Abstract
The present study tested the influence of rumination and impulsivity on experimentally induced negative mood among a sample of smokers with a lifetime history of major depression (MDD Hx+). Participants (N = 40) were categorized into four vulnerability groups: nonvulnerable (low rumination, low impulsivity), ruminative (elevated rumination, low impulsivity), impulsive (low rumination, elevated impulsivity), and vulnerable (elevated rumination, elevated impulsivity). Participants were counterbalanced to four experimental conditions, using a combination of a mood induction (negative mood induction vs. control) and smoking cue (in vivo cigarette vs. control cue). Although all participants reported greater anger responses when exposed to the negative mood induction versus control, vulnerable and ruminative smokers reported significantly greater anger responses than impulsive and nonvulnerable smokers [F(9,87) = 2.93, p = .038, Mse = 79.38]. Implications are discussed.
The association between cigarette smoking and major depression is clearly marked. Depressed smokers smoke at higher rates compared to smokers without depression1 and have an elevated risk for both smoking and nonsmoking-related cancers.2 In addition, smokers vulnerable to depression (eg, those with a lifetime history of depression) experience higher negative affect than nonvulnerable smokers3,4 and greater mood disturbances.5 For example, when exposed to stress, MDD Hx+ smokers respond with increased negative affect, while MDD Hx− smokers respond with decreased negative affect.6 Provided that smoking is posited to be reinforced via self-medicating/negative affect reduction processes,7 and that increases in negative affect are associated with relapse,8 increased mood reactivity among MDD Hx+ is especially concerning. In efforts to identify factors that may increase mood distress among MDD Hx+ smokers, the present study examined the interactive effects of two factors (ie, rumination, impulsivity) associated with a vulnerability to stress as well as cue induced responses.
Rumination is linked with depression history among smokers9 and defined as “repetitively focusing on the fact that one is depressed and on the causes, meanings, and consequences of depressive symptoms.”10 Rumination is also associated with less general control,11 and greater difficulty controlling negative emotion.12 Despite these findings, whether rumination may interact with factors associated with dysregulated behavior such as impulsivity, a characteristic that typifies smoker, remains unknown.
Impulsivity is defined as a tendency to react to internal and external stimuli without considering possible consequences.13 Impulsivity is linked to smoking,14 with nicotine acting as a negative reinforcer for impulsive smokers.15 Internal stimuli such as ruminating on negative mood, may be particularly salient for impulsive smokers by increasing their susceptibility to smoke as a means of curtailing emotional responses. Given that under situations of stress rumination is associated with increases in negative mood,12 it is expected that impulsive smokers high in rumination would be more reactive to the internal stimulus of rumination, resulting in increases in self-report of negative mood. As such, researchers hypothesize that vulnerable smokers (elevated rumination and elevated impulsivity) would experience a greater increase in negative mood when exposed to a stressor coupled with an in vivo cigarette cue, compared to all other groups. Specifically, of the six mood states (anxiety, depression, anger, vigor, fatigue, and confusion), we expect greater self-report post induction in negative mood states (anxiety, depression, anger).
METHOD
Participants
The current study analyzed data from an experimental study conducted with smokers with and without a lifetime history of depression from a large midwestern city. Exclusionary criteria included meeting criteria for a current Axis I disorder other than nicotine dependence, current use of Nicorette, or other nicotine quitting substance, being abstinent less than 6 months from individuals recovering from substance dependence other than nicotine, inability to read questionnaires, use of psychiatric medications, and those not within the age range of 21–55. For information on participant flow, see McChargue et al.16 Current smokers with a lifetime history of depression and who completed all necessary measures (N = 40) were included in this analysis. Refer to Table 1 for univariate statistics and between group mean differences on demographic (ie, age) and patient characteristics (ie, rumination, impulsivity).
TABLE 1.
Summary of univariate statistics by vulnerability group and total
| Mean (SD) or frequency (%) |
|||||
|---|---|---|---|---|---|
| Variables | Nonvulnerable | Impulsive | Ruminator | Vulnerable | |
| (n = 11) | (n = 8) | (n = 7) | (n = 14) | ||
| Age | 41.4(11.8) | 44.6(11.7) | 44.9(10.1) | 40.0(12.8) | |
| Gender | Female | 7(63.6%) | 2(25%) | 6(85.7.0%) | 5(35.7%) |
| Race | African American | 8(72.7%) | 4(50%) | 4(57.1%) | 8(57.1%) |
| Caucasian | 2(18.2%) | 4(50.0%) | 2(28.6%) | 5(35.7%) | |
| Other | 1(9.1%) | – | 1(14.3% | 1(7.1%) | |
| Education | Some high school | 2(18.2%) | 1(12.5%) | – | 1(7.1%) |
| High-school degree | 3(27.3%) | 2(25.0%) | 3(42.9%) | 3(21.4%) | |
| Some college | 4(36.4%) | 4(50.0%) | 3(42.9%) | 8(57.1%) | |
| AA and higher degree | 2(18.2%) | 1(12.5%) | 1(14.3%) | 2(14.2%) | |
| Hx of substance abuse | Yes | 5(45.5%) | 5(62.5%) | 3(42.9%) | 10(71.4%) |
| Longest time (days) ever abstinence | 4.0(10.71) | 3.13(4.4) | 2.57(3.46) | 1.14(1.61) | |
| Family Hx of nicotine dep | Yes | 10(90.9%) | 6(75%) | 7(100%) | 13(92.9%) |
| FTND total score* | 5.09(2.0) | 6.1(1.7) | 5.7(2.0) | 7.4(1.9) | |
| Rumination total score* | 27.9(3.8) | 30.1(1.6) | 38.7(3.6) | 40.0(6.0) | |
| Impulsivity total score* | 55.4(6.1) | 74.1(6.6) | 60.7(4.8) | 75.1(8.6) | |
| Depression Proneness Inventory total* | 25.8(5.9) | 37.9(5.6) | 38.4(10.0) | 41.4(12.3) | |
Significant mean differences (p > .05) on (a) FTND score for nonvulnerable vs. vulnerable; (b) impulsivity total score for nonvulnerable vs. impulsive, nonvulnerable vs. vulnerable, and impulsive vs. ruminators; (c) rumination total score for nonvulnerable vs. ruminators, nonvulnerable vs. vulnerable, impulsive vs. ruminator, and impulsive vs. vulnerable; (d) depression proneness total score for nonvulnerable vs. all three groups. Differences in mean age were not observed. Chi-square analysis could not be conducted for qualitative variables given small sample size of each cell.
Measures
Trait impulsivity was measured by the Barratt Impulsivity Scale version 1117 (BIS-11). The BIS is a 30-item self-report questionnaire rated on a 4-point Likert scale with scores ranging from 30 to 120. Tobacco dependence was operationalized with the Fageström Test for Nicotine Dependence (FTND).18 Mood states (outcome variables) were assessed with the Profile of Mood States (POMS).19 Ruminative response style was assessed by the Response to Depression Questionnaire (RTD).20 Depression proneness was measured by the Depression Proneness Inventory.21 The Structured Clinical Interview for DSM-IV Non-Patient version (SCID-NP)22 was used to assess for history of MDD and administered by Dr. McChargue.
Procedures
Screening Session
Participants were screened over the phone and those who met study criteria were scheduled for the screening session. Smoking status was assessed by self-report and expired carbon monoxide samples (CO readings > 10). Those found eligible completed baseline questionnaires. Participants also completed a guided imagery interview and were asked to describe several events within the past year which had caused them to feel “upset, very anxious, angry, or sad” as well as four events which did not evoke “upset or unhappy” feelings.23 The interviewer encouraged participants to provide a comprehensive summary of each situation including factors preceding the event, emotions regarding the event, and the outcome. Events were rated on a 10-point Likert scale of distress indicating the degree of anxiety, anger, or sadness experienced as well as a 10-point Likert scale indicating the degree of vividness of the memory. Memories with a score greater than 7 were used in the negative mood induction, and those scored 0 or 1 were used in the neutral condition.
Experimental Session
Participants were asked to not consume caffeine after 9:00 am. Measures for caffeine, food, alcohol intake, and exercise were taken at start of session, given their potential for influencing cue reactivity. Consistent with other studies,24 subjects were allowed to smoke one cigarette and then rest for 30 minutes to minimize possible nicotine withdrawal and to standardize time to last cigarette. After the resting period, mood + cue exposure induction procedures were put into operation. Each participant was exposed to four counterbalanced exposure trials: neutral mood induction + neutral cue (NEUTRAL); neutral mood + smoking cue (NU/NEG CUE); negative mood + neutral cue (NEG/NU CUE); negative mood + smoking cue (NEGATIVE). A Latin-square design was used to randomize the order of exposure trials. To ensure sufficient engagement in memory recall and response to the induction technique, memory vividness was rated on a 100-point Likert, asking participants to rate the level of memory vividness directly after the mood induction procedures.25
Mood Induction Procedures
For the negative mood induction condition, participants were asked to recall a negative memory they had previously rated a 7–10 on a 10-point Likert scale of distress during the screening session. Participants were then asked to listen to audiotape pieces of classical music to evoke depressed states (ie, Russia Under the Mongolian Yoke and Adiago Pour Codes26). In the neutral mood condition, participants did not listen to music, given that music has been shown to evoke both negative and positive mood states.27 Participants were instead asked to recall a neutral memory rated as 0 or 1 at the screening session that held no affective value (ie, doing the dishes). Inductions lasted 10 minutes with physiological measures sampled throughout and self-reported mood and level of memory vividness assessed directly after each exposure period. Before leaving the experimental session, participants were exposed to a positive mood induction to dispel possible acquired negative affect.
Cue Exposure Procedure
For the in vivo cigarette condition, participants were provided with a tray that had their brand of cigarette, a lighter, and an ashtray. Cigarettes for the cue exposure condition were provided by the experimenter. Participants were instructed to light one cigarette in their dominant hand by holding the cigarette in the flame of the lighter until it burned without putting it to their mouth, and then asked to extinguish it at the end of the 10-minute exposure period. For the neutral cue condition, participants were instructed to hold a roll of scotch tape in lieu of a cigarette and instructed to hold in their dominant hand.15
Analytic Plan
Given the potential to minimize error variance between independent and dependent variables,28 a mixed group factorial analysis of covariance (ANCOVA) was chosen to test the interaction between exposure condition and vulnerability group. Vulnerability groups were created based on median cut-off scores for each impulsivity and rumination variable. Outcomes included pre-exposure to postexposure mood change score on the POMS subscales (anger, anxiety, confusion, depression, vigor, and tension) taken at pre-exposure and postexposure. Covariates, based on their theoretic potential to influence results, included nicotine dependence, history of substance abuse, ethnicity, longest time (in days) ever abstinence from nicotine, family history of nicotine dependence, depression proneness inventory total score, and sum of coffee and alcohol consumption in the last 24 hours.
RESULTS
As a mood-manipulation check, paired sample t-test showed that significant changes in anger were observed in the NEG/NU CUE [t(34) = −4.153, p < .001] and NEGATIVE condition [t(34) = −5.450, p < .001]. Similarly, depression pre–post scores in the NEG/NU CUE [t(34) = −4.387, p < .001] and NEGATIVE condition [t(34) = −6.902, p < .001] were also observed. Finally, POMS anxiety pre–post scores in the NEG/NU CUE [t(34) = −4.578, p < .001] and NEGATIVE condition [t(34) = −5.438, p < .001] were noted. Pre–post change scores in anger, depression, and anxiety where nonsignificant in the neutral conditions, respectively: NEUTRAL [t(34) = 1.030, p = .310; t(34) = 1.598, p = .118; t(34) = .493, p = .625], and NU/ CIG[t(34) = .647, p <.521; t(34) = .505, p < .616; t(34) = −.946, p < .35]. In summary, anger, depression, and anxiety significantly increased in the negative conditions, but not within the neutral mood conditions. Memory engagement was evaluated by assessing mean vividness ratings for each exposure condition. Data evidenced that all participants were adequately engaged with all rating vividness 7 or greater.
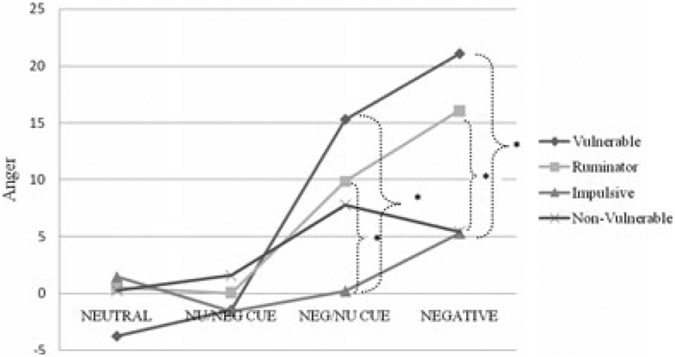
Primary results revealed a significant two-way interaction between exposure condition and vulnerability group on anger [F(9,87) = 2.1, p = .038, Mse = 166.8]. Findings on depression [F(9,81) = 1.53, p = .15], anxiety [F(9,81) = 1.25, p = .36], vigor [F (9,81) = .60, p = .79], fatigue [F(9,81) = .69, p = .71], and confusion [F(9,81) = 1.0, p = .44] were nonsignificant. See Fig. 1 for mean change in anger by vulnerability group and condition.
FIGURE 1.
Summary of cell mean changes in anger responses. Neutral mood/neutral cue (NEUTRAL); neutral mood/cigarette cue (NU/CIG); negative mood/neutral cue (NEG/NU CUE); negative mood/cigarette cue (NEGATIVE). Bold brackets denote between group differences using an HSD of 10.53. * < .05.
Pairwise comparisons were conducted using a Tukey’s honestly significant difference (HSD) value of 10.53. Post hoc tests revealed that vulnerability group differences were nonexistent when exposed to neutral mood condition, regardless of holding a lit cigarette. Significant group differences were observed in the negative mood induction conditions (NEG/NU CUE; NEGATIVE), particularly in the presence of a lit cigarette. Most notably, vulnerable and ruminative smokers reported significantly greater increases in anger than impulsive and nonvulnerable smokers when exposed to the combination of a stressor in the presence of a cigarette cue.
DISCUSSION
To our knowledge, this is the first study to link impulsivity and rumination among MDD Hx+ smokers using an experimental cue reactivity design. While we hypothesized participants would report increases in all three negative mood states (anxiety, depression, anger) during a negative mood induction, our findings appear selective to anger. This unexpected outcome provides supporting evidence for anger as a prominent mood state among vulnerable smokers. For instance, among prequit smokers with and without a history of MDD, significant differences in POMS subscales were also selective to anger, with MDD Hx+ scoring higher.29
The association between anger and smoking outcomes has been documented. MDD Hx+ smokers show higher POMS anger scores at baseline, 1 and 2 weeks post quitting compared to MDD Hx− smokers.30 In turn, greater levels of anger experienced at baseline are also associated with greater smoking urges,31 difficulty with continuous abstinence,32 relapse,33 and a lower likelihood to abstain from smoking.34 Furthermore, among highly hostile people receiving intradermal nicotine administration, nicotine results in decreased reports of anger.35 Given that anger states among smokers are an “important withdrawal symp-om that influences liability to relapse”36 our findings draw attention to a subpopulation of MDD Hx+ smokers possibly recalcitrant to cessation efforts. Yet the degree to which anger sensitivity translates to behaviors (ie, relapse) among MDD Hx+ smokers with impulsive and ruminative tendencies, warrants further research. At best, research on nonsmokers suggests that anger is also related to both impulsivity37 and rumination.38
Despite these interesting findings, some limitations are noted. Although we had an adequate sample size for the entire study, sample size for each group was relatively small. The small sample size may have also contributed to the lack of findings among the less activating emotions, such as, depression. In addition, the sample consisted of nontreatment-seeking smokers who participated in a controlled laboratory study, which may limit the ability to generalize our findings. Future research should address the aforementioned limitations of this study and continue to investigate the impact of individual difference factors on clinically significant mood distress among MDD Hx+ smokers. These findings may aid cessation efforts and the development of specialized interventions for vulnerable subgroups of smokers.
Acknowledgments
This research was supported in part by grant DA00467 from the National Institutes of Health, Bethesda, MD (Dr. McChargue).
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. [DOI] [PubMed] [Google Scholar]
- 2.Linkins RW, Comstock GW. Depressed mood and development of cancer. Am J Epidemiol. 1990;132:962–972. [DOI] [PubMed] [Google Scholar]
- 3.Dalack GW, Glassman AH, Rivelli S, et al. Mood, major depression, and fluoxetine response in cigarette smokers. Am J Psychiatry. 1995;152:398–403. [DOI] [PubMed] [Google Scholar]
- 4.Niaura R, Britt DM, Borrelli B, et al. History and symptoms of depression among smokers during self-initiated quit attempt. Nicotine Tob Res. 1999;1:251–257. [DOI] [PubMed] [Google Scholar]
- 5.Haas AL, Muñoz RF, Humfleet GL, et al. Influences of mood, depression history, and treatment modality outcomes in smoking cessation. J Consult Clin Psychol. 2004;72:563–570. [DOI] [PubMed] [Google Scholar]
- 6.Spring B, Cook JW, Appelhans B, et al. Nicotine effects on affective responses in depression-prone smokers. Psychopharm. 2008;196:461–471. [DOI] [PubMed] [Google Scholar]
- 7.Lerman C, Audrian J, Orleans CT, et al. Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav. 1996;21:9–19. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. J Consult Clin Psychol. 2004;72:192–201. [DOI] [PubMed] [Google Scholar]
- 9.McChargue DE, Werth Cook J. Depression vulnerability within smoking research: How accurate are one-item screening items? Addict Behav. 2007;32:404–409. [DOI] [PubMed] [Google Scholar]
- 10.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–582. [DOI] [PubMed] [Google Scholar]
- 11.Lyubomirsky S, Tucker KL, Caldwell ND, et al. Why ruminators are poor problem solvers: Clues from the phenomenology of dysphoric rumination. J Pers Soc Psychol. 1999;77:1041–1060. [DOI] [PubMed] [Google Scholar]
- 12.Nolen-Hoeksema S, Jackson B. Mediators of the gender difference in rumination. Psychol Women Q. 2001;25:37–47. [Google Scholar]
- 13.Moeller FG, Barratt ES, Dougherty DM, et al. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharm. 1999;146:455–464. [DOI] [PubMed] [Google Scholar]
- 15.Doran N, Spring B, McChargue DE. Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharm. 2007;194:279–288. [DOI] [PubMed] [Google Scholar]
- 16.McChargue DE, Klanecky AK, Walsh K, et al. Trauma exposure influences cue elicited affective responses among smokers with and without a history of major depression. Addict Behav. 2008; 33:1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsivity scale. J Clin Psychol. 1995;51:768–774. [DOI] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fageström test for nicotine dependence: A revision of the Fageström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 19.McNair DM, Lorr M, Droppleman LF. BITS Manual for the Profile of Mood States, San Diego, CA: Educational & Industrial Testing Service; 1971. [Google Scholar]
- 20.Nolen-Hoeksema S, Morrow J, Fredrickson BL, Response styles and the duration of episodes of depressed mood. J Abnorm Psychol. 1993;102:20–28. [DOI] [PubMed] [Google Scholar]
- 21.Alloy LB, Hartlage S, Metalsky GI, et al. The Depression Proneness Inventory: A Brief Face Valid Scale of Vulnerability to Depressive Reactions in Response to Stress, Unpublished Manuscript. Philadelphia, PA: Temple University; 1987. [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders-Non-Patient Edition, New York: State Psychiatric Institute; 1995. [Google Scholar]
- 23.Litt MD, Cooney NL, Kadden RM, et al. Reactivity to alcohol cues and induced moods in alcoholics. Addict Behav. 1990;15:137–146. [DOI] [PubMed] [Google Scholar]
- 24.Tiffany ST, Drobes DJ. Imagery and smoking urges: The manipulation of affective content. Addict Behav. 1990;15:531–539. [DOI] [PubMed] [Google Scholar]
- 25.Tiffany ST, Hakenewerth DM. The production of smoking urges through imagery manipulation: Psychological and verbal manifestations. Addict Behav. 1991;16:389–340. [DOI] [PubMed] [Google Scholar]
- 26.Clark DM, Teasdale JD. Constraint on the effects of affect on memory. J Pers Soc Psychol. 1985;48:1595–1608. [Google Scholar]
- 27.Vaestfjaell D. Emotion induction through music: A review of the musical mood induction procedures. Musicae Scientiae. 2002;173–211. [Google Scholar]
- 28.Tabachnick BG, Fidell LS. Using Multivariate Statistics, 4th edn. Needham Heights, MA: Pearson Educational Company; 2001. [Google Scholar]
- 29.Sukhodolsky DG, Golub A, Cromwell EN. Development and validation of the anger rumination scale. Pers Individ Differ. 2001;31:689–700. [Google Scholar]
- 30.Ginsberg D, Hall SM, Reus VI, et al. Affect and depression diagnosis in smoking cessation. Exp Clin Psychopharmacol. 1995;3:389–395. [Google Scholar]
- 31.Delfino RJ, Jammer LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine Tob Res. 2001;3:235–248. [DOI] [PubMed] [Google Scholar]
- 32.Hass AL, Muñoz R, Humfleet GL, et al. Influences of affect, depression history, and treatment modality on outcomes in smoking cessation. J Consult Clin Psychol. 2004;72(4):563–570. [DOI] [PubMed] [Google Scholar]
- 33.Doherty K, Kinnuen T, Militello FS, et al. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology. 1995;119:171–178. [DOI] [PubMed] [Google Scholar]
- 34.Kahler CW, Strong DR, Niaura R, et al. Hostility in smokers with past major depressive disorder: Relation to smoking patterns, reasons for quitting, and cessation outcomes. Nicotine Tob Res. 2004;6:809–818. [DOI] [PubMed] [Google Scholar]
- 35.Jammer LD, Shapiro D, Jarvik ME. Nicotine reduces the frequency of anger reports in smokers and nonsmokers with high but not low hostility: An ambulatory study. Exp Clin Psychopharm. 1999;7:454–463. [DOI] [PubMed] [Google Scholar]
- 36.Patterson F, Kerrin K, Wileyto EP, et al. Increase in anger symptoms after smoking cessation predicts relapse. Drug Alcohol Depend. 2008;95:173–176. [DOI] [PubMed] [Google Scholar]
- 37.Bond AJ, Verheyden SL, Wingrove J, et al. Angry cognitive bias, trait aggression and impulsivity in substance users. Psychopharmacology. 2004:171;1432–2072. [DOI] [PubMed] [Google Scholar]
- 38.Sukhodolsky DG, Golub A, Cromwell EN. Development and validation of the anger rumination scale. Pers Individ Differ. 2001;31:689–700. [Google Scholar]