Abstract
Background/Aim: The influence of surgical interventions and anaesthesiological procedures on tumour progression was investigated as early as the 1920s. In current cancer management, the perioperative phase is increasingly being considered a vulnerable period with an increased risk of tumour cell dissemination due to medication, surgical manipulation, and immunosuppression. The extent to which narcotics administered in the perioperative setting influence the oncological outcomes of patients with pancreatic cancer is still unclear.
Materials and Methods: To investigate the effect of propofol and etomidate on the proliferation, cell-cycle distribution, apoptosis, and necrosis of pancreatic tumour cells in vitro, PaTu 8988t and Panc-1 pancreatic cancer cells were treated with 0-1,000 μM propofol or etomidate for 24 h each. Cell proliferation was measured with enzyme-linked immunosorbent–bromodeoxyuridine assay. The apoptosis rate was analysed with annexin V staining and the cell-cycle distribution with flow cytometry.
Results: Propofol at 1,000 μM induced apoptosis and inhibited cell proliferation. The cell cycle showed an increased S-phase and reduced cells in the G1-phase. At 100 μM, propofol significantly inhibited proliferation of the pancreatic cancer cell line PaTu 8988t and reduced cells in the G2-phase in the cell cycle. Etomidate had no effects on cell-cycle distribution, proliferation, apoptosis, and necrosis at the concentrations used.
Conclusion: In this study, propofol was shown to have anticancer effects by induction of apoptosis and inhibition of cell proliferation, while etomidate did not affect pancreatic cancer cells. However, it is too early to make any recommendation for changes in clinical practice and further clinical studies are warranted to investigate the effect of anaesthetics on cancer progression.
Keywords: Propofol, etomidate, pancreatic cancer, cancer, proliferation, apoptosis, necrosis, cell-cycle distribution
The influence of surgical interventions and anaesthesiological procedures on tumour progression was investigated as early as the 1920s. In 1916, Gaylord and Simpson showed that repeated anaesthesia accelerates the growth of breast carcinoma (1). In 1977, a retrospective study by Fried et al. investigated the effect of anaesthetic gases on the long-term prognosis of patients with cancer (2). In current cancer management, the perioperative phase is assumed to play a key role in the progression of malignant tumours (3). The combination of surgical manipulation and perioperative impairment of the immune defence increases the risk of tumour cell dissemination, with negative consequences for the course of the disease (4).
During the perioperative phase, mechanical manipulation of tumour tissue and traumatisation of tumour vessels may result in the infiltration of tumour cells into the lymphatic and vascular system (5). The increased secretion of growth factors and the imbalance between pro-angiogenetic and anti-angiogenetic factors during wound healing may facilitate the progression of disseminated cells and micro-metastases (6). In addition, the increase in catecholamine level via the perioperative stress axis impedes the antitumour defence mechanisms of the immune system and reduces the number of natural killer cells, T-helper cells, and cytotoxic T-cells in the postoperative period (7). β-Adrenergic signals regulate multiple cellular processes in tumour tissue, thereby directly facilitating the proliferation, metastasis, and progression of the tumour (8). Overall, tumorigenesis is a multistep process, in which several mutations are responsible for the transformation from a normal cell into a highly aggressive tumour cell. Although the first mutations mostly cause only subtle changes in cell morphology, a carcinoma in situ develops in the further course of the disease. During further transformation, aggressive tumours develop, which are marked by destructive growth and later also by invasion and metastasis (9). This process also applies to pancreatic adenocarcinoma, one of the most aggressive types of malignant tumour in humans (10).
During the past two decades, increasing interest has been focused on the impact of anaesthesia on cancer progression and oncological outcome (11). A large number of preclinical and clinical studies examined whether a patient`s oncological outcome can be influenced by the choice of specific anaesthetic technique [reviewed in (12)]. While the effect of propofol was investigated in several tumour entities [reviewed in (13)], knowledge of the influence of etomidate on cancer cells is limited [reviewed in (14)]. For this reason, the aim of this study was to compare the effects of both these intravenous anaesthetic agents on two pancreatic cancer cell lines.
Materials and Methods
Cell lines. The human pancreatic cancer cell lines PaTu 8988t and PANC-1 were obtained from Professor Ellenrieder (Philipps University of Marburg, Germany). PaTu 8988t and PANC-1 cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Gallen, Switzerland) supplemented with 10% foetal calf serum (Sigma-Aldrich) and 5% Myco Zap (Lonza Verviers SPRL, Verviers, Belgium). Cells were cultured at 37˚C in humidified atmosphere with 5% CO2 and maintained in monolayer culture. Experiments were carried out with cells at ~70-80% confluence.
Reagents. Commercially available propofol was purchased from Sigma-Aldrich and etomidate from Piramal Critical Care (Hallbermoss, Germany). Final concentrations were obtained by diluting drugs in standard growth media. All solutions were prepared freshly prior to use.
Cell proliferation. Quantification of cell proliferation was based on the measurement of bromodeoxyuridine (BrdU) incorporation during DNA synthesis. The test was performed according to the manufacturer’s protocol (cell proliferation ELISA-BrdU; Roche Applied Science, Basel, Switzerland). In brief, cells were incubated with 0, 1, 10, 100 or 1,000 μM propofol or etomidate in a serum-free medium for 24 h. After incubation, cells were additionally treated with BrdU-labelling solution for 16 h. The culture medium was then removed, the cells were fixed, and DNA was denatured. Afterwards, the cells were incubated with anti-BrdU-peroxidase solution for 90 min and washed three times to remove antibody conjugates. Immune complexes were detected using TMB substrate for 15 min and quantified by measuring absorbance at 405 nm and 490 nm. All tests were performed in duplicates; eight wells per treatment group were used, and tests were repeated at least three times.
Cell cycle analysis. For flow cytometric analysis, cancer cells were incubated with 0, 1, 10, 100 or 1,000 μM propofol or etomidate in serum-free medium for 24 h. After incubation, detachment by standard trypsinisation and cell counting, the cells were fixed in 100% ethanol at room temperature for 30 min. The cells were then treated with 1 mg/ml RNase A. After incubation for 30 min, cells were stained with 100 μg/ml propidium iodide (Sigma-Aldrich) and analysed with flow cytometry using FACS Calibur (BD Bioscience, Haryana, India) and Cellquest Pro software (BD Bioscience); 104 cells were counted for each sample. All tests were performed in duplicate and repeated three times.
Apoptosis analysis. Apoptosis assays by annexin V staining were performed according to the manufacturer’s instructions (BD Pharming, Franklin Lakes, NJ, USA). In brief, PaTu 8988t and PANC-1 cells were incubated with 0, 1, 10, 100 or 1,000 μM propofol or etomidate in serum-free medium. Staurosporine was used for positive control and standard growth medium for negative control. After 24 h incubation, the supernatant was decanted from the cells to preserve floating cells. Adherent cells were rinsed with warm Dulbecco’s phosphate-buffered saline (Sigma-Aldrich) and harvested by standard trypsinisation. Afterwards, harvested and floating cells were mixed, washed and re-suspended in binding buffer at a final density of 106 cells/ml. Cell suspension containing 105 cells (100 μl) was re-suspended in 5 μl fluorescein isothiocyanate-conjugated annexin plus 5 μl propidium iodide, followed by 15 min incubation at room temperature in the dark. Cells were washed and re-suspended with 400 μl binding buffer. Finally, the cells were analysed by flow cytometry using FACS Calibur and Cellquest Pro software (BD Bioscience). All tests were performed in duplicate and repeated three times.
Statistical analysis. Data are presented as the mean±standard deviation. The non-parametric Mann-Whitney U-test was used for statistical evaluation of the data. Values of p<0.05 were considered significant. IBM SPSS Statistics (Version 26; IBM, Armonk, NY, USA) and Excel Version 2019 (Microsoft, Redmond, WA, USA) packages were employed for statistical analysis.
Results
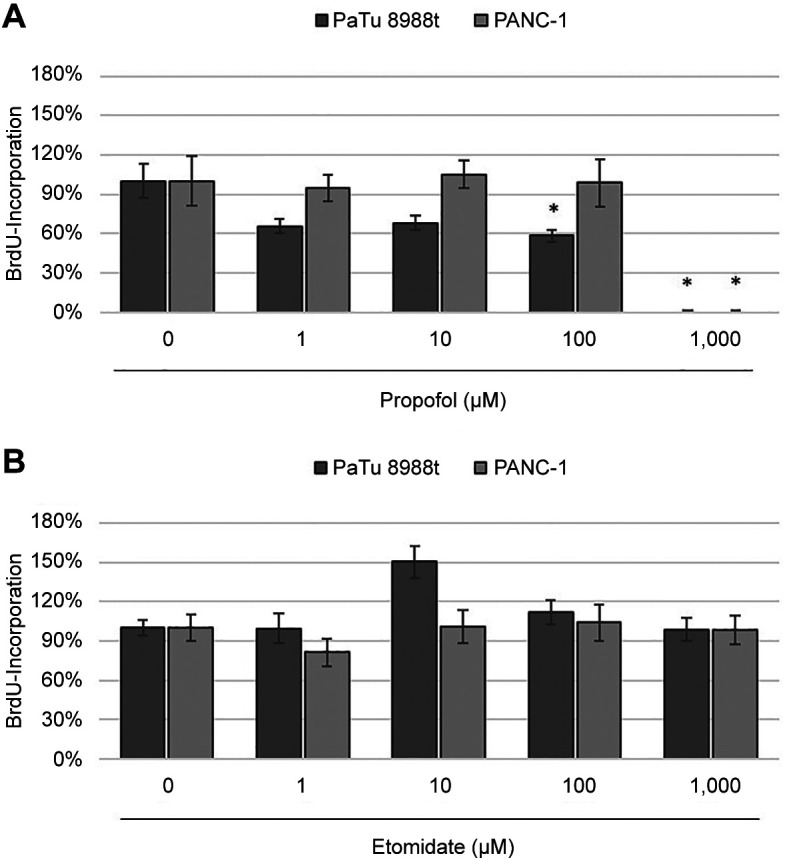
Proliferative behaviour of cells. PaTu 8988t and Panc-1 pancreatic cancer cells were stimulated with 0-1,000 μM propofol or etomidate in serum-free medium for 24 h each (Figure 1). At concentrations of 100 and 1,000 μM, propofol significantly inhibited cell proliferation in the PaTu 8988t pancreatic carcinoma cell line (Figure 1A) and 1,000 μM propofol significantly reduced cell proliferation in PANC-1 cells compared to the untreated control (Figure 1A). In contrast, etomidate did not significantly affect growth of either cell line (Figure 1B).
Figure 1. The effects of propofol (A) and etomidate (B) on the proliferation of the pancreatic carcinoma cell lines PaTu 8988t and PANC-1 in vitro. Cell proliferation was quantified by measuring bromodeoxyuridine (BrdU) incorporation. *Significantly different at p<0.05 compared to the untreated control.
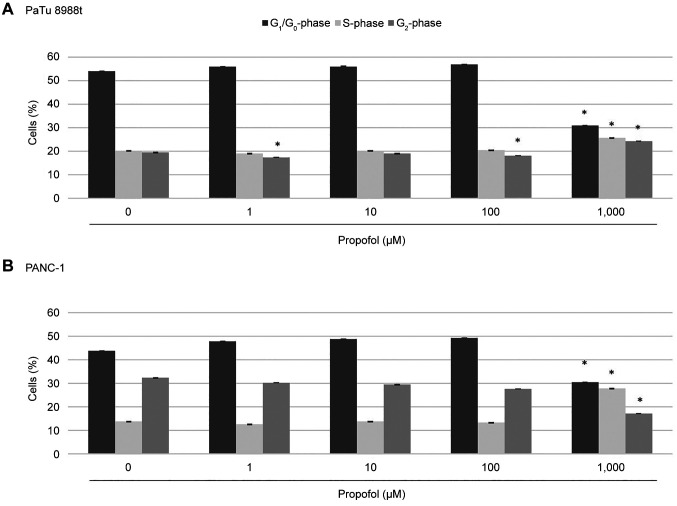
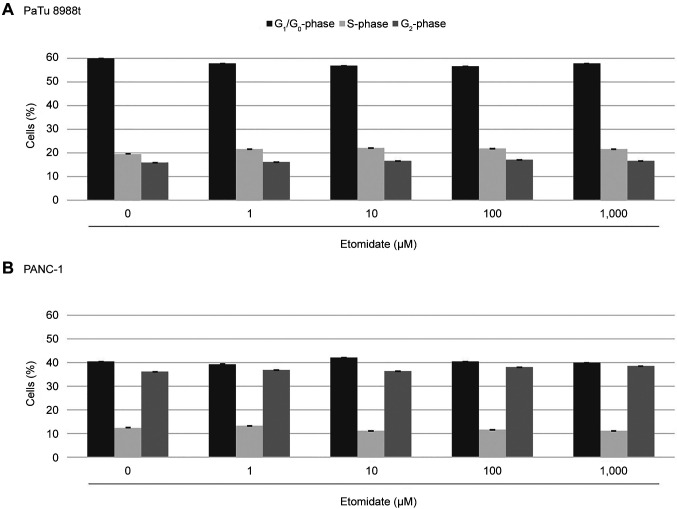
Effects of propofol and etomidate on the cell-cycle progression of pancreatic cancer cells. The aim of this study was to investigate the effects of propofol and etomidate on the cell-cycle behaviour of pancreatic cancer cells. Figure 2 shows a typical histogram after marking the cell nuclei with propidium iodide. At concentrations of 1 and 100 μM, propofol significantly changed the cell distribution in the PaTu 8988t pancreatic cancer cell line, and the fraction of cells in the G2-phase was reduced compared to the untreated control cells (Figure 3A). At 1,000 μM, propofol led to a significant increase in the S-phase, a significant decrease in the G1-phase and a change in the G2-phase in both the PaTu 8988t cell line and the PANC-1 cell line (Figure 3). Treatment with etomidate did not result in any changes in cell distribution, neither in the PaTu 8988t cell line nor in the PANC-1 cell line (Figure 4).
Figure 2. Typical histogram of the cell-cycle distribution after marking the cell nuclei with propidium iodide in standard growth medium.
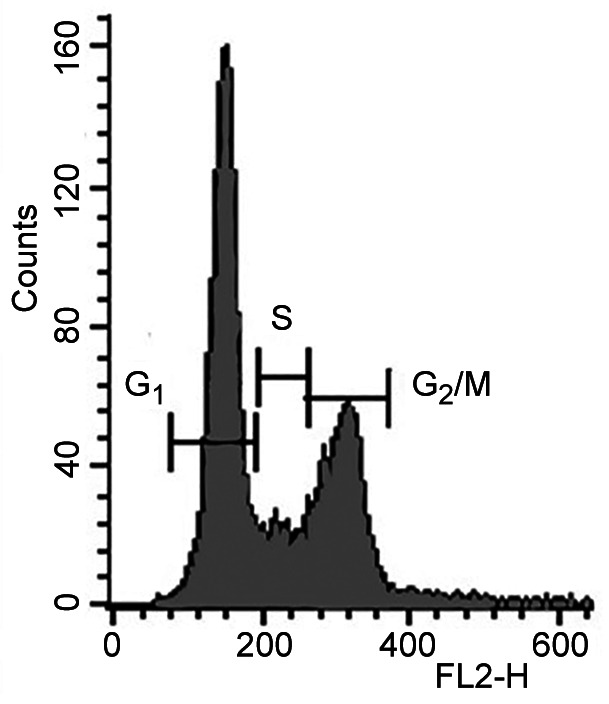
Figure 3. Cell-cycle distribution in PaTu 8988t (A) and PANC-1 (B) pancreatic cancer cell lines after treatment with 0 μM, 1 μM, 10 μM, 100 μM and 1,000 μM propofol for 24 h. Cell cycle was analysed by means of flow cytometry after staining with propidium iodide. *Significantly different at p<0.05 compared to the untreated control.
Figure 4. Cell-cycle distribution in PaTu 8988t (A) and PANC-1 (B) pancreatic cancer cell lines after treatment with etomidate for 24 h. The cellcycle distribution was analysed by means of flow cytometry after staining with propidium iodide.
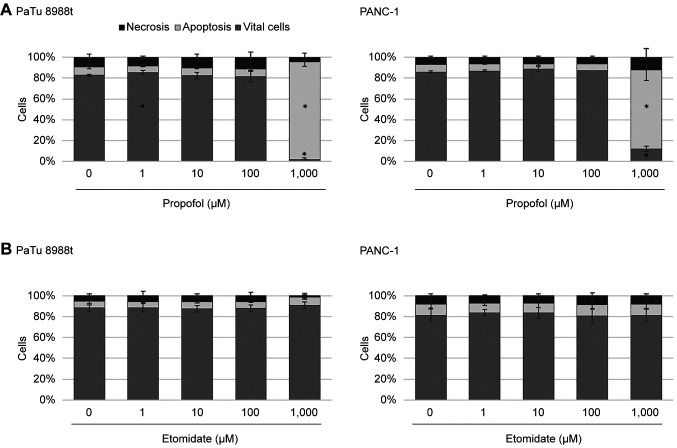
Analysis of apoptosis and necrosis. The annexin V-staining apoptosis assay was used to determine whether treatment with propofol or etomidate induced apoptosis or necrosis. Figure 5 shows typical dot plots after double staining with annexin V and propidium iodide. In the PaTu 8988t cell line, the number of vital cells was significantly increased after 24 h incubation with 1 μM propofol; 24 h incubation with 1,000 μM propofol (Figure 6A) induced significant apoptosis and reduced the vital cell fraction from 83% to 2% compared to the untreated control samples. In the PANC-1 cell line, 24 h incubation with 1,000 μM propofol significantly increased apoptosis and reduced the vital cell fraction compared to the untreated control samples (Figure 6A). Propofol at other concentrations did not result in any significant changes in the apoptosis rate. Etomidate did not induce any changes in cell death behaviour, neither in the PaTu 8988t cell line nor in the PANC-1 cell line (Figure 6B). Staurosporine, often employed for inducing apoptosis, was used as positive control for the testing procedure and induced significant apoptosis in pancreatic cancer cells (data not shown).
Figure 5. Typical dot plots after double staining with annexin V and propidium iodide in standard growth medium.
Figure 6. The effects of propofol (A) and etomidate (B) on apoptosis in the pancreatic carcinoma cell lines PaTu 8988t (left) and PANC-1 (right) in vitro. For analysis of apoptosis, cancer cells were stained with annexin V. *Significantly different at p<0.05 compared to the untreated control.
Discussion
To date, only a few studies have investigated the effect of narcotics on tumour cells, and some of them have yielded contradictory results.
Propofol (2,6-diisopropylphenol), a rapid short-acting intravenous general anaesthetic without any analgesic effect, acts as an allosteric modulator at pentameric ion channels such as gamma-aminobutyric acid (GABA)-A receptors and nicotinic acetylcholine receptors (15), is able to stimulate protein kinase C (16,17) and has been classified as antioxidant (18). Propofol also inhibits the entry of calcium into muscle cells (19) and increases myofilament calcium sensitivity in ventricular myocytes (20). Propofol is the most commonly used hypnotic drug worldwide and is non-toxic for humans, even at high concentrations (3-8 μg/ml; 20-50 μM) (21). Propofol has also been classified as direct vasodilator and bronchodilator with anti-inflammatory and anticonvulsant properties (22). Although the exact molecular mechanism of action for these different properties is unknown, propofol is thought to be able to modulate not only intracellular signalling pathways but also cellular processes that influence tumour dissemination (21).
In recent years, several animal studies have shown a positive effect of propofol on tumour progression (22-24). Mammoto et al. showed that clinically relevant concentrations of propofol reduced the invasion and metastatic potential of human cancer cells, including HeLa, HT1080, HOS and RPMI-7951 (23). In mice, continuous infusion of propofol inhibited lung metastasis of murine osteosarcoma cells by modulating Rho A (24). In human HL-60 promyelocytic leukaemia cells, propofol inhibited tumour growth, induced the formation of apoptotic bodies, increased DNA fragmentation and activated caspases 3, 6, 8 and 9. Moreover, cytosol enhanced the release of cytochrome c (25). The conclusion of these studies was that propofol induces apoptosis via a cell-surface death receptor (extrinsic), as well as through the mitochondrial intrinsic pathway. In a study by Kushida et al. in 2007, tumour growth in mice was significantly reduced after the administration of propofol in comparison to the administration of saline (24). Propofol inhibited the invasion of lung carcinoma cells by reducing the expression of matrix metalloproteinases 2, 7 and 9, and by increasing the expression of TIMP metallopeptidase inhibitors 1 and 2 (26). Propofol also inhibited cell invasion in breast and colon carcinoma in vitro (27,28).
On the other hand, Garib et al. found that the administration of propofol increased the potential of cell migration in breast carcinoma (29). Other studies showed it induced cell proliferation in gallbladder carcinoma (30) and neuroblastoma (31). In the present study, at 1,000 μM, propofol induced apoptosis, halted cell proliferation, and increased the S-phase whilst reducing the G1-phase in the cell cycle. At 100 μM, propofol also significantly inhibited proliferation and reduced the G2-phase in the cell cycle of the pancreatic cancer cell line PaTu 8988t. Thus, this study shows once again that propofol influences tumour spread, likely through modulation of intracellular signalling pathways and cellular processes.
Etomidate is known for its short-acting properties, as well as its low cardiovascular risk profile. In contrast to other hypnotics, etomidate causes only a relatively small drop in blood pressure and is therefore suitable for patients with impaired cardiac performance (32). It has a GABAA-mimetic effect by attenuating the reticular formation (33). Even a single dose of etomidate impairs adrenal cortex function, causing a drop in serum levels of both cortisol and aldosterone (34). Although etomidate is a common and widely used intravenous anaesthetic, studies on its effects on cancer cells are rare. Previous studies have shown that etomidate impeded migration and invasion of A549 lung adenocarcinoma cells by inhibiting the expression of matrix metalloproteinases 1, 2, 7 and 9 (35). Furthermore, etomidate exerted antiproliferative effects on adrenocortical carcinoma (36) and induced apoptosis in neuroblastoma (37). In contrast, Deng et al. showed that etomidate increased migration of colon carcinoma cells via the phosphatidylinositol-4,5-bisphosphate 3-kinase–AKT serine/threonine kinase 1 pathway (38).
In our study, the concentrations of etomidate used had no effects on cell-cycle distribution or proliferation. Moreover, etomidate did not induce apoptosis and necrosis, neither in the PaTu 8988t cell line nor in the PANC-1 cell line.
Conclusion
The perioperative phase is suggested to be a vulnerable phase for cancer cell dissemination and progression. For this reason, it is under discussion whether the choice of anaesthetic agents can affect a patient’s oncological outcome. In our study, propofol was shown to have anticancer effects by induction of apoptosis and inhibition of cell proliferation, while etomidate did not affect pancreatic cancer cells. However, it is too early to make any recommendation for changes in clinical practice and further clinical studies are warranted to investigate the effect of anaesthetics on cancer progression.
Conflicts of Interest
The Authors declare that they have no competing interests.
Authors’ Contributions
All Authors have made substantial contributions to the conception, design, analysis, and the interpretation of this research article; they were involved in the critical revision of the article with regard to important intellectual content. All Authors gave their final approval for the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
The Authors thank Sigrid Bamberger, Regina Lindner, Gabriele Bollwein, Marion Schindler, and Ruth Spaeth for technical assistance. We thank Monika Schoell for linguistic support.
References
- 1.Gaylord HR, Simpson BT. The effect of certain anesthetics and loss of blood upon the growth of transplanted mouse cancer. J Can Res. 1916;1:379–380. [Google Scholar]
- 2.Fried IA. The influence of the anaesthetic on survival rates of breast cancer patients after surgery. Int J Cancer. 1977;20(2):213–218. doi: 10.1002/ijc.2910200208. [DOI] [PubMed] [Google Scholar]
- 3.Lai HC, Kuo YW, Huang YH, Chan SM, Cheng KI, Wu ZF. Pancreatic cancer and microenvironments: implications of anesthesia. Cancers (Basel) 2022;14(11):2684. doi: 10.3390/cancers14112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–150. doi: 10.1016/j.bja.2019.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114. doi: 10.3233/bd-2007-26109. [DOI] [PubMed] [Google Scholar]
- 6.de Castro Junior G, Puglisi F, de Azambuja E, El Saghir NS, Awada A. Angiogenesis and cancer: A cross-talk between basic science and clinical trials (the “do ut des” paradigm) Crit Rev Oncol Hematol. 2006;59(1):40–50. doi: 10.1016/j.critrevonc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60(3):319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 11.Chang CY, Wu MY, Chien YJ, Su IM, Wang SC, Kao MC. Anesthesia and long-term oncological outcomes: a systematic review and meta-analysis. Anesth Analg. 2021;132(3):623–634. doi: 10.1213/ANE.0000000000005237. [DOI] [PubMed] [Google Scholar]
- 12.Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2017;124(5):1697–1708. doi: 10.1213/ANE.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Cheng CS, Lu Y, Ding X, Zhu M, Miao C, Chen J. Novel findings of anti-cancer property of propofol. Anticancer Agents Med Chem. 2018;18(2):156–165. doi: 10.2174/1871520617666170912120327. [DOI] [PubMed] [Google Scholar]
- 14.Buddeberg BS, Seeberger MD. Anesthesia and oncology: friend or foe. Front Oncol. 2022;12:802210. doi: 10.3389/fonc.2022.802210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahinovic MM, Struys MMRF, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57(12):1539–1558. doi: 10.1007/s40262-018-0672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmings HC Jr, Adamo AI. Effects of halothane and propofol on purified brain protein kinase C activation. Anesthesiology. 1994;81(1):147–155. doi: 10.1097/00000542-199407000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Lei Z, Zhao M, Hou Y, Wang D, Xu X, Lin X, Li J, Tang S, Yu J, Meng T. Propofol inhibits ischemia/reperfusion-induced cardiotoxicity through the protein kinase C/nuclear factor erythroid 2-related factor pathway. Front Pharmacol. 2021;12:655726. doi: 10.3389/fphar.2021.655726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zuo Y, Li B, Xie J, Ma Z, Thirupathi A, Yu P, Gao G, Shi M, Zhou C, Xu H, Chang Y, Shi Z. Propofol prevents oxidative stress and apoptosis by regulating iron homeostasis and targeting JAK/STAT3 signaling in SH-SY5Y cells. Brain Res Bull. 2019;153:191–201. doi: 10.1016/j.brainresbull.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Horibe M, Kondo I, Damron DS, Murray PA. Propofol attenuates capacitative calcium entry in pulmonary artery smooth muscle cells. Anesthesiology. 2001;95(3):681–688. doi: 10.1097/00000542-200109000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Kanaya N, Murray PA, Damron DS. Propofol increases myofilament Ca2+ sensitivity and intracellular pH via activation of Na+-H+ exchange in rat ventricular myocytes. Anesthesiology. 2001;94(6):1096–1104. doi: 10.1097/00000542-200106000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui RA, Zerouga M, Wu M, Castillo A, Harvey K, Zaloga GP, Stillwell W. Anticancer properties of propofol-docosahexaenoate and propofol-eicosapentaenoate on breast cancer cells. Breast Cancer Res. 2005;7(5):R645–R654. doi: 10.1186/bcr1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10(29):3639–3649. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 23.Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, Kishi Y, Nakamura H. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184(2):165–170. doi: 10.1016/s0304-3835(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 24.Kushida A, Inada T, Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol. 2007;29(3-4):477–486. doi: 10.1080/08923970701675085. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya M, Asada A, Arita K, Utsumi T, Yoshida T, Sato EF, Utsumi K, Inoue M. Induction and mechanism of apoptotic cell death by propofol in HL-60 cells. Acta Anaesthesiol Scand. 2002;46(9):1068–1074. doi: 10.1034/j.1399-6576.2002.460903.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu KC, Yang ST, Hsia TC, Yang JS, Chiou SM, Lu CC, Wu RS, Chung JG. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Res. 2012;32(11):4833–4842. [PubMed] [Google Scholar]
- 27.Li Q, Zhang L, Han Y, Jiang Z, Wang Q. Propofol reduces MMPs expression by inhibiting NF-ĸB activity in human MDA-MB-231 cells. Biomed Pharmacother. 2012;66(1):52–56. doi: 10.1016/j.biopha.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Wang H, Shi ZA, Li Y. Propofol prevents the growth, migration, invasion, and glycolysis of colorectal cancer cells by downregulating lactate dehydrogenase both in vitro and in vivo. J Oncol. 2022;2022:8317466. doi: 10.1155/2022/8317466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garib V, Niggemann B, Zänker KS, Brandt L, Kubens BS. Influence of non-volatile anesthetics on the migration behavior of the human breast cancer cell line MDA-MB-468. Acta Anaesthesiol Scand. 2002;46(7):836–844. doi: 10.1034/j.1399-6576.2002.460714.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Wang N, Zhou S, Ye W, Jing G, Zhang M. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. J Exp Clin Cancer Res. 2012;31:66. doi: 10.1186/1756-9966-31-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu GJ, Chen WF, Hung HC, Jean YH, Sung CS, Chakraborty C, Lee HP, Chen NF, Wen ZH. Effects of propofol on proliferation and anti-apoptosis of neuroblastoma SH-SY5Y cell line: new insights into neuroprotection. Brain Res. 2011;1384:42–50. doi: 10.1016/j.brainres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Paris A, Philipp M, Tonner PH, Steinfath M, Lohse M, Scholz J, Hein L. Activation of alpha 2B-adrenoceptors mediates the cardiovascular effects of etomidate. Anesthesiology. 2003;99(4):889–895. doi: 10.1097/00000542-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Campagna-Slater V, Weaver DF. Anaesthetic binding sites for etomidate and propofol on a GABAA receptor model. Neurosci Lett. 2007;418(1):28–33. doi: 10.1016/j.neulet.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 34.Allolio B, Dörr H, Stuttmann R, Knorr D, Engelhardt D, Winkelmann W. Effect of a single bolus of etomidate upon eight major corticosteroid hormones and plasma ACTH. Clin Endocrinol (Oxf) 1985;22(3):281–286. doi: 10.1111/j.1365-2265.1985.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 35.Chu CN, Wu KC, Chung WS, Zheng LC, Juan TK, Hsiao YT, Peng SF, Yang JL, Ma YS, Wu RS, Chung JG. Etomidate suppresses invasion and migration of human A549 lung adenocarcinoma cells. Anticancer Res. 2019;39(1):215–223. doi: 10.21873/anticanres.13100. [DOI] [PubMed] [Google Scholar]
- 36.Fassnacht M, Hahner S, Beuschlein F, Klink A, Reincke M, Allolio B. New mechanisms of adrenostatic compounds in a human adrenocortical cancer cell line. Eur J Clin Invest. 2000;30 Suppl 3:76–82. doi: 10.1046/j.1365-2362.2000.0300s3076.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen HT, Zhou J, Fan YL, Lei CL, Li BJ, Fan LX, Xu L, Xu M, Hu XQ, Yu ZY. Anesthetic agent etiomidate induces apoptosis in N2a brain tumor cell line. Mol Med Rep. 2018;18(3):3137–3142. doi: 10.3892/mmr.2018.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng F, Ouyang M, Wang X, Yao X, Chen Y, Tao T, Sun X, Xu L, Tang J, Zhao L. Differential role of intravenous anesthetics in colorectal cancer progression: implications for clinical application. Oncotarget. 2016;7(47):77087–77095. doi: 10.18632/oncotarget.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]