Abstract
Background
Ionizing radiation (IR) is high-energy radiation that has the potential to displace electrons from atoms and break chemical bonds. It has the ability to introduce mutations, DNA strand breakage, and cell death. Being a radiosensitive organ, exposure of the thyroid gland to IR can lead to significant changes in its function.
Aim of the work
Was to measure the levels of thyroid hormones panel and ultrasonography abnormalities in medical staff occupationally exposed to IR.
Subjects and methods
A total of 120 subjects were divided into three main groups: Group I: radiation-exposed workers occupationally exposed to radioiodine (131I) (n = 40), Group II: radiation-exposed workers occupationally exposed to X-ray (n = 40), and Group III: non-exposed healthy professionals matched in age and sex with the previous groups (n = 40). Thyroid hormones panel including free triiodothyronine (fT3), free thyroxine (fT4), thyroid-stimulating hormone (TSH), anti-thyroperoxidase antibodies (anti-TPO), and thyroglobulin (Tg) were measured. Thyroid ultrasonography was performed. Oxidative stress markers such as malondialdehyde (MDA), hydrogen peroxide (H2O2), and total antioxidant capacity (TAC) were measured.
Results
Group I had significantly higher fT3 levels than the control group. fT3 levels were considerably higher, while TSH was substantially lower in group II participants than in the control group. Tg was markedly lower in radiation-exposed workers. However, anti-TPO levels in radiation-exposed workers were significantly higher than in the control group. MDA and H2O2 were substantially higher; TAC was significantly lower in radiation-exposed workers compared to the control group. According to ultrasonographic examination, thyroid volume and the percentage of thyroid nodules in all radiation workers were significantly higher than in the control group.
Conclusion
Despite low exposure doses, occupational exposure to IR affects the thyroid hormones and links with a higher likelihood of developing thyroid immune diseases.
Keywords: Ionizing radiation, Thyroid gland, Radioiodine, X-ray, Ultrasound
Background
Ionizing radiation (IR) is energy in the form of waves or particles that knocks atoms’ electrons out of place. It is capable of causing DNA strand breaks and mutations. IR is mostly genotoxic agent and a known carcinogen. Ionizing radiation’s impact on human health has been thoroughly documented throughout the past century. There is a growing evidence from researches indicating the association between IR and cancer. It is best represented by a linear nonthreshold model [1, 2]. There is a general agreement that exposure to large doses of IR poses a serious risk to human health. On the other hand, numerous scientists have voiced rising scepticism and put up various hypotheses on the dangers associated with long-term exposures to low doses of IR, which occur more frequently than exposure to large doses [3].
Ionizing radiation has become a necessary component of modern life, particularly in the medical field. In other circumstances, it is utilized for both diagnostic and therapeutic purposes, such as cancer. Radiation workers are exposed to IR in the workplace. With developments in modern medicine, radio-diagnosis and radiotherapy are being utilised more frequently which lead to an increase in the number of occupationally exposed persons [4].
Our previous findings revealed that, workers occupationally exposed to low doses of IR showed higher incidence of all types of chromosomal aberrations and elevated levels of serum 8-OHdG [5]. Moreover, Occupational exposure to IR alters circulating redox and inflammatory biomarkers [6]. Recently, significant increase in methemoglobin levels and significant decrease in MCV and ferritin levels were found among radiation-exposed workers [7].
The thyroid gland, which receives a significant radiation dosage from scatter radiation due to its anatomical location, is one of the target organs for radiation-related disease [8, 9]. The thyroid gland is the largest endocrine gland, with two lobes beside the trachea and a lower larynx. The thyroid gland produces hormones and regulates basal metabolic rate, protein synthesis, and several other processes, including development. Iodine and tyrosine make thyroid hormones T3 and T4 in follicular cells. Calcitonin hormone is produced by the thyroid and is involved in calcium homeostasis [10]. While the relation between thyroid irradiation and an increased risk of thyroid cancer is well known, the effects of radiation on thyroid gland function have received less attention [11]. Low-dose radiation’s effects on thyroid hormone levels have only been studied in few studies [12, 13].
Radiation causes oxidative stress, which happens when there is an imbalance between reactive oxygen species (ROS) and antioxidants. Cells boost defensive enzymes and proteins to counteract the oxidant property and redox balance [14–16]. Although oxidative reactions occur in all tissues and organs, oxidative activities are essential for thyroid hormone synthesis in the thyroid gland. Under normal conditions, the thyroid creates substantial ROS, mainly hydrogen peroxide (H2O2) [17]. On the other hand, increased oxidative stress caused by ionizing radiation causes more damage to macromolecules, potentially leading to thyroid problems and cancer. Free radicals including hydroxyl, superoxide, nitric oxide, and hydrogen peroxide radicals are produced by ionizing radiation. These free radicals are chemically very active acting as oxidizing agents causing morphological and physiological changes in the cells. In thyroid gland cells, these radicals have the potential to interact with other macromolecules in thyroid cells and alter their structure and function, leading to hypo- or hyperthyroid disorders [2]. Therefore, the present study aimed to investigate whether exposed medical personnel are more likely to develop thyroid hormones and gland abnormalities. To achieve this goal, a thyroid hormones panel including free triiodothyronine (fT3), free thyroxine (fT4), thyroid-stimulating hormone (TSH), anti-thyroperoxidase antibodies (anti-TPO), and thyroglobulin (Tg) were measured in the serum samples of all studied groups. In addition, oxidative stress markers such as malondialdehyde (MDA), hydrogen peroxide (H2O2), and total antioxidant capacity (TAC) were assayed.
Methods
The present prospective study included a total of 120 subjects divided into three main groups: Group I: radiation-exposed workers occupationally exposed to radioactive Iodine-131(n = 40). Group II: radiation-exposed workers occupationally exposed to X-ray (n = 40). Group III: non-exposed healthy professionals matched in age and sex with both groups (n = 40), in the period between April 2019 till January 2020. Group I participants were selected from Nuclear Medicine Department, while group II participants were selected from Diagnostic Radiology and Radiotherapy Departments, National Cancer Institute, Cairo University, Egypt.. The Group III participants were health professionals selected from the different departments not exposed to ionizing radiation. After approval of The Ethical Committee of the Medical Research Institute, Alexandria University, Alexandria, Egypt, on the protocol of the present study, informed consent was taken from every participant. The study was done according to The Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans. All subjects were interviewed and completed a questionnaire including demographic data, lifestyles, medical records and radiation exposure history. The radiation workers in groups I and II were included in the study if their current jobs required them to be exposed regularly to radioiodine or X-ray. They were working 6 hours per day for 6 days per week in two rotating shifts. None of them received chemotherapeutic drugs or subjected to ionizing radiation for diagnostic or therapeutic purposes in the 6 months before blood collection. The annual accumulated effective dose was measured during the person’s entire working time using personal pocket dosimeters. Participants who met the inclusion criteria were included in the study. Exclusion criteria included participants in either group who had a history or confirmed diagnosis of thyroid cancer, hypothyroidism, hyperthyroidism, or thyroid parenchymal disease. Pregnant women and smokers were also not included in the study.
Blood samples collection
One venous blood sample was collected from radiation workers and healthy controls. The blood sample (5 ml) was allowed to clot for 10–20 min at room temperature. It was centrifuged at 2000–3000 RPM for 20 min. The supernatants were collected carefully. Serum was stored at − 80 °C until used. Thyroid hormone panel (fT3, fT4, TSH), serum anti-TPO, and Tg levels were measured using the Enzyme-Linked Immunosorbent Assay (ELISA), by the recommendations of the manufacturer (Diagnostic Automation, USA). A colorimetric approach was used to detect oxidative stress markers such as MDA, H2O2, and TAC according to manufacturers’ instructions (Bio diagnostic, Egypt).
Thyroid ultrasonography
Thyroid ultrasonographic evaluation was performed for all subjects who participated in the study. Ultrasonography was used to determine the thyroid parenchymal echo structure, thyroid volume, and the presence of thyroid nodules in workers from three groups. The thyroid parenchymal echo structure was detected as homogeneous and heterogenous by ultrasound [18]. Thyroid volume was calculated according to this formula: TV = RL [T × W × L × CF] + LL [T × W × L × CF] and the volume of the isthmus was not included [19]. The thyroid ultrasound examinations were performed by a single radiology consultant with 22 years of experience in ultrasound (Y. A.) using SIEMENS-G40 ultrasound equipment and a 7–10 MHz (MHz) linear probe.
Statistical analyses
The IBM SPSS software programmer version 20.0 was used to examine the data (Armonk, NY: IBM Corp). Numbers and percentages were used to describe qualitative data. The normality of the data distribution was examined by the Kolmogorov-Smirnov test. The range (minimum and maximum), mean, and standard deviation characterize quantitative data. To compare categorical variables between groups, the Chi-square test was used. The student t-test was used to compare two normally distributed quantitative data groups. The Mann-Whitney test was created to compare two groups of quantitative data that were abnormally distributed. The Spearman coefficient was used to determine a relationship between two abnormally quantitative variables. The significance of the obtained results was decided at a 5% level.
Results
Demographic data
Demographic data of the studied groups was illustrated in Table 1. The difference in mean age and sex status between participants.
Table 1.
Demographic data of the studied groups
| Participants exposed to radioiodine (Group I) (n = 40) |
Participants exposed to X-ray (Group II) (n = 40) |
Control group (Group III) (n = 40) |
p | |
|---|---|---|---|---|
| Age (years) | ||||
| Min. – Max. | 24.0–57.0 | 22.0–51.0 | 22.0–51.0 | 0.291 |
| Mean ± SD. | 33.91 ± 10.30 | 31.16 ± 7.92 | 30.59 ± 7.12 | |
| Sex: n (%) | ||||
| Female | 18 (45%) | 14 (35%) | 16 (40%) | 0.321 |
| Male | 22 (55%) | 26 (65%) | 24 (60%) | |
| Working period (years) | ||||
| Min. – Max. | 2–20 | 1–38 | – | |
| Mean ± SD. | 7.50 ± 5.46 | 14.83 ± 10.91 | ||
| Annual effective dose (mSv) | ||||
| Min. – Max. | 0.44–2.89 | 0.5–3.6 | – | |
| Mean ± SD. | 1.22 ± 0.88 | 1.53 ± 0.91 | ||
p, p-value for comparing each radiation workers group and control group. Student t test was used
There was no significant difference in mean age and sex between Group I or Group II with Group III (p = 0.291 and p = 0.321, respectively) (Table 1).
Thyroid function tests
Thyroid function tests of radiation workers groups and control group were shown in Table 2. Regarding participants occupationally exposed to radioiodine (Group I), mean values of TSH and fT4 showed an insignificant difference in comparison to the control group (Group III) (2.12 ± 0.90 vs. 2.16 ± 0.73, p = 0.896 and 1.28 ± 0.14 vs. 1.25 ± 0.25, p = 0.212, respectively). Contrariwise, participants from group I had significantly higher fT3 mean values than participants from group III (3.01 ± 0.41 versus 2.76 ± 0.38, p = 0.047*). Meanwhile, Tg values in participants from group I were significantly lower than in participants from group III (12.86 ± 11.53 vs. 18.29 ± 11.29, p = 0.028). Regarding anti-TPO, their mean values were substantially higher in participants from group I than in participants from group III (35.61 ± 82.35 vs. 8.40 ± 1.26, p < 0.001*, respectively).
Table 2.
Comparison between the studied groups according to the thyroid function tests
| Thyroid function tests | Participants exposed to radioiodine (Group I) (n = 40) |
Participants exposed to X-ray (Group II) (n = 40) |
Control group (Group III) (n = 40) |
p* | p** |
|---|---|---|---|---|---|
| TSH (μIU/ml) | |||||
| Min. – Max. | 0.85–4.61 | 0.31–3.12 | 0.92–3.24 | 0.896 | 0.010* |
| Mean ± SD. | 2.12 ± 0.90 | 1.80 ± 1.22 | 2.16 ± 0.73 | ||
| Free T3 (pg/ml) | |||||
| Min. – Max. | 2.35–3.56 | 2.44–4.25 | 2.09–3.43 | 0.047* | < 0.001* |
| Mean ± SD. | 3.01 ± 0.41 | 3.49 ± 0.53 | 2.76 ± 0.38 | ||
| Free T4 (ng/dl) | |||||
| Min. – Max. | 0.90–1.47 | 0.77–1.76 | 0.88–1.55 | 0.212 | 0.101 |
| Mean ± SD. | 1.28 ± 0.14 | 1.32 ± 0.20 | 1.25 ± 0.25 | ||
| Thyroglobulin (ng/ml) | |||||
| Min. – Max. | 0.69–43.30 | 0.86–37.60 | 4.12–49.30 | ||
| Mean ± SD. | 12.86 ± 11.53 | 10.81 ± 8.86 | 18.29 ± 11.29 | 0.028* | 0.005* |
| Anti-Thyroid Peroxidase Ab (IU/ml) | |||||
| Min. – Max. | 5.33–397.0 | 5.0–249.0 | 6.89–11.50 | ||
| Mean ± SD. | 35.61 ± 82.35 | 31.93 ± 57.58 | 8.40 ± 1.26 | < 0.001* | < 0.001* |
p*, p-value for comparing between group I and group III. p**, p-value for comparing between group II and group III. *: Statistically significant at p ≤ 0.05. Student t test was used
Regarding participants occupationally exposed to X-rays (Group II), TSH levels were significantly lower than participants from group III (1.80 ± 1.22 versus 2.16 ± 0.73, p = 0.010* respectively). Contrariwise, fT4 showed an insignificant difference in participants of Group II in comparison to participants from group III (1.32 ± 0.20 vs. 1.25 ± 0.25, p = 0.101 respectively). Participants from group II had significantly higher mean fT3 values than participants from group III (3.49 ± 0.53 versus 2.76 ± 0.38, p < 0.001* respectively). Meanwhile, the mean serum Tg levels in participants from group II were significantly lower than in the participants from group III (10.81 ± 8.86 vs. 18.29 ± 11.29, p = 0.005* respectively). Regarding anti-TPO, their mean values were substantially higher in participants from group II than in the participants from group III (31.93 ± 57.58 vs. 8.40 ± 1.26, P < 0.001* respectively) (Table 2).
Oxidative stress markers
The mean values of MDA and H2O2 levels were significantly higher in radioiodine-exposed workers (Group I), than in the participants from group III (5.76 ± 7.09 vs. 1.14 ± 0.67, p = 0.006* and 1.36 ± 0.40 vs. 0.48 ± 0.23, p < 0.001*, respectively). However, the mean values of TAC were significantly lower in participants from group I than in the participants from group III (0.74 ± 0.56 vs. 1.59 ± 0.51, p < 0.001*). For participants from group II exposed to X-ray, the mean values of MDA and H2O2 levels were significantly higher than in the participants from group III (4.04 ± 2.32 vs. 1.14 ± 0.67, p < 0.001* and 1.25 ± 0.39 vs. 0.48 ± 0.23, p < 0.001*, respectively). However, compared to the control group, the mean values of TAC were significantly lower (0.56 ± 0.23 vs. 1.59 ± 0.51, p < 0.001*) (Table 3).
Table 3.
Comparison between the studied groups according to oxidative stress markers
| Participants exposed to radioiodine (Group I) (n = 40) |
Participants exposed to X-ray (Group II) (n = 40) |
Control group (Group III) (n = 40) |
p* | p** | |
|---|---|---|---|---|---|
| MDA (nmol/ml) | |||||
| Min. – Max. | 0.13–21.84 | 1.52–11.10 | 0.26–2.50 | 0.006* | < 0.001* |
| Mean ± SD. | 5.76 ± 7.09 | 4.04 ± 2.32 | 1.14 ± 0.67 | ||
| H2O2 (mM/L) | |||||
| Min. – Max. | 1.01–2.42 | 1.20–2.34 | 0.16–0.93 | < 0.001* | < 0.001* |
| Mean ± SD. | 1.36 ± 0.40 | 1.25 ± 0.39 | 0.48 ± 0.23 | ||
| TAC (mM/L) | |||||
| Min. – Max. | 0.08–1.74 | 0.19–0.94 | 1.08–2.71 | < 0.001* | < 0.001* |
| Mean ± SD. | 0.74 ± 0.56 | 0.56 ± 0.23 | 1.59 ± 0.51 | ||
p*, p-value for comparing between group I and group III. p**, p-value for comparing between group II and group III. *: Statistically significant at p ≤ 0.05. Student t test and Mann Whitney test were used
Thyroid ultrasonography
The mean thyroid volume (ml) was significantly larger in participants from group I, in comparison to participants from group III (10.32 ± 3.42 vs. 4.62 ± 1.13, p < 0.001*). The thyroid nodule percentage in Group I was significantly higher than in the control group (p = 0.005*). An insignificant difference was found between the two studied groups regarding the disease rate of diffuse thyroid parenchymal disease (p = 0.172). The mean thyroid volume (ml) was significantly larger in participants from group II, in comparison to participants from group III (11.65 ± 5.95 vs. 4.62 ± 1.13, p < 0.001*). The percentage of thyroid nodules was significantly higher in participants from group II than in participants from group III (p= =0.021*). Moreover, the rate of diffuse thyroid parenchymal disease among participants from group II was considerably higher (p < 0.001*) (Table 4 and Figs. 1, 2, and 3).
Table 4.
Comparison between the studied groups according to Thyroid Ultrasonography
| Participants exposed to radioiodine (Group I) (n = 40) |
Participants exposed to X-ray (Group II) (n = 40) |
Control group (Group III) (n = 40) |
p* | p** | |
|---|---|---|---|---|---|
| Thyroid volume (ml) | |||||
| Min. – Max. | 7.0–18.0 | 4.50–28.20 | 3.0–8.0 | < 0.001* | < 0.001* |
| Mean ± SD. | 10.32 ± 3.42 | 11.65 ± 5.95 | 4.62 ± 1.13 | ||
| Thyroid nodules | |||||
| Normal | 27 (67.5%) | 26 (65%) | 36 (90%) | 0.005* | 0.021* |
| Cystic | 7 (17.5%) | 4 (10%) | 3 (7.5%) | ||
| Solid | 4 (10%) | 6 (15%) | 1 (2.5%) | ||
| Mixed | 2 (5%) | 4 (10%) | 0 (0.0%) | ||
| % of Diffuse thyroid parenchymal disease | 0% | 30% | 0% | 0.172 | < 0.001* |
p*, p-value for comparing between group I and group III. p**, p-value for comparing between group II and group III. *: Statistically significant at p ≤ 0.05. Student t test and Chi square test were used
Fig. 1.
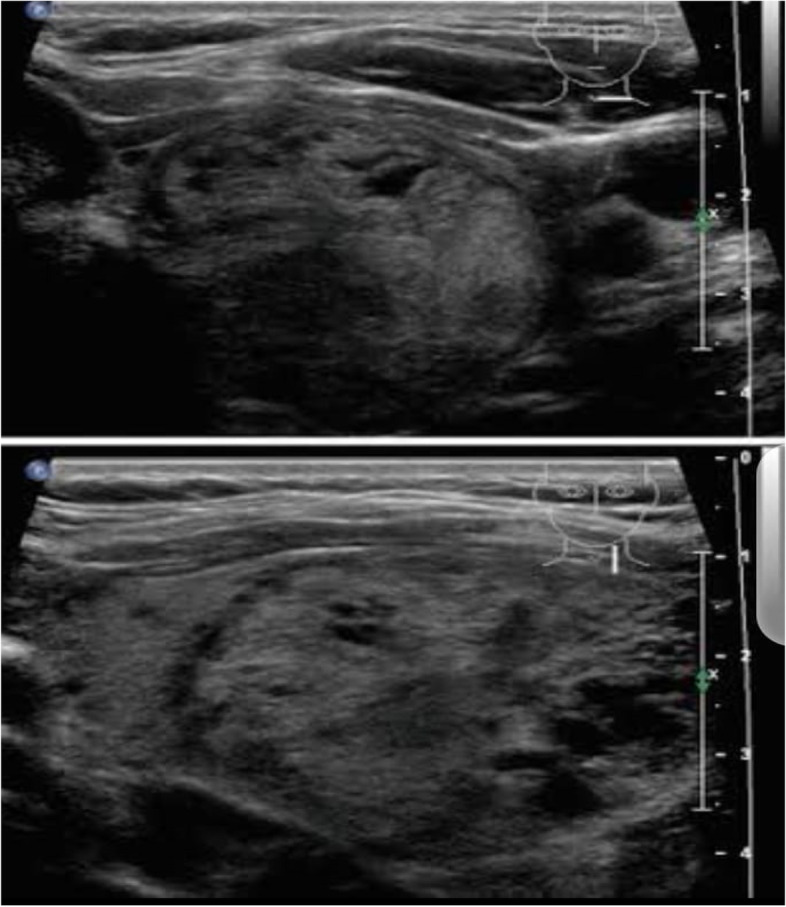
Gray-scale ultrasound images show a right thyroid nodule described as TIRADS IV in a Group I participant
Fig. 2.
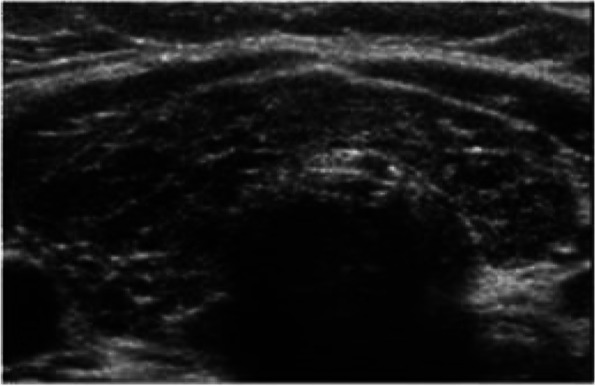
Gray-scale ultrasound images show heterogeneous thyroid parenchyma in a Group II participant
Fig. 3.
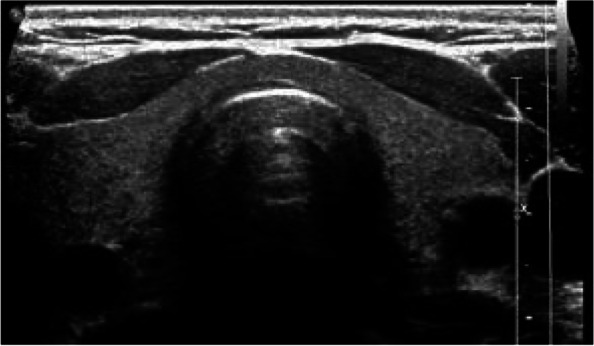
Gray-scale ultrasound images show homogeneous thyroid parenchyma in a Group III participant
Correlation between working period and annual effective dose with studied biomarkers in radiation workers
MDA levels and working period were significantly correlated (p = 0.013*) in participants from group I. An insignificant difference between the working period and annual dose with other studied biomarkers is illustrated in Table 5.
Table 5.
Correlation between working period and annual absorbed dose with different studied biomarkers in radiation workers
| Participants exposed to radioiodine (Group I) (n = 40) |
Working period (years) | Annual effective dose (mSv) | Participants exposed to X-ray (Group II) (n = 40) |
Working period (years) | Annual effective dose (mSv) | ||||
|---|---|---|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | rs | p | ||
| TSH | 0.249 | 0.263 | 0.112 | 0.618 | TSH | −0.075 | 0.693 | 0.085 | 0.657 |
| Free T3 | 0.002 | 0.992 | −0.123 | 0.584 | Free T3 | 0.108 | 0.569 | 0.267 | 0.154 |
| Free T4 | 0.257 | 0.248 | −0.085 | 0.708 | Free T4 | 0.226 | 0.230 | 0.176 | 0.352 |
| Thyroglobulin | 0.166 | 0.461 | 0.130 | 0.564 | Thyroglobulin | 0.293 | 0.611 | −0.145 | 0.445 |
| Thyroid-Anti-peroxidase Ab | 0.047 | 0.837 | 0.310 | 0.098 | Thyroid-Anti-peroxidase Ab | 0.137 | 0.469 | 0.238 | 0.205 |
| MDA | 0.520 | 0.013* | 0.320 | 0.147 | MDA | 0.121 | 0.526 | 0.344 | 0.062 |
| H2O2 | 0.008 | 0.972 | −0.084 | 0.711 | H2O2 | 0.141 | 0.457 | 0.258 | 0.169 |
| TAC | 0.170 | 0.449 | 0.173 | 0.441 | TAC | −0.005 | 0.980 | −0.137 | 0.470 |
rs: Spearman coefficient*: Statistically significant at p ≤ 0.05
Discussion
Several studies have established ionizing radiation’s effects on the thyroid, particularly as a significant reason for thyroid carcinoma and nodules [20–22]. The severity of the disorders linked to radiation dose has led to the conclusion that acute radiation exposure is more damaging than chronic radiation exposure. Ionizing radiation at high doses has undeniable detrimental consequences involving cancer induction. Although low-dose radiation risk is substantial due to its linkages to cancer screening tests and occupational radiation exposure, the situation is less evident at very low radiation doses [23]. The current study revealed that the mean TSH levels in radiation workers, especially exposed to X-rays, were considerably lower than in healthy controls. Contrariwise, radiation workers had a significant increase in the mean fT3 values than in the control group. Regarding ultrasonography imaging, radiation workers had a greater thyroid volume (ml), diffuse thyroid parenchymal disease, and an increased percentage of thyroid nodules. These findings point to hyperthyroidism because of occupational ionizing radiation exposure. Oxidative stress and the generation of reactive oxygen species (ROS) due to ionizing radiation exposure during work shifts may be implicated in these changes induced in the thyroid gland.
Our results were in agreement with Alawneh K et al. [24] who revealed that thyroid hormone levels might be elevated due to radiation exposure. On the contrary to our results, Wong YS et al. [25] concluded that despite low exposure doses, occupational exposure to IR in medical workers still may be linked with the declines in the serum levels of T3 and T4. In a previous study, the authors suggested that occupationally exposed medical personnel to IR have iodine deficiency and higher thyroid nodules [26]. Furthermore, Chen et al. conducted a study [27] which assumed that thyroid disorder among radiologists might be considered related to other risk factors, including working night shifts and being under heavy work stress.
A recent study conducted by Guo et al., [28] showed that T3 and T4 levels in the participants decreased slightly but significantly during the follow-up years. This study agrees in part with our results, in which T3 levels increased as the radiation dose increased, implying the existence of a dose threshold above which T3 synthesis and secretion are promoted. This study, however, found no link between radiation doses and thyroid hormone level decreases, which is consistent with the current research. This may be due to the negative feedback regulation mechanism of the thyroid system. The dynamic equilibrium of TSH, T3, and T4 levels can be maintained and the relative stability of thyroid hormone secretion can be controlled through the hypothalamus-pituitary-thyroid regulation loop which enhances TSH production to support T4 and T3 secretion when the serum T4 concentration and T3 are diminished [28].
We explain that occupational exposure, especially to radioiodine, may result in radioiodine accumulation in the thyroid. Radioiodine results in a significant β-decay component. Contrariwise, for nuclear medical personnel, γ-emission represents the primary source of external exposure [29]. Radioiodine emitted radiation directly induces DNA damage or generates reactive oxygen species (ROS). The thyroid tissue has a high concentration of NADPH oxidases (NOX), specialized ROS-generating enzymes defined as NOX. Radiation exposure increases NOX1 expression, resulting in significant ROS production in the thyroid gland after radiation exposure, demonstrating its high sensitivity to radiation. This DNA damage includes single-strand or double-strand breaks that will result in chromosomal aberrations [5, 30]. The International Commission on Radiation Protection (ICRP) and the National Council on Radiation Protection and Measurements (NCRP) have set a yearly exposure limit and preventive advice against overexposure. On the other hand, medical personnel take thyroid protection shields lightly and do not take them seriously, according to our observations in everyday practice. Staff not wearing thyroid shield are currently being exposed to ionizing radiation on a regular basis and the thyroid gland is more vulnerable to harmful effects of ionizing radiation [24]. The present study viewed that serum anti-TPO levels in radiation workers were significantly higher than in the control group. Meanwhile, serum Tg levels in radiation workers were considerably lower than in the control group. Suggesting autoimmune thyroid disease (AITD) induced by exposure to radioiodine or X-ray during work shifts.
A recent study by Albehairy A et al. [31] is constant with the present study, indicating that working personnel in the radiation field are positive for anti-TPO. In autoimmune thyroid disorders, thyroid autoantibodies such as a thyroid-stimulating antibody, anti-thyroglobulin antibody, and anti-thyroperoxidase antibody can be found. The latter is a sensitive method for detecting early subclinical autoimmune thyroid illnesses, monitoring immunotherapy response, and identifying autoimmune thyroid disease at-risk cases. The iodination of tyrosine residues in the thyroglobulin molecule is carried out by thyroid peroxidase. Anti-TPO antibodies are inductors of oxidative stress and mediate thyroid cell death in vitro [32–34].
The current study revealed that MDA and H2O2 levels increased substantially more in radiation workers than in the control group while TAC levels are decreased, reflecting that chronic low dose ionizing radiation can cause systemic oxidative stress. In addition, ionizing radiation exposure at work alters the redox status. These findings agree with previous reports [35, 36]. It has been demonstrated that ionizing radiation causes the immediate generation of ROS in eukaryotic cells via the radiolysis of water, which is an indirect consequence of radiation. This rapid increase in ROS causes oxidative stress damage to biological macromolecules such as lipids, proteins, and DNA. Radiation-induced ROS include O2•, hydrogen peroxide (H2O2), and the hydroxyl radical (OH•). Enzymatic and non-enzymatic detoxify ROS and protect cells from oxidative damage. The decrease in TAC could be attributed to the consumption of endogenous antioxidants because of free radical generation after radiation exposure [37]. In summary, professionals who work in a job that exposes them to radiation regularly should follow the recommendations of radiation protection, which include worker radiation safety education, dose monitoring of radiation, and the use of all protective shielding devices. Moreover, radiation exposure should be kept to a minimum (ALARA).
The limitation of this study is the different exposure times between participants from group I and group II which most likely affected the results of the study. Also this was a single-center study, further studies are needed.
Conclusion
Occupational ionizing radiation exposure impacts the thyroid hormone panel and increased the risk of autoimmune thyroid disease, even at low doses. Biological monitoring of thyroid hormones and anti-TPO levels detects early affection of the thyroid gland among radiation-exposed workers.
Acknowledgments
Not Applicable.
Abbreviations
- AITD
Autoimmune Thyroid Disease
- Anti-TPO
Anti-thyroperoxidase antibodies
- ELISA
Enzyme-Linked Immunoassay
- fT3
Free Triiodothyronine
- fT4
Free Thyroxine
- H2O2
Hydrogen Peroxide
- ICRP
International Commission on Radiation Protection
- IR
Ionizing Radiation
- MDA
Malondialdehyde
- MHZ
Megahertz
- NCRP
National Council on Radiation Protection and Measurements
- ROS
Reactive Oxygen Species
- TAC
Total Antioxidant Capacity
- Tg
Thyroglobulin
- TSH
Thyroid-stimulating hormone
Authors’ contributions
Study concept and design: Sanaa A. El-Benhawy; Sherien M. Mahdy,; Enayat I. Fahmy, Tarek M.Salem. Searching the literature; Galal H. Khedr, Alyaa S. Sarhan, Tarek M.Salem. Data collection: Sanaa A. El-Benhawy, Galal H. Khedr; Alyaa S. Sarhan; Yousef A. Yousef selim. Thyroid hormone tests: Sanaa A. El-Benhawy, Hany A. El-Khodary; Galal H. Khedr; Alyaa S. Sarhan; Nehal Abu -Samra. Ultrasound examinations: Yousef A. Yousef selim. Statistical analysis: Sanaa A. El-Benhawy, Mohamed H. Nafady; Manuscript revision: Sanaa A. El-Benhawy, Yousef A. Yousef selim; Galal H. Khedr; Alyaa S. Sarhan; Sherien M. Mahdy; Tarek M.Salem. Supervision: Sanaa A. El-Benhawy; Enayat I. Fahmy; Sherien M. Mahdy. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was obtained from this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are not publicly available but available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
After approval of The Ethical Committee of the Medical Research Institute, Alexandria University, Alexandria, Egypt, on the protocol of the present study, an informed consent was taken from every participant. The study was done according to The Code of Ethics of the World Medical Association (Declaration of Helsinki) for studies involving humans.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sanaa A. El-Benhawy, Email: sanaa.elbanhawi@alexu.edu.eg
Enayat I. Fahmy, Email: Enayat.Fahmy@yahoo.com
Sherien M. Mahdy, Email: sherry72m@yahoo.com
Galal H. Khedr, Email: Galal.khedr@must.edu.eg
Alyaa S. Sarhan, Email: alyaasoudy62@gmail.com
Mohamed H. Nafady, Email: Mohamed.nafady@must.edu.eg
Yousef A. Yousef Selim, Email: Yousef.Selim@must.edu.eg
Tarek M. Salem, Email: tareksalemalexuni@yahoo.com
Nehal Abu-Samra, Email: nehal_chemistry2005@yahoo.com.
Hany A. El Khadry, Email: dr.hanyahmed@alexu.edu.eg
References
- 1.Mavragani IV, Nikitaki Z, Kalospyros SA, Georgakilas AG. Ionizing radiation and complex DNA damage: from prediction to detection challenges and biological significance. Cancers. 2019;11(11):1789. doi: 10.3390/cancers11111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai G, Kumar A, Mahobiya P. The effect of radiation on the thyroid gland. Int J Biol Res. 2018;3:217–222. [Google Scholar]
- 3.Burgio E, Piscitelli P, Migliore L. Ionizing Radiation and Human Health: Reviewing Models of Exposure and Mechanisms of Cellular Damage. Int J Environ Res Public Health. 2018;15(9):1971. doi: 10.3390/ijerph15091971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desouky O, Ding N, Zhou G. Targeted and non-targeted effects of ionizing radiation. J Radiat Res Appl Sci. 2015;8:247–254. doi: 10.1016/j.jrras.2015.03.003. [DOI] [Google Scholar]
- 5.El-Benhawy SA, Sadek NA, Behery AK, Issa NM, Ali OK. Chromosomal aberrations and oxidative DNA adduct 8-hydroxy-2-deoxyguanosine as biomarkers of radiotoxicity in radiation workers. J Radiat Res and APP Sci. 2016;9:249–258. [Google Scholar]
- 6.El-Benhawy SA, El-Tahan RA, Nakhla SF. Exposure to radiation during work shifts and working at night act as occupational stressors Alter redox and inflammatory markers. Arch Med Res. 2021;52(1):76–83. doi: 10.1016/j.arcmed.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 7.El-Benhawy SA, Al Roba MK, Fahmy EI, Khalifa HF, Rabie MAF. Impact of occupational exposure to low dose ionizing radiation versus high dose exposure during radiotherapy on met Hb levels. Arab J Nucl Sci Appl. 2022;55(3):1–9. [Google Scholar]
- 8.Donya M, Radford M, ElGuindy A, Firmin D. Yacoub M H (2015) radiation in medicine: origins, risks and aspirations. Glob Cardiol Sci Pract. 2014;4:57. doi: 10.5339/gcsp.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinnott B, Ron E, Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010;31:756–773. doi: 10.1210/er.2010-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrangoiz R, Cordera F, Caba D, et al. Comprehensive review of thyroid embryology, anatomy, histology, and physiology for surgeons. Int J Otolaryngol Head and Neck Surg. 2018;7:160–188. doi: 10.4236/ijohns.2018.74019. [DOI] [Google Scholar]
- 11.Fiore M, Oliveri Conti G, Caltabiano R, et al. Role of emerging environmental risk factors in thyroid cancer: a brief review. Int J Environ Res Public Health. 2019;16:1186. doi: 10.3390/ijerph16071185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WJ, Preston DL, Cha ES, Ko S, Lim H. Thyroid cancer risks among medical radiation workers in South Korea, 1996-2015. Environ Heal A Glob Access Sci Source. 2019;18:1–10. doi: 10.1186/s12940-019-0460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitahara CM, Preston DL, Neta G, et al. Occupational radiation exposure and thyroid cancer incidence in a cohort of U.S. radiologic technologists, 1983–2013. Int J Cancer. 2018;143:2145–2149. doi: 10.1002/ijc.31270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing radiation as a source of oxidative stress—the protective role of melatonin and vitamin d. Int J Mol Sci. 2020;21:1–22. doi: 10.3390/ijms21165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbari A, Jelodar G, Nazifi S, Afsar T, Nasiri K. Oxidative stress as the underlying biomechanism of detrimental outcomes of ionizing and non-ionizing radiation on human health: antioxidant protective strategies. Zahedan J Res Med Sci. 2019;21(4).
- 16.Khattab MG, Sayed ZS, Altaf RA, et al. The prophylactic roles of dietary antioxidants for medical radiology workers: a mini-review. Nat Resour Hum Heal. 2022. 10.53365/nrfhh/146248.
- 17.Mancini A, Di Segni C, Raimondo S, et al. Thyroid hormones, oxidative stress, and inflammation. Mediat Inflamm. 2016;2016. [DOI] [PMC free article] [PubMed]
- 18.Çolak E, Özkan B. The association between thyroid parenchymal echogenicity and thyroid function in pediatric population. Iran J Pediatr. 2022;32(1):e116175. doi: 10.5812/ijp.116175. [DOI] [Google Scholar]
- 19.Çolak E, Özkan B, Genç S, Polat B. Ultrasonographic determination of thyroid volume in infants and children from Aegean region of Turkey and comparison with national and international references. J Pediatr Endocrinol Metab. 2021;34(4):457–464. doi: 10.1515/jpem-2020-0514. [DOI] [PubMed] [Google Scholar]
- 20.Albi E, Cataldi S, Lazzarini A, et al. Radiation and thyroid cancer. Int J Mol Sci. 2017;18:911. doi: 10.3390/ijms18050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari SM, Fallahi P, Antonelli A, Benvenga S. Environmental issues in thyroid diseases. Front Endocrinol. 2017;8:50. doi: 10.3389/fendo.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drozdovitch V. Radiation exposure to the thyroid after the Chernobyl accident. Front Endocrinol. 2021;11:569041. doi: 10.3389/fendo.2020.569041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali YF, Cucinotta FA, Ning-Ang L, Zhou G. Cancer risk of low dose ionizing radiation. Front Phys. 2020;8:234. doi: 10.3389/fphy.2020.00234. [DOI] [Google Scholar]
- 24.Alawneh K, Alshehabat M, Al-Ewaidat H, et al. Asymptomatic effect of occupational radiation exposure on thyroid gland hormones and thyroid gland ultrasonographic abnormalities. J Clin Med. 2018;7:72. doi: 10.3390/jcm7040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong YS, Cheng YY, Cheng TJ, et al. The relationship between occupational exposure to low-dose ionizing radiation and changes in thyroid hormones in hospital workers. Epidemiol. 2019;30:32–38. doi: 10.1097/EDE.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 26.Elzaki AAE, Osman H, Lawz O. Thyroid nodules development among radiographers. J Adv Med Res. 2012;2:79–89. [Google Scholar]
- 27.Chen TY, Hsu CC, Feng IJ, et al. Higher risk for thyroid diseases in physicians than in the general population: a Taiwan nationwide population-based secondary analysis study. Qjm. 2017;110:163–168. doi: 10.1093/qjmed/hcw140. [DOI] [PubMed] [Google Scholar]
- 28.Guo QS, Ruan P, Huang WX, Huang DZ, Qiu JC. Occupational radiation exposure and changes in thyroid hormones in a cohort of Chinese medical radiation workers. Biomed Environ Sci. 2021;34:282–289. doi: 10.3967/bes2021.037. [DOI] [PubMed] [Google Scholar]
- 29.Al-Mohammed HI, Sulieman A, Mayhoub FH, et al. Occupational exposure and radiobiological risk from thyroid radioiodine therapy in Saudi Arabia. Sci Rep. 2021;11:14557. doi: 10.1038/s41598-021-93342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglesias ML, Schmidt A, Ghuzlan AA, et al. Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab. 2017;61:180–187. doi: 10.1590/2359-3997000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albehairy A, Fathy S, Bahriz R. Thyroid peroxidase antibody (TPO) as a predictor of radiation induced thyroid dysfunction among nurses and technicians working in Mansoura specialized medical hospital: cross sectional study. Endocrine Metab Immune Disord - Drug Targets. 2019;20:288–294. doi: 10.2174/1871530319666190626143301. [DOI] [PubMed] [Google Scholar]
- 32.Kemp EH. Autoantibodies as diagnostic and predictive markers of vitiligo. Autoimmunity. 2004;37:287–290. doi: 10.1080/08916930410001710857. [DOI] [PubMed] [Google Scholar]
- 33.Cioffi DL, Fontana L, Leso V, Dolce P, Vitale R, Vetrani I, et al. Low dose ionizing radiation exposure and risk of thyroid functional alterations in healthcare workers. J European Journal of Radiology. 2020;132:109279. doi: 10.1016/j.ejrad.2020.109279. [DOI] [PubMed] [Google Scholar]
- 34.Luna-Sánchez S, Del Campo M, Morán JV, Fernández IM, Checa FJS, Rafael E. Thyroid function in health care workers exposed to ionizing radiation. J Health Physics. 2019;117(4):403–407. doi: 10.1097/HP.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 35.Celik H, Koyuncu I, Karakilcik AZ, Gonel A, Musa D. Effects of ionizing and non-ionizing radiation on oxidative stress and the Total antioxidant status in humans working in radiation environments. Bezmialem Sci. 2016;4:106–109. doi: 10.14235/bs.2016.872. [DOI] [Google Scholar]
- 36.Ahmad IM, Temme JB, Abdalla MY, Zimmerman MC. Redox status in workers occupationally exposed to long-term low levels of ionizing radiation: a pilot study. Redox Rep. 2016;21:139–145. doi: 10.1080/13510002.2015.1101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are not publicly available but available from the corresponding author on reasonable request.


