Abstract
Aotearoa New Zealand experienced a wave of the Omicron variant of SARS-CoV-2 in 2022 with around 200 confirmed cases per 1000 people between January and May. Waning of infection-derived immunity means people become increasingly susceptible to re-infection with SARS-CoV-2 over time. We investigated a model that included waning of vaccine-derived and infection-derived immunity under scenarios representing different levels of behavioural change relative to the first Omicron wave. Because the durability of infection-derived immunity is a key uncertainty in epidemiological models, we investigated outcomes under different assumptions about the speed of waning. The model was used to provide scenarios to the New Zealand Government, helping to inform policy response and healthcare system preparedness ahead of the winter respiratory illness season. In all scenarios investigated, a second Omicron wave was projected to occur in the second half of 2022. The timing of the peak depended primarily on the speed of waning and was typically between August and November. The peak number of daily infections in the second Omicron wave was smaller than in the first Omicron wave. Peak hospital occupancy was also generally lower than in the first wave but was sensitive to the age distribution of infections. A scenario with increased contact rates in older groups had higher peak hospital occupancy than the first wave. Scenarios with relatively high transmission, whether a result of relaxation of control measures or voluntary behaviour change, did not necessarily lead to higher peaks. However, they generally resulted in more sustained healthcare demand (>250 hospital beds throughout the winter period). The estimated health burden of Covid-19 in the medium term is sensitive to the strength and durability of infection-derived and hybrid immunity against reinfection and severe illness, which are uncertain.
Keywords: COVID-19, Mathematical model, Modelling-policy interface, Vaccination, Endemicity
1. Introduction
The B.1.1.529 (Omicron) variant of SARS-CoV-2 was designated a variant of concern by the World Health Organisation on 26 November 2021 (World Health Organisation, 2021) following a rapid growth in cases of Covid-19 in southern Africa (Viana et al., 2022). Aotearoa New Zealand experienced a wave of the Omicron variant starting in February 2022. Prior to this wave, the total number of confirmed cases of Covid-19 in New Zealand had been limited to around 0.3 % of the population by a combination of border restrictions, vaccination, and strong public health measures. This meant that the population had negligible levels of immunity from prior infection at the start of the wave.
By 1 February 2022, 77 % of the population (90 % of those aged over 12 years) had received at least two doses of the Pfizer/BioNTech BNT162b2 vaccine and 27 % of the population (35 % of those aged over 18 years) had received a third dose. By 1 April 2022, third dose coverage had increased to 51 % of the population (66 % of those aged over 18 years). In addition, those aged 5–11 years became eligible for vaccination on 17 January 2022 and by 1 April, 54 % of this age group had received at least one dose and 17 % had received two doses. The Omicron variant has substantial immune escape from the protection provided the vaccine (Cheng et al., 2022, Cromer et al., 2022a). Although vaccine effectiveness against severe disease and death remains high, particularly after three doses, effectiveness against acquisition and transmission is much lower for Omicron than for previous SARS-CoV-2 variants (Andrews et al., 2022).
The number of new confirmed cases peaked at a seven-day rolling average of 20,500 cases per day (4000 per million population) around 5 March 2022. The number of hospital patients reported with Covid-19 peaked at 1016 (198 per million) on 22 March 2022. Between 25 January and 12 May 2022, there were a total of 1.01 million confirmed cases (197,000 per million) and 926 deaths (181 per million) within 28 days of a positive Covid-19 test result. The wave was initially a mixture of BA.1 and BA.2 lineages, but the BA.2 subvariant became dominant by the end of February and accounted for an estimated 88 % of new cases by the end of March (Chen et al., 2021).
Public health measures to control the spread of the virus were applied as part of New Zealand’s Covid-19 Protection Framework (New Zealand Government, 2021). The whole country was moved to the Red setting of the framework on 23 January 2022. This setting imposed widespread mask mandates, gathering size limits, vaccine pass requirements for many businesses, and encouraged working from home. These restrictions were relaxed in stages with outdoor gathering restrictions removed and indoor gathering size limits increased from 100 to 200 on 25 March. Vaccine pass requirements were removed on 4 April. The country was moved to the Orange setting on 13 April, meaning that all gathering restrictions were lifted and masks were no longer mandatory in schools. During this period, isolation requirements were also progressively reduced from 14 days to 7 days and quarantine requirements were narrowed to just household contacts.
We have previously modelled the spread and impact of the Omicron variant of SARS-CoV-2 in New Zealand using an age-structured model (Vattiato et al., 2022). This model included the effects of age-specific vaccination rates, different vaccine effectiveness against different clinical endpoints, and waning of vaccine-derived immunity. The model results were reasonably consistent with the observed numbers of cases, hospitalisations and deaths during the first Omicron wave up to early April 2022 (Vattiato et al., 2022). However, the model did not include the effects of waning of infection-derived immunity, meaning that people could not be infected with the virus for a second time. This assumption meant that, after the first wave, the epidemic died out in the model as the number of susceptible people became too low to sustain transmission.
In reality, infection-derived immunity against SARS-CoV-2 likely wanes over time, meaning it is possible for people to be infected more than once. Mathematically, this means that transmission of the virus does not die out and instead gives rise to a stable equilibrium state in which a steady fraction of the population is infected with the virus per day, balancing loss of immunity through waning (Anderson and May, 1992). In reality, the system is unlikely to reach true equilibrium because transmission is affected by factors such as changing behaviour, age distribution of new infections, antigenic evolution, and seasonality. In particular, since the emergence of Omicron in late 2021, the arrival of new subvariants has been a major factor driving transmission dynamics internationally and disrupting the trend towards equilibrium (Tegally et al., 2022). However, attraction towards the equilibrium is a strong driving force in the epidemic dynamics (Bjørnstad et al., 2020). In the absence of significant new variants of concern, it is expected that waves would become gradually smaller over time, or that transmission would settle towards a more predictable seasonal cycle.
Here, we extend our previous model to include waning of infection-derived immunity and consequent re-infection. We investigate scenarios with different levels of contact rates in different age groups and how these affect the size and timing of a second Omicron wave. We also conduct a sensitivity analysis to the strength and rate of waning of infection-derived immunity. This provides a useful tool to explore the effects of different waning assumptions, as there is currently significant uncertainty around these. The model was used as the basis for policy advice to the New Zealand government and for operational planning in the healthcare system in April and May 2022.
2. Methods
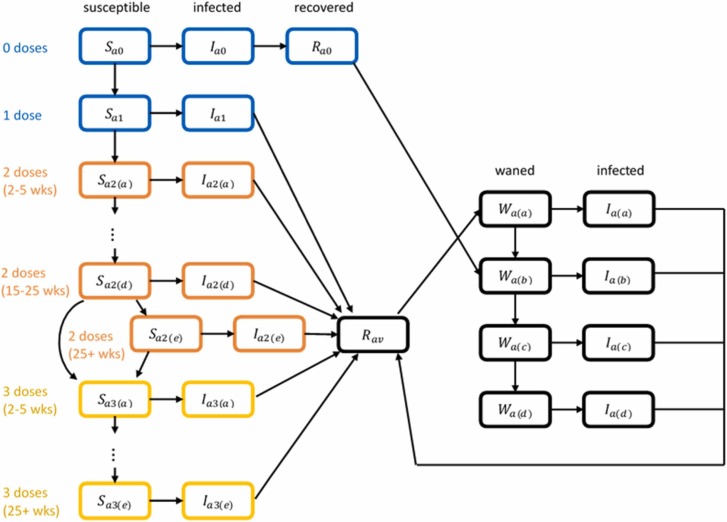
We used an age-structured stochastic branching process model (Haccou et al., 2005). This was a hybrid of a compartment model and individual-based model in which the susceptible population in each 5-year age band was divided into compartments depending on vaccination status, prior infection status, and the amount of waning that has occurred ( Fig. 1). Infected individuals were modelled explicitly, which allowed individual variation in transmission and the effect of test-trace-isolate measures to be included. The model was simulated in discrete time steps of 1 day.
Fig. 1.
Schematic diagram showing how individuals transition between model compartments. Compartments are coloured according to the type of immunity: 0–1 doses and no prior infection (blue); two doses and no prior infection (orange); three doses and no prior infection (yellow); prior infection (black). Transitions between compartments with different colours occur as a result of receiving a dose of the vaccine or recovering from infection. Transitions between susceptible compartments of the same colour occur as a result of waning immunity over time. For each of the 2-dose and 3-dose categories, there are five compartments representing people who are 2–5 weeks, 5–10 weeks, 10–15 weeks, 15–25 weeks and more than 25 weeks since their last dose. People whose first infection occurs after they have had at least one dose of the vaccine transition initially into compartment W(a) following recovery; people whose first infection occurs when they are unvaccinated transition initially into compartment W(b) following recovery reflecting lower levels of infection-derived immunity in unvaccinated people; following recovery from second or subsequent infection, people transition initially into compartment W(a) regardless of vaccination status. The model consists of 16 five-year age bands; for simplicity the diagram only shows compartments for one age group .
Mixing within and between age groups is described in the model by an age-dependent contact matrix based on pre-pandemic survey data (Prem et al., 2017) and adjusted for the New Zealand population (Steyn et al., 2022). However, the age distribution of cases in first Omicron wave was more skewed towards younger age groups than this matrix would suggest (only 9% of cases between 25 January and 10 May 2022 were in over 60-year-olds despite them representing 22% of the population). Reported cases are subject to testing biases but there is no evidence that ascertainment rates are lower in older age groups and the age gradient of disease severity would, if anything, suggest the opposite is likely to be true. The age distribution of cases will also be affected by the fact that vaccine coverage is higher in older groups, although this effect is included in the model. Instead, we hypothesise that the observed age distribution of cases partly reflects heightened levels of precautionary behaviour in high-risk demographic groups. To model this, we used an adjusted form of this pre-pandemic contact matrix in which contact rates within older groups (over 35-year-olds) and between older and younger groups are reduced relative to contact rates within younger groups (under 35-year-olds) (see Supplementary Table S2(a)). This was an ad hoc adjustment that was observed to give a reasonable match with empirical data, however other assumptions about contact patterns between age groups were explored in sensitivity analyses (see below).
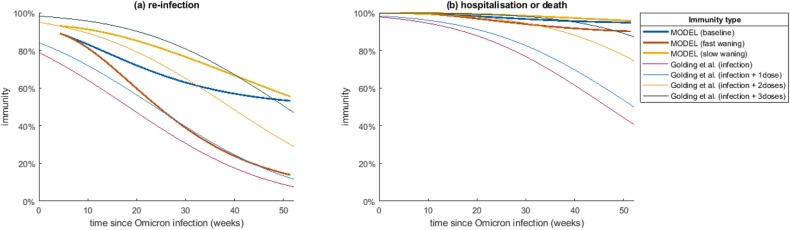
Following infection, individuals were assumed to remain completely immune to re-infection for a fixed period of 30 days. At the end of this period, immunity was assumed to decline over time, modelled via a series of compartments with increasing levels of susceptibility (Fig. 1). The waning model was similar conceptually to (Keeling et al., 2021) but the inclusion of multiple post-recovery compartments meant the model was not restricted to exponential waning curves and could capture differing dynamics of immunity against infection and immunity against severe disease. Since the strength and durability of infection-derived immunity and hybrid immunity following infection with Omicron are highly uncertain, we investigated three models with different speeds of waning of infection-derived immunity, which we refer to as the baseline, fast waning and slow waning scenario. The average immunity as a function of time since recovery from prior infection under these three scenarios are shown in Fig. 2 (see Supplementary Information Section 4 for more details). We assumed in all cases that immunity to severe disease and death is higher and longer-lasting than immunity to infection. Our assumptions are broadly within the range of values estimated for the Omicron variant by (Khoury et al., 2021, Cromer et al., 2022b, Golding and Lydeamore, 2022). These estimates were based on the model of (Khoury et al., 2021, Cromer et al., 2022b) for the relationship between neutralising antibody titres and immunity against different clinical endpoints estimated from population-level epidemiological data. However, the waning predictions of (Khoury et al., 2021, Cromer et al., 2022b) were calibrated against data for the Delta variant and then extrapolated by (Golding and Lydeamore, 2022) for Omicron, so these estimates are subject to significant uncertainty. The curves in Fig. 2(b) correspond to an assumption that immunity to severe illness and death as a result of prior infection with Omicron is somewhat more durable than estimated by (Golding and Lydeamore, 2022)(though see Supplementary Fig. S6 and Table S4 for a sensitivity analysis).
Fig. 2.
Three model scenarios for average post-infection immunity against: (a) re-infection and (b) hospitalisation or death, over time since previous infection, for people who have had at least one dose of the vaccine. People who are unvaccinated start with a lower level of immunity following their first infection. For comparison, we show the immunity estimates of (Golding and Lydeamore, 2022), based on the model of (Khoury et al., 2021, Cromer et al., 2022b), following infection with Omicron (note as a simplifying assumption our model assumes the same level of infection-derived immunity for vaccinated people regardless of the number of doses received).
As a simplifying assumption, we assumed that infection-derived immunity is the same for individuals who have had one, two or three doses of the vaccine. However, unvaccinated people were assumed to start at a lower level of immunity following their first infection (see Supplementary Table S1), reflecting evidence that infection with Omicron elicits a substantially weaker antibody response in unvaccinated people compared to vaccinated people (Khan et al., 2022). Following recovery from a second or subsequent infection, all individuals’ immunity were assumed to follow the curves shown in Fig. 2 regardless of vaccination status. The risks of hospitalisation and death are age-dependent in the model (see Supplementary Table S3) but the relative reduction in risk due to immunity was assumed to be age-independent.
The generation interval, in the absence of interventions, was assumed to have mean 3.3 days and standard deviation 1.3 days (Abbott et al., 2022, Kim et al., 2022, Ito et al., 2022). We assumed that, at the beginning of the outbreak, 30% of symptomatic infections become confirmed cases, with an average of 4 days from symptom onset to positive test result. However, in late February, New Zealand introduced widespread use of rapid antigen tests (RATs) following a large backlog of unprocessed RT-PCR tests. The switch to RATs as the main form of testing was associated with a jump in reported cases and a reduction in the time from symptom onset to test result ( days for 577,370 cases between 1 March and 30 April 2022 with onset date recorded). As an approximate model of this, from 23 February onwards we increased the proportion of symptomatic infections that become confirmed cases to 50% and reduced the average time from symptom onset to positive test result to 1.5 days. These parameters gave a reasonable empirical match with the change in trends in reported cases that occurred at this time, although other parameter combinations are also possible (see Supplementary Information Section 1 for details).
Vaccine coverage in each 5-year age group over time was based on Ministry of Health data on the number of first, second and third doses of the vaccine given each day up to 4 May 2022 (Supplementary Fig. S1). Model assumptions around vaccine effectiveness and disease severity for Omicron were as described in (Vattiato et al., 2022) based on estimates from (Andrews et al., 2022, Herrera-Esposito, 2022, Nyberg et al., 2022). We assumed that the infection fatality rate for the over 75 year age group was a factor of 1.6 higher than assumed by (Vattiato et al., 2022) so that the ratio of deaths to reported cases in the model is closer to the empirically observed case fatality rate in this age group (3.1%).
Immunity from infections that occurred prior to the start of the simulated time period (5 January 2022) was ignored. This assumption is not likely to have a large effect on model results given that, prior to the introduction of Omicron, New Zealand had approximately 12,600 confirmed community cases of Covid-19, which is around 0.25% of the total population. The effects of seasonality were not included in the model.
We considered four scenarios for changes in contact rates following the peak in reported cases for the first Omicron wave, which occurred around 5 March 2022. These represent relaxation of public health measures as well as voluntary changes in precautionary behaviour. The exact magnitude of these was unknown in advance, so we investigated the effects of contact rates increasing by differing amounts during March and April 2022 ( Table 1). During the first Omicron wave, reported cases of Covid-19 were concentrated in younger age groups. As described above, to reproduce this observed age distribution, we used a modified contact matrix in which contact rates for older groups were reduced relative to pre-pandemic estimates. We interpret this as representing heightened levels of precautionary behaviour among older age groups. However, it might be expected that this precautionary behaviour would reduce over time due to fatigue with precautions or reduction in perceived risk. To model this possibility, we also investigated a scenario in which the contact matrix was re-adjusted back towards its pre-pandemic estimates from 1 July 2022 (see Supplementary Table S2(b)). This represents a scenario where contact patterns were intermediate between those of the first Omicron wave and those corresponding to the matrix used by (Steyn et al., 2022) based on pre-pandemic estimates.
Table 1.
Summary of assumptions in the four main scenarios about overall transmission rate, measured by the reproduction number, and age-specific contact patterns in the four main model scenarios. Changes in reproduction number are reported as relative changes to the reproduction number excluding immunity , which was assumed to be in the period prior to 10 March 2022 (see Supplementary InformationSection 3 for more details). The value of was increased linearly over a specified period of time starting on 10 March. This date was chosen as it followed the peak in reported cases, modelling a component of reactive voluntary behaviour change, and gave a reasonable match with the observed decline in cases following the peak, which was slower than would be expected with a fixed value of . The switch from the Red to the Orange setting of the Covid-19 Protection Framework on 13 April 2022 was not explicitly linked to the timing of these scenarios, but likely contributed to the increase in , particularly in scenarios C.1 and C.2.
| Scenario | Reproduction number | Age-specific contact rates |
|---|---|---|
| A | 13% increase over 10 days from 10 March |
Constant throughout simulated period |
| B | 40% increase over 30 days from 10 March |
Constant throughout simulated period |
| C.1 | 67% increase over 50 days from 10 March |
Constant throughout simulated period |
| C.2 | 67% increase over 50 days from 10 March |
Contact rates of older age groups increase on 1 July |
Simulations were initialised with 500 seed infections introduced over a one-week time period starting on 5 January 2022. Key model outputs were not highly sensitive to seeding assumptions, which were chosen to give a reasonable match to the initial growth phase of the first Omicron wave. We also assumed an average of 50 daily infections arriving at the border. This prevented simulations from stochastically eliminating, but did not otherwise have a significant effect on model results. In Results, we report the median of 15 independent simulations for reach parameter combination investigated. The relatively small number of simulations is reasonable as model outputs did not vary greatly from between simulations. Further details are provided in Supplementary Information and model parameters are shown in Supplementary Table S3. Software to run the model is available at https://github.com/Giorgia93/NZ-COVID19-reinfectionModel.
3. Results and discussion
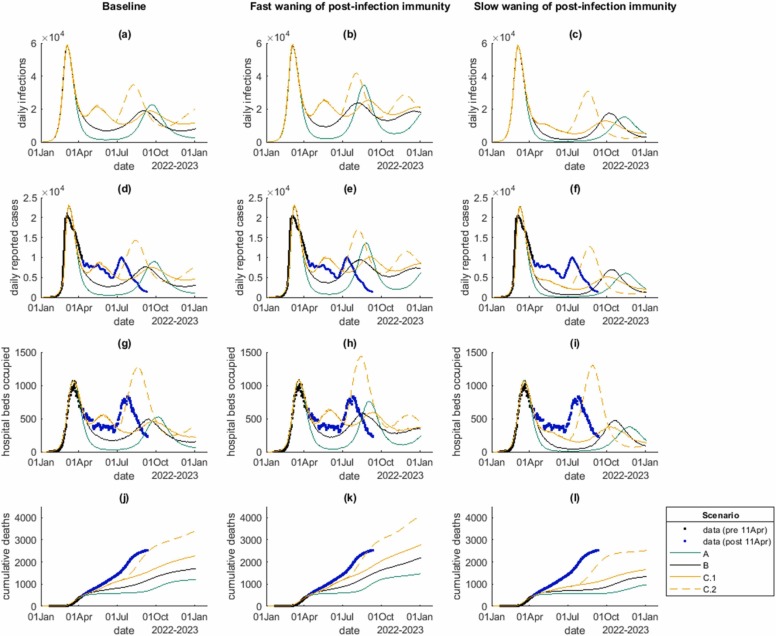
Results for the four contact rate scenarios and three models of infection-derived immunity are shown in Fig. 3 and Table 2. These results were produced in mid-April 2022 and are shown alongside subsequent data available up to 12 September 2022. In all scenarios, a second Omicron wave of infections occurred in 2022, driven by a mixture of people not infected in the first Omicron wave and re-infection of people whose immunity has waned (Supplementary Fig. S5). In all scenarios, infections and cases followed very similar trajectories because the case ascertainment rate in the model varied relatively little through time. However, case ascertainment did increase after 23 February as a result of the assumed increase in testing probability and, in scenario C.2 from 1 July, of the shift in the age distribution of infections into older age groups, which have a higher probability of symptomatic disease.
Fig. 3.
Model results for: (a,b,c) daily infections; (d,e,f) daily reported cases; (g,h,i) number of hospital beds occupied; (j,k,l) cumulative deaths, for the four scenarios A (green), B (black), C.1 (solid yellow) and C.2 (dashed yellow), under the baseline model for post-infection immunity (first column), fast waning of post-infection immunity (second column), and slow waning of post-infection immunity (third column). Black dots show data that was available at the time the analysis was done (11 April 2022), blue dots show data from 12 April to 11 September 2022. Curves show the medians of 15 independent model simulations.
Table 2.
Total number of infections, reinfections, cases, hospitalisations, deaths over the simulated time period (365 days starting on 5 January 2022) for each of the scenarios described in Table 1. Note that the “reinfections” column shows the proportion of all infections that are reinfections in the model. The empirically observed reinfection rate may be lower than this because a case can only be classified as a reinfection if both the first infection and the reinfection were ascertained.
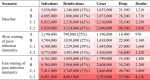 |
The peak number of new daily cases in the second wave in the model was significantly smaller than in the first wave (in which the seven-day rolling average number of reported cases peaked at around 20,500 per day). The peak hospital occupancy was also generally smaller than in the first wave (in which the peak occupancy was 1016), with the exception of the scenario (C.2) with increased contact rates among older groups. The size and timing of the second wave in the model was sensitive to assumptions about the speed of waning of immunity from prior infection, with faster waning generally associated with earlier waves, slightly higher peaks and significantly larger numbers of cumulative infections, hospitalisations and deaths. Results of a sensitivity analysis with faster waning of immunity to severe disease are shown in Supplementary Table S4 and Fig. S6.
In the scenario with the largest increase in contact rates (scenario C), there was a small secondary wave in May/June 2022 driven by the increased ability of the virus to find new hosts that had not been previously infected. This had the effect of reducing the size of the subsequent wave that occurred later in the year, as there was more immunity and fewer naive hosts at that time. However, the cumulative numbers of infections, hospitalisations and deaths were still larger in scenario C than in scenarios A and B (Table 2).
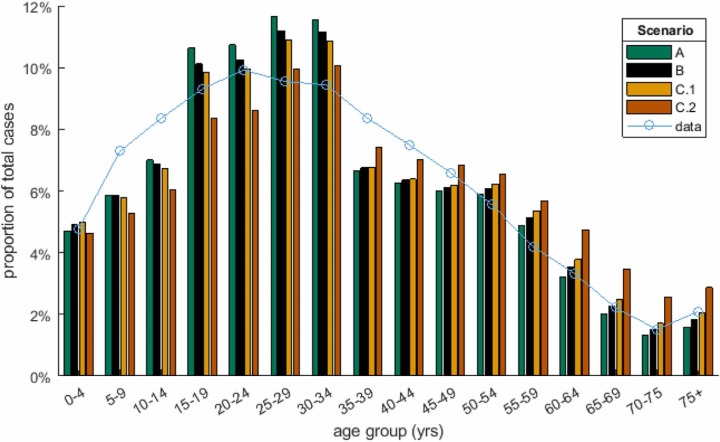
In the scenario with increased contact rates in older age groups (C.2), peak hospitalisations and total deaths exceeded the first Omicron wave. This was due to a shift in the age distribution of infections ( Fig. 4): although the overall distribution was still dominated by under-35-year-olds, relatively small increases in the proportion of infections aged over 60 years had a major impact on hospitalisations and deaths due to the steep age gradient in risk. This scenario was modelled via a change in the contract matrix, which was assumed to take effect on 1 July 2022. This led to a sudden start to the second wave in model results. This was a simplifying assumption and it is likely that, in reality, any change would take place more gradually and could occur earlier or later than assumed.
Fig. 4.
Age distribution of cases in model scenarios A, B, C.1 and C.2 (coloured bars), alongside data on the age distribution of reported cases from 25 January to 10 May 2022 (blue curve).
Nevertheless, these results show that the overall health burden is highly sensitive to the number of infections in high-risk groups. It should be remembered that Māori and Pacific people are known to have higher risk of severe illness after controlling for age (Steyn et al., 2021). Ministry of Health data includes ethnicity and other covariates such as deprivation index for cases, hospitalisations and deaths so, in principle, differences in transmission and risk among these groups could be modelled. There are challenges in parameterising a compartment model of the type studied here to include multiple demographic groups and including this level of detail may be better suited to an agent-based modelling (e.g (Thompson et al., 2022).) or network contagion modelling approach (see (Turnbull et al., 2022)).
The model results for scenario C provide a reasonable match with age-stratified data on cases, hospitalisations and deaths (Supplementary Fig. S7). However, cases in over 70-year-olds were becoming higher than model predictions towards the end of this time period, suggesting that some of the shift in age distribution of infections modelled by scenario C.2 may have already been taking place. The model appears to underestimate hospital admissions in some age groups, notably over 70-year-olds and 10–40-year-olds. However, it should be noted that hospitalisation data includes a mixture of people who are being treated for Covid-19 and people who are being treated for other conditions but test positive before or during they stay in hospital. In addition, age-stratified data on hospital admissions (Supplementary Fig. S7) was only available for the Northern Region (Auckland, Counties Manukau, Waitematā and Northland District Health Boards). More recent Ministry of Health data suggests that the number of recorded admissions in the Northern Region was significantly higher than the actual number of patients who received hospital treatment for Covid-19 (see (Lustig et al., 2022)).
At the time the analysis was done, data on deaths included everyone who has died within 28 days of a positive Covid-19 test result. More recent Ministry of Health data categorises deaths as either “Covid as underlying”, “Covid as contributory” or “Not related to Covid”. This shows that approximately 35% of deaths with 28 days of a positive test result are unrelated to Covid-19 (Ministry of Health, 18 May, 2022). Model outputs should be interpreted with caution in the context of these known data limitations.
Reported cases represent an unknown fraction of total infections, and this means that the amount of infection-derived immunity that has built up in the population through the first Omicron wave is uncertain. In the results presented in Fig. 3, the case ascertainment rate, calculated as the total number of reported cases divided by the total number of infections, was between 37 % and 39 %. In order to explore the effects of different case ascertainment rates, we carried out a sensitivity analysis with different values for the probability of testing. To match the observed height of the peak in reported cases in March 2022, we increased or decreased the assumed reproduction number in the low/high case ascertainment scenarios respectively (Supplementary Fig. S4). The alternative case ascertainment scenarios shown in Supplementary Fig. S4 do not match the trajectories for cases and hospital occupancy up to 11 April 2022 as well as the baseline scenario does. However, the timing of the second Omicron wave and peak number of infections and hospitalisations are quite similar across scenarios (although unsurprisingly higher case ascertainment leads to a greater number of cases). This suggests that model projections are not highly sensitive to the assumed case ascertainment rate.
The scenarios shown in Fig. 3 attempt to capture some of the uncertainty in two key variables driving epidemic dynamics: (i) changing contact rates as a result of relaxing public health measures and voluntary behaviour change; and (ii) the strength and durability of infection-derived immunity. The model parameters used in these scenarios cover a reasonable range of possible outcomes based on previous estimates of changes in contact rates and estimates of the dynamics of waning immunity from different sources against different clinical endpoints (Khoury et al., 2021, Cromer et al., 2022b, Golding et al., 2021). However, it is possible that outcomes will fall outside these ranges. In particular, there is a lack of population-level data at present on time-varying immunity (with or without vaccination) against re-infection with the same or an antigenically similar variant of Omicron. The estimates of (Golding et al., 2021) were based on extrapolating immunity estimates for the Delta variant of SARS-CoV-2 using laboratory data on neutralisation titre for the Omicron variant, which means they are subject to significant uncertainty. Other modelling groups have used the modelled relationship of (Khoury et al., 2021) between neutralisation titre and level of protection to derive immunity estimates for Omicron (Barnard et al., 2022), but others have used different assumptions (Keeling et al., 2021) and there is no universally agreed upon set of immunity parameters.
Uncertainty concerning model scenarios is compounded by the emergence of new sub-variants of Omicron with different transmissibility and immune escape characteristics. For example, New Zealand's first Omicron wave was dominated by BA.2 with a significant minority of BA.1 infection (Chen et al., 2021). If a new subvariant becomes dominant, such as BA.4 or BA.5, which can more easily re-infect people previously infected with BA.1 or BA.2 (Tegally et al., 2022, Khan et al., 2022), this could cause a wave to happen earlier than it would otherwise. This wave's health impact would largely depend on the degree to which prior infection with BA.1 or BA.2 protects against severe illness with the new sub-variant. The scenarios presented here do not attempt to capture the effects of a new variant of concern with a substantial increase in transmissibility, immune escape and/or virulence. However, this model framework is being used in a separate piece of work to investigate the potential impact of hypothetical new variants with specific epidemiological characteristics in New Zealand.
The model ignores important sources of heterogeneity in the New Zealand population that could affect rates of transmission and health burden over time. Model results show the national picture but this is likely to be unevenly distributed across the population. Communities with low vaccination rates, high comorbidity rates, poorly served by healthcare systems, or other risk factors are likely to be disproportionately affected. Māori and Pacific people in particular experienced very high transmission rates during the 2021 Delta outbreak and the first Omicron wave in 2022, and are at higher risk of severe illness if infected with SARS-CoV-2 (Steyn et al., 2021). Reinfections are likely to be clustered in particular communities at different times due to the persistence of particular transmission networks, which could lead, for example, to acute worker shortages in some workplaces or sectors. Our results show that the health impacts of a second wave will be highly sensitive to the number of infections occurring in older age groups. This will also apply to other high-risk groups including Māori and Pacific people (McLeod et al., 2020, Whitehead et al., 2021, Waitangi Tribunal Wai, 2021) and people who are immunocompromised or have other comorbidities, although these factors were not explicitly included in the model.
3.1. Addendum: comparison of model results with subsequent data
The analysis described above was done using data available up to 11 April 2022. Subsequent data on cases, hospitalisations and deaths up 11 September 2022 (blue points in Fig. 3) agree reasonably well with scenario C.2 under the baseline waning assumptions up to around August 2022. The sizes of the peaks in daily cases and hospitalisations in the July wave were intermediate between model scenarios C.1 and C.2. Cumulative deaths were very close to scenario C.2 by the end of August 2022.
The second wave that occurs in scenario C.2 in the model is a result of a step-change in the assumed contact matrix on 1 July 2022, in combination with waning immunity. In reality, the wave that occurred in July 2022 was caused by a combination of a shift in the age distribution of cases and the BA.5 sub-variant displacing BA.2 as the dominant variant (Lustig et al., 2022). It is unsurprising that the model did not predict the size and timing of this wave completely correctly: the effects of new variants were explicitly excluded from the model given the uncertainty in April as to which sub-variant would become dominant and when. Nevertheless, by exploring the effects of a change in age-structured contact patterns, the model provided important scenarios about how the second Omicron wave might result in different levels of healthcare burden from the first wave. This was useful to policymakers and healthcare analysts, independently of whether the model produced a quantitatively accurate prediction of the cases, hospitalisation and deaths time series. The model results showed that it was important to consider how the emergence of new susceptibility was likely to interact with a shift in age distribution of infections to older groups. This turned out to have a substantial effect on the July wave, particularly for hospitalisations and deaths.
For future modelling of SARS-CoV-2, potential simplifications could include using a deterministic model, since the effect of stochasticity in our model simulations was small and likely dominated by other sources of uncertainty not included in the model, such as parameter uncertainty and potential model misspecification. The effect of testing, tracing and isolation in the model could also be simplified as this component of the model required several parameter values, results were not highly sensitive to these, and the significance of contact tracing as a control measure has diminished over time. The inclusion of age structure in the model is essential because of strong age gradients in key variables such as disease severity, vaccine coverage, and contact rates. Further work on factors that affect age-specific contact patterns, for example using national or regional social survey data and household size data, would help inform more accurate contact matrices for use in epidemiological models.
CRediT authorship contribution statement
Giorgia Vattiatio: Conceptualization; Methodology; Software; Validation; Data curation; Writing – original draft, Writing – review & editing, Visualization. Audrey Lustig: Conceptualization; Methodology; Software; Writing – original draft, Writing – review & editing, Visualization. Oliver J. Maclaren: Conceptualization; Software; Validation; Writing – original draft, Writing – review & editing. Michael J. Plank: Conceptualization; Methodology; Data curation; Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Conflict of interest statement
The author declares no conflict of interest in relation to this article.
Acknowledgements
The authors acknowledge the support of the New Zealand Ministry of Health, StatsNZ, and the Institute of Environmental Science and Research in supplying data in support of this work. The authors are grateful to Emily Harvey, Nick Golding, James McCaw, Dion O’Neale and Freya Shearer for discussions about modelling waning immunity, and to Samik Datta, Nigel French, Anja Mizdrak, Matt Parry, the Covid-19 Modelling Government Steering Group, and three anonymous reviewers for feedback on earlier versions of this manuscript. Kannan Ridings helped with data management. This work was funded by the New Zealand Department of Prime Minister and Cabinet.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2022.100657.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Matlab code to run the model is available at https://github.com/Giorgia93/NZ-COVID19-reinfectionModel.
References
- Abbott S., Sherratt K., Gerstung M., Funk S. Estimation of the test to test distribution as a proxy for generation interval distribution for the Omicron variant in England. medRxiv. 2022 doi: 10.1101/2022.01.08.22268920. [DOI] [Google Scholar]
- Anderson R.M., May R.M. Infectious Diseases of Humans: Dynamics and Control: OUP Oxford; 1992.
- Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) Variant. New Engl. J. Med. 2022 doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R.C., Davies N.G., Pearson C.A.B., Jit M., Edmunds W.J. Projected epidemiological consequences of the Omicron SARS-CoV-2 variant in England, December 2021 to April 2022. medRxiv. 2021:2021.12.15.21267858.
- Bjørnstad O.N., Shea K., Krzywinski M., Altman N. The SEIRS model for infectious disease dynamics. Nat. Methods. 2020;17(6):557–559. doi: 10.1038/s41592-020-0856-2. [DOI] [PubMed] [Google Scholar]
- Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., Ng S.S., Chan K.C.K., Ko F.W., et al. Neutralizing antibodies against the SARS-CoV-2 omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat. Med. 2022;28(3):486–489. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nadeau S., Yared M., Voinov P., Xie N., Roemer C., et al. CoV-Spectrum: analysis of globally shared SARS-CoV-2 data to identify and characterize new variants. Bioinformatics. 2021;38(6):1735–1737. doi: 10.1093/bioinformatics/btab856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D., Steain M., Reynaldi A., Schlub T.E., Sasson S.C., Kent S.J., et al. Neutralising antibodies predict protection from severe COVID-19. medRxiv. 2022a:2022.06.09.22275942.
- Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding N., Lydeamore M. Analyses to predict the efficacy and waning of vaccines and previous infection against transmission and clinical outcomes of SARS-CoV-2 variants. Accessed 5 April 2022. 2022 [Available from: 〈https://github.com/goldingn/neuts2efficacy〉.
- Golding N., Price D.J., Ryan G.E., McVernon J., McCaw J.M., Shearer F.M. Estimating the transmissibility of SARS-CoV-2 during periods of high, low and zero case incidence. medRxiv. 2021 doi: 10.7554/eLife.78089. 2021.11.28.21264509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haccou P., Haccou P., Jagers P., Vatutin V.A., Vatutin V. Cambridge University Press; 2005. Branching Processes: Variation, Growth, and Extinction of Populations. [Google Scholar]
- Herrera-Esposito D. de los Campos G. Age-specific rate of severe and critical SARS-CoV-2 infections estimated with multi-country seroprevalence studies. BMC Infect. Dis. 2022;22:311. doi: 10.1186/s12879-022-07262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Piantham C., Nishiura H. Estimating relative generation times and relative reproduction numbers of Omicron BA.1 and BA.2 with respect to Delta in Denmark. medRxiv. 2022 doi: 10.3934/mbe.2022418. 2022.03.02.22271767. [DOI] [PubMed] [Google Scholar]
- Keeling M.J., Brooks-Pollock E., Challen R., Danon L., Dyson L., Gog J.R., et al. Short-term projections based on early omicron variant dynamics in England. medRxiv. 2021 2021.12.30.21268307. [Google Scholar]
- Khan K., Karim F., Ganga Y., Bernstein M., Jule Z., Reedoy K., et al. Omicron sub-lineages BA.4/BA.5 escape BA.1 infection elicited neutralizing immunity. medRxiv. 2022 2022.04.29.22274477. [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Kim D., Ali S.T., Kim S., Jo J., Lim J.-S., Lee S., et al. Estimation of serial interval and reproduction number to quantify the transmissibility of SARS-CoV-2 omicron variant in South Korea. Viruses. 2022;14:533. doi: 10.3390/v14030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig A., Vattiato G., Maclaren O., Watson L., Datta S., Plank M.J. Modelling the effects of Omicron sub-variant BA.5 in New Zealand. Covid-19 Modelling Aotearoa pre-print. 2022; 〈https://www.covid19modelling.ac.nz/modelling-ba5/〉.
- McLeod M., Gurney J., Harris R., Cormack D., King P. COVID-19: we must not forget about Indigenous health and equity. Aust. N. Z. J. Public Health. 2020;44(4):253–256. doi: 10.1111/1753-6405.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health. COVID-19: Case demographics, Accessed 18 May 2022. 2022: 〈https://www.health.govt.nz/covid-19-novel-coronavirus/covid--data-and-statistics/covid--case-demographics〉.
- New Zealand Government. COVID-19 Public Health Response (Protection Framework) Order 2021 New Zealand Legislation. 2021;(SL 2021/386): 〈https://www.legislation.govt.nz/regulation/public/2021/0386/latest/LMS563461.html〉.
- Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem K., Cook A.R., Jit M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017;13(9) doi: 10.1371/journal.pcbi.1005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn N., Binny R.N., Hannah K., Hendy S., James A., Lustig A., et al. Māori and Pacific People in New Zealand have higher risk of hospitalisation for COVID-19. N. Z. Med. J. 2021;134(1538):28–43. [PubMed] [Google Scholar]
- Steyn N., Plank M.J., Binny R.N., Hendy S., Lustig A., Ridings K. A COVID-19 vaccination model for Aotearoa New Zealand. Sci. Rep. 2022;12:2720. doi: 10.1038/s41598-022-06707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Moir M., Everatt J., Giovanetti M., Scheepers C., Wilkinson E., et al. Continued emergence and evolution of omicron in South Africa: new BA.4 and BA.5 lineages. Nat. Med. 2022 doi: 10.1038/s41591-022-01911-2. [DOI] [Google Scholar]
- Thompson J., McClure R., Blakely T., Wilson N., Baker M.G., Wijnands J.S., et al. Modelling SARS‐CoV‐2 disease progression in Australia and New Zealand: an account of an agent‐based approach to support public health decision‐making. Aust. N. Z. J. Public Health. 2022 doi: 10.1111/1753-6405.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull S., Hobbs M., Gray L., Harvey E., Scarrold W., O'Neale D. Investigating the transmission risk of infectious disease outbreaks through the Aotearoa co-incidence Network (ACN): a population-based study. Lancet Reg. Health-West. Pac. 2022;20 doi: 10.1016/j.lanwpc.2021.100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattiato G., Maclaren O., Lustig A., Binny R.N., Hendy S.C., Plank M.J. An assessment of the potential impact of the Omicron variant of SARS-CoV-2 in Aotearoa New Zealand. Infect. Dis. Model. 2022;7:94–105. doi: 10.1016/j.idm.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022 doi: 10.1038/d41586-021-03832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitangi Tribunal Wai 2575. Haumaru: The Covid-19 Priority Report. Waitangi Tribunal, Wellington. 2021: 〈https://waitangitribunal.govt.nz/assets/Documents/Publications/Covid-Priority-W.pdf〉.
- Whitehead J., Scott N., Carr P.A., Lawrenson R. Will access to COVID-19 vaccine in Aotearoa be equitable for priority populations? N. Z. Med. J. 2021;134(1535):25–34. [PubMed] [Google Scholar]
- World Health Organisation. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021: 〈https://www.who.int/news/item/26–11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern〉.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Matlab code to run the model is available at https://github.com/Giorgia93/NZ-COVID19-reinfectionModel.