Abstract
Objective:
To evaluate the prevalence and major comorbidities of ADHD, using different operational definitions, in a newly available national data set and to test the utility of operational definitions against genetic and cognitive correlates.
Method:
The U.S. Adolescent Behavior Cognition Development (ABCD) study enrolled 11,878 children aged 9–10 years at baseline. ADHD prevalence, comorbidity, and association with polygenic risk score (PRS) and laboratory-assessed executive functions were calculated at four thresholds of ADHD phenotype restrictiveness. Bias from missingness, sampling, and nesting were addressed statistically.
Results:
Prevalence of current ADHD for 9–10 year old children was 3.53% (95% CI: 3.14–3.92%) when computerized Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-COMP) and parent and teacher ratings were required to converge. Of ADHD cases so defined, 70% had a comorbid psychiatric disorder. After control for overlapping comorbidity, and ruling out for psychosis or low IQ, 30.9% (95% CI: 25.7–36.7%) had a comorbid disruptive behavior disorder, 27.4% (95% CI: 22.3–33.1%) an anxiety or fear disorder, and 2.1% (95% CI: 1.2%−3.8%) a mood disorder. Children in the top decile of polygenic load incurred a 63% increased chance of having ADHD versus the bottom half of polygenic load (p<.01)—an effect only detected with a stringent phenotype definition. Dimensional latent variables for irritability, externalizing, and ADHD yielded convergent results for cognitive correlates.
Conclusions:
This fresh estimate of national prevalence in the United States suggests that the DSM-5 definition requiring multiple informants yields a prevalence of about 3.5%. Results may inform further ADHD studies in the ABCD sample.
Keywords: ADHD, comorbidity, prevalence, polygenic score, executive function
INTRODUCTION
Attention-deficit / hyperactivity disorder (ADHD) is of major importance due to its developmental and causal linkages to subsequent psychopathology, poor life outcome, and premature death.1,2 This paper addresses two fundamental, yet related, challenges that impede progress on ADHD: (a) unclear prevalence and comorbidity in light of varying operational definitions of ADHD and a dearth of multi-informant national surveys, and (b) associated uncertainty about whether, for detection of genetic and other mechanistic signals, it is more effective to define ADHD narrowly (multiple informants and multi-measure confirmation as in the DSM-5 text, thus lowering prevalence) or broadly (requiring only one measure or reporter to confirm symptoms, often done).
Prevalence and comorbidity.
Prevalence of ADHD has been unclear.3 Previous information on ADHD prevalence and comorbidity in the U.S. has come primarily from two types of data. First, national surveys of parents, such as those conducted by the Centers for Disease Control,4,5 as well as the National Comorbidity Survey Replication Adolescent Supplement (NCS-A)6 relied on versions of parent report alone, estimating prevalence at 8% to over 9%. Local but non-representative studies using more stringent evaluation of ADHD in children have yielded noticeably lower estimates of 2–4%.7 Standard meta-analyses have reached an interim value of 5–7% in children8,9 but are limited by combining studies using different methods of varying rigor. Two Bayesian meta-analyses from the global Burden of Disease Study estimated DSM-qualifying, multi-informant prevalence at ~2% worldwide and ~3% in North America10 and at 3.7% worldwide for high income countries like the U.S.11 (Further details on that literature are provided in the online supplement, Section I). Thus, new multi-method, multi-informant national data are needed to further evaluate prevalence and comorbidity in the U.S.
Phenotype refinement.
Secondly, however, that same literature underscores the need to attend to phenotype definition, because ADHD is defined in different ways in different studies, obscuring prevalence estimates. The DSM-5 defines ADHD in relation to a literature that views it as a polygenic, multifactorial, neurodevelopmental condition. It is essential to clarify how ADHD should be operationalized—broadly (as in national parent surveys) or more stringently (using multi-method, multi-informant procedures), to best detect genetic liability and cognitive markers. Evaluation of different definitions in the same cohort is necessary to do so.
Utility of the ABCD data.
The multi-institute NIH-funded Adolescent Behavior Cognition Development (ABCD)12 offers a unique opportunity to address these issues by virtue of nationwide sampling, the availability of multiple informants, and prior work on propensity weighting to estimate bias-corrected prevalence.13 It thus is well suited to evaluate competing ways of defining ADHD in relation to comorbidity, genetic risk, and cognitive correlates. Because of the ABCD’s uniqueness and widespread scientific use, determining the effect of different operational definitions of ADHD in the ABCD sample for different purposes is timely as well. The key test of detecting genetic liability can be done using polygenic scores. These scores, based on genotyping millions of locations on the genome for common variants, sum up the contributions of all disorder-associated variants into a single liability score for a disorder.
Goals of the Current Study.
The present paper aimed to (a) estimate ADHD prevalence and comorbidity from the new ABCD sample, (b) evaluate different thresholds of restrictive phenotype definition for identifying external correlates of ADHD as a category, with particular emphasis on polygenic risk, (c) evaluate a multi-measure dimensional measures of ADHD in this sample, and (d) offer guidance for operationalizing ADHD as a clinical entity in the ABCD sample.
METHODS
Description of the ABCD sample and ADHD Evaluation
The ABCD study is the largest longitudinal study of child-adolescent neurodevelopment and mental health in the U.S.12 The ABCD cohort enrolled 11,878 participants between 9–10 years of age from among community volunteers, at 21 sites around the nation. Participants were screened for basic inclusion eligibility information prior to enrollment. Wave 1 data collection included measures of mental health status with a recently developed and validated computerized parent-answered Kiddie Schedule of Affective Disorders and Schizophrenia for DSM-5 (KSADS-COMP).14 It coded a positive diagnosis of ADHD when the parent report met DSM-5 criteria including duration and impairment. (Note, however, that this version of the KSADS-COMP required impairment in only one setting for the diagnosis of ADHD, whereas DSM-5 requires impairment in two settings. Also, the baseline KSADS-COMP assessment of major depression failed to utilize impairment criteria. Corrections to both errors, while underway, were not publicly available at this writing.)
Also available were two well-validated, nationally normed scales—the parent report Childhood Behavioral Checklist (CBCL)15 and the teacher-report Brief Problem Monitor (BPM)16. Child participants completed a computerized version of the WISC-V17 Matrix Reasoning subtest as an estimate of non-verbal IQ. They also completed the youth self-report KSADS-COMP for selected mood and anxiety modules, but not for all conditions. For testing of dimensional latent variable models, demographically matched split-half samples known as the ABCD Reproducible Matched Samples (ARMS)18 were used for replicability analyses (ARMS-1; N=5,786, ARMS-2; N= 5,786).
ADHD restrictive phenotypes defined
Four ADHD phenotypes were created for the current report using increasingly more stringent filters called ADHD Tier 1, Tier 2, Tier 3, and Tier 4 in this paper, as follows.
ADHD-Tier 1:
Met ADHD-current on the KSADS-COMP. (DSM-5 Criterion A). Exclude ADHD-past-only. This was hypothesized to yield a prevalence similar to other parent-report-only studies (e.g. about 8–9%).
ADHD-Tier 2:
ADHD-1 + rule out unspecified schizophrenia spectrum and other psychotic disorder (hereafter, “psychosis”), bipolar disorder, or estimated IQ<70 (DSM-5 Criterion E). This was not expected to much change the prevalence estimate.
ADHD-Tier-3:
ADHD-2 + teacher BPM T-score ≥ 65 (DSM-5 Criterion C).
ADHD-Tier 4:
ADHD-3 + parent CBCL attention scale or ADHD DSM5 scale T ≥ 65 (DSM-5 Criterion E). Here we expected prevalence to drop to that predicted by the more stringent studies cited earlier (3–4%).
For the specific variable names and cutoffs used for ADHD see the online supplement section II.A, and for comorbid disorders, below, section II.B.
Comorbid Disorders
Comorbid psychiatric disorders were estimated by parent-report KSADS-COMP, and by youth self-report on the KSADS-COMP for bipolar disorder, depressive disorders, and anxiety and fear disorders (again, other disorders were not assessed by youth report). When both parent and youth reports were available, we used an “or” rule, in which the disorder was considered present if full criteria were endorsed by either reporter. Parent KSAD alone was used for the comorbidities when no youth KSADS was available.
Substance use was exclusionary at baseline for ABCD participation at baseline, and autism spectrum disorder was insufficiently evaluated for its inclusion here. To calculate rates of comorbidity, three broad categories of comorbid disorders were used for ease of interpretation in the manuscript: Mood Disorders, Disruptive Behavior Disorders, and Anxiety and Fear Disorders. Complete listing of all comorbid disorders is also provided, in the online supplement.
Creation of composite dimensional variables for secondary analysis and validity checks
In addition, to evaluate external correlates, dimensional scores were created for ADHD, irritability, disruptive behavior, and internalizing behavior, as well as for cognitive measures, as follows. (See the online supplement for details as to the specific ABCD variables used to create these composite scores).
ADHD dimension.
Extensive evidence suggests that for some purposes ADHD can be treated as a trait dimension in the population rather than only as a categorical disorder. Therefore, dimensional measures of ADHD were created as a resource for the field and alternative to categorical ADHD analysis. These were validated by fitting structural equation models in a replication analysis using the split-half of the ABCD data set or ARMS samples.18 The primary ADHD latent variable included the parent-rated CBCL attention problems t-score, the teacher BPM attention problems t-score, and inattention and hyperactivity symptom scores from the KSADS-COMP.
Irritability dimensions.
A parent-rated child irritability score was created using the average of five CBCL and three KSADS-COMP items (alpha=0.833) selected after asking three experts to independently rate the relevance of CBCL items to the irritability construct (Ellen Leibenluft, M.D., Philip Shaw, B.M.B.Ch., Ph.D., Argyris Stringaris, M.D., Ph.D., FRCPsych). All included items were rated as either definite or possible symptoms of irritability by all three expert raters and all were rated as definite by at least one rater (See online supplement section II.C for more detail including the specific items included).
Disruptive Behavior Disorder (DBD) dimension.
A teacher-rated ODD/CD composite utilized four BPM items.
Internalizing Mood Dimension.
The internalizing mood composite utilized six teacher BPM items.
Cognitive measures.
Latent variables were created for general cognitive ability (GA), executive function (EF), and learning and memory using the laboratory measures identified in a principal component analysis in19 and summarized in the online supplement, section II.D.
External correlates: Polygenic score
The final external correlate was polygenic risk for ADHD. Saliva samples were collected at the baseline visit and sent to the Rutgers University Cell and DNA Repository for DNA isolation.20 Genotyping21,22 was performed using the Smokescreen Array.23 For the present study, processed genotypes were downloaded from the NIMH Data Archive (dx.doi.org/10.15154/1503209), and standard QC checks were performed. Details are provided in the online supplement Section II.E. For analyses involving the PRS scores, the first three genomic principal components were covaried to control for ancestry stratification.
The polygenic risks score (PRS) was constructed using the 2016–2017 PGC+iPSYCH ADHD GWAS meta-analysis24 as the discovery data set (20,183 ADHD cases; 35,191 controls). For the primary analysis, the PRS was calculated using the LDpred method.25 Only SNPs with INFO (imputation quality) score ≥ 0.8 in both the PGC meta-analysis and the ABCD data were considered. SNPs were further limited to the ~1.2 million HapMap SNPs as suggested for LDpred.25 Linkage disequilibrium was estimated using all unrelated individuals in the ABCD cohort, and the PRS was created with the proportion of causal SNPs set to 0.3. For sensitivity analysis, we examined a PRS constructed using standard methods as reported previously26 and checked results relying only on the majority (European-ancestry) discovery set (see online supplement section II.E for further methodological details).
Data Handling: Complex sampling corrections
All analyses were completed on data from ABCD release 2.0 (accessed 12/2018). The complex sampling design required special handling as described in this section. Standard errors were adjusted for family and within site nesting using the TYPE = COMPLEX setting in Mplus. SEM analyses were completed in Mplus27 (vers. 8.3) or R28 (vers. 3.6.1) as noted later.
Missing Data.
The proportion of missingness for the variables utilized in this report ranged from 0.0% to 70.1% (M = 8.1%, SD = 17.7%). In general, parent measures (KSADS-COMP, CBCL) had minimal missing data, but teacher ratings on the BPM were missing for over half the baseline sample. To address bias that would arise from listwise deletion, we employed multiple imputation, a well-recognized method that effectively addresses missingness bias (under certain assumptions), even with the amount of missing teacher data noted here. All analyses were completed for each of 100 imputed datasets and combined using the TYPE = MI setting in Mplus, which averages the parameter estimates per Rubin’s rules.29 Data and results were transferred between R and Mplus using the Mplus Automation package30 (vers. 0.7–3). (Readers wishing more background on this method may consult the online supplement Section II.F).
Population and Nesting Adjustments.31
To address sampling bias, propensity scores31 that were developed and recommended for ABCD32,33 were utilized to align the parameter estimates with the American Communities Survey. Propensity weights are a well-recognized method that can reduce bias introduced by unequal likelihood of selection in observational studies, such as ABCD, by using participant characteristics to calculate the weights.32,34 See online supplement (section II.G.) for additional details.
Data analysis
ADHD prevalence.
For ADHD, the proportion of the entire sample that met each set of criteria was estimated and weighted as described above.
Comorbid psychiatric disorders.
We evaluated the prevalence of individuals meeting each ADHD criterion and major psychiatric comorbidities in the ABCD sample in two ways. First, we used the [Y ON] syntax in Mplus, and exponentiated the resulting logit coefficients and confidence intervals to produce proportion estimates. Separate analyses were conducted for each ADHD criterion subset, the full sample, and individuals categorized as non-ADHD. For comorbidities the prevalence was calculated within each ADHD tier.
Second, to adjust for the fact that many children with ADHD present with multiple comorbidities, a second set of logistic regressions was computed utilizing the binary diagnostic variables. The outcomes were the three major comorbidity sets (any anxiety or fear disorder, any mood disorder, and any disruptive behavior disorder). Two models for each ADHD-comorbidity set pairing were computed: one unadjusted, and one adjusted for other comorbidity sets. Thus, the second model provided the pairwise relationship adjusting for the relationships among all major comorbidities. To ensure that estimates of the overlap among comorbidities was not due to disorder-specific variation in sex or age, those models were conducted with age and sex covaried. Parameters were then converted to prevalence estimates for ease of interpretation.
External correlates.
We examined theorized external correlates as an estimate of utility and construct validity of the ADHD criterion definitions. We did so via a series of logistic regression analyses with the binary ADHD variable as the outcome and, respectively, the polygenic risk score (PRS), the cognitive principal components (executive function, general cognitive ability, and learning and memory),19 and the WISC matrix reasoning scale score, as the individual predictor in each model (sex and age were covariates). Separate models were assessed for each ADHD tier-predictor pair. Then, to probe the differences in these associations between ADHD tiers, the equivalence of predictor means for non-overlapping groups of individuals from each ADHD tier (phenotype definition) were assessed using a series of Wald tests.35
External correlates of dimensional measure of ADHD.
To estimate the utility and construct or convergent validity of the dimensional ADHD score and the effect size of key theorized correlates of ADHD on an ADHD dimension, we conducted a series of linear regression and SEM models with the same external correlates. However, we added as correlates dimensional irritability, ODD/CD, and internalizing/mood composites (controlling for age and sex). As before, separate analyses were conducted for each ADHD outcome-predictor pair for ease of interpretation.
RESULTS
Characteristics of ADHD after phenotype refinement.
Table 1 provides the prevalence as well as descriptive and clinical data for ADHD as defined by refined phenotypes (the four Tiers) here. It shows that the ABCD KSADS-COMP estimates current ADHD at 9.17% (8.54% after rule outs—Tier 2). Prevalence was 5.41% when teacher convergence was required (Tier 3), and 3.53% when parent standardized ratings in the clinical range were added (Tier 4). Statistical consideration of variable changes across the tiers are provided later in results (see section “Correlates of ADHD Tiers” below).
Table 1.
Demographics and Group Descriptions for the Full ABCD baseline sample and four ADHD phenotype refinements
| % of Full Sample | % of Non-ADHD | % of ADHD 1 | % of ADHD 2 | % of ADHD 3 | % of ADHD 4 | |
|---|---|---|---|---|---|---|
| Variable | (N = 11875) | (n = 10784) | (n = 1091) | (n = 1029) | (n = 642) | (n = 420) |
| ADHD prevalence | 9.17 | 0.00 | 9.17 | 8.54 | 5.41 | 3.53 |
| %Male | 52.11 | 50.41 | 68.92 | 68.63 | 64.90 | 67.10 |
| Race: | ||||||
| % White Non-Hispanic | 60.10 | 59.73 | 63.82 | 65.46 | 62.88 | 61.36 |
| % Black | 15.94 | 15.76 | 17.71 | 16.62 | 17.28 | 18.40 |
| % Asian | 2.49 | 2.65 | 0.83 | 0.88 | 0.61 | 0.62 |
| % Native American/AK Native | 0.55 | 0.55 | 0.56 | 0.50 | 0.79 | 0.98 |
| % Native Hawaiian/Pacific Is. | 0.14 | 0.16 | 0.01 | 0.01 | 0.01 | 0.01 |
| % More than one race | 12.58 | 12.34 | 14.94 | 14.79 | 14.53 | 16.18 |
| Ethnicity: % Latinx/Hispanic | 20.68 | 21.09 | 16.59 | 15.86 | 18.12 | 18.86 |
| % Prescribed ADHD Medication | 8.05 | 5.39 | 34.28 | 34.00 | 39.84 | 44.65 |
| M Age (months) | 118.94 (0.07) | 118.98 (0.07) | 118.58 (0.22) | 118.53 (0.23) | 118.23 (0.30) | 118.12 (0.37) |
| M Income Group (1–10) | 7.12 (0.02) | 7.13 (0.02) | 7.02 (0.07) | 7.15 (0.07) | 6.93 (0.10) | 6.70 (0.12) |
| M CBCL Externalizing T-score | 45.72 (0.09) | 44.60 (0.09) | 56.82 (0.33) | 56.44 (0.34) | 58.60 (0.41) | 61.40 (0.48) |
| M CBCL Internalizing T-score | 48.43 (0.10) | 47.55 (0.10) | 57.09 (0.33) | 56.76 (0.33) | 57.48 (0.42) | 60.21 (0.48) |
| M CBCL Attention T-score | 53.87 (0.06) | 52.74 (0.04) | 65.0 (0.27) | 64.70 (0.27) | 66.63 (0.33) | 70.76 (0.36) |
| M Executive Function | −0.006 (0.007) | 0.012 (0.007) | −0.189 (0.024) | −0.167 (0.025) | −0.20 (0.032) | −0.216 (0.041) |
| M General Cognitive Ability | −0.008 (0.007) | 0.007 (0.007) | −0.155 (0.024) | −0.125 (0.024) | −0.207 (0.031) | −0.237 (0.038) |
| M Learning and Memory | −0.003 (0.006) | 0.016 (0.007) | −0.187 (0.021) | −0.164 (0.022) | −0.222 (0.027) | −0.284 (0.033) |
| M WISC-V Matrix Reasoning | 9.85 (0.03) | 9.90 (0.03) | 9.31 (0.09) | 9.53 (0.09) | 9.43 (0.12) | 9.30 (0.15) |
| M PRS (traditional, standardized) | 0.024 (0.009) | 0.013 (0.010) | 0.133 (0.030) | 0.116 (0.031) | 0.156 (0.039) | 0.234 (0.048) |
| M PRS LD_Pred (standardized) | 0.027 (0.009) | 0.013 (0.010) | 0.160 (0.031) | 0.140 (0.032) | 0.184 (0.039) | 0.259 (0.048) |
Note: Values are pooled estimates of mean and standard error across all imputation sets. N is estimated after imputation. For race, the ABCD variable codes were combined as follows: White Non-Hispanic utilized the White category, excluding anyone that met the Hispanic/Latinx ethnic variable; Asian included Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, and Other Asian; Native Hawaiian/Pacific Islander included Native Hawaiian, Guamanian, Samoan, and Other Pacific Islander. Matrix reasoning is the scaled score. ABCD=Adolescent Brain Cognitive Development Study. ADHD=Attention-Deficit/Hyperactivity Disorder. CBCL=Child Behavior Checklist. PRS=Polygenic Risk Score. WISC-V=Wechsler Intelligence Scale for Children. See text for methods and definitions.
Conditional prevalence and conditional comorbidity of ADHD.
Table 2 shows the prevalence of three major comorbidity classes in the ADHD sample for each of the four increasingly restrictive tiers (age and sex adjusted). It conveys the magnitude of reduction in estimated comorbidity after accounting for overlaps among the different classes of comorbidity (e.g., some children have both disruptive and anxiety disorder). As described in the note to Table 2, the prevalence for individual disorders and their frequency by ADHD tier are all available (without the need to adjust for age and sex) in Table S1 online.
Table 2.
Estimated Rate within ADHD Population of Three Classes of Comorbid Psychiatric Disorder before and After Adjusting for Other Comorbidity Classes
| % of ADHD with Any Disruptive Behavior Disorder | % of ADHD with Any Mood Disorder | % of ADHD with Any Anxiety Disorder | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Criterion | Covariate Inclusion Status | Est. | Lower CI | Upper CI | Est. | Lower CI | Upper CI | Est. | Lower CI | Upper CI |
| ADHD1 (n = 1091) | Unadjusted | 36.1 | 32.1 | 40.2 | 5.4 | 3.6 | 7.9 | 33.0 | 29.4 | 36.8 |
| Adjusted | 31.5 | 27.7 | 35.6 | 2.9 | 1.8 | 4.4 | 26.3 | 22.8 | 30.1 | |
| ADHD2 (n = 1029) | Unadjusted | 33.1 | 29.2 | 37.2 | 4.6 | 3.0 | 6.9 | 30.4 | 26.9 | 34.2 |
| Adjusted | 28.9 | 25.2 | 32.9 | 2.4 | 1.5 | 7.8 | 23.8 | 20.5 | 27.5 | |
| ADHD3 (n = 642) | Unadjusted | 34.8 | 30.2 | 39.8 | 4.7 | 2.9 | 7.6 | 32.2 | 27.8 | 37.0 |
| Adjusted | 30.0 | 25.4 | 35.0 | 2.3 | 1.3 | 3.9 | 24.2 | 20.1 | 28.9 | |
| ADHD4 (n = 420) | Unadjusted | 36.9 | 31.5 | 42.7 | 4.9 | 2.9 | 8.2 | 36.8 | 31.3 | 42.6 |
| Adjusted | 30.9 | 25.7 | 36.7 | 2.1 | 1.2 | 3.8 | 27.4 | 22.3 | 33.1 | |
Note: Prevalence was estimated using the risk ratio (converted from the logistic regression odds ratio) with the control group as the baseline after controlling for age and sex. Adjusted prevalence represents the likelihood of demonstrating the comorbidity given the ADHD criterion diagnosis after controlling for other comorbidities, age, and sex. Unadjusted only controlled for age and sex. N is estimated after imputation. Any disruptive includes Oppositional Defiant Disorder and Conduct Disorder. Any anxiety disorder includes agoraphobia, generalized anxiety disorder, panic disorder, specific phobia, post-traumatic stress disorder, unspecified anxiety disorder, separation anxiety, and social anxiety. Any mood disorder includes major depressive disorder, disruptive mood dysregulation disorder, and unspecified depressive disorder. See Table S2 in the online supplement for a detailed breakdown of the % of ADHD cases by each definition having each of the disorders assessed. Results without controlling for age and sex are in Table S2; Table S3 provides the statistical for odds ratio results from the logistic regression analyses presented here in Table 2. ADHD=Attention-deficit/hyperactivity disorder.
As shown in Table 2, total ADHD comorbidity increased slightly as diagnosis became more restrictive from Tier 2 to Tier 4 (after removal of psychosis, bipolar, and IDD). The adjusted comorbidities within each ADHD tier remained notable, ranging from an adjusted 28.9%−31.5% for disruptive behavior disorders and from 23.8%−27.4% for anxiety/fear disorders across tiers. Mood disorders displayed relatively low estimated prevalence within the ADHD tiers, ranging from 2.1%−2.9%. Total comorbidity (not shown in Table 2) ranged from 61.0% for Tier 2 to 70.2% for Tier 4 (again see Table S1). Odds ratios for Table 2 are in Table S2.
Correlates of ADHD Tiers.
Table 1 provided the mean scores on the external validators. How do these compare statistically? Which ADHD tier most sensitively separates ADHD from non-ADHD youth? The cases with psychosis, bipolar, and/or IDD (ADHD-1) present obvious confounds for the cognitive measures (and also have elevated ADHD PRS scores). When they are removed, then Tier 2, Tier 3, and Tier 4 present a clear pattern of results shown in Figure 1 and Figure 2.
Figure 1. Risk Ratio for ADHD by PRS Score in the Top 10% vs. Bottom 50% of Scores.
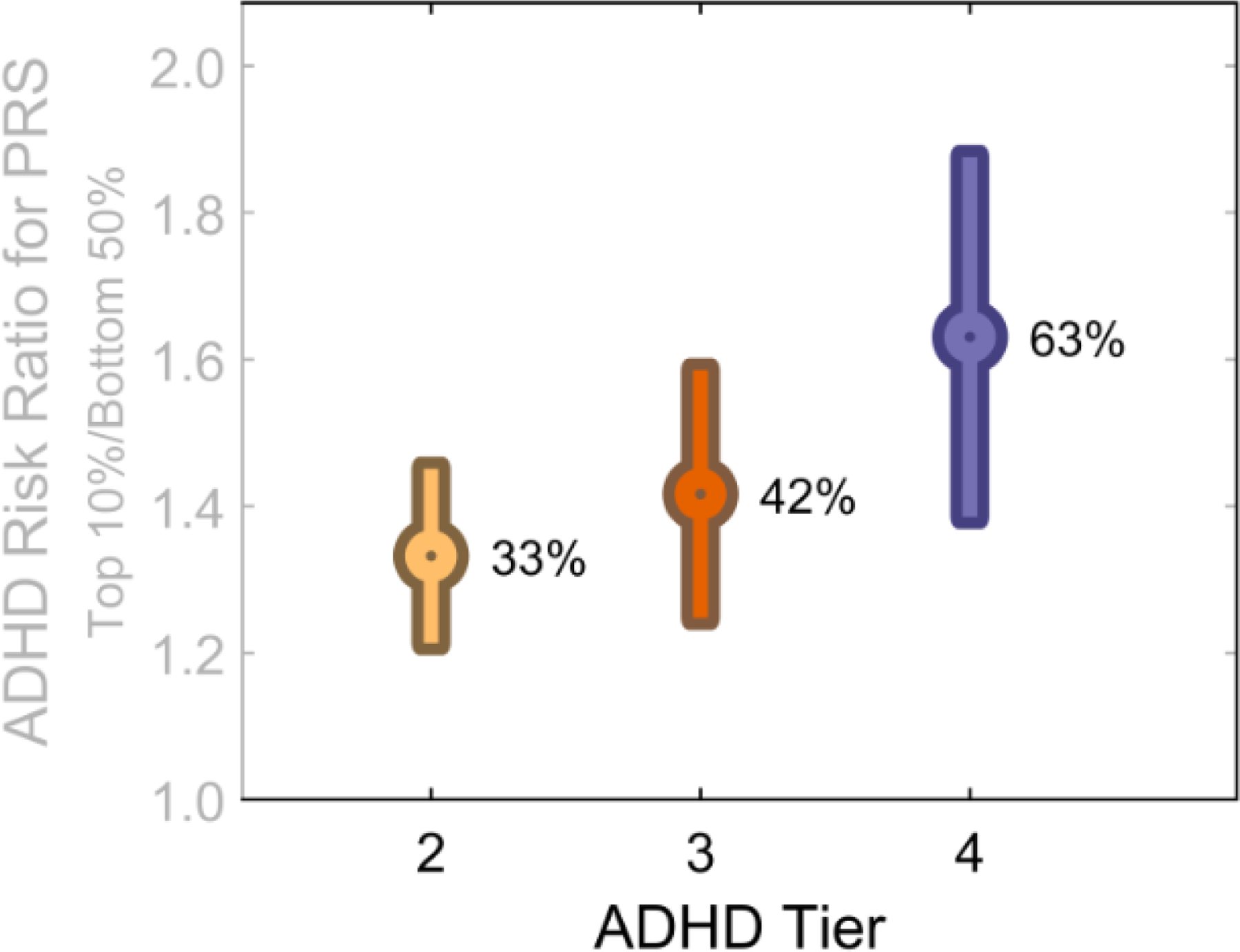
Each ADHD tier is increasingly restrictive. The yellow bar represents ADHD Tier 2 (risk ratio and its standard error) that is, the KDADS alone after rule outs; the red bar is Tier 3, (adding BPM.65); the blue bar is ADHD-Tier 4 (adding parent CBCL screen). The figure excludes cases of schizophrenia, bipolar disorder, and intellectual development disorder (hence, excludes “ADHD-1”) for clarity. The percentages represent odds ratios that have been converted to percentage of increased risk. Thus, an OR=1.33 is represented here as 33%. It represents the increased risk of being in the top 10% of PRS scores based on meeting criteria for each ADHD tier versus being in the bottom half of ADHD-PRS scores. ADHD-Tier 2 (KSAD only after rule outs, n = 1029); ADHD-3 (requires teacher BPM T>=65, n = 642); ADHD-4 (also requires parent CBCL attention problems T score >=65; n= 420.) PRS=LD pred polygenic risk for ADHD. Figure demonstrates that the restrictive phenotype more clearly detects polygenic risk.
Figure 2. Distribution of Values for External Variables by ADHD Tier excluding rule out cases.
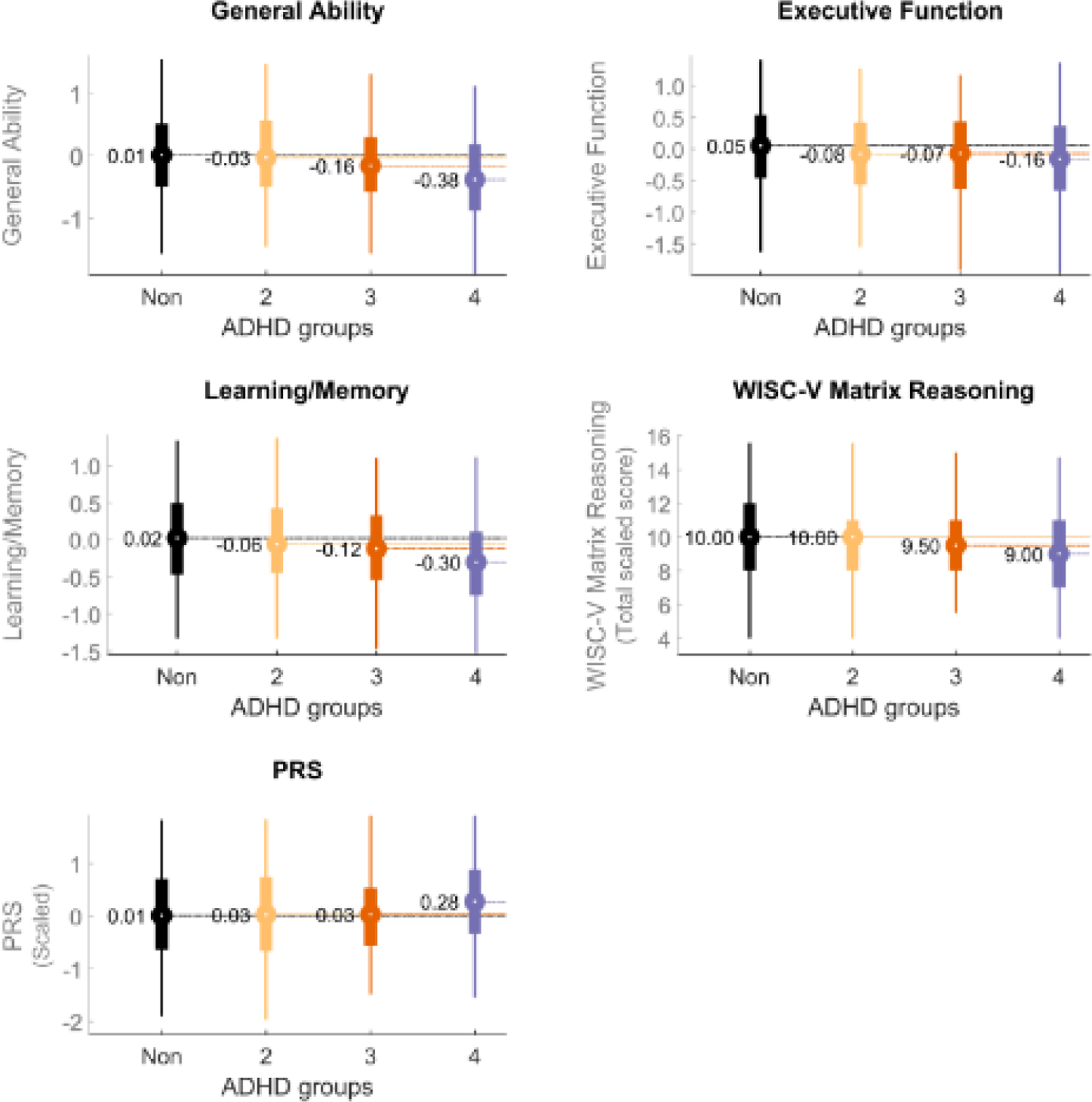
For clarity of presentation, cases of schizophrenia, bipolar disorder, or intellectual development disorder are excluded (“ADHD-1” is thus excluded, as it includes those cases. ADHD-2 is just ADHD-1 with those rule out cases removed). The black bar represents the non-ADHD youth; the yellow is ADHD-Tier 2; the red is ADHD Tier 3, and the blue is ADHD Tier 4. The values graphed here represent the standardized mean within each group for General Ability, Executive Function, and Learning/Memory. WISV-V Matrix reasoning is the total scaled score. PRS is polygenic risk for ADHD computed by the LD-pred method (see Methods).
Figure 1 shows relative risk of ADHD based on polygenic score for each of the phenotype refinements defined as the top decile of PRS risk (“high risk”) compared to the bottom 50% of PRS risk (“low to average risk”). It shows that the ADHD definitions have similarly weak genetic signal. Only ADHD-4, the most restrictive, yields a sharply increased genetic signal. When ADHD is defined by Tier 4, then those in the top decile of polygenic risk have a 63% increased probability for ADHD versus low to average risk individuals.
Figure 2 shows that the mean scores for all three cognitive scores (General Ability, Executive Function, Working Memory) and WISC-V Matrix Reasoning scores decrease as the ADHD tiers increase from non-ADHD to Tier 4. (Again, ADHD Tier 1 is excluded because it includes children with low estimated IQ who, by definition, have impaired cognitive scores). Figure 2 also includes the PRS scores in standardized form for completeness. The ability of ADHD to be differentiated from non-ADHD youth was thus improved when ADHD was defined more restrictively. See Table S3 for specific parameters.
Figure 3 illustrates this in terms of effect size. It shows the relative magnitude of the effect size for all of the external neuropsychological correlates and for the PRS score of each ADHD tier versus non-ADHD as non-overlapping samples. It shows that in relative terms, the effect parameter for ADHD versus non-ADHD comparisons increased dramatically and qualitatively in a non-linear fashion, using the more restrictive definitions of ADHD. (Table S4 has the statistical parameters for Figure 3).
Figure 3. Wald Test Statistic for Difference in Estimated Means Between Non-ADHD and ADHD Tier Groups scaled by relative effect magnitude.

For clarity of presentation, cases of schizophrenia, bipolar disorder, or intellectual development disorder are excluded (“ADHD-1” is thus excluded, as it includes those cases. ADHD-2 is just ADHD-1 with those rule out cases removed). The X axis represents ADHD Tier 2, Tier 3, and Tier 4 respectively. Values in the grid represent Wald test statistics comparing each attention-deficit/hyperactivity disorder (ADHD) phenotype definition to non-ADHD cases, with larger numbers representing a larger effect size. The color scale thus depicts the difference in magnitude of the effect between the ADHD-Tier 2 comparison to non-ADHD, and the effect for ADHD-3 and ADHD-4 respectively versus non-ADHD. Thus, the effect for PRS detection is 7x as great for ADHD-4 as ADHD-2 or ADHD, whereas the effect for executive function (EF) for Tier 4 is roughly double the other tiers. PRS is polygenic risk score using the LDPRED method as noted in the text and supplement. The most restrictive definition most effectively captures hypothesized mechanistic cognitive features associated with ADHD.
External correlates of ADHD dimensional score.
ADHD can be assessed as a dimension using single or multiple measures. Here we present results for the multi-measure ADHD latent variable (indicators for the best fit model were parent CBCL attention problems; teacher BPM attention problems, and KSADS-COMP inattention and hyperactivity symptom counts); although fit was not excellent, it was adequate (RMSEA=.12, SRMR=.03, CFI=.952, TLI=.856).
As expected, all of the convergent validity measures were significantly associated with ADHD composite scores using individual parent or teacher rating scales or the latent variables that combined them (Table S5). The Parent Irritability Composite (B=.47, SE=.02), Teacher ODD/CD Composite (B=.49, SE=.03), and Teacher Internalizing/Mood Composite (B=.31, SE=.03) provided the strongest associations with ADHD, again as expected. (As documented in the online supplemental material, associations with ADHD were similar for the 5 CBCL items, alone B=.56, but notably smaller for the 3 KSAD items alone, B=.28). The association between the cognitive latent variables and the ADHD latent variable were highly reliable (all p<1E-10), although modest in effect size. ADHD symptoms were associated with weaker performance on executive function (B= −.17, SE=.02), general cognitive ability (B= −.17, SE=.02), and learning and memory (B= −.18, SE=.02). The association of the ADHD dimension with the polygenic risk score was small but reliable (B=.07, SE=.02, p=.00047). (See Table S6 for all fit statistics; see Figure S-1 for depiction of the latent variable measurement models and Figure S-2 for depiction of the full structural equation model.)
Sensitivity Analyses.
First, in Table 1, each refinement removes children from the ADHD group. For readers interested in non-overlapping, exclusive groupings, see Table S4 and Figure 3. Second, we limited our analysis to cases meeting current ADHD diagnosis, not those only meeting past ADHD. Children who are past ADHD but currently treated were omitted, but could in fact be true cases that are partially or wholly treatment responsive—perhaps especially those cases still exhibiting clinical range symptoms. What if they are counted? Data on this question are available in Tables S7 and S8. Table S7 shows that children with past ADHD by the KSADS-COMP, but currently treated with medication and still showing clinical range symptoms on teacher rating forms, would add 0.54% to prevalence; .20% if restricted to youth with both parent and teacher ratings elevated. Table S8 shows that those cases also have markedly elevated PRS scores. If all of those were counted as true cases partially treated, the prevalence estimate would rise from 3.53% to 4.07%.
Third, we excluded children with psychosis, bipolar, or IDD (defined here crudely as a low single test score). What if they had been included? That question is answered in Table S8 as well, showing data with and without them included with minimal changes in any results. However, that small group did have elevated ADHD symptom and PRS scores; see Table S9.
Fourth, although the associations between the external correlates and the ADHD latent variables were all significant, they were not numerically consistent across the different ADHD measures, with some (e.g., the cognitive measures) more closely associated with teacher report and others (e.g., parent irritability) more closely associated with parent report. Table S5 showed earlier that the parent CBCL attention problems T-score appeared the most sensitive single measure overall. Finally, readers wishing to replicate the multiple imputation design here may consult Table S10.
Finally, we briefly note the pattern of race and ethnicity effects in Table 1. African American and Multiracial youth were slightly over-represented in all ADHD tiers, both with the KSADS alone and then, slightly increasing when standardized ratings were added. In contrast, Hispanic/Latino youth were somewhat under-represented by the KSADS interview, although this was partially reversed by addition of standardized parent and teacher ratings. Likewise, Asian youth were under-represented in the ADHD cases relative to their presence in the sample overall. Further examination was not undertaken due to small absolute numbers and limited space.
DISCUSSION
Results here provide four major findings. First, the prevalence of ADHD in the important national ABCD study depends crucially on how ADHD is operationalized, and a best estimate using a restrictive definition that best fits the DSM-definition is 3.5% for current ADHD. This estimate, beyond the limitations noted later, improves on many prior estimates by capitalizing on the national ABCD study with a multi-method, multi-information assessment of ADHD. Careful attention was paid to addressing missing data and volunteer sampling bias and those methods are made available in the online supplement. As a resource, all the derived categorical and dimensional variables here, along with derived PRS scores, are available as detailed in the online supplement section on Data Sharing (Section II.H).
The estimates here can be reconciled well with other estimates using multiple criteria. Even for the U.S. National Survey of Children’s Health, a re-analysis of the 2012 survey36 suggested that if the available questions about severity and duration within that survey are incorporated, the prevalence of ADHD would be 4% to 5%, not the more commonly cited 9%. Similarly, the estimate of 3.5% here is in line with major meta-analytic results as noted in the introduction, particularly two high-quality Bayesian meta-analysis.10,11
Second, within the limitations noted below, the results suggest that a refined phenotype that augments computerized structured interview and key rule outs with parent and teacher standardized ratings, sharply increases the detection of biological signal, represented here by the ADHD polygenic score—and was the only categorical definition we examined that was able to detect a polygenic effect. The increased sensitivity of more restrictive definitions was qualitatively non-linear.
The third, related finding, is that this same story holds although somewhat less sharply for executive functioning and learning and memory. The finding that cognitive associates were highly reliable but had modest effect sizes is broadly consistent with the literature, although effect size estimates here are somewhat smaller than in the literature to date. The result for executive functioning, for example, would equal d=~.35 for the dimensional analysis and d=~.31 for the categorical analysis. This is slightly smaller than the d=.5 or greater for some executive function measures in authoritative meta-analyses relying on convenience samples.37 It may be that ABCD did not utilize the most sensitive measures of executive functioning for ADHD or that convenience samples over-estimate the population-representative effect size. Regardless, given the large sample size, and effort to statistically estimate national representation, the present results will help add precision to estimates of population level effect sizes and should contribute to efforts to characterize ADHD heterogeneity.38
Taking all this together, when ADHD is to be considered as a category and conceptualized as a polygenic, neurodevelopmental disorder, as defined in DSM-5 for clinical purposes, then a restrictive definition appears to have the most validity for study in the ABCD sample. Results thus support the importance of multi-informant data, which DSM-5 advises but does not require, and the value of utilizing valid and normed rating scales in addition to symptom count. (On the other hand, the difficulty of obtaining teacher ratings in the ABCD study may support the continuation of a strong recommendation rather than a requirement for a second informant). Nonetheless, it is important to note again that many children who are below threshold for this definition are still impaired enough to potentially require clinical attention.39 Further, the sensitivity analysis revealed that an argument could be made for regarding as current ADHD cases a subset of the past-only KSADS-identified cases who are (a) actively being treated and (b) still exhibiting clinical range symptoms. These could be considered partially treated true cases. The case for including other past-only ADHD cases appears weaker.
Fourth, findings confirm substantial ADHD comorbidity. In the most restrictive phenotypes with the greatest construct validity here, fully 70% of children with ADHD have at least one comorbid condition. The most common comorbidity, as expected, was disruptive behavior disorders. However, excepting an unusually high estimate for OCD, anxiety and mood overlaps at this age were quite low. It was notable that DMDD had very low prevalence relative to ODD, which may warrant further investigation regarding its measurement. It is likely that these ratios will increase as the sample ages into adolescence and the peak age of onset for internalizing disorders.40 This latter point is critically important to prevention, and is part of the reason it is important that researchers and clinicians not treat mood and anxiety disorders as automatic rule outs for ADHD. Indeed, recent studies provide evidence that ADHD is on the causal pathway for comorbid cases of anxiety and mood disorders.1,2 Ruling out ADHD at those later ages would obscure the developmental pathway those children have followed.
While the study has unique strengths and provides valuable new information at the national level for the U.S., results still have to be viewed in light of remaining limitations. First, the propensity weighting here should largely correct demographic sampling bias, but we cannot rule out other unmeasured sampling bias. Results depend on multiple imputation of over half of the teacher ratings. Although this is less than ideal, an extensive simulation literature indicates that multiple imputation has among the best validities of all methods and is superior to most other methods (such as listwise deletion) even with this level of missingness.41 In the worst case, the imputation adds little or no power (fails to prevent type II error), but does not increase Type I error. Thus, the consensus in the field, followed here, is that amount of missingness in itself is not a criterion for whether to impute.29,42 For more discussion see the missing data section in the online supplement.
Race and ethnicity were included in the imputation and propensity weighing models, so results are not biased by unrepresentative race or ethnic groupings in sampling or missingness. However, race and ethnic differences in ADHD prevalence and comorbidity remain crucially important43,44 yet were not able to be explored here within the length limits and cell sizes available. It was notable that the percent of the ADHD population that was Black or Multiracial was higher than expected based on population representation and increased as criteria became more stringent. In contrast, Hispanic/Latino youth were under-represented in the ADHD population compared to their representation overall (after exclusion of rule outs), although this was partially corrected with the addition of informant ratings. Although the percent estimate for Native American participants suggests marked over-representation when rating scales are added, the absolute numbers here mandate caution—a difference of only 10 or 20 children would change the conclusion. Likewise, no comment is possible for Hawaiian/Pacific Islander youth, as the estimated ADHD count is less than 3 children. Overall, these initial counts suggest the urgent need to further investigate potential racial and ethnic variation in KSADS and ratings scale performance for different informants for ADHD evaluation. They also suggest the need for further evaluation of the most appropriate multi-method algorithms for combining information to identify ADHD across racial and ethnic groups. Racially and ethnically sensitive diagnosis must continue to consider potential differential over- and under-identification by race and ethnicity for ratings and interviews.45 At the same time, due to the very small absolute number of children in these groups, these observations should be considered as hypothesis-generating, and certainly not as definitive. This remains a critical topic for future study with sophisticated consideration of multiple factors that may be in play.
Secondly, we lacked robust measures of impairment outside of the parent KSAD here46 (which only required impairment in one setting while DSM-5 requires two settings) or of direct clinician interview of the parents or children. Third, comorbid disorders were not as refined by consideration of rating scales or other measures the way we did with ADHD, thus comorbidities may be over- or under-estimated. Although we partially compensated by including youth report for internalizing disorders, those self-reports may have questionable validity or reliability.
Substantial data support the idea that ADHD may also be considered like a trait dimension. The latent variable validation supplied here, and the correlates demonstrated, suggest that utilization of the composite or latent variables proposed should be productive as a means of evaluating ADHD correlations in neuroimaging and other studies. The observed correlations with executive functioning, general cognitive ability, and polygenic risk are consistent with the literature and lend support to the utility of this highly reliable composite. However, for investigators wishing to use a single simple dimensional measure of ADHD liability in the ABCD data set, the data presented here suggest that the parent CBCL attention scale t-score is the most useful—and is as useful as the composite dimensional score for some purposes including detection of polygenic risk. That result supports the extensive work done on evaluating problem dimensions in children over the past several decades by Achenbach and others47.
Similarly, the new irritability composite introduced here for the ABCD data has strong face validity via expert consensus ratings and internal validity on latent variable validation. Results here confirm a very strong association of irritability with ADHD and further support the importance of irritability as a clinical correlate and/or feature of ADHD.48 For all composites, fit and validity replicated in a matched split-half sample within the ABCD data set.
In conclusion, the ABCD study provides a valuable resource for estimation of population needs and correlates for ADHD. The analysis here suggests that the most dependable findings for clinical application will benefit from utilization of a refined categorical phenotype for ADHD cases.
Supplementary Material
Acknowledgements:
They are grateful for helpful comments on this work by Deanna Barch, Ph.D., Joan Kaufman, Ph.D., Stefanie Bodison, O.T.D., O.T.R./L., Anthony Dick, Ph.D., and advice on the irritability measures by Ellen Leibenluft, M.D., Philip Shaw, B.M.B.Ch., Ph.D., Argyris Stringaris, M.D., Ph.D. The authors also thank Hannah Morton, Ph.D. for supplemental assistance on revisions. Effort on this project was supported by NSF GRP fellowship 2020239717 (Cordova) and NIH grant R37-MH59105 (Nigg). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or of those who generously commented on aspects of the work.
Footnotes
The authors have no reportable conflicts of interest to disclose.
Contributor Information
Ms. Michaela Cordova, UC San Diego (UCSD)/San Diego State University (SDSU)
Dr. Dylan Antovich, Oregon Health & Science University (OHSU)
Mr. Peter Ryabinin, OHSU
Mr. Christopher Neighbor, OHSU
Dr. Michael A. Mooney, OHSU
Dr. Nathan F. Dieckmann, OHSU
Dr. Oscar Miranda-Dominguez, University of Minnesota (UMN)
Dr. Bonnie J. Nagel, OHSU
Dr. Damien Fair, UMN
Dr. Joel T. Nigg, OHSU
Literature Cited
- 1.Treur JL, Demontis D, Smith GD, et al. Investigating causality between liability to ADHD and substance use, and liability to substance use and ADHD risk, using Mendelian randomization. Addict Biol Jan 2021;26(1):e12849. doi: 10.1111/adb.12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riglin L, Leppert B, Dardani C, et al. ADHD and depression: Investigating a causal explanation. Psychological Medicine Apr 6 2020:1–8. doi: 10.1017/s0033291720000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortese S Pharmacologic Treatment of Attention Deficit-Hyperactivity Disorder. N Engl J Med Sep 10 2020;383(11):1050–1056. doi: 10.1056/NEJMra1917069 [DOI] [PubMed] [Google Scholar]
- 4.Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J Clin Child Adolesc Psychol Mar-Apr 2018;47(2):199–212. doi: 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med Sep 2007;161(9):857–64. doi: 10.1001/archpedi.161.9.857 [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Avenevoli S, McLaughlin KA, et al. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychol Med Sep 2012;42(9):1997–2010. doi: 10.1017/s0033291712000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry Aug 2003;60(8):837–44. doi: 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- 8.Polanczyk GV, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry Jun 2007;164(6):942–8. doi: 10.1176/ajp.2007.164.6.942 [DOI] [PubMed] [Google Scholar]
- 9.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics Jul 2012;9(3):490–9. doi: 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erskine HE, Ferrari AJ, Nelson P, et al. Epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the Global Burden of Disease Study 2010. J Child Psychol Psychiatry Dec 2013;54(12):1263–74. doi: 10.1111/jcpp.12144 [DOI] [PubMed] [Google Scholar]
- 11.Barican JL, Yung D, Schwartz C, Zheng Y, Georgiades K, Waddell C. Prevalence of childhood mental disorders in high-income countries: a systematic review and meta-analysis to inform policymaking. Evid Based Ment Health Feb 2022;25(1):36–44. doi: 10.1136/ebmental-2021-300277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci Aug 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chorpita BF, Reise S, Weisz JR, Grubbs K, Becker KD, Krull JL. Evaluation of the Brief Problem Checklist: child and caregiver interviews to measure clinical progress. J Consult Clin Psychol Aug 2010;78(4):526–36. doi: 10.1037/a0019602 [DOI] [PubMed] [Google Scholar]
- 14.Townsend L, Kobak K, Kearney C, et al. Development of Three Web-Based Computerized Versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary Validity Data. J Am Acad Child Adolesc Psychiatry Feb 2020;59(2):309–325. doi: 10.1016/j.jaac.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 15.Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev Aug 2000;21(8):265–71. doi: 10.1542/pir.21-8-265 [DOI] [PubMed] [Google Scholar]
- 16.Manual for the ASEBA Brief Problem Monitor (BPM) https://aseba.org/wp-content/uploads/School-age-bpm-manual.pdf.pdf. Burlington, VT.
- 17.Wechsler D WISC-V: Technical and Interpretive Manual Pearson; 2014. [Google Scholar]
- 18.Feczko E, Conan G, Marek S, et al. Adolescent Brain Cognitive Development (ABCD) Community MRI Collection and Utilities. bioRxiv 2021;doi: 10.1101/2021.07.09.451638 [DOI] [Google Scholar]
- 19.Thompson WK, Barch DM, Bjork JM, et al. The structure of cognition in 9 and 10 year-old children and associations with problem behaviors: Findings from the ABCD study’s baseline neurocognitive battery. Dev Cogn Neurosci Apr 2019;36:100606. doi: 10.1016/j.dcn.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uban KA, Horton MK, Jacobus J, et al. Biospecimens and the ABCD study: Rationale, methods of collection, measurement and early data. Dev Cogn Neurosci Aug 2018;32:97–106. doi: 10.1016/j.dcn.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohi K, Ochi R, Noda Y, et al. Polygenic risk scores for major psychiatric and neurodevelopmental disorders contribute to sleep disturbance in childhood: Adolescent Brain Cognitive Development (ABCD) Study. Transl Psychiatry Mar 26 2021;11(1):187. doi: 10.1038/s41398-021-01308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loughnan RJ, Palmer CE, Thompson WK, Dale AM, Jernigan TL, Fan CC. Polygenic Score of Intelligence is More Predictive of Crystallized than Fluid Performance Among Children. bioRxiv 2019:637512. doi: 10.1101/637512 [DOI] [Google Scholar]
- 23.Baurley JW, Edlund CK, Pardamean CI, Conti DV, Bergen AW. Smokescreen: a targeted genotyping array for addiction research. BMC Genomics Feb 27 2016;17:145. doi: 10.1186/s12864-016-2495-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet Jan 2019;51(1):63–75. doi: 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilhjálmsson BJ, Yang J, Finucane HK, et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am J Hum Genet Oct 1 2015;97(4):576–92. doi: 10.1016/j.ajhg.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nigg JT, Gustafsson HC, Karalunas SL, et al. Working Memory and Vigilance as Multivariate Endophenotypes Related to Common Genetic Risk for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry Mar 2018;57(3):175–182. doi: 10.1016/j.jaac.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthén LK, Muthén BO. Mplus User’s Guide Muthén & Muthén; 1998–2012. [Google Scholar]
- 28.R: A language and environment for statistical computing. https://R-project.org/.
- 29.van Ginkel JR, Linting M, Rippe RCA, van der Voort A. Rebutting Existing Misconceptions About Multiple Imputation as a Method for Handling Missing Data. J Pers Assess May-Jun 2020;102(3):297–308. doi: 10.1080/00223891.2018.1530680 [DOI] [PubMed] [Google Scholar]
- 30.Murray JS. Multiple Imputation: A Review of Practical and Theoretical Findings. Statistical Science 5/1 2018;33(2):142–159. doi: 10.1214/18-STS644 [DOI] [Google Scholar]
- 31.Dick AS, Lopez DA, Watts AL, et al. Meaningful associations in the adolescent brain cognitive development study. Neuroimage Jun 18 2021;239:118262. doi: 10.1016/j.neuroimage.2021.118262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res Feb 2014;49(1):284–303. doi: 10.1111/1475-6773.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heeringa SG, Berglund PA. A Guide for Population-based Analysis of the Adolescent Brain Cognitive Development (ABCD) Study Baseline Data. bioRxiv 2020:2020.02.10.942011. doi: 10.1101/2020.02.10.942011 [DOI] [Google Scholar]
- 34.Okoli GN, Sanders RD, Myles P. Demystifying propensity scores. Br J Anaesth Jan 2014;112(1):13–5. doi: 10.1093/bja/aet290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox J Applied regression analysis, linear models, and related methods Sage Publications; 1997. [Google Scholar]
- 36.Song M, Dieckmann NF, Nigg JT. Addressing Discrepancies Between ADHD Prevalence and Case Identification Estimates Among U.S. Children Utilizing NSCH 2007–2012. J Atten Disord Dec 2019;23(14):1691–1702. doi: 10.1177/1087054718799930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willcutt EG, Nigg JT, Pennington BF, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol Nov 2012;121(4):991–1010. doi: 10.1037/a0027347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A Apr 24 2012;109(17):6769–74. doi: 10.1073/pnas.1115365109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong SB, Dwyer D, Kim JW, et al. Subthreshold attention-deficit/hyperactivity disorder is associated with functional impairments across domains: a comprehensive analysis in a large-scale community study. Eur Child Adolesc Psychiatry Aug 2014;23(8):627–36. doi: 10.1007/s00787-013-0501-z [DOI] [PubMed] [Google Scholar]
- 40.Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: Toward biologically based nosologic criteria. JAMA Psychiatry Sep 2014;71(9):1015–24. doi: 10.1001/jamapsychiatry.2014.763 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Enders CK. Applied missing data analysis Guilford Press; 2010. [Google Scholar]
- 42.Madley-Dowd P, Hughes R, Tilling K, Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol Jun 2019;110:63–73. doi: 10.1016/j.jclinepi.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zablotsky B, Alford JM. Racial and Ethnic Differences in the Prevalence of Attention-deficit/Hyperactivity Disorder and Learning Disabilities Among U.S. Children Aged 3–17 Years. NCHS Data Brief Mar 2020;(358):1–8. [PubMed] [Google Scholar]
- 44.Morgan PL, Staff J, Hillemeier MM, Farkas G, Maczuga S. Racial and ethnic disparities in ADHD diagnosis from kindergarten to eighth grade. Pediatrics Jul 2013;132(1):85–93. doi: 10.1542/peds.2012-2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DuPaul GJ. Adult Ratings of Child ADHD Symptoms: Importance of Race, Role, and Context. J Abnorm Child Psychol May 2020;48(5):673–677. doi: 10.1007/s10802-019-00615-5 [DOI] [PubMed] [Google Scholar]
- 46.Sibley MH, Pelham WE, Molina BSG, et al. Diagnosing ADHD in adolescence. J Consult Clin Psychol Feb 2012;80(1):139–150. doi: 10.1037/a0026577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achenbach TM. Bottom-Up and Top-Down Paradigms for Psychopathology: A Half-Century Odyssey. Annu Rev Clin Psychol May 7 2020;16:1–24. doi: 10.1146/annurev-clinpsy-071119-115831 [DOI] [PubMed] [Google Scholar]
- 48.Nigg JT, Karalunas SL, Gustafsson HC, et al. Evaluating chronic emotional dysregulation and irritability in relation to ADHD and depression genetic risk in children with ADHD. Journal of Child Psychology and Psychiatry Feb 2020;61(2):205–214. doi: 10.1111/jcpp.13132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


