Abstract
Intravenous inoculation of CD1 mice with 107 CFU of type IV group B Streptococcus (GBS IV) results in a high incidence of diffuse septic arthritis. In this study the roles of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in articular pathology were evaluated. Cytokine levels were quantified in the serum and joints by enzyme-linked immunosorbent assay in mice injected with GBS IV and tested or not tested with pentoxifylline (PTF), a methylxanthine that affects cytokine production. PTF was administered intraperitoneally at a dose of 1 mg/mouse (50 mg/kg of body weight) 1 h after GBS infection and then at 24-h intervals for 4 days. High levels of IL-1β and IL-6, but not TNF-α, were detected in the joints of mice injected with GBS IV from 5 to 15 days after infection, when articular lesions were most frequent and severe. IL-1β and IL-6 concentrations in the joints significantly (P < 0.001) exceeded those detected in the serum, confirming a strong local production. PTF treatment resulted in a strong reduction of cytokine production and in a marked decrease in both the incidence and severity of arthritis. Inoculation of exogenous murine recombinant IL-1β or IL-6 in mice treated with GBS IV plus PTF resulted in an incidence and severity of articular lesions similar to those obtained with inoculation of GBS IV alone. No significant effect was obtained with TNF-α administration. These data show a strong involvement of IL-1β and IL-6, but not TNF-α, in the pathogenesis of GBS arthritis.
Group B streptococci (GBS) are a leading cause of life-threatening infection in neonates and young infants (3). Invasive neonatal GBS infection has either an early (usually the first 24 h after birth) or late (7 days after birth) onset. The source of the organism is the genital tract of the infected infant’s mother (3). Common manifestations of GBS disease in neonates include pneumonia, septicemia, meningitis, bacteremia, and bone or joint infections (3, 6, 19, 24). Invasive disease caused by GBS has also been recognized in adults (8, 32).
Septic arthritis has been described as a clinical manifestation of late-onset GBS infection in neonates (3, 6) and requires prolonged antibiotic treatment to ensure an uncomplicated outcome. In adults, septic arthritis due to GBS has also been documented (32, 34) and is often associated with age and risk factors, such as diabetes mellitus, cancer, cardiovascular disease, chronic renal insufficiency, alcoholism, intravenous drug abuse, human immunodeficiency virus infection, neurological disease, and cirrhosis (14).
We have recently described a mouse model of hematogenously induced GBS arthritis (39). Mice inoculated with the reference serotype IV GBS strain manifested clinical arthritis characterized by an early onset and the evolution from acute exudative synovitis to permanent lesions with irreversible joint damage and ankylosis. Subsequently, our studies demonstrated that GBS serotypes II, III, V, VI, and VII are also able to induce septic arthritis (40). The presence and amount of capsule as well as sialic acid in the capsular polysaccharide influenced the incidence of articular lesions. However, other factors, not related to the bacterial components, could contribute to the establishment of arthritis. The role of cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), on cartilage degradation and bone resorption has been well documented in different experimental models of inflammatory arthritis (11, 17, 27, 29, 30, 37, 38). TNF-α and IL-1β are produced primarily by macrophages and fibroblasts in the inflamed synovia and by neutrophils in the synovial fluid. IL-1 and TNF-α appear to contribute directly to tissue damage through induction of the release of tissue-damaging enzymes from synovial cell and articular chondrocytes and by activation of osteoclasts (2, 37). In addition to IL-1 and TNF-α, other cytokines, such as IL-6, gamma interferon, granulocyte macrophage colony-stimulating factor, and transforming growth factor beta have been incriminated in the mechanisms of synovial proliferation and joint destruction in rheumatoid arthritis (1). An altered cytokine profile, with high production of TNF-α and IL-6, has also been demonstrated in a mouse model of Staphylococcus aureus septic arthritis (4, 46).
The ability of GBS to induce cytokine production has been carried out in vitro and in vivo with human monocytes or whole-blood cultures (41–43) and experimental infections in rodent models (7, 22, 35, 36). However, the role of cytokines in GBS arthritis has not yet been defined.
The aim of the present study was to perform a detailed investigation of cytokine production in mice with GBS septic arthritis. Thus, TNF-α, IL-1β, and IL-6 concentrations were quantified in the joints and sera of mice at various time points during infection by using an enzyme-linked immunosorbent assay (ELISA). The cytokine profile and incidence and severity of arthritis were also studied after inoculation with pentoxifylline (PTF), a methylxanthine known to inhibit TNF-α production (20, 21), as well as that of other inflammatory cytokines, such as IL-1β and IL-6 (25).
MATERIALS AND METHODS
Mice.
Outbred CD-1 mice of both sexes, 8 weeks old, were obtained from Charles River Breeding Laboratories (Calco, Milan, Italy).
Microorganism.
Type IV GBS, reference strain GBS 1/82 (GBS IV), was used throughout the study. For experimental infection, the microorganisms were grown overnight at 37°C in Todd-Hewitt broth (Oxoid Ltd., Basingstoke, Hampshire, England) and then washed and diluted in RPMI 1640 medium (GIBCO, Life Technologies, Milan, Italy). The inoculum size was estimated turbidimetrically, and viability counts were performed as previously described (39). A bacterial suspension was prepared in RPMI 1640 medium. Mice were inoculated intravenously (i.v.) via the tail vein with 5 × 106 or 1 × 107 CFU of GBS/mouse in a volume of 0.5 ml. Control mice were injected in the same way with 0.5 ml of RPMI 1640 medium.
Drug and cytokines.
PTF was obtained from Sigma-Aldrich (Milan, Italy). To determine whether PTF per se exhibits antimicrobial activity, the MIC was measured by a standard dilution broth method (45). PTF was tested in twofold dilution, the final concentration ranging from 0.125 to 4 mg/ml, using an inoculum of 5 × 105 or 5 × 106 CFU/ml of GBS IV in Müller-Hinton broth (Oxoid). One set of control tubes contained bacteria but no drug. Test and control tubes were incubated at 37°C for 18 h. MICs were evaluated in four separate experiments. PTF, diluted in phosphate-buffered saline (PBS; 0.01 M phosphate, 0.15 M NaCl, pH 7.2), was injected intraperitoneally at a dosage of 1 mg/mouse (corresponding to 50 mg/kg) 1 h and 1, 2, 3, and 4 days after GBS infection with 107 CFU/mouse. Murine recombinant TNF-α, IL-1β, and IL-6 were purchased from Sigma. All cytokines, diluted in PBS supplemented with 0.1% bovine serum albumin (BSA) (Sigma), were given intraperitoneally at a dosage of 0.2 μg/mouse at 2 h and on days 1, 2, 3, and 4 after GBS infection (107 CFU/mouse). Cytokines were always injected in mice 1 h after PTF administration. In another set of experiments, mice were injected with cytokines (0.2 μg/mouse) at 1 h and 1, 2, 3, and 4 days after GBS infection with 5 × 106 CFU/mouse. Cumulative survival rates were recorded for each experimental group at 24-h intervals for 60 days.
Clinical evaluation of arthritis.
Mice injected with GBS IV and treated or not treated with cytokines as described above were examined two or more times during day 1 after challenge and then daily for 2 months to evaluate the presence of joint inflammation. Arthritis was defined as a visible erythema or swelling of at least one joint. To evaluate the intensity of arthritis, the following clinical scoring (arthritic index) was used for each limb: 1 point, mild swelling and erythema; 2 points, moderate swelling and erythema; 3 points, marked swelling, erythema, and/or ankylosis. Thus, a mouse could have a maximum score of 12. The arthritic index was constructed by dividing the total score by the number of animals used in each experimental group.
Histological studies.
Groups of mice inoculated i.v. with 107 CFU of GBS IV and treated or not treated with PTF were examined at selected intervals starting 2 days after infection for histopathological features of arthritis. Joints were removed aseptically, fixed in formalin (10% [vol/vol]) for 24 h, and then decalcified in trichloroacetic acid (5% [vol/vol]) for 7 days, dehydrated, embedded in paraffin, sectioned at 5 to 7 μm, and stained with hematoxylin eosin.
Sample preparation for cytokine assessment.
Blood samples from mice injected with 107 CFU of GBS IV or 107 CFU of GBS IV plus PTF and from uninfected, untreated control mice were obtained by retroorbital sinus bleeding before sacrifice at selected intervals; the sera were stored at −80°C until analysis. Joint tissues were prepared as described by Kasama et al. (16). Briefly, articular samples were removed and then homogenized in 1 ml of lysis medium (RPMI 1640 containing 2 mM polymethylsulfonyl fluoride and 1 μg of apoprotinin, leupeptin, and pepstatin A/ml, final concentration)/100-mg joint weight. The homogenized tissues were then centrifuged at 2,000 × g for 10 min, and the supernatants were sterilized with a Millipore filter (0.45-μm pore size) and stored at −80°C until analysis.
TNF-α, IL-6, and IL-1β ELISA.
Polystyrene 96-well flat-bottom plates were coated overnight at 4°C with 50 μl of purified rat anti-mouse TNF-α (4 μg/ml) or rat anti-mouse IL-6 (2 μg/ml) resuspended in 0.1 M Na2HPO4, pH 9.0/well. Both antibodies were purchased from PharMingen (San Diego, Calif.). The coated plates were washed with PBS–0.05% Tween 20 and saturated with 1% BSA in PBS for 1 h at room temperature. After being washed, the plates were incubated overnight at 4°C with different sample dilutions (100 μl/well). The plates were washed again, and 100 μl of biotinylated rat anti-mouse TNF-α or rat anti-mouse IL-6 (2 μg/ml) (PharMingen) diluted in PBS-BSA-Tween 20/well was added. After 1 h of incubation at room temperature, the plates were washed and filled with 100 μl of 2.5 μg/ml of avidin-horse radish peroxidase (Sigma)/well. The plates were incubated for 30 min at room temperature and then washed, and 100 μl of 0.3 mg of enzyme substrate 2,2-azino-bis-(3-ethylbenzothiazoline sulfonic acid) (Sigma)/ml was added to each well. Absorbance was read at 405 nm in a microplate reader (Mago; Diamedix Corporation, Miami, Fla.). The concentrations of TNF-α or IL-6 were calculated with standard curves based on known quantities of recombinant mouse TNF-α or IL-6 (Sigma). IL-1β levels in sera and joints were measured with a commercial ELISA kit (Biosource International, Camarillo, Calif.) according to the manufacturer’s recommendations. Results were expressed for all cytokines as picograms of serum or of supernatants from the joint homogenates per milliliter. The detection limit of the assays was 25 pg/ml for TNF-α, 15 pg/ml for IL-6, and 15.6 pg/ml for IL-1β.
Statistical analysis.
Differences in cytokine concentrations between the groups injected with 107 CFU of GBS IV and treated or not treated with PTF were analyzed by Student’s t test. Comparison of the incidence of arthritis was performed by the χ2 test, and differences in the arthritis index were analyzed by Student’s t test. Each experiment was repeated three to five times. A P value of <0.05 was considered significant.
RESULTS
Clinical course of arthritis.
The clinical signs of joint swelling were observed as early as 24 h after injection of 107 CFU of GBS IV in 40% of the mice. The incidence of arthritis increased to 80% by day 5, and the maximal prevalence was observed on day 10 after inoculation, when about 90% of the mice displayed clinical arthritis. In the same way, the arthritic index reached the maximum 7 to 10 days after GBS challenge (mean value, 6.2 ± 0.3), and most of the animals showed articular lesions in both the hindpaws and forepaws. Thirty percent of the mice died during the course of infection.
Microbiological effect of PTF.
PTF had no antimicrobial activity against type IV GBS up to a concentration of 4 mg/ml, whether an inoculum of 5 × 105 or 5 × 106 CFU/ml was used. GBS susceptibility was not influenced when a culture in the late exponential or stationary phase of growth was used (data not shown).
Kinetics of cytokine appearance.
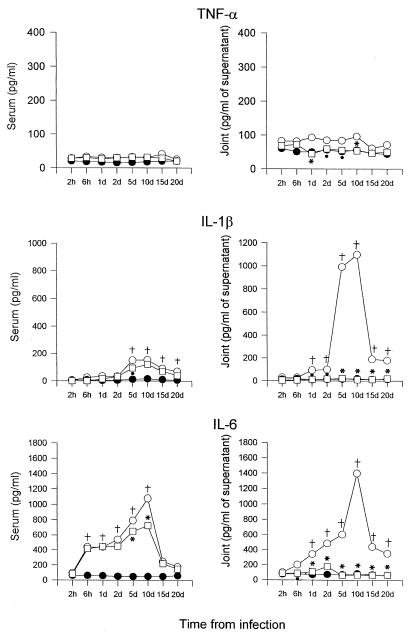
Joint tissues from uninfected or infected mice were dissected at defined time periods, and samples were prepared as described for ELISA assays. Blood samples were taken at the same intervals. Figure 1 shows that TNF-α concentrations were always higher than control levels both in serum and joints at each time point examined. However, a high production of TNF-α was never observed during the whole cytokine detection period. Different results were obtained for IL-1β. A significant (P < 0.001) increase in the level of this cytokine in the serum was evident 5 days after infection, while in the joints, IL-1β concentration began to grow 1 day after GBS injection and reached the maximal value on day 10 (1,100 pg/ml of supernatants from the joints). A progressive decrease was observed from day 15 after infection.
FIG. 1.
Kinetics of appearance and cytokine concentrations in the serum and joints of CD1 mice injected i.v. with 107 CFU of GBS IV (○) or 107 CFU of GBS IV + PTF (□) and in control mice (●). The values represent the means of three separate experiments. Standard deviations, always <10%, have been omitted. Five mice per group were sacrificed at each time point. †, P < 0.001 (GBS IV-infected mice versus uninfected controls); ∗, P < 0.001 (GBS IV + PTF-treated mice versus GBS IV-treated mice); •, P < 0.05 (GBS IV + PTF-treated mice versus GBS IV-treated mice); d, day(s).
IL-6 production was evident 6 h after infection in both the serum and joints. Peak values were reached on day 10. Notably, the IL-6 concentration in joint supernatants was higher than that detected in serum (1,440 pg/ml versus 1,080 pg/ml). As for IL-1β, a progressive decrease in IL-6 concentration was observed on subsequent days.
Effect of PTF administration on cytokine appearance and incidence of arthritis.
Figure 1 shows that PTF administration, after GBS IV challenge, influenced cytokine production in a variable way. TNF-α levels, already low in mice injected with GBS only, further decreased in both the serum and joints. The inhibitory effect of PTF injection was more evident for IL-1β production. In the serum, IL-1β concentrations were always below the values detected in mice injected with GBS only, whereas in the joints cytokine levels were similar to those observed in untreated, uninfected controls at each time point examined.
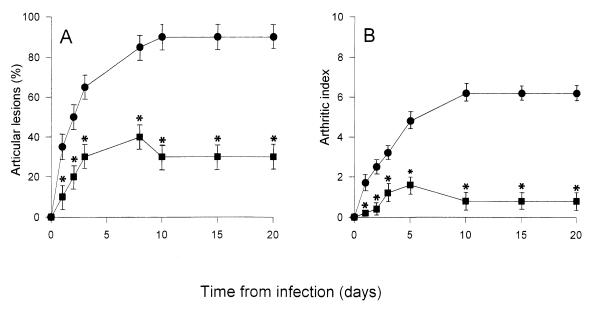
Finally, a lower production of IL-6 was observed in the sera and joints of mice treated with PTF than in the animals injected with GBS alone. In particular, in the joints, IL-6 concentrations returned to baseline levels. The decrease or abolition of cytokine production corresponded to a decrease in both the incidence and severity of arthritis (Fig. 2). In fact, the arthritic index of mice treated with PTF, after infection with 107 CFU of GBS IV, reached a maximum value of 1.6, and only 40% of the animals manifested articular lesions. On the other hand, in mice injected with GBS alone, an arthritic index of up to 6.3 was observed, and 90% of the animals showed articular lesions. Furthermore, no death occurred after PTF treatment (data not shown).
FIG. 2.
Incidence (A) and severity (B) of arthritis in CD1 mice injected i.v. with 107 CFU of GBS IV (●) or GBS IV + PTF (■). The results represent the means ± standard deviation of three separate experiments. In each experiment, 20 mice were used. ∗, P < 0.001 (PTF-treated mice versus untreated mice).
Histopathology.
As previously described (39), the major histopathological changes in mice injected with GBS IV were the presence of an acute exudative synovitis, starting as early as 48 h after infection, and a polymorphonuclear leukocyte-monocyte infiltrate of the subsynovium and periarticular connective tissues. One week later, the articular cavities of infected joints were filled with purulent exudate. Subsequently, joint destruction progressed rapidly, with loss of cartilage and proliferation of granulation tissues. Fibrous ankylosis was observed almost 2 months after GBS inoculation. No great differences in cell infiltrate were observed between the joints of mice treated with GBS plus PTF and those of mice injected with GBS only in the first week after infection. However, reduced histopathological severity of arthritis was observed later, and no bone destruction or loss of joint integrity was observed throughout the observation period (data not shown).
Effect of cytokine administration on arthritis.
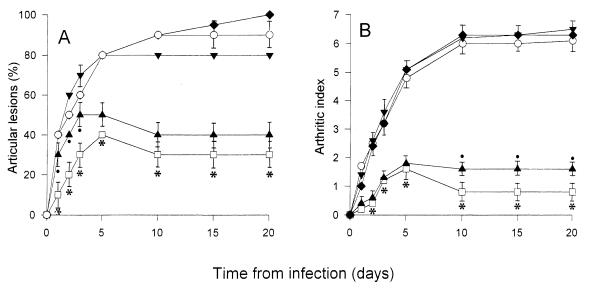
Because of the remarkable effect of PTF treatment on cytokine production and on the incidence and severity of arthritis, mice treated with GBS (107 CFU/mouse) were subsequently inoculated with exogenous TNF-α, IL-1β, or IL-6 to assess the possible roles of these cytokines in articular pathology. As shown in Fig. 3, IL-1β or IL-6 administration in mice pretreated with PTF resulted in an arthritic index and an incidence of articular lesions similar to those obtained with the inoculation of GBS alone. A slight increase in severity and frequency of arthritis was observed after treatment with TNF-α. Mortality rates in the groups of mice injected with cytokines were about 30% (data not shown).
FIG. 3.
Incidence (A) and severity (B) of arthritis in CD1 mice injected with 107 CFU of GBS IV (○), GBS IV + PTF (□), GBS IV + PTF + TNF-α (▴), GBS IV + PTF + IL-1β (▾), or GBS IV + PTF + IL-6 (⧫). The results are the means ± standard deviations of three separate experiments. In each experiment, 20 mice were used. ∗, P < 0.001 (GBS IV + PTF-treated mice versus GBS IV-, GBS IV + PTF + IL-1β-, and GBS IV + PTF + IL-6-treated mice). •, P < 0.05 (GBS + PTF + TNF-α-treated mice versus GBS IV + PTF-treated mice).
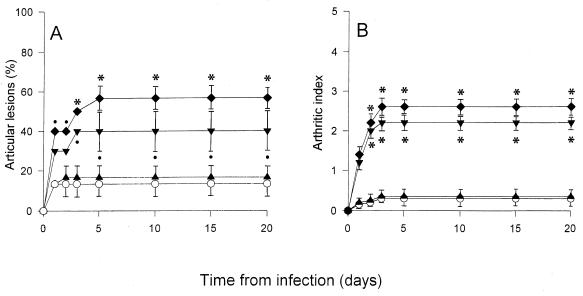
The influence of cytokine administration was also investigated in mice injected with a suboptimal arthritogenic dose of GBS. In this case, 5 × 106 CFU/mouse was inoculated, and the animals were subsequently treated with five doses of each cytokine. Only about 13% of the mice injected with GBS, but not with cytokines, manifested articular lesions, with a mean arthritic index of 0.4 (Fig. 4). Inoculation with IL-1β or IL-6 resulted in an increase of up to 40 and 56.6% (mean values) in the incidence of arthritis, with arthritic indexes of 2.2 and 2.6, respectively. No significant effect was observed with administration of TNF-α, and no mortality was observed in any of the experimental groups (data not shown).
FIG. 4.
Incidence (A) and severity (B) of arthritis in CD1 mice injected with 5 × 106 CFU of GBS IV (○), GBS IV + TNF-α (▴), GBS IV + IL-1β (▾), or GBS IV + IL-6 (⧫). The results represent the means ± standard deviations of three separate experiments. In each experiment, 10 mice were used. ∗, P < 0.001 (GBS + IL-1β- and GBS + IL-6-treated mice versus GBS-treated mice); •, P < 0.05 (GBS + IL-1β- and GBS + IL-6-treated mice versus GBS-treated mice).
DISCUSSION
Induction of GBS arthritis depends on the viability and number of microorganisms injected (39) and on the presence and amount of capsular as well as sialic acid in the capsular polysaccharide (40). In this study we demonstrated that cytokines could also participate in the pathogenesis of GBS arthritis. High levels of IL-1β and IL-6, but not of TNF-α, were found in the sera and joints of mice during infection with type IV GBS. The low circulating levels of TNF-α were unexpected because GBS are good inducers of this cytokine, as demonstrated in different experimental in vivo studies (9, 23, 35). In fact, the peak serum TNF-α level was reached 20 h after infection with type III GBS in neonatal rats (35), and the purified group- and type-specific antigens of this GBS serotype were able to induce TNF-α production (23). However, in an adult-mouse model of type III GBS infection, no serum TNF-α was detected with a sublethal dose consisting of 0.5 50% lethal dose (36). This is in agreement with our findings, in that a dose of 107 CFU/mouse was used throughout this study and the 50% lethal dose of this type IV GBS strain was 1.97 × 107 (±0.2 standard deviation) (39). Production of TNF-α has been documented in experimental models of collagen-induced arthritis (38) and in streptococcal cell wall arthritis (18). Neutralization of TNF-α by specific monoclonal antibodies results in a decrease of inflammation and joint destruction (15, 28, 38, 44).
In a mouse model of septic arthritis induced by S. aureus a rapid, pronounced, sustained accumulation of TNF-α and IL-1β mRNA-expressing cells occurred in the joints (47). The authors stressed the importance of these cytokines as mediators of inflammation and joint destruction in septic arthritis as well. Although we never found high levels of TNF-α in the supernatants from the joints of GBS-infected mice, the concentrations of this cytokine were always higher than those detected in uninfected controls.
In a mouse model of streptococcal cell wall arthritis, IL-1 (α and β) had a dominant role in cartilage destruction and inflammatory-cell influx (18). In our model, a high production of IL-1β was evident, and the highest values were detected in the joints between 5 and 15 days after infection, when articular lesions were more frequent and severe. At that time, histological examination showed that the articular cavities were filled with purulent exudate, and loss of cartilage progressed rapidly. Thus, we can hypothesize that in GBS-induced arthritis as well IL-1β actively participates in joint injury. During infection with GBS, IL-6 levels exceeded those of IL-1β and TNF-α in both the serum and joints. IL-6 production can be induced directly by microbial products or indirectly through IL-1 (33) or TNF-α (5). In our case, IL-6 production appeared to be TNF-α and IL-1 independent, since significant IL-6 concentrations were evident as early as 6 h after infection, before the appearance of significant levels of the other cytokines. High IL-6 levels were found in sera of neonates with GBS sepsis or meningitis (42), and elevated concentrations of this cytokine correlated with disease severity and mortality in animal models (35, 36). In rheumatoid arthritis, high levels of IL-6 were found in the synovial fluid of patients (12). Synoviocytes are a potent source of IL-6 in vitro, and IL-1 and TNF-α increase the release of this cytokine (10). It has been suggested that IL-6 may participate together with IL-1 in catabolism of connective tissue components at sites of inflammation (13, 26).
In the mouse model of S. aureus arthritis, the local presence of IL-6 in the joints was not investigated, but systemic, high IL-6 production was observed throughout the course of arthritis (46). Furthermore, when S. aureus was injected in X-linked immunodeficient (xid) mice, a correlation between low IL-6 response and a mild course of arthritis was observed (46). In our model not only was IL-6 found directly in the joints but its concentration at this site exceeded that detected in the serum, confirming a strong local production. As with IL-1β, the highest levels of IL-6 were detected when the severity of arthritis was at the maximum.
The participation of cytokines in joint injury was emphasized by PTF treatment. PTF inoculation resulted in a decrease or abolition of cytokine production and in a consequent reduction in both the incidence and severity of GBS arthritis. PTF has been used in other experimental studies of GBS infection to inhibit TNF-α production (9, 21). However, its down regulation on TNF-α release as well as on IL-1β and IL-6 synthesis by human peripheral blood mononuclear cells has been reported (25). In our study, the effect of PTF was much more pronounced in the joints than in peripheral cytokine production. This difference was particularly evident with IL-6. This is consistent with the data of Schandené et al. (31), who indicated that PTF could modulate IL-6 production in different ways, depending on its cellular origin. Thus, chondrocytes, synoviocytes, osteoclasts, and all IL-6-producing cells which are present in the inflamed joints could be more susceptible to the PTF effect than peripheral blood mononuclear cells.
Clear proof that mild arthritis observed in PTF-treated mice was due to the drop in cytokine production was given by the administration of exogenous cytokines. In fact, systemic inoculation with IL-1β and IL-6 in PTF-treated mice resulted in a rapid and strong worsening of articular lesions. The minor role of TNF-α in joint injury was also confirmed in these experiments. A further demonstration of the participation of IL-1β and in particular IL-6 in GBS arthritis was carried out with a subarthritogenic dose. Also, in this case an increase in severity and incidence of articular lesions was obtained after inoculation with each of these cytokines.
In conclusion, our results account for a strong involvement of IL-1β and IL-6, but not of TNF-α, in the pathogenesis of GBS arthritis. Further studies are in progress to assess the roles of other cytokines in the establishment of articular damage.
ACKNOWLEDGMENTS
We are grateful to Eileen Mahoney Zannetti for dedicated editorial assistance.
This study was supported by M.U.R.S.T. 1997–1998, Progetto Finalizzato (infections in the immunocompromised host: modulation of the immune response), Italy.
REFERENCES
- 1.Arend W P, Dayer J M. Cytokine and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33:305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 2.Arend W P, Dayer J M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 3.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders; 1995. pp. 980–1054. [Google Scholar]
- 4.Bremmel T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouckaert P, Spriggs D R, Demetri G, Kufe D W, Fiers W. Circulating interleukin 6 during a continuous infusion of tumor necrosis factor and interferon γ. J Exp Med. 1989;169:2257–2262. doi: 10.1084/jem.169.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dan M. Neonatal septic arthritis. Isr J Med Sci. 1983;19:967–971. [PubMed] [Google Scholar]
- 7.Derrico C A, Goodrum K J. Interleukin-12 and tumor necrosis factor alpha mediate innate production of gamma interferon by group B Streptococcus-treated splenocytes of severe combined immunodeficiency mice. Infect Immun. 1996;64:1314–1320. doi: 10.1128/iai.64.4.1314-1320.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farley M M, Harvey R C, Stull T, Smith J D, Schucat A, Wenger J D, Stephens D. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant women. N Engl J Med. 1993;328:1807–1811. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 9.Gibson R L, Redding G J, Henderson W R, Truog W E. Group B Streptococcus induces tumor necrosis factor in neonatal piglets. Effect of the tumor necrosis factor inhibitor pentoxifylline on hemodynamics and gas exchange. Am Rev Respir Dis. 1991;143:598–604. doi: 10.1164/ajrccm/143.3.598. [DOI] [PubMed] [Google Scholar]
- 10.Guerne P-A, Zuraw B L, Vaughan J H, Carson D A, Lotz M. Synovium as a source of interleukin-6 in vitro. J Clin Investig. 1989;83:585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson B, Pettipher E R. Arthritogenic actions of recombinant IL-1 and tumor necrosis factor α in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol. 1989;75:306–310. [PMC free article] [PubMed] [Google Scholar]
- 12.Houssiau F A, Devogelaer J-P, van Damme J, Nagant De Deuchaisnes C, van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 13.Ito A, Itoh Y, Sasaguri Y, Morimatsu M, Mori Y. Effects of interleukin-6 on the metabolism of connective tissue component in rheumatoid synovial fibroblasts. Arthritis Rheum. 1992;35:1197–1201. doi: 10.1002/art.1780351012. [DOI] [PubMed] [Google Scholar]
- 14.Jackson L A, Hilsdon R, Farley M M, Harrison H, Reingold A L, Plikaytis B D, Wenger D, Schucat A. Risk factors for group B streptococcal disease in adults. Ann Intern Med. 1995;123:415–420. doi: 10.7326/0003-4819-123-6-199509150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Joosten L A B, Helsen M M A, van de Loo F A J, van den Berg W B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNFα, anti-IL-1α/β and anti-IL-1Rα. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 16.Kasama T, Strieter R M, Lukacs N W, Lincoln P M, Burdick M D, Kunkel S L. Interleukin-10 expression and chemokine regulation during the evaluation of murine type II collagen-induced arthritis. J Clin Investig. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killar L M, Dunn C J. Interleukin-1 potentiates the development of collagen-induced arthritis. Clin Sci. 1989;76:535–538. doi: 10.1042/cs0760535. [DOI] [PubMed] [Google Scholar]
- 18.Kuiper S, Joosten L A B, Bendelf A M, Edwards III C K, Arntz O J, Helsen M M A, Van de Loo F A J, van den Berg W B. Different roles of tumor necrosis factor α and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine. 1998;10:690–702. doi: 10.1006/cyto.1998.0372. [DOI] [PubMed] [Google Scholar]
- 19.Lai T K, Hingston J, Scheifele D. Streptococcal neonatal osteomyelitis. Am J Dis Child. 1980;134:711. doi: 10.1001/archpedi.1980.02130190077025. [DOI] [PubMed] [Google Scholar]
- 20.Maderazo E G, Breaux S, Woronick C L, Krause P J. Efficacy, toxicity, and pharmacokinetics of pentoxifylline and its analogs in experimental Staphylococcus aureus infections. Antimicrob Agents Chemother. 1990;34:1100–1106. doi: 10.1128/aac.34.6.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancuso G, Cusumano V, Cook J A, Smith E, Squadrito F, Blandino G, Teti G. Efficacy of tumor necrosis factor α and eicosanoid inhibitors in experimental models of normal sepsis. FEMS Immunol Med Microbiol. 1994;9:49–54. doi: 10.1111/j.1574-695X.1994.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 22.Mancuso G, Cusumano V, Genovese F, Gambuzza M, Beninati C, Teti G. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect Immun. 1997;65:3731–3735. doi: 10.1128/iai.65.9.3731-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancuso G, Tomasello F, von Hunolstein C, Orefici G, Teti G. Induction of tumor necrosis factor alpha by the group- and type-specific polysaccharides from type III group B streptococci. Infect Immun. 1994;62:2748–2753. doi: 10.1128/iai.62.7.2748-2753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memon I A, Jacobs N M, Yeh T F, Lilien L D. Group B streptococcal osteomyelitis and septic arthritis. Its occurrence in infants less than 2 months old. Am J Dis Child. 1979;133:921–923. doi: 10.1001/archpedi.1979.02130090049009. [DOI] [PubMed] [Google Scholar]
- 25.Neuner P, Klosner G, Schauer E, Pourmojib M, Macheiner W, Grünwald C, Knobler R, Schwartz A, Luger T A, Schwartz T. Pentoxifylline in vivo down-regulates the release of IL-1β, IL-6, IL-8 and tumor necrosis factor-α by human peripheral blood mononuclear cells. Immunology. 1994;83:262–267. [PMC free article] [PubMed] [Google Scholar]
- 26.Nietfeld J J, Wilbrink B, Helle M, van Roy J L A M, den Otter W, Swank A J G, Huber-Bruning O. Interleukin-1-induced interleukin-6 is required for the inhibition of proteoglycan synthesis by interleukin-1 in human articular cartilage. Arthritis Rheum. 1990;33:1695–1701. doi: 10.1002/art.1780331113. [DOI] [PubMed] [Google Scholar]
- 27.Pettipher E R, Higgs G A, Henderson B. Interleukin 1 induced leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci USA. 1986;83:8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piquet P F, Grau G E, Vesin G, Loetscher H, Gentz R, Leslauer W. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumor necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology. 1992;77:510–514. [PMC free article] [PubMed] [Google Scholar]
- 29.Ridderstad A, Abedi-Valugerdi M, Moller E. Cytokines in rheumatoid arthritis. Ann Med. 1991;23:219–223. doi: 10.3109/07853899109148051. [DOI] [PubMed] [Google Scholar]
- 30.Sàez-Llorens X, Jafari H S, Olsen K D, Nariuchi H, Hansen E J, McCraken G H., Jr Induction of suppurative arthritis in rabbit by Haemophilus endotoxin, tumor necrosis factor-α, and interleukin-1β. J Infect Dis. 1991;163:1267–1272. doi: 10.1093/infdis/163.6.1267. [DOI] [PubMed] [Google Scholar]
- 31.Schandené L, Vandenbussche P, Crusiaux A, Alègre M-L, Abramowicz D, Dupont E, Content J, Goldman M. Differential effects of pentoxifylline on the production of tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) by monocytes and T cells. Immunology. 1992;76:30–34. [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz B, Schuchat A, Oxtoby M J, Cochi S I, Hightower A, Broome C V. Invasive group B streptococcal disease in adults. A population-based study in metropolitan Atlanta. JAMA. 1991;266:1112–1114. [PubMed] [Google Scholar]
- 33.Shalaby M R, Waage A, Espevic T. Cytokine regulation of interleukin 6 production by human endothelial cells. Cell Immunol. 1989;121:372–382. doi: 10.1016/0008-8749(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 34.Small C B, Slater L N, Lowy F D, Small R D, Salvati E A, Casey J I. Group B streptococcal arthritis in adults. Am J Med. 1984;76:367–375. doi: 10.1016/0002-9343(84)90653-3. [DOI] [PubMed] [Google Scholar]
- 35.Teti G, Mancuso G, Tomasello F. Cytokine appearance and effects of anti-tumor necrosis factor alpha antibodies in a neonatal rat model of group B streptococcal infection. Infect Immun. 1993;61:227–235. doi: 10.1128/iai.61.1.227-235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teti G, Mancuso G, Tomasello F, Chiopalo M S. Production of tumor necrosis factor-α and interleukin-6 in mice infected with group B streptococci. Circ Shock. 1992;38:138–144. [PubMed] [Google Scholar]
- 37.Thomas B M, Mundy G R, Chambers J J. Tumor necrosis factor alpha and beta induce osteoblastic cells to stimulate osteoclast bone resorption. J Immunol. 1987;138:775–779. [PubMed] [Google Scholar]
- 38.Thorbecke G J, Shah R, Leu C H, Kuruvilla A P, Haridson A M, Palladino M A. Involvement of endogenous tumor necrosis factor α and transforming growth factor β during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tissi L, Marconi P, Mosci P, Cornacchione P, Rosati E, Recchia S, von Hunolstein C, Orefici G. Experimental model of type IV Streptococcus agalactiae (group B Streptococcus) infection in mice with early development of specific arthritis. Infect Immun. 1990;58:3093–3100. doi: 10.1128/iai.58.9.3093-3100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tissi L, von Hunolstein C, Bistoni F, Marangi M, Parisi L, Orefici G. Role of group B streptococcal capsular polysaccharides in the induction of septic arthritis. J Med Microbiol. 1998;47:717–723. doi: 10.1099/00222615-47-8-717. [DOI] [PubMed] [Google Scholar]
- 41.Vallejo J G, Baker C J, Edwards M S. Role of the bacterial cell wall and capsule in induction of tumor necrosis factor α by type III group B streptococci. Infect Immun. 1996;64:332–337. doi: 10.1128/iai.64.12.5042-5046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallejo J G, Baker C J, Edwards M S. Interleukin-6 production by human neonatal monocytes stimulated by type III group B streptococci. J Infect Dis. 1996;174:332–337. doi: 10.1093/infdis/174.2.332. [DOI] [PubMed] [Google Scholar]
- 43.von Hunolstein C, Totolian A, Alfarone G, Mancuso G, Cusumano V, Teti G, Orefici G. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;10:4017–4021. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams R O, Feldman M F, Ravinder N M. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods G L, Washington J A. Antibacterial susceptibility: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 1327–1341. [Google Scholar]
- 46.Zhao Y-X, Abdelnour A, Holmdahl R, Tarkowski A. Mice with the xid B cell defect are less susceptible to developing Staphylococcus aureus-induced arthritis. J Immunol. 1995;155:2067–2076. [PubMed] [Google Scholar]
- 47.Zhao Y-X, Ljungdahl A, Olsson T, Tarkowski A. In situ hybridization analysis of synovial and systemic cytokine messenger RNA expression in superantigen-mediated Staphylococcus aureus arthritis. Arthritis Rheum. 1996;39:959–967. doi: 10.1002/art.1780390613. [DOI] [PubMed] [Google Scholar]