Suppl 1):233. [Google Scholar]
Ghadmi 2015 {published data only}
- Ghadmi K, Hashmi N, Levy J, Ortel TL, Welsby IJ. Prothrombin complex concentrates and cardiac surgery. Blood 2015;126(23):3. [Google Scholar]
Green 2019 {published data only}
- Green L, Roberts N, Cooper J, Field J, Gill R, Klein A, et al. A pragmatic pilot phase II randomised controlled trial of prothrombin complex concentrates (PCC) versus fresh frozen plasma (FFP) in adult patients who are undergoing heart surgery (PROPHESY). Trials 2019;20(1):684. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2020 {published data only}
- Harris JE, Varnado S, Herrera E, Salazar E, Colavecchia AC. Evaluation of postoperative clinical outcomes in Jehovah's Witness patients who receive prothrombin complex concentrate during cardiac surgery. Journal of Cardiac Surgery 2020;35(4):801-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jooste 2016 {published data only}
- Jooste EH, Machovec KA, Einhorn LM, Ames WA, Homi HM, Jaquiss RD, et al. 3-factor prothrombin complex concentrates in infants with refractory bleeding after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2016;30(6):1627-31. [DOI: ] [DOI] [PubMed] [Google Scholar]
Katz 2021 {published data only}
- Katz A, Ahuja T, Arnouk S, Lewis TC, Marsh K, Papadopoulos J, et al. A comparison of prothrombin complex concentrate and recombinant activated factor VII for the management of bleeding with cardiac surgery. Journal of Intensive Care Medicine 2021;37(2):231-9. [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee JK, Ing C. Prothrombin complex concentrate and methylene blue for treatment of coagulopathy and vasoplegia in a pediatric heart transplant patient. A and A Case Reports 2016;6(5):127-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee S, De Leon N, Thompson A, Baumgartner C, Fang M, Prasad P. Use of 4-factor prothrombin complex concentrate in cardiothoracic surgery. Critical Care Medicine 2018;46(1):608. [DOI: 10.1097/01.ccm.0000529252.47169.fe] [DOI] [Google Scholar]
Levy 2014 {published data only}
- Levy JH. Editorial comment: "coronary artery bypass grafting in 2 thrombophilic patients with protein s deficiency" and "fulminant intraoperative right heart and pulmonary artery thrombosis following prothrombin complex concentrate infusion after complex open heart surgery with cardiopulmonary bypass". A and A Case Reports 2014;2(8):95. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lin 2013 {published data only}
- Lin DM, Murphy LS, Tran MH. Use of prothrombin complex concentrates and fibrinogen concentrates in the perioperative setting: a systematic review. Transfusion Medicine Reviews 2013;27(2):91-104. [DOI: ] [DOI] [PubMed] [Google Scholar]
Maynard 2020 {published data only}
- Maynard K, Falvey J, Groth C. Use of four-factor prothrombin complex concentrate for coagulopathic bleeding during cardiac surgery. Critical Care Medicine 2020;48:1. [Google Scholar]
Mo 2016 {published data only}
- Mo A, Matalanis G, Hong F, Smith C. Impact of prothrombin complex concentrate (PCC) use on transfusion requirements and blood loss in cardiac surgery. Haematologica 2016;101:134. [Google Scholar]
Ortmann 2013 {published data only}
- Ortmann E, Besser MW, Sharples LD, Gerrard C, Berman M, Jenkins DP, et al. Efficacy and safety of prothrombin complex concentrate vs fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anaesthesia 2013;68(10):1088. [DOI] [PubMed] [Google Scholar]
Ortmann 2015 {published data only}
- Ortmann E, Besser MW, Sharples LD, Gerrard C, Berman M, Jenkins DP, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesthesia and Analgesia 2015;121(1):26-33. [DOI: ] [DOI] [PubMed] [Google Scholar]
Phillips 2019 {published data only}
- Phillips J, Nasef R, Kablaoui F, Cherfan A. Use of factor concentrates for the management of perioperative bleeding in cardiac surgery. Critical Care Medicine 2019;47(1):48. [Google Scholar]
Pollock 2017 {published data only}
- Pollock KA, McMillan Z, Pretorius GD, Cronin B. Low cardiac output after orthotopic heart transplant secondary to large inferior vena cava thrombus after prothrombin complex: a case report. A and A Case Reports 2017;8(3):43-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ranucci 2017 {published data only}
- Ranucci M, Baryshnikova E, Pistuddi V, Menicanti L, Frigiola A, Surgical and Clinical Outcome REsearch (SCORE) Group. The effectiveness of 10 years of interventions to control postoperative bleeding in adult cardiac surgery. Interactive Cardiovascular and Thoracic Surgery 2017;24(2):196-202. [DOI] [PubMed] [Google Scholar]
Rao 2016 {published data only}
- Rao SL, Salamanca YP, Pae WE. Use of prothrombin complex concentrates (PCCs) in patients undergoing heart transplant surgery: clinical experience at a university hospital. Journal of Heart and Lung Transplantation 2016;35(4):S290-S291. [DOI: 10.1016/j.healun.2016.01.828] [DOI] [Google Scholar]
Robblee 2012 {published data only}
- Robblee JA, Wilkes PR, Dickie SJ, Rubens FD, Bormanis J. Bleeding in a Jehovah's Witness patient undergoing a redo aortic valve replacement controlled with cryoprecipitate and a prothrombin complex concentrate. Canadian Journal of Anaesthesia 2012;59(3):299-303. [DOI: ] [DOI] [PubMed] [Google Scholar]
Roman 2019 {published data only}
- Roman M, Biancari F, Ahmed AB, Agarwal S, Hadjinikolaou L, Al-Sarraf A, et al. Prothrombin complex concentrate in cardiac surgery: a systematic review and meta-analysis. Annals of Thoracic Surgery 2019;107(4):1275-83. [DOI: ] [DOI] [PubMed] [Google Scholar]
Santibanez 2018 {published data only}
- Santibanez M, Lesch CA, Lin L, Berger K. Tolerability and effectiveness of 4-factor prothrombin complex concentrate (4F-PCC) for warfarin and non-warfarin reversals. Journal of Critical Care 2018;48:183-90. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sarode 2009 {published data only}
- Sarode R, Matevosyan K. Prothrombin complex concentrate and fatal thrombosis: poor evidence to implicate a relatively safe drug. Annals of Emergency Medicine 2009;54(3):481-2. [DOI: ] [DOI] [PubMed] [Google Scholar]
Stuklis 2001 {published data only}
- Stuklis RG, O'Shaughnessy DF, Ohri SK. Novel approach to bleeding in patients undergoing cardiac surgery with liver dysfunction. European Journal of Cardio-Thoracic Surgery 2001;19(2):219-20. [DOI] [PubMed] [Google Scholar]
Urbanowicz 2013 {published data only}
- Urbanowicz T, Puslecki M, Budniak W, Buczkowski P, Tomczyk J, Walczak M, et al. Plasma hemostasis disturbances after heart transplantation procedure corrected by of human prothrombin complex. Acta Haematologica Polonica 2013;44(1):63-6. [DOI: ] [Google Scholar]
Van den 2020 {published data only}
- Van den Brink DP, Wirtz MR, Neto AS, Schochl H, Viersen V, Binnekade J, et al. Effectiveness of prothrombin complex concentrate for the treatment of bleeding: a systematic review and meta-analysis. Journal of Thrombosis and Haemostasis 2020;18(10):2457-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02557672 {unpublished data only}
- NCT02557672. PCC vs. FFP for post cardiopulmonary bypass coagulopathy and bleeding. clinicaltrials.gov/show/NCT02557672 (first received 23 September 2015).
NCT04244981 {unpublished data only}
- NCT04244981. Efficacy of prothrombin complex concentrate reducing perioperative blood loss in cardiac surgery. clinicaltrials.gov/show/NCT04244981 (first received 28 January 2020).
NCT04434001 {published data only}
- NCT04434001. ZEPLAST- PED: zEro_PLASma trial in small infants undergoing cardiac surgery. protect-au.mimecast.com/s/Uq9fCyoj4JiL8JwysR34FhX?domain=clinicaltrials.gov (first received 16 June 2020).
Additional references
Achneck 2010
- Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: from vascular endothelium to circulatory assist device surface. Circulation 2010;122(20):2068-77. [DOI] [PubMed] [Google Scholar]
Arias‐Morales 2017
- Arias-Morales CE, Stoicea N, Gonzalez-Zacarias AA, Slawski D, Bhandary SP, Saranteas T, et al. Revisiting blood transfusion and predictors of outcome in cardiac surgery patients: a concise perspective. F1000Research 2017;6:168-75. [DOI: 10.12688/f1000research.10085.1. eCollection 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Behring
- CS Behring. Product Information - Prothrombinex-VF. www.cslbehring.com.au/-/media/cslb-australia/documents/aus-pis-and-cmis/prothrombinexvf-au-pi-1300.pdf?la=en-us&hash=BC1625CF859B45E164CA759E7B871216243CFFBE (date accessed 16 December 2019).
Bordeleau 2015
- Bordeleau S, Poitras J, Marceau D, Breton C, Beaupre P, Archambault PM. Use of prothrombin complex concentrate in warfarin anticoagulation reversal in the emergency department: a quality improvement study of administration delays. BMC Health Services Research 2015 Mar 15 [Epub ahead of print];15:106. [DOI: 10.1186/s12913-015-0775-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bortolussi 2019
- Bortolussi G, McNulty D, Waheed H, Mawhinney JA, Freemantle N, Pagano D. Identifying cardiac surgery operations in hospital episode statistics administrative database, with an OPCS-based classification of procedures, validated against clinical data. BMJ Open 2019;9(3):e023316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradford 2015
- Bradford CD, Stahovich MJ, Dembitsky WP, Adamson RM, Engelbert JJ, Perreiter AS. Safety of Prothombin Complex Concentrate to control excess bleeding during continuous flow LVAD insertion. American Society for Artificial Internal Organs Journal 2015;61(5):509-13. [DOI] [PubMed] [Google Scholar]
Cappabianca 2016
- Cappabianca G, Mariscalco G, Biancari F, Maselli D, Papesso F, Cottini M, et al. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Critical Care 2016 Jan 6 [Epub ahead of print];20(5):5. [DOI: 10.1186/s13054-015-1172-6] [DOI] [PMC free article] [PubMed]
Cartmill 2000
- Cartmill M, Dolan G, Byrne JL, Byrne PO. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. British Journal of Neurosurgery 2000;14(5):458-61. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 5 March 2020. Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Denault 2014
- Denault A, Lamarche Y, Rochon A, Cogan J, Liszkowski M, Lebon JS, et al. Innovative approaches in the perioperative care of the cardiac surgical patient in the operating room and intensive care unit. Canadian Journal of Cardiology 2014;30(12 Suppl):S459-77. [DOI] [PubMed] [Google Scholar]
Erdoes 2021
- Erdoes G, Koster A, Ortmann E, Meesters MI, Bolliger D, Baryshnikova E, et al. A European consensus statement on the use of four-factor prothrombin complex concentrate for cardiac and non-cardiac surgical patients. Anaesthesia 2021;76(3):381-92. [DOI] [PubMed] [Google Scholar]
Estrada 2016
- Estrada VH, Franco DL, Moreno AA, Gambasica JA, Nunez CC. Postoperative right ventricular failure in cardiac surgery. Cardiology Research 2016;7(6):185-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
European Medicine Authority
- European Medicine Authority. Committee for Medical Products for Human Use (CHMP) core SPC for human prothrombin complex products (CPMP/BPWG/3735/02). www.ema.europa.eu/en/documents/scientific-guideline/core-spc-human-prothrombin-complex-products-cpmp/bpwg/3735/02_en.pdf (accessed 5 March 2020).
Felton 2016
- Felton D, Foley EM, Traub SJ, Vodonos A, Ganetsky M. Risk of venous thromboembolism after receiving prothrombin complex concentrate for warfarin-associated intracranial hemorrhage. Journal of Emergency Medicine 2016;50(1):1-6. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2018
- Fitzgerald J, Lenihan M, Callum J, McCluskey SA, Srinivas C, Van Rensburg A, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. British Journal of Anaesthesia 2018;120(5):928-34. [DOI] [PubMed] [Google Scholar]
Franchini 2010
- Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfusion 2010;8(3):149-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghadimi 2016
- Ghadimi K, Levy JH, Welsby IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesthesia and Analgesia 2016;122(5):1287-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed after 2 March 2020. Available at gradepro.org.
Green 2019
- Green L, Roberts N, Cooper J, Field J, Gill R, Klein A, et al. A pragmatic pilot phase II randomised controlled trial of prothrombin complex concentrates (PCC) versus fresh frozen plasma (FFP) in adult patients who are undergoing heart surgery (PROPHESY). Trials 2019;20(1):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanke 2013
- Hanke AA, Joch C, Gorlinger K. Long-term safety and efficacy of a pasteurized nanofiltrated prothrombin complex concentrate (Beriplex P/N): a pharmacovigilance study. British Journal of Anaesthesia 2013;110(5):764-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harper 2018
- Harper PC, Smith MM, Brinkman NJ, Passe MA, Schroeder DR, Said SM, et al. Outcomes following three-factor inactive prothrombin complex concentrate versus recombinant activated factor VII administration during cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2018;32(1):151-7. [DOI] [PubMed] [Google Scholar]
Hashmi 2019
- Hashmi NK, Ghadimi K, Srinivasan AJ, Li YJ, Raiff RD, Gaca JG, et al. Three-factor prothrombin complex concentrates for refractory bleeding after cardiovascular surgery within an algorithmic approach to haemostasis. Vox Sanguinis 2019;114(4):374-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions, version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019), Cochrane 2019. Available from www.training.cochrane.org/handbook.
Höfer 2016
- Höfer J, Fries D, Solomon C, Velik-Salchner C, Ausserer J. A snapshot of coagulopathy after cardiopulmonary bypass. Clinical and Applied Thrombosis/Hemostasis 2016;22(6):505-11. [DOI] [PubMed] [Google Scholar]
Ishikura 2017
- Ishikura H, Kitamura T. Trauma-induced coagulopathy and critical bleeding: the role of plasma and platelet transfusion. Journal of Intensive Care 2017;5(2):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaspereit 2010
- Kaspereit F, Hoffmann S, Pragst I, Dickneite G. Prothrombin complex concentrate mitigates diffuse bleeding after cardiopulmonary bypass in a porcine model. British Journal of Anaesthesia 2010;105(5):576-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilic 2014
- Kilic A, Whitman GJ. Blood transfusions in cardiac surgery: indications, risks, and conservation strategies. Annals of Thoracic Surgery 2014;97(2):726-34. [DOI] [PubMed] [Google Scholar]
Köhler 1999
- Köhler M. Thrombogenicity of prothrombin complex concentrates. Thrombosis Research 1999;95(4 Suppl 1):S13-7. [DOI] [PubMed] [Google Scholar]
Koster 2014
- Koster A, Meyer-Jark T, Schirmer U, Sandica E. Fulminant intraoperative right heart and pulmonary artery thrombosis following prothrombin complex concentrate infusion after complex open heart surgery with cardiopulmonary bypass. A & A Case Reports 2014;2(8):89-91. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nascimento 2010
- Nascimento B, Callum J, Rubenfeld G, Neto JR, Lin Y, Rizoli S. Clinical review: fresh frozen plasma in massive bleedings - more questions than answers. Critical Care 2010;14(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nashef 2012
- Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. European Journal of Cardiothoracic Surgery 2012;41(4):734-45. [DOI] [PubMed] [Google Scholar]
NBAA
- National Blood Authority Australia. Point of care coagulation testing. www.blood.gov.au/system/files/documents/%5BFinal%5D%20Point%20of%20Care%20Coagulation%20Testing%20Case%20Study.pdf (accessed 5 March 2020).
O'Carroll‐Kuehn 2007
- O'Carroll-Kuehn BU, Meeran H. Management of coagulation during cardiopulmonary bypass. Continuing Education in Anaesthesia, Critical Care and Pain 2007;7(6):195-8. [Google Scholar]
Ortmann 2015
- Ortmann E, Besser MW, Sharples LD, Gerrard C, Berman M, Jenkins DP, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anesthesia and Analgesia 2015;121(1):26-33. [DOI] [PubMed] [Google Scholar]
Ostermann 2007
- Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thrombosis and Haemostasis 2007;98(4):790-7. [PubMed] [Google Scholar]
Pabinger 2010
- Pabinger I, Tiede A, Kalina U, Knaub S, Germann R, Ostermann H. Impact of infusion speed on the safety and effectiveness of prothrombin complex concentrate: a prospective clinical trial of emergency anticoagulation reversal. Annals of Hematology 2010;89(3):309-16. [DOI] [PubMed] [Google Scholar]
Pagano 2018
- Pagano D, Milojevic M, Meesters MI, Benedetto U, Bolliger D, Heymann C, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery: the task force on patient blood management for adult cardiac surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiology. European Journal of Cardio-thoracic Surgery 2018;53(1):79-111. [DOI] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019), Cochrane 2019. Available from www.training.cochrane.org/handbook.
Raphael 2019
- Raphael J, Mazer CD, Subramani S, Schroeder A, Abdalla M, Ferreira R, et al. Society of Cardiovascular Anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Journal of Cardiothoracic and Vascular Anesthesia 2019;33(11):2887-99. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Riess 2007
- Riess HB, Meier-Hellmann A, Motsch J, Elias M, Kursten FW, Dempfle CE. Prothrombin complex concentrate (Octaplex) in patients requiring immediate reversal of oral anticoagulation. Thrombosis Research 2007;121(1):9-16. [DOI] [PubMed] [Google Scholar]
Roman 2019
- Roman M, Biancari F, Ahmed AB, Agarwal S, Hadjinikolaou L, Al-Sarraf A, et al. Prothrombin complex concentrate in cardiac surgery: a systematic review and meta-analysis. Annals of Thoracic Surgery 2019;107(4):1275-83. [DOI] [PubMed] [Google Scholar]
Schünemann 2019a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019), Cochrane 2019. Available from www.training.cochrane.org/handbook.
Schünemann 2019b
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2019;111:105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddon 2016
- Siddon AJ, Tormey CA. Successful use of four factor-prothrombin complex concentrate for congenital factor X deficiency in the setting of neurosurgery. Laboratory Medicine 2016;47(3):e35-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2014
- Song HK, Tibayan FA, Kahl EA, Sera VA, Slater MS, Deloughery TG, et al. Safety and efficacy of prothrombin complex concentrates for the treatment of coagulopathy after cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 2014;147(3):1036-40. [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sørensen 2011
- Sørensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: prothrombin complex concentrates - evaluation of safety and thrombogenicity. Critical Care 2011;15(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanaka 2010
- Tanaka KA, Szlam F. Treatment of massive bleeding with prothrombin complex concentrate: argument for. Journal of Thrombosis and Haemostasis 2010;8(12):2589-91. [DOI] [PubMed] [Google Scholar]
Unold 2015
- Unold D, Tormey CA. Clinical applications of 4-factor prothrombin complex concentrate: a practical pathologist's perspective. Archives of Pathology and Laboratory Medicine 2015;139(12):1568-75. [DOI] [PubMed] [Google Scholar]
Van den Brink 2020
- Van den Brink DP, Wirtz MR, Neto AS, Schöchl H, Viersen V, Binnekade J, et al. Effectiveness of prothrombin complex concentrate for the treatment of bleeding: a systematic review and meta-analysis. Journal of Thrombosis and Haemostasis 2020;18(10):2457-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Veen 2007
- Van Veen JJ, Hampton KK, Maclean R, Fairlie F, Makris M. Blood product support for delivery in severe factor X deficiency: the use of thrombin generation to guide therapy. Blood Transfusion 2007;5(4):204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veluz 2017
- Veluz JS, Leary MC. Cerebrovascular complications of cardiac surgery. In: Primer on Cerebrovascular Diseases. 2nd edition. Elsevier, 2017:650-5. [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazer 2010
- Yazer MH. The how's and why's of evidence based plasma therapy. Korean Journal of Hematology 2010;45(3):152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


