Abstract
Hirsuteine is extracted from Uncaria rhynchophylla, the bark of which has traditionally been used to treat hypertension, cancer, convulsions, hemorrhage, auto-immune disorders, and other ailments. The anticancer properties of hirsuteine are of significant importance to the research community; however, its underlying mechanism of action is not well understood. The aim of the present study was to examine the antiproliferative ability of hirsuteine using human breast cancer MDA-MB-453 cells and to determine the underlying molecular mechanism involved in its therapeutic efficacy. The effects of hirsuteine on cell viability were determined using CCK-8 and colony formation assays, while apoptosis was assessed using flow cytometry. Cell cycle distribution was assessed using flow cytometry, and apoptotic cell quantification was performed using via Annexin V-FITC/PI staining and flow cytometry. Reverse transcription-quantitative PCR and western blotting were used to assess the expression of cell cycle progression and apoptosis associated genes and proteins. MDA-MB-453 cell proliferation was significantly reduced by hirsuteine in a concentration and time-dependent manner. Hirsuteine-treated cells exhibited G2/M phase arrest, as evidenced by the increase in G2/M phase cells and a decrease in the G0/G1 phase cells, and this was related to cyclin B1 and CDK1 downregulation. Furthermore, hirsuteine accelerated MDA-MB-453 cell apoptosis by downregulating Bcl-2 while upregulating cytoplasmic cytochrome c, Bax, Apaf1, cleaved caspase-3, and cleaved caspase-9 levels, which together drove apoptotic cell death. Thus, hirsuteine suppressed MDA-MB-453 cancer cell proliferation by inducing cell cycle arrest and promoting apoptosis.
Keywords: hirsuteine, breast cancer, cell cycle arrest, MDA-MB-453 cells, apoptosis
Introduction
The 2020 Global Cancer Statistics report published by the International Agency for Research on Cancer showed that in 2020, female breast cancer (BC) was the leading cause of cancer-associated death in women worldwide, with 2,261,419 new cases, accounting for 24.5% of all new cancer cases in women. Additionally, there were 684,996 deaths, accounting for 15.5% of all cancer-associated deaths in 2020 (1). Metastatic BC remains a major contributor to cancer-associated mortality in women. Multiple reports have shown that 20–30% of BC patients have distant metastases when first diagnosed (2–4). Gene expression profiling has had a considerable impact on our understanding of breast cancer biology. In the past 15 years, technological progress has revealed the emergence of at least five different molecular subtypes (Luminal A, Luminal B, HER-2 enriched, Basal-like and Claudin low) and normal breast like groups, which are based on gene expression clustering (5). According to the biological characteristics of the different subtypes, different targeted therapeutics are used in the clinic. For example, trastuzumab, an anti-HER2 drug, has significantly changed the therapeutic field of BC management in the past 20 years (6,7). However, its concomitant cardiotoxicity and the chances of acquired drug resistance remain significant challenges that need to be overcome. Trodelvy is a targeted drug for the treatment of triple-negative BC, although it is associated with adverse reactions such as neutropenia, a decrease in white blood cell count and anaemia (8–11). Cell cycle arrest has been used in the field of BC treatment with CDK inhibitors such as Palbociclib, Ribociclip, and Abemaciclib (12). Since the approval of Palbociclib by the FDA in 2015, CDK4/6 inhibitors have become a first-line treatment option for patients with metastatic hormone receptor-positive (HR+)/HER2-negative (HER-2−) BC (13,14).
The use of CDK4/6 inhibitors alone or in combination with other drugs has also become a research hotspot in recent years (15–18). Despite significant progress in the field of chemotherapy-based treatments for BC, as well as anti-angiogenic therapy, immunotherapy, targeted therapy, and other emerging therapies, the clinical effects of these approaches remain unsatisfactory, and the effective rate of immunotherapy is generally low (19,20). Therefore, there is an urgent need to identify novel treatment strategies for BC.
Phytochemicals are promising sources for the development of novel cancer therapeutics, due to their potential efficiency and low toxicity profiles (21,22), and they have generally been shown to be promising for the development of novel agents in the management of numerous diseases (23,24). Plant phytochemicals represent an exciting opportunity for improving an individual's general health through a balanced and appropriate diet, and have also been considered suitable options for identifying novel therapeutic agents.
Traditional Chinese Medicines (TCMs) have been used to treat numerous human diseases in China for thousands of years (25,26), and have served as a remarkable source for drug discovery (27). In the present study, the effect of hirsuteine (Fig. 1), an active compound extracted from the traditional well-known Chinese herb Uncaria rhynchophylla, on BC was assessed in vivo. Several studies have shown that hirsuteine possesses a number of therapeutically relevant properties, especially for treating central nervous system and cardiovascular disorders, namely, hypertension, epilepsy, dizziness, convulsion, preeclampsia, and tremor, amongst others, initially confirming the efficacy and safety (28–30).
Figure 1.
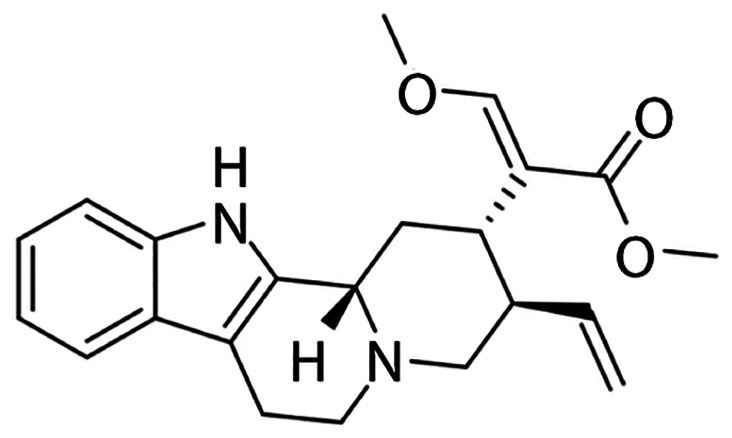
Chemical structure of hirsuteine (MW=366.45).
However, the antiproliferative activity and the underlying mechanisms by which hirsuteine reduces cancer development and progression have not been determined. Cancer progression is dependent on its unique ability to escape programmed cell death (31). Apoptosis is usually initiated by the death receptor (DR) (extrinsic) or mitochondrial (intrinsic) axis, and it functions to eliminate injured cells to maintain homeostasis (32). In both axes, active caspase 3 and 7 cleave poly-ADP ribose polymerase 1 (PARP1) following DNA degeneration (33). The intrinsic network is mitochondrial-based apoptosis, which involves cytochrome c release and activation of caspase-9, which in turn activates caspase-3. The extrinsic network is dependent on DR stimulation, which in turn induces the FAS-related death domain (FADD) and generates the death-induced signal complex, which regulates downstream caspases-8, −7, −6, and −3 (34,35).
Research on the active ingredients of botanicals have received increasing attention. Our research group has primarily studied the TCM Uncaria rhynchophylla, and hirsuteine is an alkaloid extracted from Uncaria rhynchophylla, which has a significant inhibitory effect on several cancer cell lines (36,37). Our previous study demonstrated that hirsuteine is cytotoxic to numerous tumor cells in vitro (38). Nevertheless, despite its valuable properties, little is known regarding its antitumor capacity and the possible mechanism of hirsuteine in BC. Therefore, determining the underlying mechanisms by which hirsuteine affects BC cancer development and progression was assessed in the present study.
The aim of the present study was to examine the possible anticancer effects of hirsuteine and the molecular mechanisms underlying its therapeutic efficacy. In particular, hirsuteine-mediated regulation of MDA-MB-453 cell apoptosis was assessed. The distribution of cells in the cell cycle and apoptosis induction were examined in MDA-MB-453 cells.
Materials and methods
Chemicals and reagents
Hirsuteine (ST17300105; 5 mg/dose; purity ≥98%) was acquired from Shanghai Standard Technology Co., Ltd. The CCK-8 Detection kit, BSA, and Annexin V-FITC Detection kit were acquired from Beyotime Institute of Biotechnology. B-cell lymphoma-2 (Bcl-2; ab32124; dilution, 1:1,000), Bcl-2-associated X protein (Bax; ab32503; dilution, 1:1,000), Cyclin B1 (ab32053; dilution, 1:1,000), CDK1 (ab133327; dilution, 1:1,000), Apaf1 (ab234436; dilution, 1:1,000), cytochrome C (ab133504; dilution, 1:1,000), cleaved-caspase3 (ab32042; dilution, 1:1,000), cleaved-caspase9 (ab2324; dilution, 1:1,000), cleaved-PARP1 (ab32064; dilution, 1:1,000), β-actin (ab8227; dilution, 1:5,000) antibodies, goat anti-rabbit IgG secondary antibody (ab6721; dilution, 1:20,000) were purchased from Abcam. Other reagents were analytical reagent grade and from commercial sources.
Cell culture
Human BC MDA-MB-453, MDA-MB-231, and MCF-7 cells were acquired from American Type Culture Collection. Cells were cultured using RPMI 1640 or DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml). Normal human breast cells Hs 578Bst, human normal lung epithelial cell BEAS-2B, and normal human hepatocyte THLE-2 cells were obtained from Nanjing KeyGen Biotech Co., Ltd. and cultured in DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml) in a humidified incubator supplied with 5% CO2 at 37°C.
CCK-8 assay
Cells were plated in 96-well culture plates (1×104 cells/well) overnight and then treated with a range of hirsuteine concentrations (0, 2.5, 5, 10, 20, 40, or 80 µM) for 24, 48 and 72 h, as described previously (38). For Hs 578Bst, THLE-2, and BEAS-2B cells, assay protocols were as above, with cells being treated for 48 h with various concentrations of hirsuteine (0, 2.5, 5, 10, 20, 40, or 80 µM) prior to addition of the CCK-8 reagent. The 50% growth inhibition (IC50) value was determined using a hirsuteine survival concentration curve. All experiments were performed at least three times.
Colony formation assay
Cells were plated in 6-well culture plates at 2–5×103 cells per well for 24 h. Cells were then exposed to varying hirsuteine concentrations (0, 5, 10, or 25 µM) for 48 h, after which the hirsuteine-containing media was removed, and fresh media was added. The cells were then cultured for 14 days in supplemented media. Finally, cells were fixed in methanol at room temperature for 15 min, dyed with 1% crystal violet solution at room temperature for 10 min, washed with PBS three times, dried in the room and observed under a microscope, with images captured. The number of colonies consisting of ≥50 cells was counted.
Apoptosis staining
Cells were exposed to varying concentrations of hirsuteine (0, 5, 10, or 25 µM) for 48 h and apoptosis was measured as described previously (38).
Cell cycle analysis
Briefly, a six-well plate was seeded with 3×105 cells per well. After incubation, the media was replaced with supplemented media containing a range of hirsuteine concentrations (0, 5, 10, or 25 µM) for an additional 48 h. Cells were then detached using 0.25% trypsin, and centrifuged at 4°C for 5 min at 300 × g, washed once with PBS, and fixed overnight at 4°C with 70% chilled ethanol. Subsequently, the cells were centrifuged again as above, fully suspending the cells after treating with 100 µl RNase A (50 µg/ml) at 37°C in a water bath for 30 min, prior to a 30 min staining at 4°C in the dark with 400 µl PI (50 µg/ml). All samples were evaluated using a FACScan flow cytometer (BD Biosciences). Data were analyzed using CellQuest Pro software, version 5.1 (BD Biosciences). All experiments were performed three times independently.
Western blotting
Following 48 h of treatment with hirsuteine (0, 5, 10, or 25 µM), chilled RIPA buffer was used for cell lysis for 30 min. Extracted proteins were resolved and transferred as described previously (38). The membranes were blocked for 1 h at 37°C with 5% milk in Tris-buffered saline (TBS) containing 0.05% Tween-20 (TBST) and then incubated with cleaved caspase-3, cleaved caspase-9, cleaved CARP, Bax, Bcl-2, Apaf1, cytochrome c, CDK1, Cyclin B1 and β-actin (Abcam) antibodies at 4°C overnight. Membranes were then washed using TBST and incubated with the goat anti-rabbit IgG secondary antibody (Abcam) at room temperature for 2 h. Immunoblotted proteins were analyzed with the ChemiDoc XRS imaging system and QuantityOne software (Version 4.6.9; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR (RT-qPCR)
Cells were treated with different concentrations of hirsuteine for 48 h. Total RNA was isolated from cells using TRIzol® reagent followed by reverse transcription to cDNA using a QuantiTect Reverse Transcription kit, according to the manufacturer's protocol. Subsequently, qPCR was performed using ChamQ™ SYBR qPCR MasterMix in triplicate according to the manufacturer's protocol. Each reaction was conducted in duplicate using the following thermocycling conditions: Initial denaturation for 10 min at 95°C, followed by 40 cycles of denaturation for 30 sec at 95°C, annealing for 30 sec at 60°C and extension for 20 sec at 72°C. The sequences of the primers were: Bax forward, 5′-AAGAAGCTGAGCGAGTGTCT-3′ and reverse, 5′-GTTCTGATCAGTTCCGGCAC-3′; Bcl-2 forward, 5′-GCCTTCTTTGAGTTCGGTGG-3′ and reverse, 5′-GAAATCAAACAGAGGCCGCA-3′; caspase-3 forward, 5′-ACTGGACTGTGGCATTGAGA-3′ and reverse, 5′-GCACAAAGCGACTGGATGAA-3′; caspase-9 forward, 5′-ACATGCTGGCTTCGTTTCTG-3′ and reverse, 5′-TCTCAAGAGCACCGACATCA-3′; and GAPDH forward, 5′-TCAAGAAGGTGGTGAAGCAGG-3′ and reverse, 5′-TCAAAGGTGGAGGAGTGGGT-3′. GAPDH was used as the loading control. Expression was quantified using the 2−∆∆Cq method (39).
Statistical analysis
Data are presented as the mean ± SD from three independent repeats. Statistical comparisons were performed by one-way analysis of variance, followed by Bonferroni's test. P<0.05 was considered to indicate a statistically significant difference. Data were analyzed using GraphPad Prism version 6.0 (GraphPad Software Inc.).
Results
Cytotoxicity of hirsuteine against human BC MDA-MB-453 cells
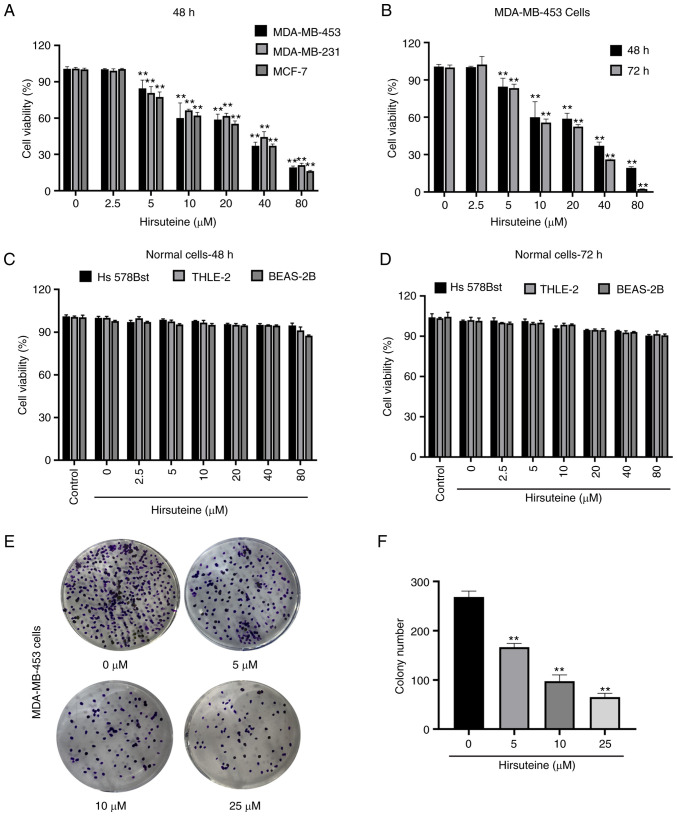
Hirsuteine-mediated regulation of MDA-MB-453, MDA-MB-231, and MCF-7 cell viability was assessed using a CCK-8 assay (Fig. 2A). Hirsuteine lowered MDA-MB-453 cell viability in a concentration and time-dependent manner (Fig. 2B). Next, we explored the impact of hirsuteine on the proliferation of Hs 578Bst, BEAS-2B, and human hepatocyte THLE-2 cells using a CCK-8 assay. The results showed that the cell viability after hirsuteine treatment in normal cells was >80%, which was considered non-toxic for the non-cancerous cell lines after 48 and 72 h of treatment at doses up to and including 80 µM (Fig. 2C and D). As indicated in Fig. 2E and F, hirsuteine markedly reduced colony formation in a concentration-dependent manner. Based on the results, hirsuteine inhibited MDA-MB-453 cell proliferation and lowered MDA-MB-453 colony formation activity, whilst not exerting a notable effect on healthy human cell lines at the same doses.
Figure 2.
Hirsuteine suppresses MDA-MB-453 cell proliferation and colony formation. (A) The proliferative capacity was assessed in MDA-MB-453, MDA-MB-231, and MCF-7 cells following treatment with hirsuteine for 48 h using CCK-8 assays. (B) Time-dependent MDA-MB-453 cell proliferation curve following treatment with varying hirsuteine concentrations. (C and D) Normal Hs 578Bst, THLE-2, and BEAS-2B were exposed for 48 and 72 h to a series of hirsuteine concentrations, prior to CCK-8 analysis. (E and F) Colony formation assays using MDA-MB-453 cells following treatment with hirsuteine. Data are presented as the mean ± SD of three repeats. **P<0.01.
Hirsuteine inhibits cell cycle progression
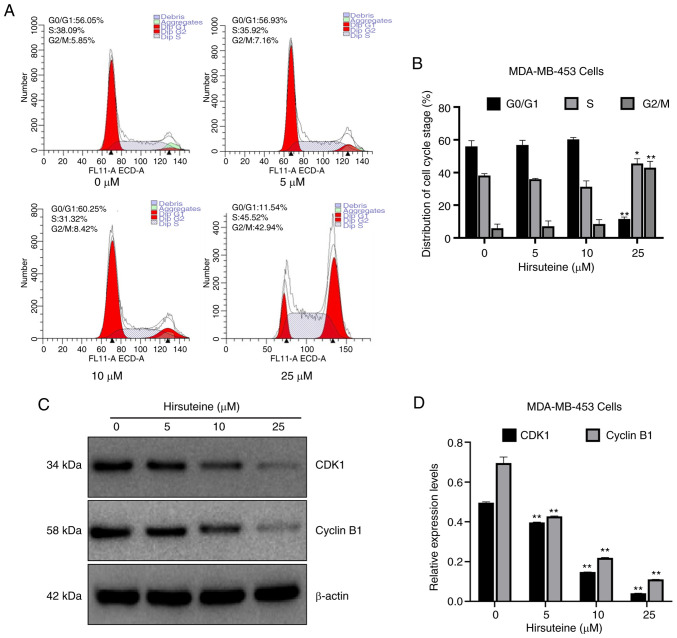
Cell-cycle distribution was assessed using flow cytometry. Apoptosis can be characterized by DNA fragmentation and damage. There are multiple phases and subphases in the cell cycle, namely, G0/G1 (DNA pre-synthesis and stationary); S (DNA synthesis); and G2/M (DNA post-synthesis and mitosis) (40–42). To elucidate whether hirsuteine altered cell cycle distribution, the number of cells in each phase of the cell cycle was evaluated following hirsuteine treatment. Based on the results, the proportion of cells in the G0/G1 phase was reduced from 56.1±3.4 to 11.5±1.3%, while the proportion of cells in the G2/M phase increased from 5.9±2.6 to 42.9±3.9% significantly, and the proportion of cells in the S phase increased from 38.1±1.2 to 45.5±2.9% following hirsuteine treatment (Fig. 3A). The proportion of cells in each phase of the cycle were compared (Fig. 3B). To determine the potential mechanism by which cell cycle arrest was induced by hirsuteine, western blotting was used to determine the expression levels of cell cycle-associated proteins in hirsuteine treated MDA-MB-453 cells. A marked decrease was observed in CDK1 and Cyclin B1 protein levels, which may underlie the G2/M phase arrest induced by hirsuteine (Fig. 3C and D). Thus, hirsuteine promoted the progression of BC cells from the S phase to the G2/M phase, prior to cell cycle arrest in G2/M phase, resulting in a reduction in proliferative ability and cell viability.
Figure 3.
Hirsuteine-mediated regulation of the cell cycle distribution. (A) Flow cytometry analysis of cell cycle phases in MDA-MB-453 cells following treatment with hirsuteine for 48 h (0, 5, 10, or 25 µM). (B) Percentage of cells in each phase of the cell cycle. (C) Western blot analysis of Cyclin B1 and CDK1 protein expression in hirsuteine-treated MDA-MB-453 cells. (D) Quantification and analysis of Cyclin B1 and CDK1 protein expression levels. Data are presented as the mean ± SD of three repeats. *P<0.05, **P<0.01.
Hirsuteine induces MDA-MB-453 cell apoptosis
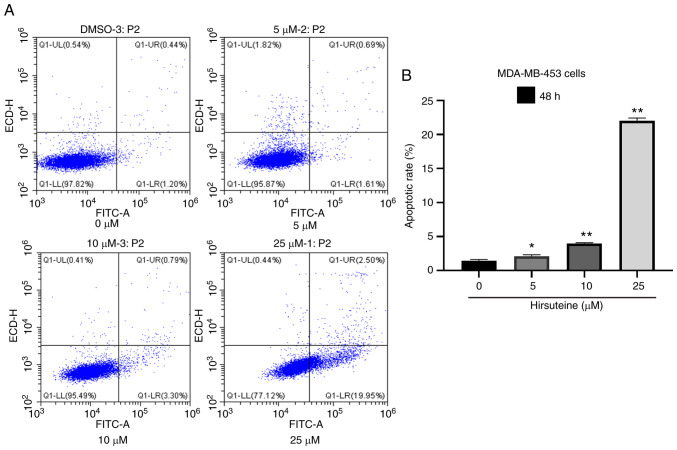
Hirsuteine-treated (0, 5, 10, and 25 µM) MDA-MB-453 cell apoptosis was quantified using flow cytometry using Annexin V labeling and PI exclusion staining. A four-quadrant schematic was used to classify the following cell states: Mechanical, necrotic, normal, early apoptotic, and late apoptotic. Relative to the control cells, the quantity of healthy cells decreased from 97.7±0.2 to 77.5±0.4%, and the quantity of early and late apoptotic cells increased from 1.4±0.2 to 22.1±0.4% with increasing hirsuteine concentrations (Fig. 4A). We also recorded the number of apoptotic cells in the control and treated groups (Fig. 4B). Thus, hirsuteine suppressed MDA-MB-453 cell proliferation whilst inducing apoptosis.
Figure 4.
Hirsuteine-mediated regulation of MDA-MB-453 cell apoptosis. (A) Annexin V- FITC/PI staining was used to stain cells using flow cytometry following treatment of MDA-MB-453 cells with hirsuteine for 48 h (0, 5, 10, or 25 µM). (B) Quantification of apoptosis of the MDA-MB-453 cells. Data are presented as the mean ± SD of three repeats. *P<0.05, **P<0.01.
Hirsuteine regulates apoptosis-related proteins
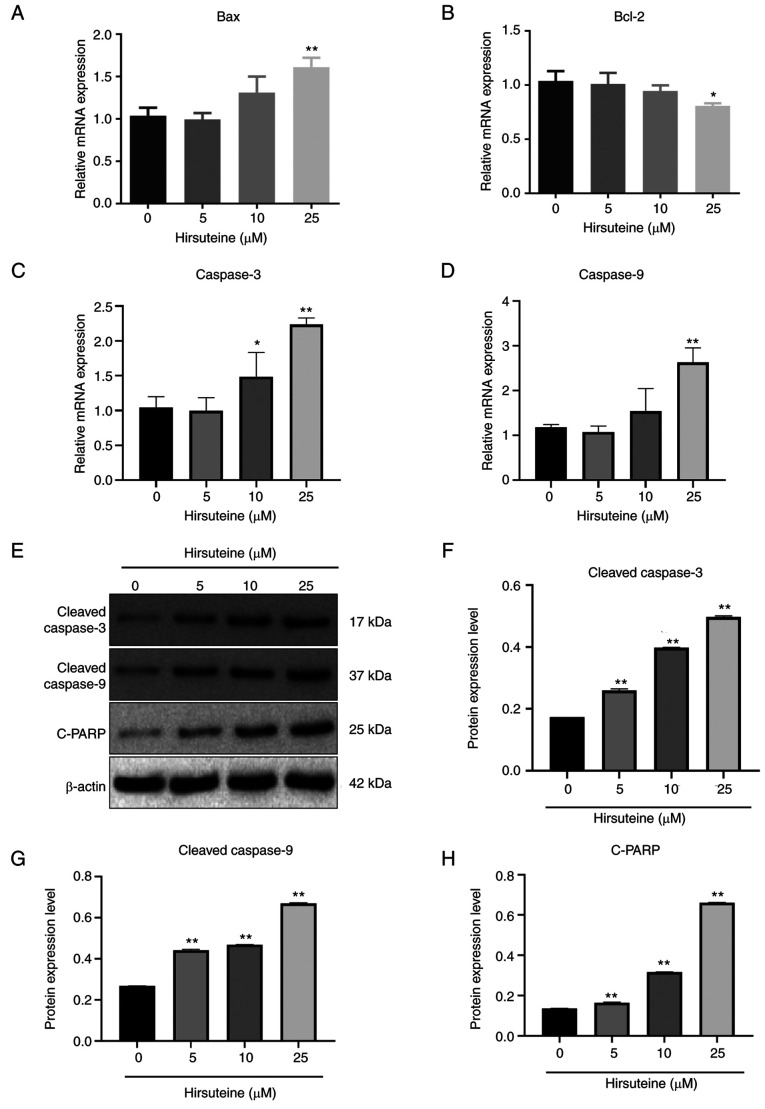
Bax, Bcl-2, cleaved caspase-3, and cleaved caspase-9 expression levels were assessed following 48 h of treatment with hirsuteine using qPCR and western blotting. Following treatment with different hirsuteine concentrations for 48 h, the RT-qPCR results showed that in the MDA-MB-453 cells, Bax, cleaved-caspase-3, and cleaved caspase-9 mRNA levels were promoted and Bcl-2 expression was decreased (Fig. 5A-D), resulting in an increase in pro-apoptotic/anti-apoptotic protein ratio. After 48 h of treatment with different doses of hirsuteine, the western blot results showed that hirsuteine increased the levels of cleaved caspase-3, cleaved caspase-9, and cleaved-PARP levels in the MDA-MB-453 cells (Fig. 5E). Additionally, densitometry analysis of the protein expression levels was performed, and the results are shown in Fig. 5F-H. These results showed that hirsuteine induced apoptosis in MDA-MB-453 cells via the intrinsic apoptotic pathway.
Figure 5.
Alterations in the expression levels of apoptosis-related proteins and mRNA in hirsuteine-treated MDA-MB-453 cells. Histogram of the mRNA levels of (A) Bax, (B) Bcl-2, (C) caspase 3, and (D) caspase 9 in MDA-MB-453 cells. (E) The relative protein levels of cleaved caspase-3, cleaved caspase-9, and cleaved-PARP in MDA-MB-453 cells treated with hirsuteine. (F-H) Protein expression levels were normalized to the control group. Data are presented as the mean ± SD of three repeats. *P<0.05, **P<0.01.
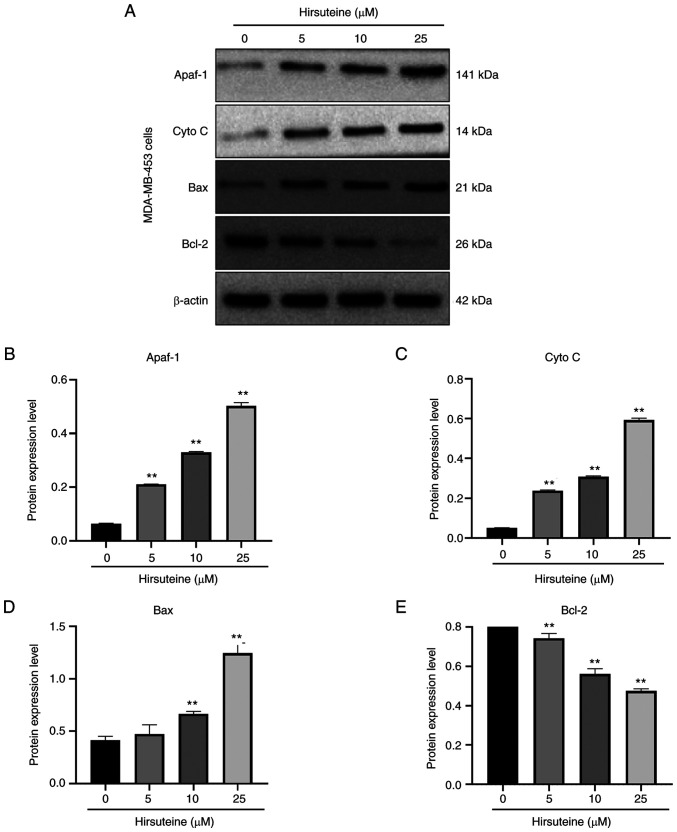
Hirsuteine induces apoptosis in MDA-MB-453 cells via the Bcl-2/Bax axis
The Bcl-2 family members induce apoptosis via the mitochondrial network (43). To examine the hirsuteine-mediated regulation of cell apoptosis, we assessed the expression levels of several apoptotic proteins. After 48 h of treatment, with hirsuteine (0, 5, 10, or 25 µM), increased expression of the proapoptotic Bax, Apaf-1, and cytoplasmic cytochrome-c levels, and reduced levels of Bcl-2 were observed, resulting in an imbalance in the Bax/Bcl-2 ratio relative to control cells in a dose-dependent manner (Fig. 6A). Furthermore, densitometry analysis of the protein expression levels was performed, and the results are shown in Fig. 6B-E. Together, these results demonstrated that hirsuteine activated the Bcl-2/Bax signaling pathway, resulting in apoptosis in MDA-MB-453 cells.
Figure 6.
Hirsuteine-mediated regulation of the Bcl-2/Bax axis. (A) Western blot analysis of the Apaf1, cytoplasmic cytochrome-c, Bax, and Bcl-2 levels in MDA-MB-453 cells exposed to varying hirsuteine concentrations (0, 5, 10, or 25 µM) for 48 h. (B) Apaf1, (C) cytochrome C, (D) Bax, and (E) Bcl-2 protein levels following normalization to Actin. Data are presented as the mean ± SD of three repeats. **P<0.01.
Discussion
At present, chemotherapy is the primary method of treatment of BC, and it can lead to several side effects (44). The common adverse reactions of traditional chemotherapeutic drugs include gastrointestinal reactions, bone marrow toxicity, hepatorenal toxicity, cardiotoxicity, neurotoxicity, and systemic reactions (45). The adverse reactions caused by immune checkpoint inhibitors differ from traditional chemotherapy-related adverse effects. Immune-related adverse events (IRAEs) caused by immune checkpoint inhibitors usually include endocrine dysfunction, skin toxicity, gastrointestinal adverse reactions, and a small number of cases of liver toxicity (46,47). The incidence of serious IRAEs is low, but if it exceeds expectations and cannot be appropriately handled, it may endanger a patient's life (46). Therefore, increasing attention is being paid to the discovery of safe and more effective novel compounds for the treatment of patients with BC.
Phytochemicals are promising compounds that have been used for several years to develop cancer medications owing to their potential efficacy and reduced toxicity profiles (21,22). Multiple reports have suggested that a majority of Uncaria rhynchophylla-mediated biological properties can be attributed to the alkaloid constituents (48,49). Hirsuteine may serve as a novel and specific SPHK1 inhibitor, exerting anti-leukemic activity by inhibiting the SPHK1/S1P/S1PR1 and BCR-ABL/PI3K/Akt pathways in CML cells (36). Similarly, we previously reported that the oxindole alkaloid purified from Uncaria rhynchophylla could effectively suppress the survival of Jurkat Clone E6-1 cells (T-cell leukemia cells) (38).
In the present study, CCK-8 assays of the effect of hirsuteine on MDA-MB-231 and MCF-7 breast cancer cells were used to determine the safe concentration range of hirsuteine. For all subsequent experiments, 25 µM was used as the upper limit as the inhibition rate of hirsuteine on the proliferation of MDA-MB-453 cells was about 45% at this concentration. However, in future studies, 50% IC50 dose, IC50 dose, and 2× IC50 dose will be used. In this study, it was shown that hirsuteine exhibited cytotoxicity against cancer cells in a concentration-dependent manner (MDA-MB-453) without affecting normal cells (Hs 578Bst, THLE-2 and BEAS-2B), which corroborates earlier investigations which showed that Uncaria rhynchophylla inhibited the proliferation of numerous tumor cell lines (50–52). The results of flow cytometry also revealed the effectiveness of hirsuteine on human MDA-MB-453 BC cells, indicating the underlying mechanisms by which hirsuteine exerts its anti-cancer-specific effects.
Cell cycle arrest is a promising approach for inhibiting tumor development, particularly since multiple studies demonstrated notable cell cycle dysregulation in several types of cancer (53–56). Based on the results of the present study, hirsuteine-treated MDA-MB-453 cells exhibited G2/M phase cell cycle arrest and displayed enhanced cleaved caspase 3 levels, indicative of an increase in the cell cycle following hirsuteine treatment. It was previously reported that CDK1 modulates cell cycle progression via interaction with cyclins B1 and A2 (57).
There is increasing evidence that numerous viral proteins can cause host G2/M cell cycle arrest. The G2/M arrest induced by some viral proteins is linked to the inhibition of cyclin B1-CDK1 kinase activity, inactivating the cyclin B1-CDK1 complex, which in turn prevents premature entry into the M phase (58,59). Hence, Cyclin B1 is responsible for entry into the M phase (60,61). Upon entry into prophase, Cyclin B1-CDK1 activity gradually increases (62), which activates the Cyclin B1-CDK1 complex, resulting in its translocation to the nucleus to promote cell division (63). Based on the western blot analysis of the aforementioned study, there was a strong association between decreased CDK1 and cyclin B1 levels with suppressed MDA-MB-453 cell proliferation following hirsuteine treatment, which eventually resulted in cell cycle arrest in the G2/M phase.
Apoptosis activation occurs in one of two methods: i) the intrinsic axis involves mitochondrial cytochrome c release, which induces various downstream caspases, and ii) the extrinsic axis which involves activation of the Fas death receptor via an external stimulus (64). The intrinsic apoptotic axis does not require the activation of a membrane receptor. In fact, the signal is generated in the mitochondria itself, and this axis is critical for drug-activated apoptosis, whereas the extrinsic apoptotic axis involves membrane-bound death receptors that belong to the TNF gene superfamily (65,66). Caspase-9 (intrinsic axis) and caspase-8 (extrinsic axis) activate caspase-3 (67–70). Caspase-9 initiates the apoptotic process by promoting the formation of the apoptosome complex within the mitochondrial network. Caspase-3 cleaves both PARP and DNA, which are hallmarks of the apoptotic process. Upon release of mitochondrial cytochrome c, it interacts with the adaptor protein Apaf-1 (71). Herein, hirsuteine substantially augmented Bax, PARP1, caspase-9, and caspase-3 expression, indicating that the mitochondria-based apoptotic network contributed to the hirsuteine-driven increase in MDA-MB-453 cell apoptosis. Collectively, the results of the present study showed that the intrinsic apoptotic axis was stimulated by hirsuteine in MDA-MB-453 cells.
In conclusion, this study revealed that hirsuteine strongly suppressed human BC cell development via activation of the caspase-3-based apoptotic network. These results highlight a novel target for the management of BC, as well as a promising agent for further assessment.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BY, YY and JM designed the present study. JM and YY performed the experiments. JM and YL analyzed the data. JM drafted the initial manuscript. BY and YY revised the initial manuscript. JM and BY confirm the authenticity of all the raw data. BY and YY helped guide JM in the whole process. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 3.Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–497. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 4.Redig AJ, McAllister SS. Breast cancer as a systemic disease: A view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Tinoco G, Warsch S, Gluck S, Avancha K, Montero AJ. Treating breast cancer in the 21st century: Emerging biological therapies. J Cancer. 2013;4:117–132. doi: 10.7150/jca.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: Delivery, duration and combination therapies. Breast. 2013;22((Suppl 2)):S152–S155. doi: 10.1016/j.breast.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak AB, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 9.Dean AQ, Luo S, Twomey JD, Zhang B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs. 2021;13:1951427. doi: 10.1080/19420862.2021.1951427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaman S, Jadid H, Denson AC, Gray JE. Targeting Trop-2 in solid tumors: Future prospects. Onco Targets Ther. 2019;12:1781–1790. doi: 10.2147/OTT.S162447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Syed YY. Sacituzumab govitecan: First approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 13.Piezzo M, Cocco S, Caputo R, Cianniello D, Gioia GD, Lauro VD, Fusco G, Martinelli C, Nuzzo F, Pensabene M, Laurentiis MD. Targeting cell cycle in breast cancer: CDK4/6 Inhibitors. Int J Mol Sci. 2020;21:6479. doi: 10.3390/ijms21186479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spring LM, Wander SA, Zangardi M, Bardia A. CDK 4/6 inhibitors in breast cancer: Current controversies and future directions. Curr Oncol Rep. 2019;21:25. doi: 10.1007/s11912-019-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future. Lancet. 2020;395:817–827. doi: 10.1016/S0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 17.Corona SP, Generali D. Abemaciclib: A CDK4/6 inhibitor for the treatment of HR+/HER2− advanced breast cancer. Drug Des Devel Ther. 2018;12:321–330. doi: 10.2147/DDDT.S137783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, Zhang QY, Rodriguez JLM, Campone M, Hamilton E, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. 1988;6:1746–1752. doi: 10.1200/JCO.1988.6.11.1746. [DOI] [PubMed] [Google Scholar]
- 20.Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, Tattersall MH. On the receiving end-patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 21.Chirumbolo S. Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: Really a promising path? J Sci Food Agric. 2012;92:1573–1577. doi: 10.1002/jsfa.5670. [DOI] [PubMed] [Google Scholar]
- 22.Thakur VS, Deb G, Babcook MA, Gupta S. Plant phytochemicals as epigenetic modulators: Role in cancer chemoprevention. AAPS J. 2014;16:151–163. doi: 10.1208/s12248-013-9548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howes MJ, Simmonds MS. The role of phytochemicals as micronutrients in health and disease. Curr Opin Clin Nutr Metab Care. 2014;17:558–566. doi: 10.1097/MCO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Feiyue Z, Gaofeng L. Traditional chinese medicine and lung cancer-from theory to practice. Biomed Pharmacother. 2021;137:111381. doi: 10.1016/j.biopha.2021.111381. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Wu Z, Le W. Traditional chinese medicine for dementia. Alzheimers Dement. 2021;17:1066–1071. doi: 10.1002/alz.12258. [DOI] [PubMed] [Google Scholar]
- 27.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 28.Ndagijimana A, Wang X, Pan G, Zhang F, Feng H, Olaleye O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia. 2013;86:35–47. doi: 10.1016/j.fitote.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Nakazawa T, Banba K, Hata K, Nihei Y, Hoshikawa A, Ohsawa K. Metabolites of hirsuteine and hirsutine, the major indole alkaloids of Uncaria rhynchophylla, in rats. Biol Pharm Bull. 2006;29:1671–1677. doi: 10.1248/bpb.29.1671. [DOI] [PubMed] [Google Scholar]
- 30.Horie S, Yano S, Aimi N, Sakai S, Watanabe K. Effects of hirsutine, an antihypertensive indole alkaloid from Uncaria rhynchophylla, on intracellular calcium in rat thoracic aorta. Life Sci. 1992;50:491–498. doi: 10.1016/0024-3205(92)90388-6. [DOI] [PubMed] [Google Scholar]
- 31.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 32.Sola S, Morgado AL, Rodrigues CM. Death receptors and mitochondria: Two prime triggers of neural apoptosis and differentiation. Biochim Biophys Acta. 2013;1830:2160–2166. doi: 10.1016/j.bbagen.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Isabelle M, Moreel X, Gagne JP, Rouleau M, Ethier C, Gagne P, Hendzel MJ, Poirier GG. Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome Sci. 2010;8:22. doi: 10.1186/1477-5956-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 35.Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153–159. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao S, Guo T, Luo S, Zhang Y, Ren Z, Lang X, Hu G, Zuo D, Jia W, Kong D, et al. Growth inhibitory and pro-apoptotic effects of hirsuteine in chronic myeloid leukemia cells through targeting sphingosine kinase 1. Biomol Ther (Seoul) 2022;30:553–561. doi: 10.4062/biomolther.2022.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang BY, Zeng Y, Li YJ, Huang XJ, Hu N, Yao N, Chen MF, Yang ZG, Chen ZS, Zhang DM, Zeng CQ. Uncaria alkaloids reverse ABCB1-mediated cancer multidrug resistance. Int J Oncol. 2017;51:257–268. doi: 10.3892/ijo.2017.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng J, Su R, Wang L, Yuan B, Li L. Inhibitory effect and mechanism of action (MOA) of hirsutine on the proliferation of T-cell leukemia Jurkat clone E6-1 cells. PeerJ. 2021;9:e10692. doi: 10.7717/peerj.10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Otsuki L, Brand AH. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science. 2018;360:99–102. doi: 10.1126/science.aan8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poncelet L, Garigliany M, Ando K, Franssen M, Desmecht D, Brion JP. Cell cycle S phase markers are expressed in cerebral neuron nuclei of cats infected by the Feline Panleukopenia virus. Cell Cycle. 2016;15:3482–3489. doi: 10.1080/15384101.2016.1249546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada T, Das Gupta TK, Beattie CW. p28-mediated activation of p53 in G2-M phase of the cell cycle enhances the efficacy of DNA damaging and antimitotic chemotherapy. Cancer Res. 2016;76:2354–2365. doi: 10.1158/0008-5472.CAN-15-2355. [DOI] [PubMed] [Google Scholar]
- 43.Handrick R, Ganswindt U, Faltin H, Goecke B, Daniel PT, Budach W, Belka C, Jendrossek V. Combined action of celecoxib and ionizing radiation in prostate cancer cells is independent of pro-apoptotic Bax. Radiother Oncol. 2009;90:413–421. doi: 10.1016/j.radonc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Odle TG. Adverse effects of breast cancer treatment. Radiol Technol. 2014;85:297M–319M. [PubMed] [Google Scholar]
- 45.Chopra D, Rehan HS, Sharma V, Mishra R. Chemotherapy-induced adverse drug reactions in oncology patients: A prospective observational survey. Indian J Med Paediatr Oncol. 2016;37:42–46. doi: 10.4103/0971-5851.177015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mina LA, Lim S, Bahadur SW, Firoz AT. Immunotherapy for the treatment of breast cancer: Emerging new data. Breast Cancer (Dove Med Press) 2019;11:321–328. doi: 10.2147/BCTT.S184710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang W, Ip SP, Liu L, Xian YF, Lin ZX. Uncaria rhynchophylla and its major constituents on central nervous system: A review on their pharmacological actions. Curr Vasc Pharmacol. 2020;18:346–357. doi: 10.2174/1570161117666190704092841. [DOI] [PubMed] [Google Scholar]
- 49.Lee J, Son D, Lee P, Kim SY, Kim H, Kim CJ, Lim E. Alkaloid fraction of Uncaria rhynchophylla protects against N-methyl-D-aspartate-induced apoptosis in rat hippocampal slices. Neurosci Lett. 2003;348:51–55. doi: 10.1016/S0304-3940(03)00613-X. [DOI] [PubMed] [Google Scholar]
- 50.Chen XX, Leung GP, Zhang ZJ, Xiao JB, Lao LX, Feng F, Mak JCW, Wang Y, Sze SCW, Zhang KYB. Proanthocyanidins from Uncaria rhynchophylla induced apoptosis in MDA-MB-231 breast cancer cells while enhancing cytotoxic effects of 5-fluorouracil. Food Chem Toxicol. 2017;107:248–260. doi: 10.1016/j.foodchem.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, Kim J, Kim BY, Lee HS, Ahn JS, Chang YS. Inhibition of phospholipase cgamma1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla. J Nat Prod. 2000;63:753–756. doi: 10.1021/np990478k. [DOI] [PubMed] [Google Scholar]
- 52.Zhang R, Li G, Zhang Q, Tang Q, Huang J, Hu C, Liu Y, Wang Q, Liu W, Gao N, Zhou S. Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 2018;9:598. doi: 10.1038/s41419-018-0641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu G, Chen G, Zhou J, Zhu H, Chu J, Zhang F. Liriodenine enhances radiosensitivity in esophageal cancer ECA-109 cells by inducing apoptosis and G2/M arrest. Oncol Lett. 2018;16:5020–5026. doi: 10.3892/ol.2018.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maharjan S, Kwon YS, Lee MG, Lee KS, Nam KS. Cell cycle arrest-mediated cell death by morin in MDA-MB-231 triple-negative breast cancer cells. Pharmacol Rep. 2021;73:1315–1327. doi: 10.1007/s43440-021-00272-w. [DOI] [PubMed] [Google Scholar]
- 56.Han YH, Mun JG, Jeon HD, Kee JY, Hong SH. Betulin inhibits lung metastasis by inducing cell cycle arrest, autophagy, and apoptosis of metastatic colorectal cancer cells. Nutrients. 2019;12:66. doi: 10.3390/nu12010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong D, Ferrell JE., Jr The roles of cyclin A2, B1, and B2 in early and late mitotic events. Mol Biol Cell. 2010;21:3149–3161. doi: 10.1091/mbc.e10-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kino T, Gragerov A, Valentin A, Tsopanomihalou M, Ilyina-Gragerova G, Erwin-Cohen R, Chrousos GP, Pavlakis GN. Vpr protein of human immunodeficiency virus type 1 binds to 14-3-3 proteins and facilitates complex formation with Cdc25C: Implications for cell cycle arrest. J Virol. 2005;79:2780–2787. doi: 10.1128/JVI.79.5.2780-2787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight GL, Turnell AS, Roberts S. Role for Wee1 in inhibition of G2-to-M transition through the cooperation of distinct human papillomavirus type 1 E4 proteins. J Virol. 2006;80:7416–7426. doi: 10.1128/JVI.00196-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 61.Kramer A, Mailand N, Lukas C, Syljuasen RG, Wilkinson CJ, Nigg EA, Bartek J, Lukas J. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–891. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- 62.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gavet O, Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J Cell Biol. 2010;189:247–259. doi: 10.1083/jcb.200909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 65.Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 66.Schmitt CA, Rosenthal CT, Lowe SW. Genetic analysis of chemoresistance in primary murine lymphomas. Nat Med. 2000;6:1029–1035. doi: 10.1038/79542. [DOI] [PubMed] [Google Scholar]
- 67.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 68.Wolf BB, Green DR. Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 69.Pal MK, Jaiswar SP, Srivastav AK, Goyal S, Dwivedi A, Verma A, Singh J, Pathak AK, Sankhwar PL, Ray RS. Synergistic effect of piperine and paclitaxel on cell fate via cyt-c, Bax/Bcl-2-caspase-3 pathway in ovarian adenocarcinomas SKOV-3 cells. Eur J Pharmacol. 2016;791:751–762. doi: 10.1016/j.ejphar.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Qu Z, Jiang C, Wu J, Ding Y. Lenalidomide induces apoptosis and inhibits angiogenesis via caspase3 and VEGF in hepatocellular carcinoma cells. Mol Med Rep. 2016;14:4781–4786. doi: 10.3892/mmr.2016.5797. [DOI] [PubMed] [Google Scholar]
- 71.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.