Abstract
Background/Aim: Prostate apoptosis response 4 (PAR4), a tumour-suppressor protein, selectively induces apoptosis of cancer cells without affecting normal cells. Its soluble form is induced by secretagogues (e.g., chloroquine), and it induces apoptosis by interacting with the receptor of glucose-regulated protein 78, which is overexpressed in cancer cells. In this study, curcumin was analyzed as an inducer of PAR4 expression in 4T1 murine breast cancer cell. and its ability to induce PAR4 secretion in Balb/c mice. In addition, the cisplatin sensitizing effect of soluble PAR4 was analyzed.
Material and Methods: The 4T1 cell line was treated in vitro using different concentrations of curcumin; cell viability was analyzed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and PAR4 expression by western blotting. The expression of soluble PAR4 in the serum of mice treated with intraperitoneal curcumin was analyzed using the dot-blot method. Moreover, MTT assay was used to analyze the effects of serum from curcumin-treated mice on cell viability. Tumor size was analyzed in mice treated with curcumin alone and in combination with cisplatin.
Results: Curcumin showed a dose- and time-dependent effects on cell viability on 4T1 cells, as well as increasing PAR4 expression. Compared with the control group (phosphate-buffered saline), mice treated with curcumin showed an increase in plasma PAR4. In the Balb/C tumor model, mice treated with curcumin and cisplatin showed greater tumor shrinkage than the control group.
Conclusion: These results indicate that curcumin induces expression of soluble PAR4 and sensitizes tumor cells to cisplatin.
Keywords: Curcumin, PAR4, cisplatin, breast cancer, triplenegative
Prostate apoptosis response 4 (PAR4) is a tumor-suppressor gene that induces apoptosis via extracellular and intracellular mechanisms in cancer cells without affecting normal cells (1,2). Its expression is decreased in some neoplasms, and it is associated with a poor prognosis (3,4). In breast cancer, metastasis, resistance, and recurrence require PAR4 dysregulation (5-7). Recently, PAR4 has been observed to be secreted spontaneously in normal cell culture and not in cancer cells by various agents that induce endoplasmic reticulum stress in both mice and humans; this increases cellular secretion of PAR4 via a P53-dependent mechanism (5,8,9). Chloroquine and hydroxychloroquine are antimalarial drugs that act as robust secretagogues of PAR4 (5). On the other hand, arylquinoline 1 disrupts the integration between PAR4-vimentin, increasing its secretion (9,10). Through the extracellular interaction with glucose-regulated protein 78 (GRP78), which is overexpressed on the surface of cancer cells and is correlated with malignancy, soluble PAR4 causes extrinsic apoptosis via FAS-associated death domain/caspase 8 and caspase 3 pathways in various tumor cell types (8,11,12). Furthermore, the overexpression of PAR4 has been observed to increase the translocation of GRP78 from the endoplasmic reticulum to the cell surface (13). Soluble PAR4 in the plasma of mice and patients with kidney cancer (treated with chloroquine) induced the inhibition of cell proliferation (5), and reduced tumor volume and inhibited metastasis in a murine model (14).
Curcumin, which is highly effective at inducing cell death in various types of cancer, is a polyphenolic yellow pigment derived from Curcuma longa (15). It can selectively chemosensitize tumor cells (16), thereby protecting normal cells from chemotherapy and radiotherapy (17). As GRP78 silencing promotes resistance to curcumin, it is possible that the GRP78 protein mediates the therapeutic effects of curcumin in hepatocellular carcinoma and colon cancer (18,19). On the other hand, curcumin has been reported to induce reactive oxygen species-dependent expression of PAR4 (20).
Therefore, this study aimed to determine whether curcumin can induce PAR4 secretion in vivo and affect viability and tumor mass volume in the 4T1 murine breast cancer model.
Materials and Methods
Breast cancer cell line culture. The murine breast cancer cell line 4T1 was cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 medium, which was supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic solution, at 37˚C in an atmosphere containing 5% CO2.
Viability testing using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT). To determine the cytotoxic effects of curcumin on the 4T1 cell line, 5×103 cells were incubated in a 96-well plate in Dulbecco’s modified Eagle’s medium/F12 with different concentrations (i.e., 0-50 μM) of curcumin (Sigma–Aldrich, Saint Louis, MO, USA) using dimethyl sulfoxide (DMSO) for solubilization. Treatments for 24 and 48 h were analyzed in triplicate. After incubation, 20 μl of MTT (5 mg/ml) was added to each well, and the plate was incubated for 1 h at 37˚C. Subsequently, the culture medium was removed, and 100 μl of DMSO was added to each well. Finally, the plate was measured using a plate reader at a wavelength of 570 nm (Microplate Autoreader EL311; BioTek Instruments Inc., Winooski, VA, USA).
PAR4 protein expression. To determine the effects of curcumin on PAR4 expression in 4T1 cells, 1×105 cells were cultured in 6-well plates, and 0, 10 and 60 μM of curcumin was added. Cells were harvested after 4 h and 24 h and lysed in 100 μl of lysis buffer (1% Triton, 150 mmol/l NaCl, 25 mmol/l Tris, pH 7.6), and the protein concentration was measured using a DC Protein Assay kit (Bio-Rad, Hercules, CA, USA). Proteins were separated using 12% polyacrylamide-sodium dodecyl sulfate gels and analyzed using western blotting with anti-PAR4 (sc-1666 HRP; Santa Cruz Biotechnology, Dallas, TX, USA). The samples were normalized using anti-β-actin (Sigma, St. Louis, MO, USA). A Western Lumi-Light sc-2048 Western blot system (Santa Cruz Biotechnology) was used for detecting protein.
In vivo secretion of soluble PAR4. Approximately 8-week-old BALB/c, mice were acquired from the Immunology and Virology Department, School of Biological Sciences, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Nuevo León, Mexico. Mice were administered curcumin (100 mg/kg) and chloroquine (50 mg/kg) as a positive control of secretagogue of PAR4, or with 1× phosphate-buffered saline (PBS) intraperitoneally, as a negative control. After 24 h, mice were anaesthetized using xylazine/ketamine (10 and 95 mg/kg) injections. Thereafter, whole blood was obtained using cardiac puncture, samples were centrifuged, and sera were inactivated at 56˚C for 30 min. Soluble PAR4 expression was determined using the immunodetection.
Immunodetection by dot-blot. Mouse serum (5 μl) was diluted in 200 μl of 1× PBS and was fixed on a nitrocellulose membrane by suction through Bio-Rad Bio-Dot 96-Well Microfiltration equipment. Proteins were detected using anti-PAR4 (sc-1666 HRP; Santa Cruz Biotechnology) and a Western Lumi-Light sc-2048 (Santa Cruz Biotechnology) transfer system was used for the detection of proteins.
Effect of curcumin on cell viability. To determine the effect of serum extracted from curcumin-treated mice on viability of 4T1 cells, 3,000 4T1 cells per well were cultured in a 96-well plate. Thereafter, they were incubated for 24 h with 10% and 20% (v/v) of serum in triplicate. Subsequently, the cell viability was assessed using MTT assay as described above.
Chemosensitization assay. A total of 3,000 cells per well were cultured in a 96-well plate and incubated with 10% (v/v) of mouse serum in combination with 10 ng/μl cisplatin (Pisa, Ciudad de México, México). After 24 h, cell viability was determined using MTT assay as described above.
In vivo assay. Groups of five 7-week-old female BALB/c mice acquired from the Immunology and Virology Department were used. They were maintained under standard conditions, with 12-hour light/dark cycles, as well as ad libitum access to water and food. Animal maintenance was approved and granted by the Animal Ethics and Management Committee of the Department of Immunology and Virology (CEIBA-2022-004).
In total, 5×105 4T1 cells were implanted in the right upper breast of each mouse. After 10 days, the measurement of the tumor mass began and administration of treatments with curcumin (100 mg/kg), chloroquine (50 mg/kg), cisplatin (70.5 μl), curcumin–cisplatin (100 mg/kg; 70.5 μl) and chloroquine–cisplatin (50 mg/kg; 70.5 μl) were performed intraperitoneally every 3 days for a period of 21 days.
Statistical analysis. The results were evaluated using one-way variance analysis and Tukey’s test, with p<0.05 being considered statistically significant.
Results
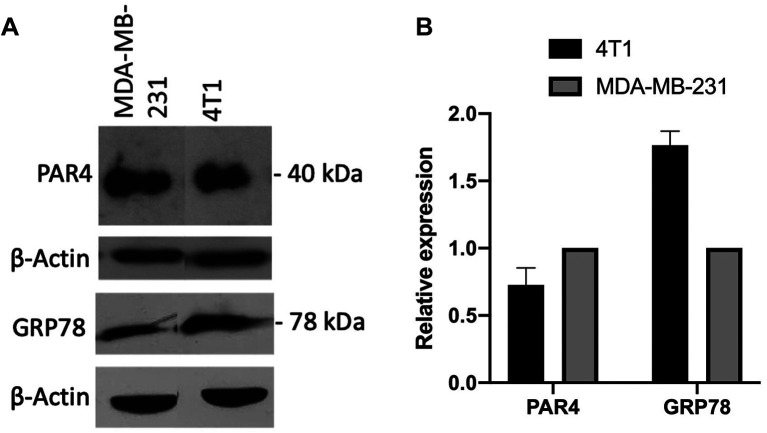
Basal expression of PAR4 and GRP78. Before initiating treatment with curcumin in the 4T1 cell line, the basal expression of PAR4 and GRP78 was determined (Figure 1a) using the MDA-MB-231 cell line as a control (21,22). Densitometric analysis revealed that compared with the MDA-MB-23 cell line, the relative expression of PAR4 in the 4T1 cell line was 0.58, whereas that of GRP78 was 1.64 (Figure 1b).
Figure 1. Basal expression of prostate apoptosis response 4 (PAR4) protein and glucose-regulated protein 78 (GRP78). A: Basal expression of PAR4 and GRP78 proteins in 4T1 and MDA-MB-231 cell lines were determined using western blot. B: Densitometric analysis of PAR4 and GRP78 expression.
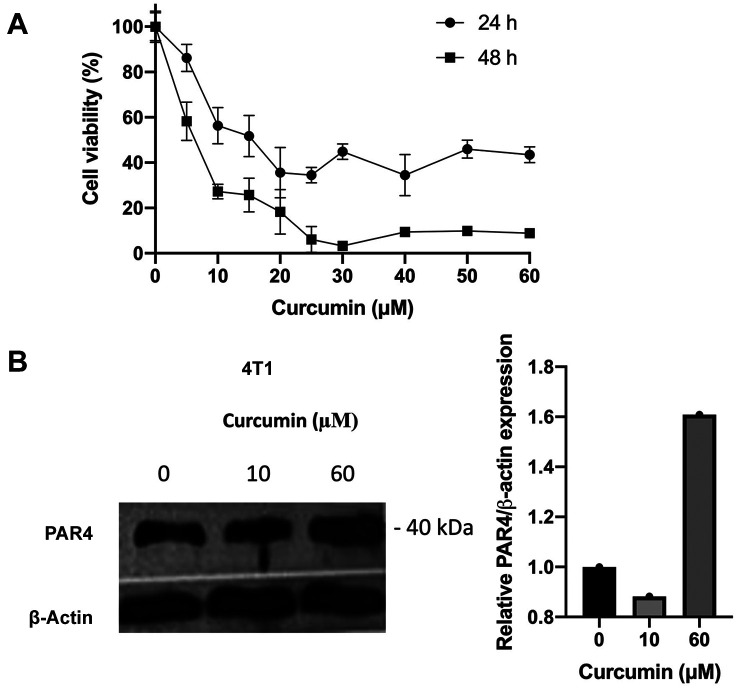
Curcumin increased PAR4 expression in 4T1 cells. Curcumin concentrations used for PAR4 analysis by western blot were determined based on cell viability assay. Cells were treated with different concentrations of curcumin (0 to 50 μM) for 24 and 48 h. Cell viability decreased depending on the dose and time (Figure 2A). Subsequently to analyze the effect of curcumin on PAR4 expression, cells were treated with curcumin at 10 and 60 μM for 24 h. The relative expression of PAR4 at 10 μM was 0.88, and its expression increased to 1.60 at 60 μM (Figure 2b).
Figure 2. Analysis of prostate apoptosis response 4 protein (PAR4) expression after curcumin treatment. A: Cell viability was analyzed after 24 and 48 h by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. B: Cells were treated with curcumin for 24 and 48 hours, and the analysis of PAR4 protein expression was conducted using western blot.
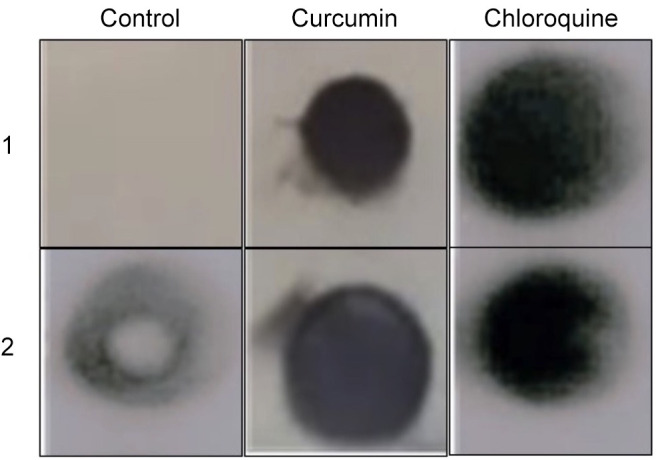
Curcumin increased PAR4 expression in mouse serum. To determine whether curcumin increases the concentration of PAR4 in mouse serum, 100 mg/kg was administered to mouse intraperitoneally; chloroquine (50 mg/kg) was used as a positive control as a PAR4 secretagogue, and PBS as a negative control. The analysis using dot-blotting revealed an increase in the PAR4 signal in the serum of mice treated with curcumin compared to the negative control. Similar results were observed for the serum from chloroquine-treated mice (Figure 3).
Figure 3. Expression of soluble prostate apoptosis response 4 protein in serum from mice treated with curcumin and chloroquine. Results from independent mice are indicated for each treatment (1 and 2).
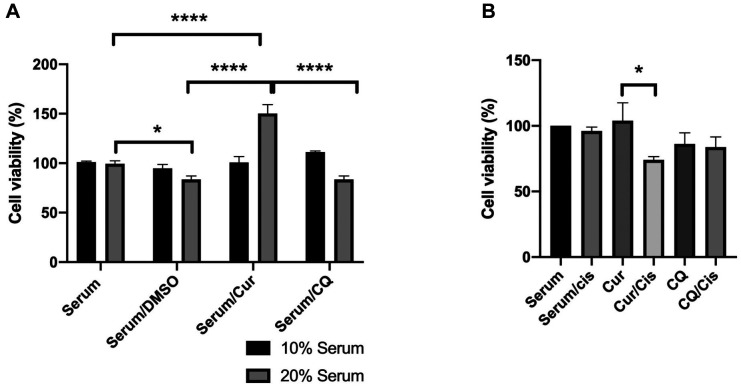
Serum from mice treated with curcumin sensitized 4T1 cells to cisplatin. To determine whether PAR4 in the serum of mice treated with curcumin affected cell viability and sensitization to cisplatin, 4T1 cells were treated with serum alone (10% and 20%) and in combination with cisplatin. No significant effects on cell viability were observed in cells treated with 10% serum from mice treated with curcumin, chloroquine, and DMSO, whereas in cells treated with 20% serum from DMSO-treated and chloroquine-treated mice, a slight decrease in viability was observed. However, in cells cultured with 20% serum, a 50.2% increase in cell proliferation compared with the control was observed (Figure 4a).
Figure 4. Analysis of viability of 4T1 cells exposed to serum from curcumin-treated mice A: Viability of cells treated with 10% and 20% serum from mice treated for 24 h with dimethyl sulfoxide (DMSO), curcumin (Cur) or chloroquine (CQ). B: Viability of cells incubated with 10% serum from mice and treated with/without cisplatin. Significantly different at *p<0.05 and ****p<0.0001.
For the analysis of the sensitization of 4T1 cells, culture was performed using 10% mouse serum, which showed no relevant effect on cell viability, in combination with 10 ng/μl cisplatin. No significant effect on cell viability was observed with sera from DMSO-treated and chloroquine-treated mice, whereas a 26% decrease in viability was observed in cells cultured with serum from curcumin-treated mice and treated with cisplatin compared without cisplatin (Figure 4b).
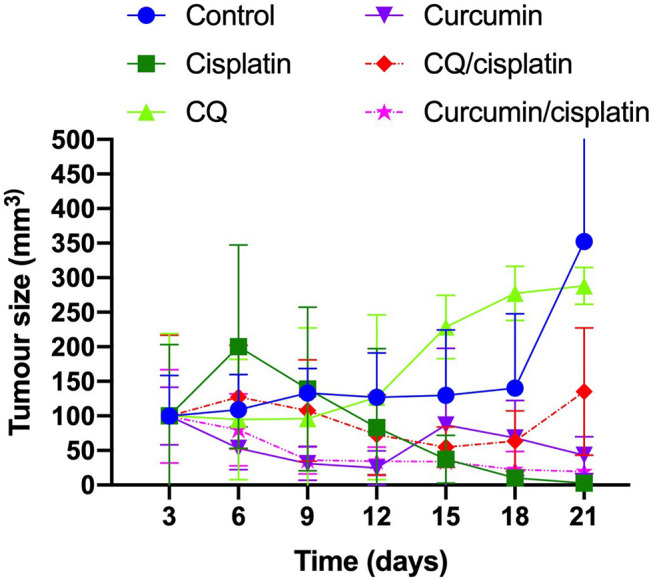
Curcumin/cisplatin reduced tumor mass in BALB/c mice. To determine whether curcumin in combination with cisplatin inhibits the growth of the 4T1 cell-derived tumors in BALB/c mice, treatment response was evaluated for 21 days. The tumor mass was significantly lower in mice treated with curcumin than in the controls (p<0.0001); however, a greater effect was observed in those treated with the curcumin–cisplatin combination than in those treated with curcumin alone, although there was no significant difference between the different treatments (Figure 5).
Figure 5. Treatment with curcumin and cisplatin in 4T1 tumor-bearing BALB/c mice. Tumor mass volume after treatment of BALB/c mice dimethyl sulfoxide (DMSO), curcumin or chloroquine (CQ).
Discussion
Currently, breast cancer is a serious public health concern, and the World Health Organization (23) reported that out of 19.3 million patients with cancer in 2020, 2.3 million had breast cancer, making it the most common type of cancer in women and the one with the highest mortality rate. Therefore, it is crucial to continue searching for novel medications and treatment approaches. The main causes of death in patients with breast cancer are drug resistance, recurrence, and metastasis (24-26). It has been observed that PAR4 is involved in these oncogenic mechanisms. PAR4 is reduced in patients in the late stages of the disease; this is considered a poor prognostic factor (3). PAR4 protein plays a fundamental role in the apoptosis of cancer cells but not in normal cells (27,28). It participates in cell apoptosis both intrinsically and extrinsically through caspase-dependent mechanisms, and it plays an important role in sensitizing cells to the action of various therapeutic agents. It is known that PAR4 at baseline expression levels is unable to induce massive apoptosis of cancer cells and generating stress in the ER is necessary for its induction and secretion (29). Rangnekar et al. have extensively studied different secretagogues for the induction of PAR4 secretion (5,9,29), including chloroquine an antimalarial drug that has been used as an inhibitor of the immune response in systemic lupus erythematosus (30,31) and against cancer (32-34). Thayyullathil et al. have reported reactive oxygen species-dependent activation of PAR4 in human malignant glioma cells (20). In this study, the effect of curcumin was analyzed as an endogenous PAR4 inducer and possible secretagogue. Before determining the effect of curcumin on the 4T1 cell line, analysis of the basal expression of PAR4 and GRP78 was performed; GRP78, an ER chaperone protein which is also found in the cell membrane, is preferentially overexpressed in aggressive, metastatic and chemoresistant cancers (13). Moreover, PAR4 interacts with GRP78 (both are expressed in the 4T1 cell line) and induces extrinsic apoptosis mediated by ER stress and activation of the FAS-associated death domain–caspase-8/caspase-3 pathway (8). Furthermore, the effect of curcumin on cell viability and PAR4 modulation was analyzed. A dose- and time-dependent decrease in cell viability was observed in the present study, as reported by Meiyanto et al. (35,36). Curcumin dose-dependently increased PAR4 expression, and this was associated with the inhibition of cell proliferation. Similar results were published by Thayyullathil et al., wherein they observed dose-dependent expression with maximal expression after 3 h (20).
Soluble PAR4 has been reported in conditioned media of mouse cells, as well as in sera of mice stimulated with different secretagogues, such as chloroquine, hydroxychloroquine and arilquin 1, and these can sensitize cancer cells (5,9,29,30). In this study, we analyzed whether curcumin stimulates secretion of soluble PAR4 in mouse serum, and an increase in PAR4 expression in the serum of mice treated with curcumin and chloroquine was identified. We found that treatment of cells with 10% mouse serum did not significantly change cell viability; these results are very similar to those found by Burikhanov et al. (5). However, in cells cultured with 20% mouse serum, an increase in cell proliferation of approximately 50% within 24 h was seen in both 4T1 cells and other breast cancer cell lines (data not shown). The trace amounts of curcumin present in serum may have induced the increase in cell proliferation, given the radio- and chemo-protective capacity of curcumin, and it may have promoted the expression of proliferation-inducing molecules (37,38). Gardane et al. reported that low concentrations of curcumin (10 μg/ml and 20 μg/ml) stimulated the proliferation of KG-1a cells, whereas high concentrations (30 μg/ml to 100 μg/ml) induced an inhibitory effect (39).
In this study, treatment of mice with curcumin led to a decrease in tumor mass. However, treatment with curcumin followed by cisplatin led to complete tumor mass regression in mice compared with the control group. Zhao et al. reported that high levels of PAR4 in the serum and plasma of mice inhibited the growth of metastatic lung tumor nodules derived from Lewis lung carcinoma cells in syngeneic mice (14). On the other hand, Burikhanov et al. reported high levels of PAR4 in plasma samples from patients with renal cell carcinoma after the use of chloroquine (5).
In our results, the mice treated with chloroquine showed an unexpected effect because tumor inhibition was not observed, rather, there was an increase in tumor mass compared with the control group without treatment. Hence, the effects of this treatment in this group of mice should be analyzed in further studies.
Curcumin is a good inducer of PAR4 expression in breast cancer cells and a secretagogue in non-tumor cells in vivo. In addition, it sensitizes cells to the effect of cisplatin; hence, curcumin, alone or in combination with chemotherapy, might be used as an alternative treatment for breast cancer.
Conflicts of Interest
The Authors declare that they have no conflicts of interest.
Authors’ Contributions
Formal analysis: Pablo Zapata Benavides, Oscar Alberto Alvarez-Quezada and Mariela Arellano-Rodríguez. Acquisition of funds: María Cristina Rodríguez-Padilla. Research: Norma Cesilia Arellano-Rodríguez, Pablo Zapata Benavides and Santiago Saavedra-Alonso. Methodology: Norma Cesilia Arellano-Rodríguez, Gerardo Vargas-Alanís, Juan Manuel Izaguirre-Alvarez and Diana Elisa Zamora-Ávila. Writing and final approval: All Authors.
References
- 1.Mundle SD. Par-4: a common facilitator/enhancer of extrinsic and intrinsic pathways of apoptosis. Leuk Res. 2006;30(5):515–517. doi: 10.1016/j.leukres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Hebbar N, Wang C, Rangnekar VM. Mechanisms of apoptosis by the tumor suppressor Par-4. J Cell Physiol. 2012;227(12):3715–3721. doi: 10.1002/jcp.24098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai MA, Gerhard R, Salaorni S, Fregnani JH, Nonogaki S, Netto MM, Soares FA. Down-regulation of the candidate tumor suppressor gene PAR-4 is associated with poor prognosis in breast cancer. Int J Oncol. 2010;37(1):41–49. doi: 10.3892/ijo_00000651. [DOI] [PubMed] [Google Scholar]
- 4.Méndez-López LF, Zapata-Benavides P, Zavala-Pompa A, Aguado-Barrera ME, Pacheco-Calleros J, Rodríguez-Padilla C, Cerda-Flores RM, Cortés-Gutiérrez EI, Dávila-Rodríguez MI. Immunohistochemical analysis of prostate apoptosis response-4 (Par-4) in Mexican women with breast cancer: a preliminary study. Arch Med Res. 2010;41(4):261–268. doi: 10.1016/j.arcmed.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Burikhanov R, Hebbar N, Noothi SK, Shukla N, Sledziona J, Araujo N, Kudrimoti M, Wang QJ, Watt DS, Welch DR, Maranchie J, Harada A, Rangnekar VM. Chloroquine-inducible Par-4 secretion is essential for tumor cell apoptosis and inhibition of metastasis. Cell Rep. 2017;18(2):508–519. doi: 10.1016/j.celrep.2016.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H, Treude F, Krämer OH, Lüscher B, Hartkamp J. PAR-4 overcomes chemo-resistance in breast cancer cells by antagonizing cIAP1. Sci Rep. 2019;9(1):8755. doi: 10.1038/s41598-019-45209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mabe NW, Fox DB, Lupo R, Decker AE, Phelps SN, Thompson JW, Alvarez JV. Epigenetic silencing of tumor suppressor Par-4 promotes chemoresistance in recurrent breast cancer. J Clin Invest. 2018;128(10):4413–4428. doi: 10.1172/JCI99481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138(2):377–388. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burikhanov R, Sviripa VM, Hebbar N, Zhang W, Layton WJ, Hamza A, Zhan CG, Watt DS, Liu C, Rangnekar VM. Arylquins target vimentin to trigger Par-4 secretion for tumor cell apoptosis. Nat Chem Biol. 2014;10(11):924–926. doi: 10.1038/nchembio.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sviripa VM, Burikhanov R, Obiero JM, Yuan Y, Nickell JR, Dwoskin LP, Zhan CG, Liu C, Tsodikov OV, Rangnekar VM, Watt DS. Par-4 secretion: stoichiometry of 3-arylquinoline binding to vimentin. Org Biomol Chem. 2016;14(1):74–84. doi: 10.1039/c5ob01980j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebbar N, Shrestha-Bhattarai T, Rangnekar VM. Cancer-selective apoptosis by tumor suppressor par-4. Adv Exp Med Biol. 2014;818:155–166. doi: 10.1007/978-1-4471-6458-6_7. [DOI] [PubMed] [Google Scholar]
- 12.Lee AS. The Par-4-GRP78 TRAIL, more twists and turns. Cancer Biol Ther. 2009;8(22):2103–2105. doi: 10.4161/cbt.8.22.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen M, Ribaux P, Epiney M, Irion O. Role of prostate apoptosis response 4 in translocation of GRP78 from the endoplasmic reticulum to the cell surface of trophoblastic cells. PLoS One. 2013;8(11):e80231. doi: 10.1371/journal.pone.0080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Burikhanov R, Brandon J, Qiu S, Shelton BJ, Spear B, Bondada S, Bryson S, Rangnekar VM. Systemic Par-4 inhibits non-autochthonous tumor growth. Cancer Biol Ther. 2011;12(2):152–157. doi: 10.4161/cbt.12.2.15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal L, Aryal UK, Camarillo IG, Raman V, Sundararajan R. Effective electrochemotherapy with curcumin in MDA-MB-231-human, triple negative breast cancer cells: A global proteomics study. Bioelectrochemistry. 2020;131:107350. doi: 10.1016/j.bioelechem.2019.107350. [DOI] [PubMed] [Google Scholar]
- 16.Jayarajan J, Angandoor S, Vedulla SH, Sritharan S, Ganesan K, War AR, Sivalingam N. Curcumin induces chemosensitization to doxorubicin in Duke’s type B coloadenocarcinoma cell line. Mol Biol Rep. 2020;47(10):7883–7892. doi: 10.1007/s11033-020-05866-w. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255(2):170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Chang YJ, Tai CJ, Kuo LJ, Wei PL, Liang HH, Liu TZ, Wang W, Tai CJ, Ho YS, Wu CH, Huang MT. Glucose-regulated protein 78 (GRP78) mediated the efficacy to curcumin treatment on hepatocellular carcinoma. Ann Surg Oncol. 2011;18(8):2395–2403. doi: 10.1245/s10434-011-1597-3. [DOI] [PubMed] [Google Scholar]
- 19.Chang YJ, Huang CY, Hung CS, Chen WY, Wei PL. GRP78 mediates the therapeutic efficacy of curcumin on colon cancer. Tumour Biol. 2015;36(2):633–641. doi: 10.1007/s13277-014-2640-3. [DOI] [PubMed] [Google Scholar]
- 20.Thayyullathil F, Rahman A, Pallichankandy S, Patel M, Galadari S. ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio. 2014;4:763–776. doi: 10.1016/j.fob.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satherley LK, Sun PH, Ji KE, Mason M, Hargest R, Jiang WG, Ye L. Prostate apoptosis response-4 (PAR4) suppresses growth and invasion of breast cancer cells and is positively associated with patient survival. Anticancer Res. 2016;36(3):1227–1235. [PubMed] [Google Scholar]
- 22.Niu S, Bremner DH, Wu J, Wu J, Wang H, Li H, Qian Q, Zheng H, Zhu L. l-Peptide functionalized dual-responsive nanoparticles for controlled paclitaxel release and enhanced apoptosis in breast cancer cells. Drug Deliv. 2018;25(1):1275–1288. doi: 10.1080/10717544.2018.1477863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Health Estimates 2020: incidence and mortality. Geneva, World Health Organization (WHO), 2022. Available at https://www.who.int/news-room/fact-sheets/detail/breast-cancer. [Last accessed on July 2, 2022]
- 24.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 25.Dent R, Valentini A, Hanna W, Rawlinson E, Rakovitch E, Sun P, Narod SA. Factors associated with breast cancer mortality after local recurrence. Curr Oncol. 2014;21(3):e418–e425. doi: 10.3747/co.21.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases. Cancer Med. 2019;8(12):5574–5576. doi: 10.1002/cam4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sells SF, Wood DP Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5(4):457–466. [PubMed] [Google Scholar]
- 28.Shrestha-Bhattarai T, Hebbar N, Rangnekar VM. Par(-4)oxysm in breast cancer. Cancer Cell. 2013;24(1):3–5. doi: 10.1016/j.ccr.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Burikhanov R, Jayswal R, Weiss HL, Arnold SM, Villano JL, Rangnekar VM. Neoadjuvant administration of hydroxychloroquine in a phase 1 clinical trial induced plasma Par-4 levels and apoptosis in diverse tumors. Genes Cancer. 2018;9(5-6):190–197. doi: 10.18632/genesandcancer.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16(3):411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 31.Esteve-Valverde E, Tapiz-Reula A, Ruiz D, Alijotas-Reig J. Systemic lupus erythematosus and hydroxychloroquine-related acute intermittent porphyria. Rheumatol Int. 2020;40(5):777–783. doi: 10.1007/s00296-019-04500-8. [DOI] [PubMed] [Google Scholar]
- 32.Ovejero-Sánchez M, González-Sarmiento R, Herrero AB. Synergistic effect of Chloroquine and Panobinostat in ovarian cancer through induction of DNA damage and inhibition of DNA repair. Neoplasia. 2021;23(5):515–528. doi: 10.1016/j.neo.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand K, Niravath P, Patel T, Ensor J, Rodriguez A, Boone T, Wong ST, Chang JC. A Phase II study of the efficacy and safety of chloroquine in combination with taxanes in the treatment of patients with advanced or metastatic anthracycline-refractory breast cancer. Clin Breast Cancer. 2021;21(3):199–204. doi: 10.1016/j.clbc.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang JR, Kim WY, Cho YJ, Ryu JY, Choi JJ, Jeong SY, Kim MS, Kim JH, Paik ES, Lee YY, Han HD, Lee JW. Chloroquine reverses chemoresistance via upregulation of p21WAF1/CIP1 and autophagy inhibition in ovarian cancer. Cell Death Dis. 2020;11(12):1034. doi: 10.1038/s41419-020-03242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farhangi B, Alizadeh AM, Khodayari H, Khodayari S, Dehghan MJ, Khori V, Heidarzadeh A, Khaniki M, Sadeghiezadeh M, Najafi F. Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur J Pharmacol. 2015;758:188–196. doi: 10.1016/j.ejphar.2015.03.076. [DOI] [PubMed] [Google Scholar]
- 36.Meiyanto E, Putri H, Arum Larasati Y, Yudi Utomo R, Istighfari Jenie R, Ikawati M, Lestari B, Yoneda-Kato N, Nakamae I, Kawaichi M, Kato JY. Anti-proliferative and anti-metastatic potential of curcumin analogue, pentagamavunon-1 (PGV-1), toward highly metastatic breast cancer cells in correlation with ROS generation. Adv Pharm Bull. 2019;9(3):445–452. doi: 10.15171/apb.2019.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46(4):1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Wu L. In vitro and in vivo cardioprotective effects of curcumin against doxorubicin-induced cardiotoxicity: a systematic review. J Oncol. 2022;2022:7277562. doi: 10.1155/2022/7277562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardane A, Poonawala M, Vaidya A. Curcumin sensitizes quiescent leukemic cells to antimitotic drug 5-fluorouracil by inducing proliferative responses in them. Journal of Cancer Metastasis and Treatment. 2016;2(7):245. doi: 10.20517/2394-4722.2016.11. [DOI] [Google Scholar]