Highlights
-
•
About 8.6% of age-eligible adults reported COVID-related delays in HPV vaccination.
-
•
Adults who preferred a language other than English reported greater delays.
-
•
Adults who are a parent/guardian reported greater delays.
-
•
Adults with a cancer history reported greater delays.
-
•
Interventions may be needed that promote HPV vaccination during the pandemic.
Keywords: Cancer prevention, HPV vaccination, Vaccination, COVID-19 pandemic
Abstract
To assess how the COVID-19 pandemic affected catch-up HPV vaccination among age-eligible adults (ages 18–45). The current study leverages a national, cross-sectional sample of US adults ages 18–45 years to assess the prevalence and determinants of COVID-19 pandemic-related disruptions to catch-up HPV vaccination in 2021. The sample was restricted to adults intending to receive the HPV vaccine. Multinomial logistic regression analysis was conducted to assess the probability of 1) pandemic-related HPV vaccination disruption and 2) uncertainty about pandemic-related HPV vaccination disruption. Report of ‘no pandemic-related HPV vaccination disruption’ served as the reference category. Among adults intending to get the HPV vaccine (n = 1,683), 8.6 % reported pandemic-related HPV vaccination disruption, 14.7 % reported uncertainty about vaccination disruption, and 76.7 % reported no disruption. Factors associated with higher odds of pandemic-related vaccination disruption included non-English language preference (OR: 3.20; 95 % CI: 1.99–5.13), being a parent/guardian (OR: 1.77; 95 % CI: 1.18–2.66), having at least one healthcare visit in the past year (OR: 1.97; 95 % CI: 1.10–3.53), being up-to-date on the tetanus vaccine (OR: 1.81; 95 % CI: 1.19–2.75), and being a cancer survivor (OR: 2.57; 95 % CI: 1.52–4.34). Catch-up HPV vaccination for age-eligible adults is a critical public health strategy for reducing HPV-related cancers. While a small percentage of adults reported pandemic-related disruptions to HPV vaccination, certain adults (e.g., individuals with a non-English language preference and cancer survivors) were more likely to report a disruption. Interventions may be needed that increase accessibility of catch-up HPV vaccination among populations with reduced healthcare access during the pandemic.
1. Introduction
Each year in the United States (U.S.), roughly 34,000 individuals are diagnosed with human papillomavirus (HPV)-related cancers (Van Dyne et al., 2018). The HPV vaccine—a prophylactic and cost-effective strategy for reducing HPV-related cancers—is recommended for adults through age 26 and encouraged for shared clinical decision-making for adults aged 27–45 (Meites et al., 2019). HPV vaccination during adulthood provides an opportunity for adults to ‘catch-up’ on HPV vaccination if they were not adequately vaccinated during their childhood or adolescence (e.g., vaccine was not available during that time). Prior to the pandemic, U.S. HPV vaccination coverage among age-eligible adults was low. In 2017, a study of 9,744 age-eligible adults found that 36.3 % of adults aged 19–26 and 9.7 % of adults aged 27–45 had completed the HPV vaccine series (Kasting et al., 2020). Given the decline in routine preventive care since the start of the COVID-19 pandemic in 2019 (Whaley et al., 2020), HPV vaccination among adults has also likely declined, similar to what has been documented among adolescents (Patel Murthy et al., 2021). The pandemic disrupted primary care and gynecological care access (Whaley et al., 2020)—settings where many adults receive the HPV vaccine (Miller et al., 2021, Prabhu et al., 2021), and has led to a reduction in other adult vaccinations, such as the pneumococcal and zoster vaccines (Hong et al., 2021). Given the extent to which healthcare delivery changed during the pandemic, research is needed to better understand how the COVID-19 pandemic has affected HPV vaccination among age-eligible adults.
Initial studies during the first wave of the COVID-19 pandemic found that HPV vaccination among individuals aged 9–26 in March and April 2020 were only 23 % of the previous years’ coverage (Daniels et al., 2021). HPV vaccination coverage reached 48 % of the previous year’s rate by August 2020, a rate still far below pre-pandemic rates (Daniels et al., 2021). Studies have not yet assessed the factors that may have affected HPV vaccination disruption during the pandemic. Pre-pandemic studies indicate disparities in adult HPV vaccination coverage based on education, race/ethnicity, sexual orientation, gender, and immigration status (Agénor et al., 2015, Bernat et al., 2013, Bird et al., 2017, Cofie et al., 2018, Fisher et al., 2013, Gerend et al., 2007, Gerend et al., 2016, Klosky et al., 2017, Lu et al., 2014, McRee et al., 2014, Pho et al., 2021, Spencer et al., 2019, Thompson et al., 2016). Additional factors such as, past vaccination behavior (e.g., receipt of other recommended vaccines), and healthcare access (e.g., usual source of care, insurance) also impact adult HPV vaccination (Bernat et al., 2013, Brewer and Fazekas, 2007, Conroy et al., 2009, Klosky et al., 2017, Lu et al., 2014, McRee et al., 2014, Reiter et al., 2020). It remains unknown whether these factors as well as others are associated with pandemic-related disruptions in HPV vaccination.
The current study leverages a national sample of US adults ages 18–45 years to assess the prevalence and determinants of COVID-19 pandemic-related disruptions to HPV vaccination. Findings from this study may inform future interventions and policies to support catch-up HPV vaccination among age-eligible adults.
2. Methods
2.1. Study sample
Participants were recruited from a panel management company that maintains a probability-based online panel. The panel management company randomly selects households listed in the U.S. Postal Service’s Delivery Sequence File, which covers nearly all U.S. households. Selected households receive an invitation letter, a reminder postcard, and follow-up letters as needed. Interested households report demographic information for all individuals in the household. For the current study, we recruited a sub-sample of panelists or participants through verified partners (response rate: 25.5 %) with internet access and English proficiency. The sample was representative of the US population for most racial/ethnic groups (12.1 % Black/African American in the sample vs 12.1 % nationally; 16.5 % Hispanic in the sample vs 18.7 % nationally; 7.2 % Asian in the sample vs 5.7 % nationally); however, American Indian and Alaskan Native adults were under-represented (<1% in sample; 1.2 % nationally) (Bureau, 2020).
The target sample included 4,000 adults stratified by sex at birth and age. Age was stratified based on current Advisory Committee on Immunization Practices (ACIP) guidelines: 1) ages 18–26, when the HPV vaccine is recommended, and 2) ages 27–45, when shared clinical decision making is recommended (Meites et al., 2019). We aimed to obtain equal representation from both age groups and sex at birth. Individuals were sent an email invitation to participate by the panel management company, with up to two reminder emails. Interested and eligible participants completed a one-time, ∼30-minute survey via Qualtrics software (Provo, UT). The survey was administered from February 25, 2021, to March 24, 2021. Participants were compensated by the panel management company with reward points, which can be redeemed for gift cards.
2.2. Measures
2.2.1. Impact of pandemic on HPV vaccination
The survey assessed individuals’ HPV vaccination history (e.g., number of doses received and age at which the first dose was received). This information was used to identify individuals who had not completed the vaccine series (e.g., unvaccinated adults and adults who had initiated but not completed the vaccine series). Among adults who had not completed the vaccine series, the survey asked if the pandemic affected receipt of the HPV vaccine and provided four response options: 1) no; 2) yes; 3) I do not plan to obtain the HPV vaccine; and 4) I’m not sure. Individuals who reported ‘I do not plan to obtain the HPV vaccine’ were excluded from the study.
2.2.2. Potential determinants of HPV vaccination
The survey measured factors previously associated with HPV vaccination (Agénor et al., 2015, Bernat et al., 2013, Bird et al., 2017, Cofie et al., 2018, Fisher et al., 2013, Gerend et al., 2007, Gerend et al., 2016, Kitur et al., 2021, Klosky et al., 2017, Lorini et al., 2018, Lu et al., 2014, McRee et al., 2014, Pho et al., 2021, Spencer et al., 2019, Thompson et al., 2016) including 1) demographics (e.g., age, gender, race/ethnicity, born in the U.S., parent born outside the U.S., parent/guardian status, relationship status, sexual orientation, religious service attendance, non-English language preference, region); 2) social determinants of health (SDOH) (e.g., education, income, employment, preferences for health information in non-English language, health literacy and numeracy); 3) healthcare access (e.g., usual source of care, insurance status, prior healthcare visits in the past year); 4) other vaccination history (e.g., flu, tetanus); and 5) cancer history.
2.3. Statistical analyses
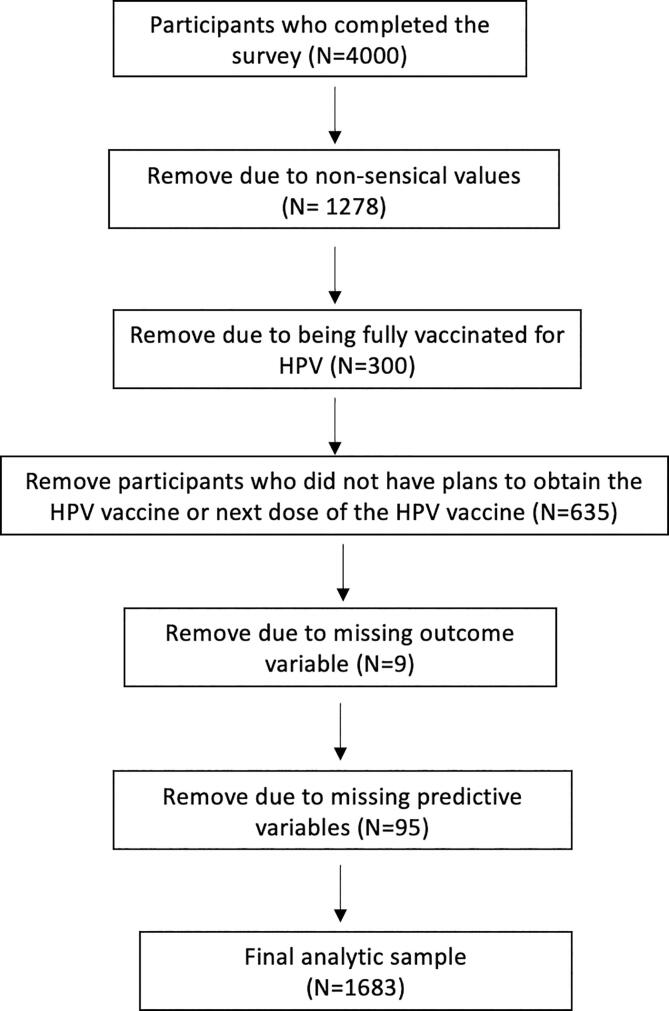
Initial descriptive statistics and quality checks were performed, and participants with unreliable data were removed (e.g., straight line responses) (Fig. 1) (Arevalo et al., 2022, Kim et al., 2019). A multinomial logistic regression analysis was conducted to model the probability of two outcomes among adults who had not completed the HPV vaccine series and intended to receive the vaccine: 1) pandemic-related HPV vaccination disruption; and 2) uncertainty about pandemic-related HPV vaccination disruption. The reference category for the multinomial model was no pandemic-related disruption in HPV vaccination. Given that many variables likely affect adult HPV vaccination (Agénor et al., 2015, Bernat et al., 2013, Bird et al., 2017, Cofie et al., 2018, Fisher et al., 2013, Gerend et al., 2007, Gerend et al., 2016, Kitur et al., 2021, Klosky et al., 2017, Lorini et al., 2018, Lu et al., 2014, McRee et al., 2014, Pho et al., 2021, Spencer et al., 2019, Thompson et al., 2016) and the exploratory nature of the study, we used backwards selection set at the 10 % significance level for variable selection. Factors likely to affect HPV vaccination (e.g., employment, income) are often highly correlated. To handle potential collinearity, we chose backward selection over other variable selection techniques given its improved performance for dealing with potential collinearity (Heinze et al., 2018, Wester et al., 2022). Data analyses were conducted from May to September 2021 using SAS Software version 9.4. We report adjusted odds ratios (OR) and 95 % confidence intervals (CI). We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting (von Elm et al., 2014). The study was approved by the Moffitt Cancer Center Scientific Review Board and the Institutional Review Board of record (Advarra).
Fig. 1.
Creation of analytic sample.
3. Results
3.1. Participant characteristics
The sample included 1,683 adults, approximately half were aged 18–26 (47.9 %) and half were aged 27–45 (52.1 %) (Table 1). About half of the sample identified as female (52.7 %), less than half identified as male (46.0 %), and 1.1 % identified as transgender. The racial/ethnic breakdown of the sample included 16.5 % Hispanic/Latinx, 12.1 % Black/African American, 7.2 % Asian, and 10.0 % from a racial group under-represented in the sample (e.g., American Indian/Alaskan Native, multiple-racial categories). Most participants had health insurance (84.1 %), a usual source of care (55.4 %), and visited a healthcare provider in the past year (74.8 %).
Table 1.
Sample characteristics, N = 1,683.
| Variable | Level | Overall sample (N = 1,683) |
|---|---|---|
| Age | 18–26 | 806 (47.9) |
| 27–45 | 877 (52.1) | |
| Gender | Female | 887 (52.7) |
| Male | 774 (46.0) | |
| Transgender | 19 (1.1) | |
| Missing | 3 (0.2) | |
| Race | White | 1190 (70.7) |
| Black/African American | 203 (12.1) | |
| Asian | 122 (7.2) | |
| Under-represented group a | 168 (10.0) | |
| Ethnicity | Hispanic/Latinx | 278 (16.5) |
| Non-Hispanic/Latinx | 1405 (83.5) | |
| Born in U.S. | No | 115 (6.8) |
| Yes | 1567 (93.1) | |
| Missing | 1 (0.1) | |
| Parents born outside U.S. | No | 1250 (74.3) |
| Yes | 415 (24.7) | |
| Missing | 18 (1.1) | |
| Education | Less than high school | 66 (3.9) |
| High school degree or equivalent | 331 (19.7) | |
| Some college/Associates degree | 532 (31.6) | |
| Bachelor's Degree | 464 (27.6) | |
| Graduate school | 289 (17.2) | |
| Missing | 1 (0.1) | |
| Annual Income | $0 - $19,999 | 200 (11.9) |
| $20,000 - $49,999 | 418 (24.8) | |
| $50,000 - $74,999 | 347 (20.6) | |
| $75,000 to $99,999 | 288 (17.1) | |
| $100,000 or more | 430 (25.5) | |
| Relationship Status | Married/Partnered | 890 (52.9) |
| Divorced/Separated/Widowed | 77 (4.6) | |
| Dating exclusively for more than 1 week | 167 (9.9) | |
| Dating but not exclusively for more than 1 week | 41 (2.4) | |
| Not currently dating and never been married | 507 (30.1) | |
| Missing | 1 (0.1) | |
| Employment Status | Employed | 1256 (74.6) |
| Unemployed | 164 (9.7) | |
| Homemaker/Student | 218 (13.0) | |
| Disabled/Retired/Other | 44 (2.6) | |
| Missing | 1 (0.1) | |
| Sexual minority status | Yes | 263 (15.6) |
| No | 1420 (84.4) | |
| Health insurance | No | 268 (15.9) |
| Yes | 1415 (84.1) | |
| Religious service attendance in past year | Never | 865 (51.4) |
| Less than once a month | 407 (24.2) | |
| Once a month or more, but less than once a week | 210 (12.5) | |
| Once a week or more | 198 (11.8) | |
| Missing | 3 (0.2) | |
| Preference for health information in non-English language | No | 1528 (90.8) |
| Yes | 155 (9.2) | |
| Parent or guardian | No | 950 (56.4) |
| Yes | 733 (43.6) | |
| Geographic Region | Midwest | 339 (20.1) |
| Northeast | 280 (16.6) | |
| South | 660 (39.2) | |
| West | 404 (24.0) | |
| Perceived difficulty with understanding written health information | Very easy | 600 (35.7) |
| Not very easy (e.g., somewhat easy, difficult, very difficult) | 1004 (59.7) | |
| Other | 72 (4.3) | |
| Missing | 7 (0.4) | |
| Perceived difficulty with understanding medical statistics | Very easy | 406 (24.1) |
| Not very easy (e.g., somewhat easy, difficult, very difficult) | 1277 (75.9) | |
| Usual source of careb | No | 751 (44.6) |
| Yes | 932 (55.4) | |
| Healthcare visit in the past year | None | 424 (25.2) |
| At least 1 time | 1259 (74.8) | |
| Receipt of flu vaccine in past year | No | 913 (54.2) |
| Yes | 770 (45.8) | |
| Receipt of tetanus vaccine in past ten years | Don’t know/Not sure | 164 (9.7) |
| No | 670 (39.8) | |
| Yes | 849 (50.4) | |
| Cancer history | No | 1563 (92.9) |
| Yes | 120 (7.1) |
Racial groups underrepresented in the study included American Indian, Alaskan Native, and multiple racial categories.
The following survey item was used to assess usual source of care, “Not including psychiatrists and other mental health professionals, is there a particular doctor, nurse, or other health professional that you see most often?”.
3.2. Pandemic-related disruptions in HPV vaccination
Among adults who had not completed the HPV vaccine series with complete data (N = 1,683), 8.6 % reported pandemic-related HPV vaccination disruption (Table 2). Controlling for other factors, adults with a non-English language preference had higher odds of reporting pandemic-related HPV vaccination disruption (OR: 3.20; 95 % CI: 1.99.513) compared to adults with an English preference. Adults who were parents/guardians (OR: 1.77; 95 % CI: 1.18–2.66) had higher odds of reporting pandemic-related HPV vaccination disruption compared to adults without children. Adults with at least one healthcare visit in the past year (OR: 1.97; 95 % CI: 1.10–3.53) had higher odds of reporting pandemic-related HPV vaccination disruption compared to adults with no healthcare visit in the past year. Adults who were up-to-date on their tetanus vaccine (OR: 1.81; 95 % CI: 1.19–2.75) had higher odds of reporting pandemic-related HPV vaccination disruption compared to adults who were not up-to-date on their tetanus vaccine. Cancer survivors (OR: 2.57; 95 % CI: 1.52–4.34) had higher odds of reporting pandemic-related HPV vaccination disruption compared to adults without a cancer history.
Table 2.
Multinomial regression of pandemic-related disruption in HPV vaccination among adults who have not completed the HPV vaccine series, N = 1,683.
| Covariatea | Reported disruptionb N = 144 |
Unsure about disruptionb N = 247 |
Overall P-value |
|---|---|---|---|
| OR (95 % CI) | OR (95 % CI) | ||
| Race | 0.003 | ||
| White (ref) | |||
| Black | 1.65 (0.93–2.90) | 1.02 (0.62–1.69) | |
| Asian | 1.89 (0.91–3.92) | 2.00 (1.23–3.25) | |
| Racial group under-represented in the study c | 1.32 (0.61–2.89) | 1.97 (1.26–3.10) | |
| Ethnicity | 0.063 | ||
| Non-Hispanic/Latinx (ref) | |||
| Hispanic/Latinx | 0.84 (0.46–1.56) | 1.55 (1.05–2.28) | |
| Non-English preference | <0.001 | ||
| No (ref) | |||
| Yes | 3.20 (1.99–5.13) | 0.94 (0.53–1.69) | |
| Parent/guardian | 0.014 | ||
| No (ref) | |||
| Yes | 1.77 (1.18–2.66) | 0.91 (0.66–1.25) | |
| Sexual minority | 0.059 | ||
| No (ref) | |||
| Yes | 1.32 (0.81–2.16) | 1.51 (1.05–2.16) | |
| Annual income | 0.048 | ||
| $100,000 or more (ref) | |||
| $0 - $19,999 | 0.73 (0.38–1.41) | 0.96 (0.56–1.64) | |
| $20,000 - $49,999 | 0.64 (0.38–1.09) | 1.13 (0.73–1.73) | |
| $50,000 - $74,999 | 0.51 (0.29–0.90) | 0.92 (0.59–1.44) | |
| $75,000 to $99,999 | 0.37 (0.20–0.69) | 0.73 (0.45–1.18) | |
| Usual source of care | 0.065 | ||
| No (ref) | |||
| Yes | 1.24 (0.83–1.85) | 0.74 (0.55–1.00) | 0.001 |
| Numeracy | |||
| Difficult (ref) | |||
| Easy | 1.10 (0.74–1.65) | 0.47 (0.31–0.71) | |
| Healthcare visit in the past year | 0.001 | ||
| None (ref) | |||
| At least 1 | 1.97 (1.10–3.53) | 0.63 (0.46–0.87) | |
| Tetanus vaccine in the past 10 years | <0.001 | ||
| No (ref) | |||
| Yes | 1.81 (1.19–2.75) | 1.79 (1.28–2.50) | |
| Don’t know | 1.29 (0.60–2.79) | 2.78 (1.78–4.35) | |
| Health insurance | 0.022 | ||
| No (ref) | |||
| Yes | 1.40 (0.73–2.69) | 0.63 (0.44–0.91) | |
| Cancer history | 0.001 | ||
| No(ref) | |||
| Yes | 2.57 (1.52–4.34) | 0.84 (0.44–1.60) |
Candidate variables for backward stepwise regression include the variables presented in Table 1.
The base category for the multinomial regression was the COVID-19 pandemic had no impact on HPV vaccination (N = 1292).
Racial groups underrepresented in the study included American Indian, Alaskan Native, and multiple racial categories.
3.3. Uncertainty about pandemic-related disruptions in HPV vaccination
Among adults who had not completed the HPV vaccine series with complete data (N = 1,683), 14.7 % reported uncertainty about HPV vaccination disruption (i.e., whether or not the pandemic had effected the timeline for completion of the HPV vaccine series; Table 2). Controlling for other factors, Asian adults (OR: 2.00; 95 % CI: 1.23–3.25) and adults identifying as a racial category under-represented in the study (e.g., American Indian/Alaskan Native) (OR: 1.97; 95 % CI: 1.26–3.10) had higher odds of reporting uncertainty about pandemic-related HPV vaccination disruption compared to White adults. Adults identifying as Hispanic/Latinx (OR: 1.55; 95 % CI: 1.05–2.28) had higher odds of reporting uncertainty about pandemic-related HPV vaccination disruption compared to non-Latinx adults. Individuals identifying as a sexual minority (e.g., lesbian, gay, bisexual, asexual) (OR: 1.51; 95 % CI: 1.05–2.16) had higher odds of reporting uncertainty about pandemic-related HPV vaccination disruption compared to non-sexual minority adults. Individuals who reported that medical statistics were very easy to understand (a proxy for numeracy) had lower odds (OR: 0.47; 95 % CI: 0.31–0.71) of reporting uncertainty about pandemic-related HPV vaccination disruption compared to individuals reporting difficulty with understanding medical statistics. Individuals with a healthcare visit in the past year (OR: 0.63; 95 % CI: 0.46–0.87) had lower odds of reporting uncertainty about pandemic-related HPV vaccination disruption compared to individuals without a healthcare visit in the past year. People with health insurance (OR: 0.63; 95 % CI: 0.44–0.91) had lower odds of reporting uncertainty about pandemic-related HPV vaccination disruption compared to individuals without insurance.
4. Discussion
The study goal was to examine the self-reported effects of the COVID-19 pandemic on HPV vaccination among a national sample of U.S adults, ages 18–45, who intended to receive the HPV vaccine. Our study found that a small percentage of adults (8.6 %) reported pandemic-related disruptions in HPV vaccination or uncertainty about disruptions (14.7 %). Like other studies reporting pandemic-related healthcare disruptions(Amram et al., 2021, Czeisler et al., 2020, Marcondes et al., 2021), our study found disparities in HPV vaccination disruption based on cancer history and language preference. Factors, such as race/ethnicity and sexual orientation, were associated with reporting uncertainty about pandemic-related disruptions.
Our study found that cancer survivors were more likely to report pandemic-related disruptions in HPV vaccination. The pandemic may have worsened healthcare access among cancer survivors who recently completed treatment, and they may have avoided healthcare to reduce COVID-19 transmission risk (Papautsky and Hamlish, 2020). Past research suggests that cancer survivors may have lower HPV vaccination rates compared to individuals without a cancer history, which may be due to lower likelihood of receiving a provider recommendation (Castellino et al., 2019, Klosky et al., 2017). A recent qualitative study found that cancer survivors reported a preference to receive HPV vaccine recommendations from their oncologist (Waters et al., 2021). Current guidelines recommend a multi-disciplinary approach to improving HPV vaccination among cancer survivors, such as encouraging oncology professionals to talk with patients about the importance of HPV vaccination (Bailey et al., 2016, Saslow et al., 2016). Oncology professionals may have more communication with cancer survivors than primary care professionals depending on the survivors’ treatment stage. Additional interventions should be tested to support HPV vaccine information dissemination for cancer survivors in cancer care and primary care settings.
Among our study participants, adults with a non-English language preference were more likely to experience disruptions in HPV vaccination. Prior studies suggest that adults who do not speak English as their primary language were more likely to experience reduced primary care access compared to primary English-speaking adults during the pandemic (Amram et al., 2021, Czeisler et al., 2020, Marcondes et al., 2021). Past research also suggests that patients who do not speak English as their primary language experience lower quality care due to lack of language-appropriate care (e.g., bilingual provider, language assistance) Diamond et al., 2010, Khoong and Fernandez, 2021, Ngo-Metzger et al., 2007). To ensure the pandemic does not increase HPV vaccination disparities, evidence-based interventions (EBIs) are needed that address multi-level barriers (e.g., patient-centered communication, language-appropriate care) among patients with a non-English language preference (Downs et al., 2010, Khoong and Fernandez, 2021, Lake et al., 2019, Rodriguez et al., 2020). Strategies are also needed to enhance interpreter access. For example, digital health companies might design information technology solutions that expand access to digital interpreters(Khoong and Fernandez, 2021), especially in under-resourced settings. Additionally, our study found that being up to date on other vaccines (e.g., tetanus) and individuals who had a healthcare visit in the past year were more likely to report HPV vaccination disruptions. One reason may be that these individuals are more likely to keep track of their preventive healthcare, such as HPV vaccination.
Our research findings suggest that certain patient characteristics, such as Asian race, Hispanic/Latinx ethnicity, and sexual minority status, were associated with greater reporting of uncertainty regarding pandemic-related HPV vaccination disruptions. There may be a few reasons for this. Prior studies have demonstrated that individuals from minoritized communities, including Hispanic/Latinx adults and Asian adults, and sexual minority individuals report lower quality patient-provider communication (Cho and Chang, 2022, Kirby et al., 2021, Palmer et al., 2014). It is possible that lower quality patient-provider communication may contribute to lower knowledge about HPV vaccination (e.g., HPV recommendation is associated with HPV knowledge(Gerend and Shepherd, 2011). Prior studies have demonstrated that Hispanic/Latinx, Asian adults, and sexual minority adults report lower HPV-related knowledge (Gilbert et al., 2011, McBride and Singh, 2018, Reimer et al., 2014, Wheldon et al., 2011). Additional targeted interventions may be needed to increase accessibility of HPV vaccination to these population subgroups. For example, studies suggest that provider recommendation for HPV vaccination, communication about sexual identity (e.g., assessment and disclosure), targeted health communication, and mobile health interventions are promising strategies for promoting HPV vaccine uptake among sexual and gender minority adults (Fontenot et al., 2020, Gerend et al., 2021, Reiter et al., 2020, Reiter et al., 2018, Stupiansky et al., 2017). Future studies should couple these strategies with communication approaches that emphasize the importance of HPV vaccination and cancer prevention during the pandemic. For example, cancer screening studies have cited the importance of using telehealth as an opportunity to promote cancer screening given the decline of in-person visits (Nodora et al., 2021). A similar approach could be used for HPV vaccination. Prior studies have also found that drive-through vaccination clinics can help to increase vaccination rates and could be explored in the future as a strategy for increasing HPV vaccination rates (Banks et al., 2013).
5. Limitations
Our findings highlight factors associated with self-reported pandemic-related disruptions in HPV vaccination. The use of a population-based online panel allowed us to collect timely and relevant information about current practices, which can guide public health efforts to increase catch-up HPV vaccination among US adults. Our study has a few limitations. First, this was a cross-sectional study, and we cannot establish causality. Second, our study relies on survey data which may be prone to self-report bias. Third, our study was unable to measure rural versus urban residence. Prior studies suggest that adults in rural areas experienced greater disruptions to primary care access than adults residing in urban areas during the pandemic (Amram et al., 2021, DeGroff et al., 2021). Future studies should compare pandemic-related disruptions in HPV vaccination across urban and rural areas. Fourth, our study was limited to the U.S context. Other studies suggest that the pandemic has greatly disrupted HPV vaccination globally (Toh et al., 2021). Further research is needed to understand cross-country differences. Fifth, our survey was administered from February 25, 2021, to March 24, 2021. Therefore, reported disruptions in HPV vaccination may be lower during this timeframe compared to the initial onset of the pandemic. Additionally, our survey was not designed to capture information on what factors contributed to COVID-related disruptions in HPV vaccination (e.g., healthcare office closure, lockdowns) or the reasons why an individual may have reported uncertainty regarding how the COVID-19 pandemic disrupted their HPV vaccination timeline.
6. Conclusion
Catch-up HPV vaccination for age-eligible adults is a critical public health strategy for reducing HPV-related cancers. Although a small percentage of adults (8.6 %) reported pandemic-related disruptions to HPV vaccination; cancer survivors and adults who prefer a language other than English were disproportionately affected, suggesting targeted HPV vaccination efforts may be needed. Interventions may be needed that increase accessibility of catch-up HPV vaccination (e.g., drive-through vaccination clinics, social marketing campaigns) among individuals who may have experienced reduced healthcare access during the pandemic.
7. Ethics approval
The study was approved by the Moffitt Cancer Center Scientific Review Board and the Institutional Review Board of record, Advarra.
Funding
The study was supported with funding from a Moffitt Center for Immunization and Infection Research in Cancer Award (PI: Christy) and a Moffitt Merit Society Award (PI: Christy). This work has been supported in part by both the Participant Research, Interventions, and Measurement Core and the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, a comprehensive cancer center designated by the National Cancer Institute and funded in part by Moffitt’s Cancer Center Support Grant (P30-CA076292). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or National Institutes of Health. Monica Kasting’s work on this project was made possible with support from Grant No, KL2TR002530 (B. Tucker Edmonds, PI), and UL1TR002529 (S. Moe and S. Wiehe, co-PIs) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Informed consent
Informed consent was obtained for all individual participants in the study.
Conflict of interest
Dr. Islam has received support to attend the following conferences: American Association for Cancer Research, and the American Society of Preventive Oncology. Dr. Kasting has received research grant funding from Merck unrelated to the current study. Dr. Brownstein has received honoraria from the Statistical Consulting Section of the American Statistical Association (ASA) for Best Paper Award at the 2019. Dr. Brownstein also received travel support to serve as an ad-hoc grant reviewer for the American Cancer Society. Dr. Brownstein currently serves on a Data Safety Monitoring Board for Moffitt Cancer Center’s Scientific Review Committee. Dr. Brownstein currently serves as Vice President of the Florida Chapter of the ASA and Section Representative for the ASA Statistical Consulting Section. Dr. Christy serves as an unpaid Medical Advisory Board Member of the HPV Cancers Alliance.
CRediT authorship contribution statement
Kea Turner: Conceptualization, Methodology, Writing – original draft. Naomi C. Brownstein: Methodology, Formal analysis, Data curation. Junmin Whiting: Methodology, Formal analysis, Data curation. Mariana Arevalo: Writing – review & editing. Susan Vadaparampil: Writing – review & editing. Anna R. Giuliano: Writing – review & editing. Jessica Y. Islam: Writing – review & editing. Cathy D. Meade: Writing – review & editing. Clement K. Gwede: Writing – review & editing. Monica L. Kasting: Writing – review & editing. Katharine J. Head: Writing – review & editing. Shannon M. Christy: Conceptualization, Methodology, Project administration, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Kea Turner, Email: kea.turner@moffitt.org.
Naomi C. Brownstein, Email: brownstn@musc.edu.
Junmin Whiting, Email: Junmin.Whiting@moffitt.org.
Mariana Arevalo, Email: Mariana.Arevalo@moffitt.org.
Susan Vadaparampil, Email: Susan.Vadaparampil@moffitt.org.
Anna R. Giuliano, Email: Anna.Giuliano@moffitt.org.
Jessica Y. Islam, Email: Jessica.Islam@moffitt.org.
Cathy D. Meade, Email: Cathy.Meade@moffitt.org.
Clement K. Gwede, Email: Clement.Gwede@moffitt.org.
Monica L. Kasting, Email: mlkastin@purdue.edu.
Katharine J. Head, Email: headkj@iupui.edu.
Shannon M. Christy, Email: Shannon.Christy@moffitt.org.
Data availability
The authors do not have permission to share data.
References
- Agénor M., Peitzmeier S., Gordon A.R., Haneuse S., Potter J.E., Austin S.B. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann. Intern. Med. 2015;163:99–106. doi: 10.7326/M14-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amram O., Robison J., Amiri S., Pflugeisen B., Roll J., Monsivais P. Socioeconomic and racial inequities in breast cancer screening during the COVID-19 Pandemic in Washington State. JAMA Netw. Open. 2021;4:e2110946. doi: 10.1001/jamanetworkopen.2021.10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo M., Brownstein N.C., Whiting J., Meade C.D., Gwede C.K., Vadaparampil S.T., Tillery K.J., Islam J.Y., Giuliano A.R., et al. Strategies and lessons learned during cleaning of data from research panel participants: cross-sectional web-based health behavior survey study. JMIR Form Res. 2022;6:e35797. doi: 10.2196/35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey H.H., Chuang L.T., duPont N.C., Eng C., Foxhall L.E., Merrill J.K., Wollins D.S., Blanke C.D. American Society of Clinical Oncology statement: human papillomavirus vaccination for cancer prevention. J. Clin. Oncol. 2016;34:1803–1812. doi: 10.1200/JCO.2016.67.2014. [DOI] [PubMed] [Google Scholar]
- Banks L.L., Crandall C., Esquibel L. Throughput times for adults and children during two drive-through influenza vaccination clinics. Disaster Med. Public Health Prep. 2013;7:175–181. doi: 10.1017/dmp.2013.3. [DOI] [PubMed] [Google Scholar]
- Bernat D.H., Gerend M.A., Chevallier K., Zimmerman M.A., Bauermeister J.A. Characteristics associated with initiation of the human papillomavirus vaccine among a national sample of male and female young adults. J. Adolesc. Health. 2013;53:630–636. doi: 10.1016/j.jadohealth.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird Y., Obidiya O., Mahmood R., Nwankwo C., Moraros J. Human papillomavirus vaccination uptake in Canada: a systematic review and meta-analysis. Int. J. Prev. Med. 2017;8:71. doi: 10.4103/ijpvm.IJPVM_49_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer N.T., Fazekas K.I. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev. Med. 2007;45:107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Bureau U.S.C. US Census Bureau; Washington D.C.: 2020. 2020. Census Illuminates Racial and Ethnic Composition of the Country. [Google Scholar]
- Castellino S.M., Allen K.E., Pleasant K., Keyes G., Poehling K.A., Tooze J.A. Suboptimal uptake of human papillomavirus (HPV) vaccine in survivors of childhood and adolescent and young adult (AYA) cancer. J. Cancer Surviv. 2019;13:730–778. doi: 10.1007/s11764-019-00791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho G., Chang V.W. Patient-provider communication quality, 2002–2016: a population-based study of trends and racial differences. Med. Care. 2022;60:324–331. doi: 10.1097/MLR.0000000000001694. [DOI] [PubMed] [Google Scholar]
- Cofie L.E., Hirth J.M., Guo F., Berenson A.B., Markides K., Wong R. HPV vaccination among foreign-born women: examining the national health interview survey 2013–2015. Am. J. Prev. Med. 2018;54:20–27. doi: 10.1016/j.amepre.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy K., Rosenthal S.L., Zimet G.D., Jin Y., Bernstein D.I., Glynn S., Kahn J.A. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J. Womens Health (Larchmt) 2009;18:1679–1686. doi: 10.1089/jwh.2008.1329. [DOI] [PubMed] [Google Scholar]
- Czeisler M., Marynak K., Clarke K.E.N., Salah Z., Shakya I., Thierry J.M., Ali N., McMillan H., Wiley J.F., et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1250–2127. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels V., Saxena K., Roberts C., Kothari S., Corman S., Yao L., Niccolai L. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39:2731–3275. doi: 10.1016/j.vaccine.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroff A., Miller J., Sharma K., Sun J., Helsel W., Kammerer W., Rockwell T., Sheu A., Melillo S., et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev. Med. 2021;151 doi: 10.1016/j.ypmed.2021.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L.C., Wilson-Stronks A., Jacobs E.A. Do hospitals measure up to the national culturally and linguistically appropriate services standards? Med. Care. 2010;48:1080–1087. doi: 10.1097/MLR.0b013e3181f380bc. [DOI] [PubMed] [Google Scholar]
- Downs L.S., Scarinci I., Einstein M.H., Collins Y., Flowers L. Overcoming the barriers to HPV vaccination in high-risk populations in the US. Gynecol. Oncol. 2010;117:486–490. doi: 10.1016/j.ygyno.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Fisher H., Trotter C.L., Audrey S., MacDonald-Wallis K., Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int. J. Epidemiol. 2013;42:896–908. doi: 10.1093/ije/dyt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot H.B., White B.P., Rosenberger J.G., Lacasse H., Rutirasiri C., Mayer K.H., Zimet G. Mobile app strategy to facilitate human papillomavirus vaccination among young men who have sex with men: pilot intervention study. J. Med. Internet Res. 2020;22:e22878. doi: 10.2196/22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerend M.A., Lee S.C., Shepherd J.E. Predictors of human papillomavirus vaccination acceptability among underserved women. Sex. Transm. Dis. 2007;34:468–471. doi: 10.1097/01.olq.0000245915.38315.bd. [DOI] [PubMed] [Google Scholar]
- Gerend M.A., Madkins K., Phillips G., Mustanski B. Predictors of human papillomavirus vaccination among young men who have sex with men. Sex. Transm. Dis. 2016;43:185–191. doi: 10.1097/OLQ.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerend M.A., Madkins K., Crosby S., Korpak A.K., Phillips G.L., Bass M., Houlberg M., Mustanski B. Evaluation of a text messaging-based human papillomavirus vaccination intervention for young sexual minority men: results from a pilot randomized controlled trial. Ann. Behav. Med. 2021;55:321–332. doi: 10.1093/abm/kaaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerend M.A., Shepherd J.E. Correlates of HPV knowledge in the era of HPV vaccination: a study of unvaccinated young adult women. Women Health. 2011;51:25–40. doi: 10.1080/03630242.2011.540744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Brewer N.T., Reiter P.L., Ng T.W., Smith J.S. HPV vaccine acceptability in heterosexual, gay, and bisexual men. Am. J. Mens Health. 2011;5:297–305. doi: 10.1177/1557988310372802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze G., Wallisch C., Dunkler D. Variable selection - a review and recommendations for the practicing statistician. Biom. J. 2018;60:431–449. doi: 10.1002/bimj.201700067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Zhou F., Tsai Y., Jatlaoui T.C., Acosta A.M., Dooling K.L., Kobayashi M., Lindley M.C. Decline in receipt of vaccines by medicare beneficiaries during the COVID-19 Pandemic - United States, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:245–329. doi: 10.15585/mmwr.mm7007a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasting M.L., Giuliano A.R., Christy S.M., Rouse C.E., Robertson S.E., Thompson E.L. Human papillomavirus vaccination prevalence among adults aged 19–45 years: an analysis of the 2017 National Health Interview Survey. Am. J. Prev. Med. 2020;59:837–849. doi: 10.1016/j.amepre.2020.05.031. [DOI] [PubMed] [Google Scholar]
- Khoong E.C., Fernandez A. Addressing gaps in interpreter use: time for implementation science informed multi-level interventions. J. Gen. Intern. Med. 2021 doi: 10.1007/s11606-021-06823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Dykema J., Stevenson J., Black P., Moberg D.P. Straightlining: overview of measurement, comparison of indicators, and effects in mail–web mixed-mode surveys. Soc. Sci. Comput. Rev. 2019;37 [Google Scholar]
- Kirby J.B., Berdahl T.A., Torres Stone R.A. Perceptions of patient-provider communication across the six largest Asian subgroups in the USA. J. Gen. Intern. Med. 2021;36:888–893. doi: 10.1007/s11606-020-06391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitur H., Horowitz A.M., Beck K., Wang M.Q. HPV knowledge, vaccine status, and health literacy among university students. J. Cancer Educ. 2021 doi: 10.1007/s13187-021-01997-1. [DOI] [PubMed] [Google Scholar]
- Klosky J.L., Hudson M.M., Chen Y., Connelly J.A., Wasilewski-Masker K., Sun C.L., Francisco L., Gustafson L., Russell K.M., et al. Human papillomavirus vaccination rates in young cancer survivors. J. Clin. Oncol. 2017;35:3582–3590. doi: 10.1200/JCO.2017.74.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake P.W., Kasting M.L., Christy S.M., Vadaparampil S.T. Provider perspectives on multilevel barriers to HPV vaccination. Hum. Vaccin. Immunother. 2019;15:1784–1793. doi: 10.1080/21645515.2019.1581554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorini C., Santomauro F., Donzellini M., Capecchi L., Bechini A., Boccalini S., Bonanni P., Bonaccorsi G. Health literacy and vaccination: a systematic review. Hum. Vaccin. Immunother. 2018;14:478–488. doi: 10.1080/21645515.2017.1392423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P.J., Rodriguez-Lainz A., O'Halloran A., Greby S., Williams W.W. Adult vaccination disparities among foreign-born populations in the U.S., 2012. Am. J. Prev. Med. 2014;47:722–733. doi: 10.1016/j.amepre.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes F.O., Cheng D., Warner E.T., Kamran S.C., Haas J.S. The trajectory of racial/ethnic disparities in the use of cancer screening before and during the COVID-19 pandemic: a large U.S. academic center analysis. Prev. Med. 2021;151(106640) doi: 10.1016/j.ypmed.2021.106640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride K.R., Singh S. Predictors of adults' knowledge and awareness of HPV, HPV-associated cancers, and the HPV vaccine: implications for health education. Health Educ. Behav. 2018;45:68–76. doi: 10.1177/1090198117709318. [DOI] [PubMed] [Google Scholar]
- McRee A.L., Katz M.L., Paskett E.D., Reiter P.L. HPV vaccination among lesbian and bisexual women: findings from a national survey of young adults. Vaccine. 2014;32:4736–4742. doi: 10.1016/j.vaccine.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites E., Szilagyi P.G., Chesson H.W., Unger E.R., Romero J.R., Markowitz L.E. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly Rep. 2019;68:698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Xu L., Qin J., Hahn E.E., Ngo-Metzger Q., Mittman B., Tewari D., Hodeib M., Wride P., et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Metzger Q., Sorkin D.H., Phillips R.S., Greenfield S., Massagli M.P., Clarridge B., Kaplan S.H. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J. Gen. Intern. Med. 2007;22(Suppl 2):324–330. doi: 10.1007/s11606-007-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodora J.N., Gupta S., Howard N., Motadel K., Propst T., Rodriguez J., Schultz J., Velasquez S., Castañeda S.F., et al. The COVID-19 pandemic: identifying adaptive solutions for colorectal cancer screening in underserved communities. J. Natl Cancer Inst. 2021;113:962–998. doi: 10.1093/jnci/djaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer N.R., Kent E.E., Forsythe L.P., Arora N.K., Rowland J.H., Aziz N.M., Blanch-Hartigan D., Oakley-Girvan I., Hamilton A.S., et al. Racial and ethnic disparities in patient-provider communication, quality-of-care ratings, and patient activation among long-term cancer survivors. J. Clin. Oncol. 2014;32:4087–4094. doi: 10.1200/JCO.2014.55.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papautsky E.L., Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res. Treat. 2020;184:249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Murthy B., Zell E., Kirtland K., Jones-Jack N., Harris L., Sprague C., Schultz J., Le Q., Bramer C.A., et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations - 10 U.S. jurisdictions, March-September 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:840–885. doi: 10.15585/mmwr.mm7023a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pho A.T., Bakken S., Lunn M.R., Lubensky M.E., Flentje A., Dastur Z., Obedin-Maliver J. Online health information seeking, health literacy, and human papillomavirus vaccination among transgender and gender-diverse people. J. Am. Med. Inform. Assoc. 2021 doi: 10.1093/jamia/ocab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu V.S., Bansal N., Liu Z., Finalle R., Sénécal M., Kothari S., Trowers K., Myers E. HPV vaccination uptake and administration from 2006 to 2016 in a commercially insured population of the United States. BMC Public Health. 2021;21:1629. doi: 10.1186/s12889-021-11664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer R.A., Schommer J.A., Houlihan A.E., Gerrard M. Ethnic and gender differences in HPV knowledge, awareness, and vaccine acceptability among White and Hispanic men and women. J. Community Health. 2014;39:274–284. doi: 10.1007/s10900-013-9773-y. [DOI] [PubMed] [Google Scholar]
- Reiter P.L., Katz M.L., Bauermeister J.A., Shoben A.B., Paskett E.D., McRee A.L. Increasing human papillomavirus vaccination among young gay and bisexual men: a randomized pilot trial of the outsmart HPV intervention. LGBT Health. 2018;5:325–339. doi: 10.1089/lgbt.2018.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P.L., Bustamante G., McRee A.L. HPV vaccine coverage and acceptability among a national sample of sexual minority women ages 18–45. Vaccine. 2020;38:4956–4963. doi: 10.1016/j.vaccine.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S.A., Mullen P.D., Lopez D.M., Savas L.S., Fernández M.E. Factors associated with adolescent HPV vaccination in the U.S.: A systematic review of reviews and multilevel framework to inform intervention development. Prev. Med. 2020;131(105968) doi: 10.1016/j.ypmed.2019.105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow D., Andrews K.S., Manassaram-Baptiste D., Loomer L., Lam K.E., Fisher-Borne M., Smith R.A., Fontham E.T., Group A.C.S.G.D. Human papillomavirus vaccination guideline update: American Cancer Society guideline endorsement. CA Cancer J Clin. 2016;66:375–385. doi: 10.3322/caac.21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.C., Calo W.A., Brewer N.T. Disparities and reverse disparities in HPV vaccination: a systematic review and meta-analysis. Prev. Med. 2019;123:197–203. doi: 10.1016/j.ypmed.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupiansky N.W., Liau A., Rosenberger J., Rosenthal S.L., Tu W., Xiao S., Fontenot H., Zimet G.D. Young men's disclosure of same sex behaviors to healthcare providers and the impact on health: results from a US National sample of young men who have sex with men. AIDS Patient Care STDS. 2017;31:342–437. doi: 10.1089/apc.2017.0011. [DOI] [PubMed] [Google Scholar]
- Thompson E.L., Vamos C.A., Vázquez-Otero C., Logan R., Griner S., Daley E.M. Trends and predictors of HPV vaccination among U.S College women and men. Prev. Med. 2016;86:92–98. doi: 10.1016/j.ypmed.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Toh Z.Q., Russell F.M., Garland S.M., Mulholland E.K., Patton G., Licciardi P.V. Human papillomavirus vaccination after COVID-19. JNCI Cancer Spectr. 2021;5(pkab011) doi: 10.1093/jncics/pkab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyne E.A., Henley S.J., Saraiya M., Thomas C.C., Markowitz L.E., Benard V.B. Trends in human papillomavirus-associated cancers - United States, 1999–2015. MMWR Morb. Mortal. Wkly Rep. 2018;67:918–924. doi: 10.15585/mmwr.mm6733a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Waters A.R., Mann K., Vaca Lopez P.L., Kepka D., Wu Y.P., Kirchhoff A.C. HPV vaccine experiences and preferences among young adult cancer survivors and caregivers of childhood cancer survivors. J. Cancer Educ. 2021 doi: 10.1007/s13187-021-01992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester R.A., Rubel J., Mayer A. Clinical Psychological Science; 2022. Covariate Selection for Estimating Individual Treatment Effects in Psychotherapy Research: A Simulation Study and Empirical Example. [Google Scholar]
- Whaley C.M., Pera M.F., Cantor J., Chang J., Velasco J., Hagg H.K., Sood N., Bravata D.M. Changes in health services use among commercially insured US populations during the COVID-19 Pandemic. JAMA Netw. Open. 2020;3:e2024984. doi: 10.1001/jamanetworkopen.2020.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheldon C.W., Daley E.M., Buhi E.R., Nyitray A.G., Giuliano A.R. Health beliefs and attitudes associated with HPV vaccine intention among young gay and bisexual men in the Southeastern United States. Vaccine. 2011;29:8060–8065. doi: 10.1016/j.vaccine.2011.08.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.