Abstract
Background/Aim: Early palliative care (EPC) intervention in patients with solid tumors can provide many benefits. However, studies on patients with hematological malignancies are limited, and there is no data on patients with lymphoma. We conducted a preliminary retrospective survey of palliative care (PC) intervention in patients with lymphoma to clarify the effect of EPC on overall survival (OS).
Patients and Methods: The first palliative care consultation (PC1) was retrospectively reviewed from medical records in Japan. Patients with lymphoma requiring inpatient PC at our institution from January 2012 to December 2018 were recruited. We conducted receiver operating characteristic (ROC) analysis; patients were divided into two groups (early and delayed), and the survival periods and palliative care team (PCT) referral details were compared.
Results: The analysis included 77 patients with lymphoma [median age, 71 (64-79)] years. The median period to PC1 from the initial diagnosis was 395 (180-1,086) days. ROC analysis revealed an optimal PC intervention timing of 140 days. OS was significantly longer in the early group than that in the delayed group. The most common counseling details for the PCT were symptom relief and palliative care transfer (36.8% and 35.2%, respectively).
Conclusion: This real-world evaluation of PC intervention for inpatients with lymphoma revealed that PC intervention was provided at approximately 13 months following initial diagnosis. EPC intervention from diagnosis to 140 days may improve OS in patients with lymphoma; however further large-scale studies are required to verify this finding.
Keywords: Palliative care, early intervention, lymphoma, overall survival
Reports from Europe and the United States have indicated that the median survival is significantly longer in patients with metastatic non-small cell lung cancer who receive early palliative care (EPC) intervention compared with patients without EPC intervention, demonstrating the effectiveness of EPC intervention in patients with lung cancer (1,2). EPC intervention is associated with various benefits, including improvements in symptoms, quality of life (QOL), mood and distress, patient satisfaction as well as benefits for caregivers (3-9). A 2015 study found that the 1-year survival rate of patients with cancer was significantly improved following EPC intervention compared with delayed intervention after 3 months (10). However, only 10 (4.8%) of 207 patients evaluated in this study were diagnosed with a hematologic malignancy (HM), which limits the accurate evaluation of the effects of EPC intervention on the survival of patients with HM.
Several arguments have been made concerning the complexity of palliative care (PC) intervention and the intervention timing in patients with HM (11-18). However, the optimal timing of palliative intervention has not been determined.
Regarding PC interventions in HMs, some studies have reported that they improved QOL and decreased psychological distress in patients with hematopoietic stem-cell transplants (19-21). Others report improved pain in patients with multiple myeloma (22) and improved QOL in patients with acute myeloid leukemia (23). However, there are no reports of patients with lymphoma. Here, we reviewed the medical records of patients with lymphoma at a single institution to elucidate the timing of PC counseling requests, consult details, and the effects of PC intervention on patient survival.
Patients and Methods
Subjects. We included inpatients with HMs who consulted the palliative care team (PCT) of our hospital between January 2012 and December 2018. Patients for whom the date of diagnosis of HM was unclear and patients with diseases other than lymphoma were excluded. All procedures in this study including human participants were performed in accordance with the ethical standards of our institution and of Japanese study groups, as well as the principles of the Declaration of Helsinki of 1964 and its later amendments. The present study was approved by the Institutional Review Board for Medical Research of the Fujita Health University School of Medicine and Aichi Gakuin University. (Approval Code of Institutional Review Board for Medical Research of the Fujita Health University School of Medicine: HM20-017).
Investigation. We collected the following demographic information from the patients’ electronic medical records: age, sex, height, weight, body surface area, body mass index, date of HM diagnosis, disease name, disease type, performance status, presence or absence of relapse, and presence or absence of a do-not-attempt-resuscitation (DNAR) order acquired at the first PCT consultation (PC1). The date of death, number of days to PC1 since the diagnosis date, number of consultations, consultation details, presence or absence of transfusion, final transfusion date, and date of final chemotherapy were retrospectively examined.
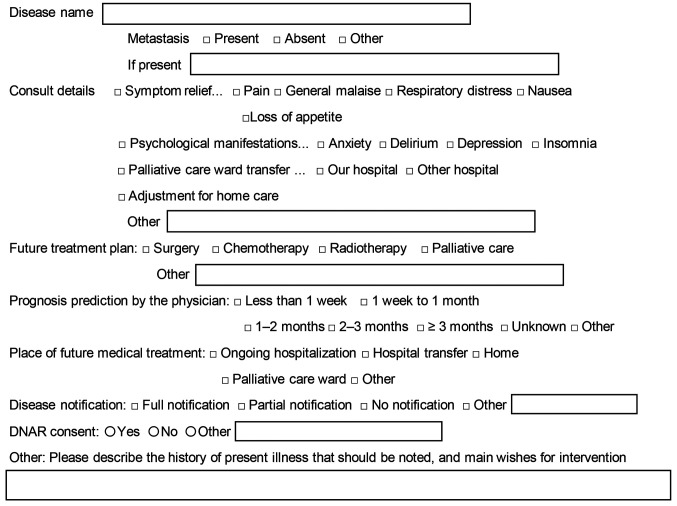
Palliative care intervention. To consult the PCT, the inpatient attending physician or ward staff, upon receiving instructions, created a PCT request form and contacted the PCT. Figure 1 shows the details of the request form. The PCT reviewed the request form. The team consisted of a PC physician, nurse, pharmacist, and clinical psychologist. The PCT followed patients longitudinally during their hospitalization and saw them at least twice per week.
Figure 1. Request form for the inpatient palliative care team. The attending physician submits consultation requests for the palliative care team via the electronic medical record using this form. Information provided includes the disease name, consultation details, future treatment plan, prognosis prediction by the physician, place of future medical treatment, disease notification, and do-not-attempt-resuscitation consent.
Evaluation methods. The reasons for patient referrals to the PCT were identified and the most common reason for referral was determined. Based on the receiver operating characteristic (ROC) analysis, subjects were divided into early and late intervention groups. The DNAR status at the time of the consultation, transfusion, date of final chemotherapy, and survival period following submission of the request form were compared between groups.
Analysis. Values indicating nonnormal distribution are shown as medians and interquartile ranges. Items with nonnormal distribution are expressed as medians (quartiles). The ROC analysis was used to calculate the cutoff for survival and PC interventions. Analyses were performed using unpaired tests to compare the two groups, including chi-squared or Mann-Whitney U-tests, with Cox’s proportional hazards model (multiple regression analysis) to calculate the hazard ratio to assess the survival period with a 95% confidence interval. We used R software (The R Foundation for Statistical Computing, Vienna, Austria; version 2.13.0) for all statistical analyses. A p-value of <0.05 was considered to indicate a statistically significant difference.
Results
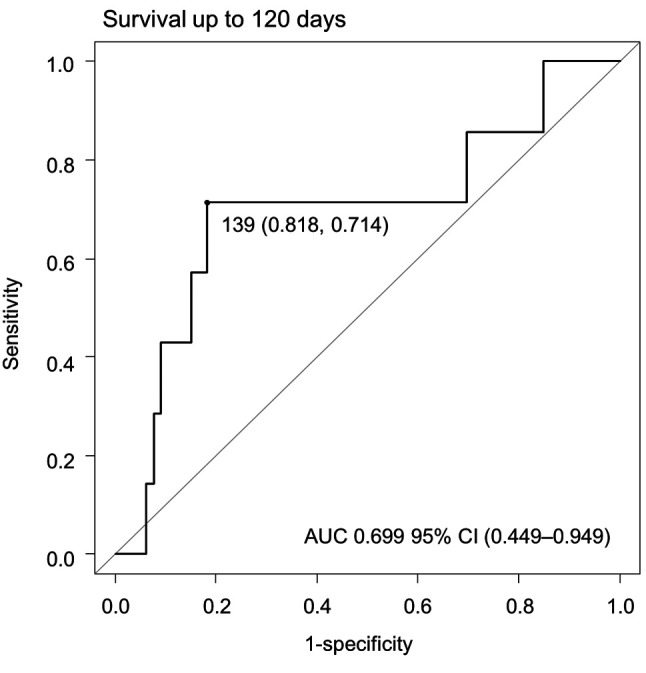
Determining palliative care intervention timing. We set the survival time at 120 days from the PC1 and analyzed the relationship between PC timing and survival using ROC analysis (Figure 2). The results were similar for 150 days and 180 days (data not shown). The ROC analysis revealed a cutoff value for the PC intervention period of 139 days from diagnosis. Based on these results, we determined that the optimal timing of palliative intervention was 140 days.
Figure 2. Receiver operating characteristic (ROC) curve analysis of time to consultation request submission for patients with lymphoma. For patients surviving 120 days after palliative care referral: cut-off, 139 days; sensitivity, 0.714; specificity, 0.818; AUC=0.699 (95%CI=0.449-0.949).
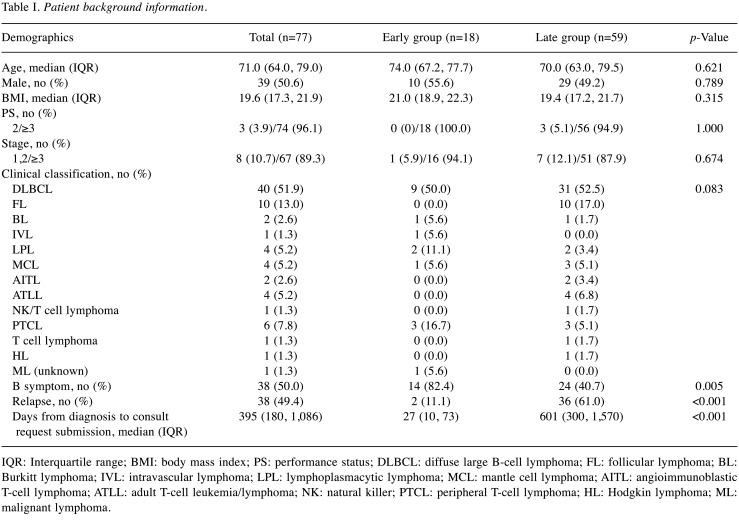
Patient background. Among 151 inpatients with HMs for whom the PCT was consulted during the study period, we excluded 10 patients with unknown dates of HM diagnosis and three patients with diseases other than leukemia, lymphoma, myeloma. We excluded 36 patients with leukemia and 25 with myeloma. Ultimately, 77 patients with lymphoma were included in the analysis. Table I shows the demographic and clinical characteristics of the 77 patients. The median period from the initial diagnosis date to PC1 was 395 (180-1,086) days; the median period was 27 (10-73) days in the early group and 601 (300-1,570) days in the delayed group, indicating a significantly shorter time to consultation in the early group. The early group consisted mostly of patients with B symptom and without relapse compared with the delayed group. Among the 38 patients with relapse, request forms were submitted prior to relapse for three patients, at the time of relapse for one patient, and after relapse for 34 (89.4%) patients. The median period from relapse to the day of consultation was 115 (71-314) days.
Table I. Patient background information.
IQR: Interquartile range; BMI: body mass index; PS: performance status; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; BL: Burkitt lymphoma; IVL: intravascular lymphoma; LPL: lymphoplasmacytic lymphoma; MCL: mantle cell lymphoma; AITL: angioimmunoblastic T-cell lymphoma; ATLL: adult T-cell leukemia/lymphoma; NK: natural killer; PTCL: peripheral T-cell lymphoma; HL: Hodgkin lymphoma; ML: malignant lymphoma.
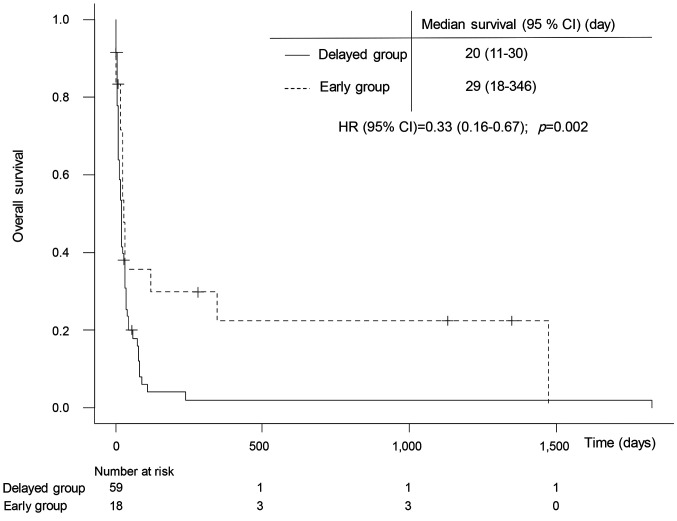
Intergroup comparison of the overall survival period. Figure 3 demonstrates the survival curve from consultation submission to death in patients with lymphoma. Comparison of the early and delayed groups revealed a longer survival period for the early group, as indicated by the dotted line.
Figure 3. Overall survival curve in delayed and early groups. The survival curve for patients with hematologic malignancies from consultation submission to death. The black dotted line represents the early group; the black line represents the delayed group. The adjusted hazard ratio was calculated using a Cox proportional hazards model (covariates: age, performance status, presence or absence of relapse, stage, and body mass index). AUC: Area under the curve; CI: confidence interval.
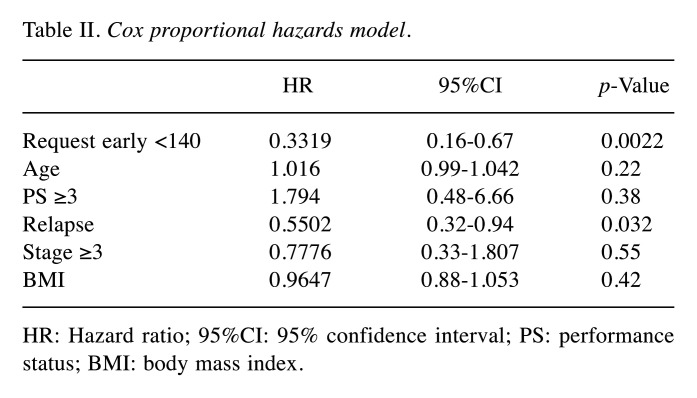
Multiple regression analysis of factors that contribute to survival between the two groups. We performed a multiple regression analysis using a Cox proportional hazards model adjusted for survival as an outcome variable and age, performance status, the presence or absence of relapse, clinical stage, and body mass index as covariates (Table II). A survival hazard ratio of 0.33 and early PC intervention from diagnosis to 140 days were found to significantly reduce mortality in patients with lymphoma.
Table II. Cox proportional hazards model.
HR: Hazard ratio; 95%CI: 95% confidence interval; PS: performance status; BMI: body mass index.
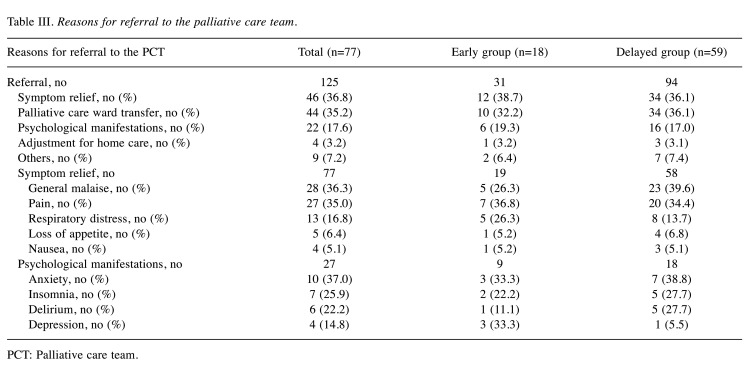
Reasons for referrals to the palliative care team. Table III lists the reasons for referrals to the PCT. Among the 77 patients, there were a total of 125 consultations (including overlapping responses). Among the reasons for referrals, physical symptom relief was most common in the early group, and physical symptom relief and transfer to a PC ward were the most common in the delayed group. Among the referrals for symptom relief, pain was the most common symptom in the early group and general malaise was the most common in the delayed group. Regarding the psychological manifestations, anxiety and depression were highest in the early group and the ratios of anxiety, insomnia, and delirium were similar in the delayed group.
Table III. Reasons for referral to the palliative care team.
PCT: Palliative care team.
DNAR consent acquisition. At the time of consultation with the PCT, DNAR consent had been obtained from 50 of 77 patients (64.9%). Consent had not been obtained from 14 patients (18.1%) and data were missing for 13 patients (16.8%). Intergroup comparison between the early and delayed groups revealed that DNAR consent had been obtained from 12 and 38 patients, but not obtained from five and nine patients, respectively. There was no statistically significant difference between the two groups (p=0.21).
Transfusion. Of 77 patients, 63 patients (81.8%) received a transfusion, and 14 patients (18.1%) did not. The consultation request for a PCT was made prior to or on the day of the final transfusion in 36 patients, which is equivalent to 57.1% of patients who underwent a transfusion. The median period from PC1 to the day of the final transfusion was 523 (190-1,121) days. A total of 27 (42.8%) patients requested a PCT consultation after their final transfusion. Among 63 patients who received transfusion, the median period from the final transfusion day to the last date of confirmed survival was 21 (7-76) days. Transfusion was performed in 13 (20.6%) and 50 patients (79.4%) in the early and delayed groups, respectively. The delayed group had a significantly higher proportion of patients who received transfusion (p<0.05).
Final chemotherapy. Data regarding the final day of chemotherapy administration was available for 70 of 77 patients (90.9%). A PCT consultation request was made on or before the final chemotherapy treatment for 19 patients (27.1%). Among these patients, the period between PC1 and the last chemotherapy treatment was ≤1 week in 10 patients (52.6%), and a total of nine patients (47.3%) received their final chemotherapy administration within one week of PCT consultation. The median period from PC1 to the final chemotherapy administration day was nine (2-109) days. Overall, 51 patients (72.8%) requested a PCT consultation after their final chemotherapy administration, and three-quarters of request forms were submitted after the final chemotherapy administration. In the between-group comparison, the percentages of requests submitted before the final chemotherapy administration were not significantly different between the early [6/14 (42.8%)] and delayed groups [13/56 (23.2%)] (p=0.353).
Discussion
The present study found, through a retrospective review of hospital records, that the median period from the initial diagnosis until the first inpatient consultation for PCT intervention was 395 (180-1,086) days in patients with lymphoma. In a comparison of the two groups using a 140-day cutoff from diagnosis to palliative intervention, the early group showed a significant prolongation of the survival curve compared with the delayed group. The most common reason for a PCT consultation was symptom alleviation at [46 of 125 (36.8%)], and the main symptoms were general malaise and pain. The second most common reason was to request transfer to the palliative care ward (35.2%), followed by psychological support (17.6%). We suggest that early intervention by PCT, including symptom relief, transfer to a palliative care ward, and psychological support, may lead to improved prognosis in patients with lymphoma.
Compared with the delayed group, the early group had a higher proportion of referrals for symptom relief. Pain was the most frequent among the symptoms, suggesting that early pain control interventions by a PCT may be important even in patients with lymphoma. In addition, the early group had high proportions of anxiety and depression, as well as pain control by PCT, indicating that early intervention of psychological manifestations may lead to prolonged OS. Compared with the early group, the delayed group had a higher proportion of referrals for palliative care ward transfer, general malaise, and delirium, suggesting worsened general condition. PCT referral in the early stage before the appearance of delirium may lead to improved OS.
Regarding HMs, administration of transfusion therapy and palliative chemotherapy may limit transfer to the palliative ward. In the study by Odejide et al. including a sample of hematologic oncologists, the majority strongly agreed that hospice care is helpful for patients with HMs. Otherwise, participants who considered home hospice to be inadequate were more likely to report an increase in the number of referrals if transfusions were readily available (24). Indeed, the American Society of Hematology released a statement recommending that hospice agencies and payers work collaboratively to ensure the availability of palliative transfusions to optimize end of life care for patients with HMs (25). Similarly, many end-stage patients with HMs in Japan may find it difficult to transfer to a palliative care ward because of the high cost of dependent blood transfusions. In our study, although there was no significant difference in the acquisition of DNAR or the presence or absence of chemotherapy, the delayed group had a significantly higher proportion of patients who received transfusion. Improving the use of blood transfusions in palliative care wards for HMs may lead to EPC interventions and prolonged patient survival.
While various randomized trials have demonstrated the benefits of providing oncology patients with PC support, the optimal timing for PC intervention has remained unclear. Thus, Bakitas et al. conducted a randomized trial examining the impact of early and delayed PC intervention on QOL, symptoms, mood, 1-year survival, and resource usage (10). They defined early PC intervention as 30-60 days from diagnosis and delayed intervention as later than 3 months from diagnosis, based on their previous findings from the ENABLE II trial. They set 3 months as the threshold for delayed intervention based on the feedback that patients experiencing enormous stress from symptoms benefitted from PC at that point. In the report by Bakitas et al. the intensity of symptoms had significantly increased by 3 months following diagnosis (26,27). In our study, the median period until PC intervention from initial diagnosis was 13.1 months. Based on the ROC analysis, we determined that the timing of palliative intervention was 140 days, which is still longer than that in the delayed group in the study by Bakitas et al. Notably, in the ENABLE II trial, the subjects presented with advanced solid tumors, and patients with HMs were not included. As with patients with solid tumors, EPC is thought to have a positive effect, although the timing should be considered separately for solid tumors and HMs owing to their specificity. In this study, we believe that determining the appropriate PC timings using ROC analysis in patients with HMs will be helpful for improving the overall survival.
This study has several limitations. First, submission of the request form to the PCT was defined as the initial PC intervention (PC1). The interventions performed by the PCT after the referral and their evaluation not surveyed. Second, treatment, procedures, and support corresponding to palliative care, thus far, have been performed by hematologists, and staff specializing in hematology who were included in the general medical care were not included. The PCT intervention was considered a specialist PC intervention. Third, this was a single-center retrospective study; therefore, interinstitutional differences such as the method of requests to PCT, criteria for PC ward transfer, and medical care that can be provided in the palliative care ward were not examined, making it difficult to exclude all biases. In this study, it was not possible to conduct a detailed examination of lymphoma types, treatment regimens, and the presence or absence of recurrence; therefore, further detailed studies including disease types and treatment regimens are required.
Conclusion
Real-world evaluation of PC intervention for inpatients with lymphoma revealed that PCT intervention was provided at approximately 13 months following initial diagnosis. EPC interventions up to 140 days after diagnosis may lead to pain control, relief of psychological stress, early transfer to an appropriate PC ward, and may improve prognosis in patients with lymphoma. This was a retrospective study that included a small number of cases at a single hospital; therefore, further detailed trials are required.
Conflicts of Interest
There are no conflicts of interest to declare regarding the publication of this study.
Authors’ Contributions
Conceptualization: Misaki Morisaku, Kaori Ito, Takenao Koseki. Data curation: Misaki Morisaku, Tatsuki Shimomura. Formal analysis: Kaori Ito, Takenao Koseki. Investigation: Misaki Morisaku, Maiko Mori, Tatsuki Shimomura, Shoko Maeda. Methodology: Kaori Ito, Takenao Koseki. Supervision: Seira Toyosato, Yosuke Ando, Masami Kawahara, Akihiro Tomita. Writing – original draft: Misaki Morisaku, Kaori Ito. Writing – review & editing: Kaori Ito, Masami Kawahara, Akihiro Tomita, Shigeki Yamada.
Acknowledgements
The Authors would like to thank Editage (https://www.editage.com/) for English editing services.
References
- 1.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 2.Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, Lennes IT, Dahlin CM, Pirl WF. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319–2326. doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, Moore M, Rydall A, Rodin G, Tannock I, Donner A, Lo C. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 4.El-Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, Jackson VA, Telles J, Rhodes A, Spitzer TR, McAfee S, Chen YA, Lee SS, Temel JS. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094–2103. doi: 10.1001/jama.2016.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grudzen CR, Richardson LD, Johnson PN, Hu M, Wang B, Ortiz JM, Kistler EA, Chen A, Morrison RS. Emergency department-initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol. 2016;2(5):591–598. doi: 10.1001/jamaoncol.2015.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, Back AL, Kamdar M, Jacobsen J, Chittenden EH, Rinaldi SP, Gallagher ER, Eusebio JR, Li Z, Muzikansky A, Ryan DP. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834–841. doi: 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, Hull JG, Li Z, Tosteson TD, Byock IR, Ahles TA. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dionne-Odom JN, Azuero A, Lyons KD, Hull JG, Tosteson T, Li Z, Li Z, Frost J, Dragnev KH, Akyar I, Hegel MT, Bakitas MA. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1446–1452. doi: 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald J, Swami N, Hannon B, Lo C, Pope A, Oza A, Leighl N, Krzyzanowska MK, Rodin G, Le LW, Zimmermann C. Impact of early palliative care on caregivers of patients with advanced cancer: cluster randomised trial. Ann Oncol. 2017;28(1):163–168. doi: 10.1093/annonc/mdw438. [DOI] [PubMed] [Google Scholar]
- 10.Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, Dionne-Odom JN, Frost J, Dragnev KH, Hegel MT, Azuero A, Ahles TA. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auret K, Bulsara C, Joske D. Australasian haematologist referral patterns to palliative care: lack of consensus on when and why. Intern Med J. 2003;33(12):566–571. doi: 10.1111/j.1445-5994.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 12.Newton S. Haematology and palliative care: blood, sweat and tears. Intern Med J. 2003;33(12):549–551. doi: 10.1111/j.1445-5994.2003.00499.x. [DOI] [PubMed] [Google Scholar]
- 13.Mander T. Haematology and palliative care: an account of shared care for a patient undergoing bone marrow transplantation for chronic myeloid leukaemia. Int J Nurs Pract. 1997;3(1):62–66. doi: 10.1111/j.1440-172x.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelly D, Ross S, Gray B, Smith P. Death, dying and emotional labour: problematic dimensions of the bone marrow transplant nursing role. J Adv Nurs. 2000;32(4):952–960. [PubMed] [Google Scholar]
- 15.Daugherty CK, Steensma DP. Overcoming obstacles to hospice care: an ethical examination of inertia and inaction. J Clin Oncol. 2003;21(9 Suppl):42s–45s. doi: 10.1200/JCO.2003.01.167. [DOI] [PubMed] [Google Scholar]
- 16.Selvaggi KJ, Vick JB, Jessell SA, Lister J, Abrahm JL, Bernacki R. Bridging the gap: a palliative care consultation service in a hematological malignancy-bone marrow transplant unit. J Community Support Oncol. 2014;12(2):50–55. doi: 10.12788/jcso.0015. [DOI] [PubMed] [Google Scholar]
- 17.Chung HM, Lyckholm LJ, Smith TJ. Palliative care in BMT. Bone Marrow Transplant. 2009;43(4):265–273. doi: 10.1038/bmt.2008.436. [DOI] [PubMed] [Google Scholar]
- 18.Manitta VJ, Philip JA, Cole-Sinclair MF. Palliative care and the hemato-oncological patient: can we live together? A review of the literature. J Palliat Med. 2010;13(8):1021–1025. doi: 10.1089/jpm.2009.0267. [DOI] [PubMed] [Google Scholar]
- 19.Loggers ET, LeBlanc TW, El-Jawahri A, Fihn J, Bumpus M, David J, Horak P, Lee SJ. Pretransplantation supportive and palliative care consultation for high-risk hematopoietic cell transplantation patients. Biol Blood Marrow Transplant. 2016;22(7):1299–1305. doi: 10.1016/j.bbmt.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 20.El-Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, Jackson VA, Telles J, Rhodes A, Spitzer TR, McAfee S, Chen YA, Lee SS, Temel JS. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094–2103. doi: 10.1001/jama.2016.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Jawahri A, Traeger L, Greer JA, VanDusen H, Fishman SR, LeBlanc TW, Pirl WF, Jackson VA, Telles J, Rhodes A, Li Z, Spitzer TR, McAfee S, Chen YA, Temel JS. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017;35(32):3714–3721. doi: 10.1200/JCO.2017.73.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porta-Sales J, Guerrero-Torrelles M, Moreno-Alonso D, Sarrà-Escarré J, Clapés-Puig V, Trelis-Navarro J, Sureda-Balarí A, Fernández De Sevilla-Ribosa A. Is early palliative care feasible in patients with multiple myeloma. J Pain Symptom Manage. 2017;54(5):692–700. doi: 10.1016/j.jpainsymman.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 23.El-Jawahri A, LeBlanc TW, Kavanaugh A, Webb JA, Jackson VA, Campbell TC, O’Connor N, Luger SM, Gafford E, Gustin J, Bhatnagar B, Walker AR, Fathi AT, Brunner AM, Hobbs GS, Nicholson S, Davis D, Addis H, Vaughn D, Horick N, Greer JA, Temel JS. Effectiveness of integrated palliative and oncology care for patients with acute myeloid leukemia: a randomized clinical trial. JAMA Oncol. 2021;7(2):238–245. doi: 10.1001/jamaoncol.2020.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odejide OO, Cronin AM, Earle CC, Tulsky JA, Abel GA. Why are patients with blood cancers more likely to die without hospice. Cancer. 2017;123(17):3377–3384. doi: 10.1002/cncr.30735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Society of Hematology ASH Statement in Support of Palliative Blood Transfusions in Hospice Setting. Washington, DC, 2019. Available at: https://www.hematology.org/advocacy/policy-news-statements-testimony-and-correspondence/policystatements/2019/palliative-blood-transfusions-in-hospice. [Last accessed on November 2, 2020]
- 26.Maloney C, Lyons KD, Li Z, Hegel M, Ahles TA, Bakitas M. Patient perspectives on participation in the ENABLE II randomized controlled trial of a concurrent oncology palliative care intervention: benefits and burdens. Palliat Med. 2013;27(4):375–383. doi: 10.1177/0269216312445188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakitas M, Lyons KD, Hegel MT, Balan S, Barnett KN, Brokaw FC, Byock IR, Hull JG, Li Z, McKinstry E, Seville JL, Ahles TA. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Support Care. 2009;7(1):75–86. doi: 10.1017/S1478951509000108. [DOI] [PMC free article] [PubMed] [Google Scholar]