Abstract
Background
The uncontrolled production of MPO promotes inflammation, oxidative stress and atherosclerosis. Serum MPO levels are increased in patients with diabetes compared with patients without diabetes.
Objectives
This study aimed to investigate whether the serum levels and activities of MPO are related to coronary plaque progression in patients with type 2 diabetes mellitus (T2DM).
Material and methods
Serum MPO levels and activities were measured in 161 patients with diabetes with plaque progression (plaque progression group) and 87 patients with diabetes with no plaque progression (no plaque progression group). These patients were eligible based on the inclusion criteria and received quantitative coronary angiography at baseline and after approximately 1 year of follow-up. The characteristics and parameters of the participants at baseline were documented.
Results
Serum MPO levels and activities were significantly higher in plaque progression group than in no plaque progression group (P < 0.001). We categorized these patients with diabetes into MPO level or activity tertile subgroups. Significant differences in the plaque progression ratio and prominent changes in the minimal lumen diameter, stenosis diameter and coronary artery stenosis score were observed across the tertile subgroups of MPO levels and activities (all P < 0.01). Moreover, serum MPO levels and activities correlated significantly with these indices of coronary artery disease severity after adjustment for other risk factors. Multivariable regression analyses revealed that serum MPO levels and activities remained independently associated with plaque progression, in addition to smoking, hypertension and CRP levels (all P < 0.05).
Conclusions
Serum MPO levels and activities are significantly associated with coronary atherosclerotic plaque progression in patients with type 2 diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-022-02953-7.
Keywords: MPO, Type 2 diabetes mellitus, Plaque progression
Introduction
It is well known that foam cells rich in cholesterol esters are characteristic of atherosclerotic plaques. High-density lipoprotein (HDL) particle-mediated cholesterol efflux is a crucial step in reverse cholesterol transport, antiatherogenesis and vascular protection [1]. Sufficient evidence has revealed that impaired HDL function by pathological modification of this lipoprotein or attenuated cholesterol transport contributes to atherosclerosis and plaque progression [1]. Atherosclerotic plaques progress to high-risk lesions, such as thin fibrous cap atherosclerosis, resulting in an increased risk of sudden death, ischemic stroke and acute myocardial infarction.
Patients with diabetes mellitus have a higher risk of vascular complications and cardiovascular events. In addition, once cardiovascular disease occurs, diabetes mellitus will aggravate the disease pathology and worsen the prognosis [2]. Importantly, the rate of atherosclerotic plaque development is faster in patients with diabetes mellitus [3]. Previous studies have suggested that metabolic disorders in the diabetic milieu cause overproduction of reactive oxygen species (ROS). ROS, via mechanisms of endothelial dysfunction and inflammation, play a major role in promoting diabetic vascular disease [4].
Myeloperoxidase (MPO) belongs to the heme-containing peroxidase family, which is mainly produced by polymorphonuclear neutrophils. Uncontrolled MPO release exacerbates inflammation, oxidative stress and metabolic disorders, leading to atherogenesis. Thus, the increased level or activity of MPO is a marker as well as a mediator involved in the pathophysiology of atherosclerosis [5]. Previous studies have demonstrated that MPO exerts a potential influence on atherosclerotic plaque rupture [6, 7]. MPO can enhance high glucose-induced vascular injury in diabetes [6, 7]. Importantly, MPO-mediated oxidation targets apolipoprotein A-I of HDL, which results in impaired reverse cholesterol transport of HDL [8]. In addition, serum MPO levels are higher in patients with diabetes than in patients without diabetes [9, 10]. These data have jointly suggested a hypothesis that MPO is closely related to atherosclerotic plaque progression in patients with diabetes.
Furthermore, most studies investigated only the total MPO levels, but rarely the MPO activity at the same time in coronary heart disease patients with T2DM. Thus, in the present study, to test the above hypothesis, we evaluated not only serum MPO levels but also MPO activity in T2DM patients with angiographically documented coronary plaque progression and in those without.
Materials and methods
The research plan was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All participants included in the study signed informed consent forms.
Patients
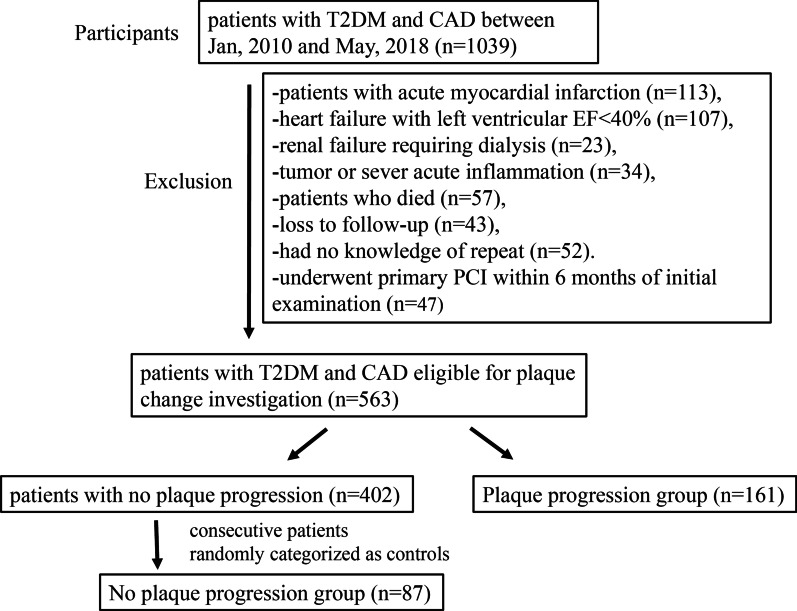
The participants were patients with type 2 diabetes mellitus (T2DM) recruited from Shanghai Ruijin Hospital. From January 2010 to May 2018, a total of 1039 consecutive T2DM patients with coronary heart disease received PCI based on drug-eluting stents (Fig. 1). Coronary angiography and PCI for symptomatic patients with stable CAD was performed according to the indication of 2011 AHA guideline [11]. These diabetic patients with CAD received regular medications including statins and aspirin as suggested by guideline [11]. Patients with severe heart failure with left ventricular ejection fraction < 40% (n = 107), acute myocardial infarction (n = 113), renal failure requiring dialysis (n = 23), and tumor or severe acute inflammation (n = 34) were excluded from this study. Patients who were lost to follow-up (n = 43), died during follow-up (n = 57), and had no knowledge of repeat angiography (n = 52) were also excluded from the analysis. Another 47 patients who underwent primary PCI within six months of initial examination were also excluded from the study. The remaining 563 patients constituted the potential study population. These patients had at least one stenosis (lumen diameter stenosis > 20%) on the baseline coronary angiography that were located in the non-PCI interventional vessels and received follow-up angiography approximately one year later.
Fig. 1.
Flow chart of patient enrollment
Biochemical assessment
Blood samples were collected after fasting overnight. Serum was separated and stored at − 80 °C before use. Serum lipid levels (triglycerides [TGs], total cholesterol [TC], high density lipoprotein cholesterol [HDL-C], low density lipoprotein cholesterol [LDL-C], apolipoprotein A, apolipoprotein B and lipoprotein [Lp](a)) and glucose levels were measured by a HITACHI 912 automatic biochemistry analyzer (Roche Diagnostics, Germany). We used enzyme-linked immunosorbent assay (ELISA) (Biocheck Laboratory, USA) to detect the levels of serum C-reactive protein (CRP). MPO levels were determined by time-resolved fluorescence lateral flow immunoassay (TRFIA), which adopts the principle of immunochromatography and double antibody sandwich (Eachy biopharma, China). MPO activities were analyzed using a colorimetric assay kit (K744-100, BioVision, USA).
Assessment of plaque progression
Coronary angiography and interventional therapy were performed by the standard Judkins technique or via the radial artery route [12]. Nitroglycerin (100 µg) was routinely injected into the coronary artery to avoid arterial spasm. In the initial and subsequent studies, coronary angiography recorded with the same projection was used for quantitative coronary artery analysis (QCA, Centricity Cardiology CA 1000. v1.0, USA). All images were analyzed by two well-experienced interventional specialists in heart diseases who knew nothing about the clinical data of the patients. The severity of coronary atherosclerosis was defined as single-vessel, double-vessel or triple-vessel disease according to the number of coronary vessels with severe stenosis (luminal stenosis > 50%).
For lesion analysis, end diastolic frames with the same angles were selected from the baseline and follow-up angiograms, which best showed the most severe stenosis, minimal anterior constriction, and branch overlap. The matching arterial segments were defined according to the images acquired from the anatomical positions of the proximal and distal branches. At follow-up, the segments with new lesions in non-PCI arteries and stenosis ≥ 20% at baseline, and all plaques with reference diameters ≥ 1.5 mm were analyzed. All coronary arteries involved in PCI were excluded to avoid including neointimal hyperplasia or restenosis after PCI. We measured the minimum lumen diameter (MLD) in diastole from multiple projections using the outer diameter of the contrast-filled catheter as a calibration. Finally, the results of a single worst view were recorded. The mean value was taken as the reference segment diameter from the 5 mm long, angiographically normal segment between the proximal and distal ends of the lesion but any major lateral branches. Atherosclerotic plaque progression was defined as: ≥ 1 lesion having a reduced MLD ≥ 0.4 mm (from baseline to follow-up angiography), which was approximately twice the SD of lesion repeated measurements [13]. The occurrence of a new coronary artery lesion was defined as no obvious stenosis on the initial angiography or stenosis diameter < 20%, but the reduction of MLD at follow-up angiography was ≥ 0.4 mm [14]. The coronary artery stenosis score (CCSS) was calculated based on the mean MLD of all measured segments in the coronary artery observed in each patient. Cumulative coronary artery occlusion was the sum of all diameter stenosis percentages expressed in standard index units (SI 50% = 0.50) [15]. Baseline QCA measurements minus follow-up measurements were defined as changes in QCA measurements.
Among 563 T2DM patients eligible for plaque progression analysis, 161 patients had plaque progression (plaque progression group). The other 402 patients had no plaque progression. Among these patients with no plaque progression, 87 consecutive patients were randomly referred to as the control (no plaque progression group).
Statistical analyses
All statistical analyses were performed using SPSS for Windows 23.0 (SPSS Inc., Chicago, IL, USA). A 2-tailed P value < 0.05 was considered statistically significant. The Kolmogorov–Smirnov test was used for normal distribution evaluation. If the data were normally distributed, continuous variables were expressed as the mean ± standard deviation (SD); otherwise, they were expressed as the median (25th–75th percentile). Categorical variables are displayed as frequencies (percentages), and the differences between groups were compared by the chi-square test. When statistical calculation of normal distribution was needed, logarithmic transformation was performed for continuous variables of nonnormal distribution. When appropriate, unpaired t test, ANOVA, or nonparametric Mann–Whitney U test were used for intergroup comparisons. Pearson correlation was used to test the relationships between continuous variables, such as MPO level and activity and changes in QCA measurements. Spearman’s correlations were used to evaluate the relationships between continuous variables (such as MPO level and activity) and changes in MLD, stenosis diameter and CCSS. We used multivariable logistic regression models to estimate the independent risk factors for plaque progression.
Results
Clinical characteristics
All information for patients with plaque progression (plaque progression group) and those without plaque progression (no plaque progression group) are detailed in Table 1. In 563 patients with diabetes, coronary artery plaque progression occurred in 161 (28.6%). The plaque progression group had a significantly higher ratio of cigarette smoking and hypertension and increased CRP levels than the no progression group (all P < 0.01), but this was not observed for the duration and other parameters (Table 1). As expected, the changes in CCSS, MLD and stenosis diameter were significantly different between these two groups (all P < 0.001).
Table 1.
Baseline characteristics and parameters in type 2 diabetic patients with plaque progression and those with no plaque progression
| No plaque progression (n = 87) | Plaque progression (n = 161) | P value | |
|---|---|---|---|
| Male, n (%) | 58 (66.7) | 114 (70.8) | 0.500 |
| Age, years | 65.71 ± 8.38 | 66.60 ± 9.77 | 0.476 |
| Body mass index, kg/m2 | 24.63 ± 2.95 | 25.24 ± 3.31 | 0.154 |
| Smoking, n (%) | 17 (19.5) | 65 (40.4) | 0.001 |
| Hypertension, n (%) | 54 (62.1) | 128 (79.5) | 0.003 |
| Dyslipidemia, n (%) | 14 (16.1) | 34 (21.1) | 0.339 |
| Severity of CHD, n (%) | 0.217 | ||
| 1-Vessel | 25 (28.7) | 38 (23.6) | |
| 2-Vessel | 32 (36.8) | 49 (30.4) | |
| 3-Vessel | 30 (34.5) | 74 (46.0) | |
| Systolic blood pressure, mm Hg | 138.74 ± 20.17 | 138.84 ± 19.85 | 0.967 |
| Diastolic blood pressure, mm Hg | 74.91 ± 16.37 | 75.42 ± 10.75 | 0.766 |
| Fasting blood glucose, mmol/L | 7.12 ± 1.80 | 7.04 ± 2.29 | 0.780 |
| HbA1c, % | 7.27 ± 1.17 | 7.43 ± 1.45 | 0.367 |
| Serum creatinine, μmol/L | 76.60 ± 19.79 | 84.14 ± 41.80 | 0.113 |
| Serum BUN, mmol/L | 6.96 ± 3.04 | 6.67 ± 3.53 | 0.509 |
| Serum uric acid, μmol/L | 334.90 ± 92.63 | 342.45 ± 91.04 | 0.536 |
| eGFR, mL/min/1.73m2 | 84.64 ± 15.69 | 80.25 ± 18.56 | 0.062 |
| Triglyceride, mmol/L | 1.69 ± 0.88 | 1.60 ± 0.98 | 0.465 |
| Total cholesterol, mmol/L | 3.84 ± 1.17 | 3.96 ± 1.18 | 0.455 |
| HDL cholesterol, mmol/L | 1.08 ± 0.28 | 1.06 ± 0.27 | 0.581 |
| LDL cholesterol, mmol/L | 2.22 ± 0.89 | 2.37 ± 0.95 | 0.216 |
| Apolipoprotein A, g/L | 1.25 ± 0.23 | 1.20 ± 0.23 | 0.095 |
| Apolipoprotein B, g/L | 0.74 ± 0.22 | 0.79 ± 0.23 | 0.109 |
| Lipoprotein (a), g/L | 0.22 ± 0.24 | 0.25 ± 0.26 | 0.458 |
| CRP, mg/mL | 0.63 (0.28–2.43) | 1.14 (0.49–4.08) | 0.001 |
| Medication, n (%) | |||
| ACE inhibitors/ARBs | 55 (63.2) | 112 (69.6) | 0.309 |
| β-blockers | 43 (49.4) | 96 (59.6) | 0.122 |
| Statins | 80 (92.0) | 146 (90.7) | 0.985 |
| Antiplatelet | 82 (94.3) | 154 (95.7) | 0.758 |
| Metformin | 31 (35.6) | 54 (33.5) | 0.740 |
| Insulin | 19 (21.8) | 36 (22.4) | 0.925 |
| MPO level, ng/mL | 20.20 (19.20–27.00) | 33.30 (21.45–60.00) | < 0.001 |
| MPO activity, mU/mL | 6.94 ± 5.20 | 10.37 ± 7.26 | < 0.001 |
| Follow-up | |||
| Duration, months | 12.18 ± 1.29 | 12.32 ± 1.24 | 0.428 |
| Changes in MLD, mm | − 0.06 ± 0.09 | 0.67 ± 0.25 | < 0.001 |
| Changes in stenosis diameter (%) | 0.88 ± 3.29 | − 22.70 ± 9.86 | < 0.001 |
| Changes in CCSS | − 0.01 ± 0.08 | − 0.33 ± 0.15 | < 0.001 |
Values are given as mean ± standard deviation (SD), median (25th–75th percentile) or number (percentage)
Change of QCA measurement is defined as baseline QCA measurement minus follow-up measurement
CHD Coronary atherosclerotic heart disease, ACE Angiotensin converting enzyme, ARB Angiotensin receptor blocker, BUN Blood urea nitrogen, CRP C-reactive protein, eGFR Estimated glomerular filtration rate, HbA1c Glycosylated hemoglobin, HDL High-density lipoprotein, LDL Low-density lipoprotein, CCSS Cumulative coronary stenosis score, MLD Minimal lumen diameter, QCA Quantitative coronary analyses
MPO levels and activities
The plaque progression group had significantly higher MPO levels than the no plaque progression group [33.30 (21.45–60.00) ng/mL vs. 20.20 (19.20–27.00) ng/mL, P < 0.001]. MPO activities in the plaque progression group were also significantly higher than those in the no plaque progression group [10.37 ± 7.26 mU/mL vs. 6.94 ± 5.20 mU/mL, P < 0.001]. We further categorized the patients into tertile subgroups according to MPO levels and activities (Tables 2, 3). Baseline data of tertile subgroups are provided in Additional file 1: Table S1 and Additional file 2: Table S2. Notably, significant differences in plaque progression ratio, change in MLD, diameter stenosis and CCSS were observed across the tertile subgroups of MPO levels and activities (all P < 0.01) (Tables 2, 3). Moreover, serum MPO levels and activities correlated significantly with changes in MLD, diameter stenosis and CCSS before and after adjustment for parameters including age, sex, body mass index (BMI), history of hypertension, smoking, estimated glomerular filtration rate (eGFR), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), Log CRP levels, antiplatelet and statin use (all P < 0.001) (Table 4). MPO levels and activities for 1-vessel vs. 2-vessel vs. 3-vessel disease in patients with CHD (coronary atherosclerotic heart disease) are shown in Additional file 3: Table S3. There was a significant difference in MPO activity among the three groups (P < 0.05).
Table 2.
Plaque progression in different tertiles of MPO levels in patients with type 2 diabetes
| Tertiles of MPO levels | n | Follow-up duration, (months) | Plaque progression, n (%) | Changes in MLD, (mm) | Changes in stenosis diameter, (%) | Changes in CCSS |
|---|---|---|---|---|---|---|
| T1 | 84 | 12.18 ± 1.44 | 34 (42.0) | 0.17 ± 0.26 | − 4.37 ± 6.82 | − 0.11 ± 0.11 |
| T2 | 82 | 12.26 ± 1.26 | 54 (65.9) | 0.34 ± 0.32 | − 13.52 ± 12.97 | − 0.18 ± 0.15 |
| T3 | 82 | 12.38 ± 1.05 | 73 (85.9) | 0.74 ± 0.40 | − 25.44 ± 12.14 | − 0.38 ± 0.22 |
| P value | 0.591 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Values are given as mean ± standard deviation (SD) or number (percentage)
Change of QCA measurement is defined as baseline QCA measurement minus follow-up measurement
Tertiles of MPO levels: T1 ≤ 20.500 ng/mL; 20.500 < T2 ≤ 38.317 ng/ mL; T3 > 38.317 ng/mL
MLD Minimal lumen diameter, CCSS Cumulative coronary stenosis score, QCA Quantitative coronary analyses
Table 3.
Plaque progression in different tertiles of MPO activity in patients with type 2 diabetes
| Tertiles of MPO activity | n | Follow-up duration, (months) | Plaque progression, n (%) | Changes in MLD, (mm) | Changes in stenosis diameter, (%) | Changes in CCSS |
|---|---|---|---|---|---|---|
| T1 | 83 | 12.22 ± 1.08 | 43 (51.8) | 0.28 ± 0.40 | − 9.08 ± 13.23 | − 0.14 ± 0.19 |
| T2 | 82 | 12.16 ± 0.84 | 52 (63.4) | 0.40 ± 0.39 | − 13.69 ± 13.57 | − 0.21 ± 0.19 |
| T3 | 83 | 12.43 ± 1.69 | 66 (79.5) | 0.58 ± 0.37 | − 20.51 ± 12.65 | − 0.31 ± 0.20 |
| P value | 0.335 | 0.001 | < 0.001 | < 0.001 | < 0.001 |
Values are given as mean ± standard deviation (SD) or number (percentage)
Change of QCA measurement is defined as baseline QCA measurement minus follow-up measurement
Tertiles of MPO activity: T1 ≤ 6.0307 mU/mL; 6.0307 < T2 ≤ 9.8684 mU/mL; T3 > 9.8684 mU/mL
MLD Minimal lumen diameter, CCSS Cumulative coronary stenosis score, QCA Quantitative coronary analyses
Table 4.
Correlation of serum levels and activity of MPO with plaque progression in patients with type 2 diabetes
| Changes in MLD | Changes in stenosis diameter | Changes in CCSS | |
|---|---|---|---|
| Serum levels of MPO, ng/mL | |||
| Unadjusted r | 0.62 | − 0.62 | − 0.59 |
| Unadjusted P | < 0.001 | < 0.001 | < 0.001 |
| Adjusted r* | 0.53 | − 0.44 | − 0.56 |
| Adjusted P* | < 0.001 | < 0.001 | < 0.001 |
| Serum MPO activity, mU/mL | |||
| Unadjusted r | 0.38 | − 0.37 | − 0.38 |
| Unadjusted P | < 0.001 | < 0.001 | < 0.001 |
| Adjusted r* | 0.30 | − 0.31 | − 0.33 |
| Adjusted P* | < 0.001 | < 0.001 | < 0.001 |
Change of QCA measurement is defined as baseline QCA measurement minus follow-up measurement
CRP C-reactive protein, HDL High-density lipoprotein, LDL Low-density lipoprotein, CCSS Cumulative coronary stenosis score, MLD Minimal lumen diameter, QCA Quantitative coronary analyses
*Adjusted for gender, age, body mass index, history of hypertension, smoking, glycated hemoglobin A1c, estimated glomerular filtration rate, HDL cholesterol, LDL cholesterol, Log CRP, antiplatelet and statins use
Multivariable logistic regression analysis
Multivariable logistic regression analysis was performed to ascertain the risk factors for plaque progression in the patients with diabetes by including all the factors in Table 1. The results revealed that smoking, hypertension and CRP were independent determinants of plaque progression (Model 1). After inclusion of MPO levels and activities and adjustment for these variables (Models 2 and 3), MPO levels and activities remained independently associated with plaque progression (MPO levels in Model 2, OR for T2: 2.10, 95% CI 1.04–4.24, P < 0.05; OR for T3: 7.41, 95% CI 3.29–16.71, P < 0.001) (MPO activities in Model 3, OR for T2: 1.78, 95% CI 0.89–3.57, P = 0.101; OR for T3: 3.53, 95% CI 1.67–7.47, P < 0.01) (Table 5). Compared with Model 1, the addition of MPO levels significantly improved the C statistic by 0.06 in Model 2 [from 0.73 (95% CI 0.66–0.79) to 0.79 (95% CI 0.73–0.85)] and MPO activities by 0.02 in Model 3 [from 0.73 (95% CI 0.66–0.79) to 0.75 (95% CI 0.69–0.81)].
Table 5.
Multivariable logistic regression analysis for the risk of plaque progression in patients with type 2 diabetes
| Variables | Adjusted OR (95%CI) | P value |
|---|---|---|
| Model 1 | ||
| Male | 1.08 (0.55–2.12) | 0.835 |
| Age | 1.00 (0.96–1.03) | 0.884 |
| Body mass index | 1.07 (0.98–1.17) | 0.158 |
| Smoking | 2.48 (1.25–4.90) | 0.009 |
| Hypertension | 2.03 (1.09–3.79) | 0.026 |
| HbA1c, % | 1.09 (0.87–1.36) | 0.464 |
| eGFR, mL/min/1.73m2 | 0.98 (0.96–1.00) | 0.069 |
| HDL cholesterol, mmol/L | 0.75 (0.25–2.24) | 0.611 |
| LDL cholesterol, mmol/L | 1.16 (0.82–1.62) | 0.403 |
| Log-transferred CRP | 2.02 (1.21–3.36) | 0.007 |
| Antiplatelet | 0.78 (0.21–2.94) | 0.713 |
| Statins | 0.77 (0.27–2.18) | 0.618 |
| Model 2 | ||
| Male | 0.88 (0.42–1.85) | 0.738 |
| Age | 1.00 (0.97–1.04) | 0.930 |
| Body mass index | 1.05 (0.95–1.15) | 0.376 |
| Smoking | 2.64 (1.28–5.42) | 0.008 |
| Hypertension | 2.21 (1.13–4.32) | 0.020 |
| HbA1c, % | 1.06 (0.84–1.34) | 0.623 |
| eGFR, mL/min/1.73m2 | 0.98 (0.96–1.00) | 0.111 |
| HDL cholesterol, mmol/L | 0.78 (0.24–2.54) | 0.684 |
| LDL cholesterol, mmol/L | 1.18 (0.82–1.70) | 0.371 |
| Log-transferred CRP | 1.72 (1.00–2.94) | 0.049 |
| Antiplatelet | 0.64 (0.17–2.43) | 0.514 |
| Statins | 0.83 (0.27–2.51) | 0.739 |
| Tertiles of MPO level | < 0.001 | |
| T1 | 1 (reference) | / |
| T2 | 2.10 (1.04–4.24) | 0.038 |
| T3 | 7.41 (3.29–16.71) | < 0.001 |
| Model 3 | ||
| Male | 1.13 (0.57–2.27) | 0.722 |
| Age | 1.00 (0.96–1.04) | 0.890 |
| Body mass index | 1.05 (0.96–1.15) | 0.299 |
| Smoking | 2.34 (1.16–4.71) | 0.017 |
| Hypertension | 1.99 (1.05–3.79) | 0.036 |
| HbA1c, % | 1.07 (0.85–1.34) | 0.564 |
| eGFR, mL/min/1.73m2 | 0.98 (0.96–1.00) | 0.047 |
| HDL cholesterol, mmol/L | 0.74 (0.24–2.29) | 0.606 |
| LDL cholesterol, mmol/L | 1.21 (0.86–1.72) | 0.277 |
| Log-transferred CRP | 1.97 (1.16–3.32) | 0.012 |
| Antiplatelet | 0.62 (0.16–2.42) | 0.488 |
| Statins | 0.67 (0.23–1.97) | 0.470 |
| Tertiles of MPO activity | 0.004 | |
| T1 | 1 (reference) | / |
| T2 | 1.78 (0.89–3.57) | 0.101 |
| T3 | 3.53 (1.67–7.47) | 0.001 |
Tertiles of MPO levels: T1 ≤ 20.500 ng/mL; 20.500 < T2 ≤ 38.317 ng/mL; T3 > 38.317 ng/mL
Tertiles of MPO activity: T1 ≤ 6.0307 mU/mL; 6.0307 < T2 ≤ 9.8684 mU/mL; T3 > 9.8684 mU/mL
CRP C-reactive protein, eGFR Estimated glomerular filtration rate, HbA1c Glycosylated hemoglobin, HDL High-density lipoprotein, LDL Low-density lipoprotein
Discussion
The uncontrolled release of MPO promotes inflammation, oxidative stress and cardiovascular diseases. Our study demonstrated that increased MPO levels and activities are associated with plaque progression. Serum MPO levels and activities were correlated with changes in MLD, stenosis diameter and CCSS before and after adjustment or conventional risk factors. Multivariable logistic regression analysis revealed that serum MPO levels and activities are independent determinants of plaque progression in patients with diabetes.
MPO is a heme-containing enzyme produced in neutrophils. It catalyzes reactions of which products and their secondary metabolites are responsible for killing bacteria and viruses. However, uncontrolled MPO release exaggerates inflammation, oxidative stress and metabolic disorders, leading to cardiovascular diseases and tissue damage [5]. Previous studies have demonstrated that MPO exerts a potential influence on many cardiovascular diseases, including atherosclerotic plaque rupture, diabetic vasculopathy, myocardial ischemia/reperfusion injury, hypertension, heart failure, pulmonary arterial hypertension, stroke, cardiac arrhythmia, venous thrombosis [6, 7, 16], and chronic kidney disease [17]. Some studies have suggested that elevated MPO levels predict future risk of coronary artery disease in apparently healthy individuals, but some do not [18, 19]. Serum MPO level is closely associated with the biological activity of MPO [20]. Thus, MPO is a promoter, mediator and marker of cardiovascular diseases.
Moreover, serum levels of MPO are elevated in patients with diabetes compared with patients without diabetes [9]. Fasting plasma glucose levels are positively correlated with plasma MPO levels [21]. MPO strongly oxidizes apolipoprotein A-I of HDL in patients with diabetes, which results in rampant impairment of cholesterol transport [8, 9]. A previous study has showed that HDL function is significantly altered in patients with diabetes with peripheral atherosclerosis disease due to multiple modifications of this lipoprotein that are aggravated by diabetes. Thus, plasma glucose and MPO levels constitute indicators of HDL dysfunction and contribute to risk stratification in patients with diabetes [22].
In the present study, we found for the first time that serum MPO activities were significantly associated with angiographically documented coronary artery plaque progression in patients with diabetes. The serum MPO levels were consistent with those of a previous study [23] and the abovementioned findings. Thus, these data suggest that MPO is closely associated with atherosclerotic plaque progression in patients with diabetes, which is significant in the prognostic implication in diabetes.
Limitations
We acknowledge that there are limitations in this study. First, our study is a cross-sectional study, aiming to investigate the relationship between MPO and coronary atherosclerotic plaque progression but not causative links. Second, FMD-closely associated apoA-I has various modification forms in patients with diabetes, such as glycation. Thus, impairment of cholesterol transport could be a combined effect of different apoA-I modifications, which jointly exert influence on plaque development. MPO-mediated oxidation is only one aspect of influence on plaques. In our future studies, a prospective study regarding the relationships between atherosclerotic plaque progression and substrates of various apoA-I modifications, including MPO, will be investigated.
Conclusion
Serum MPO levels and activities are associated with coronary atherosclerotic plaque progression in T2DM patients, suggesting that MPO is a crucial mediator or marker of atherosclerotic plaque development.
Supplementary Information
Additional file 1. Table S1. Baseline characterize of patients with type 2 diabetes in different tertiles of MPO levels.
Additional file 2. Table S2. Baseline characterize of patients with type 2 diabetes in different tertiles of MPO activity.
Additional file 3. Table S3. MPO levels and activities for 1-vessel versus 2-vessel versus 3-vessel disease in patients with CHD.
Acknowledgements
None.
Author contributions
WP, LL and WH conceptualized and designed the study. QC, SC, YD and XW collected, organized, and drafted the information. QC and SC analyzed the data. LL wrote the manuscript. FD, RZ and WS performed manuscript revision. All the authors have read and approved the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 82070358, 81770430).
Data availability
The datasets analyzed in this study are available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
The study has been performed in accordance with the Declaration of Helsinki. The study protocol was approved by hospital Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All participants included in the study had signed informed consent.
Consent for publication
There are no details on individuals within the manuscript. Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiujing Chen and Shuai Chen contributed equally to this work.
Contributor Information
Lin Lu, Email: rjlulin1965@163.com.
Wenqi Pan, Email: drpanwenqi@163.com.
References
- 1.Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport: basic mechanisms and their roles in vascular health and disease. Circ Res. 2019;124:1505–1518. doi: 10.1161/CIRCRESAHA.119.312617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118:1771–1785. doi: 10.1161/CIRCRESAHA.115.306884. [DOI] [PubMed] [Google Scholar]
- 3.Insull W., Jr The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122:S3–S14. doi: 10.1016/j.amjmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical theraphy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AA, Alsahli MA, Rahmani AH. Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci. 2018;6:33. doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng N, Maghzal GJ, Talib J, Rashid I, Lau AK, Stocker R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017;22:51–73. doi: 10.1080/13510002.2016.1256119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian R, Ding Y, Peng YY, Lu N. Myeloperoxidase amplified high glucose-induced endothelial dysfunction in vasculature: role of NADPH oxidase and hypochlorous acid. Biochem Biophys Res Commun. 2017;484:572–578. doi: 10.1016/j.bbrc.2017.01.132. [DOI] [PubMed] [Google Scholar]
- 8.Hewing B, Parathath S, Barrett T, et al. Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:779–789. doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobbert P, Rauch U, Stratmann B, et al. High molecular weight adiponectin correlates positively with myeloperoxidase in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;82:179–184. doi: 10.1016/j.diabres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Rovira-Llopis S, Rocha M, Falcon R, et al. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid Redox Signal. 2013;19:1452–1458. doi: 10.1089/ars.2013.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 12.Pu LJ, Lu L, Shen WF, et al. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. 2007;71:1067–1073. doi: 10.1253/circj.71.1067. [DOI] [PubMed] [Google Scholar]
- 13.Waters D, Lesperance J, Craven TE, Hudon G, Gillam LD. Advantages and limitations of serial coronary arteriography for the assessment of progression and regression of coronary atherosclerosis. Implications for clinical trials. Circulation. 1993;87:38–47. [PubMed] [Google Scholar]
- 14.Berry C, L'Allier PL, Gregoire J, et al. Comparison of intravascular ultrasound and quantitative coronary angiography for the assessment of coronary artery disease progression. Circulation. 2007;115:1851–1857. doi: 10.1161/CIRCULATIONAHA.106.655654. [DOI] [PubMed] [Google Scholar]
- 15.Waters D, Higginson L, Gladstone P, et al. Effects of monotherapy with an HMG-CoA reductase inhibitor on the progression of coronary atherosclerosis as assessed by serial quantitative arteriography. The Canadian Coronary Atherosclerosis Intervention Trial. Circulation. 1994;89:959–968. doi: 10.1161/01.CIR.89.3.959. [DOI] [PubMed] [Google Scholar]
- 16.Ndrepepa G. Myeloperoxidase—a bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta. 2019;493:36–51. doi: 10.1016/j.cca.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Kisic B, Miric D, Dragojevic I, Rasic J, Popovic L. Role of myeloperoxidase in patients with chronic kidney disease. Oxid Med Cell Longev. 2016 doi: 10.1155/2016/1069743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meuwese MC, Stroes ES, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50(2):159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Hasanpour Z, Javanmard SH, Gharaaty M, Sadeghi M. Association between serum myeloperoxidase levels and coronary artery disease in patients without diabetes, hypertension, obesity, and hyperlipidemia. Adv Biomed Res. 2016;5:103. doi: 10.4103/2277-9175.183663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacchetti T, Simonetti O, Ricotti F, Offidani A, Ferretti G. Plasma oxidation status and antioxidant capacity in psoriatic children. Arch Dermatol Res. 2020;312:33–39. doi: 10.1007/s00403-019-01976-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Dong L, Wang Q, Xie X. The relationship between fasting plasma glucose and MPO in patients with acute coronary syndrome. BMC Cardiovasc Disord. 2015;15:93. doi: 10.1186/s12872-015-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanda GM, Toma L, Barbalata T, et al. Clusterin, paraoxonase 1, and myeloperoxidase alterations induce high-density lipoproteins dysfunction and contribute to peripheral artery disease; aggravation by type 2 diabetes mellitus. BioFactors. 2021 doi: 10.1002/biof.1800. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka Y, Shao M, Wolski K, et al. Myeloperoxidase levels predict accelerated progression of coronary atherosclerosis in diabetic patients: insights from intravascular ultrasound. Atherosclerosis. 2014;232:377–383. doi: 10.1016/j.atherosclerosis.2013.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Baseline characterize of patients with type 2 diabetes in different tertiles of MPO levels.
Additional file 2. Table S2. Baseline characterize of patients with type 2 diabetes in different tertiles of MPO activity.
Additional file 3. Table S3. MPO levels and activities for 1-vessel versus 2-vessel versus 3-vessel disease in patients with CHD.
Data Availability Statement
The datasets analyzed in this study are available from the corresponding authors upon reasonable request.