Abstract
Background/Aim: Primary osteosarcoma of the mammary gland is a very rare disease, accounting for under 1% of all mammary gland malignancies. There is no established first-line treatment, and prognosis is poor compared to conventional breast cancer. We previously demonstrated the efficacy of cisplatinum and eribulin in a patient-derived orthotopic xenograft (PDOX) mouse model of primary breast osteosarcoma. However, these drugs show significant clinical toxicity. All cancers are addicted to methionine (Hoffman effect). In the present study, we determined whether methionine restriction with oral recombinant methioninase (o-rMETase) would lower the effective dose of cisplatinum in a PDOX model of primary osteosarcoma of the mammary gland, thereby reducing its toxicity.
Materials and Methods: Mouse PDOX models of primary osteosarcoma of the breast were randomized into the following groups: control; cisplatinum (weekly at 3 or 6 mg/kg); twice-daily o-rMETase; or o-rMETase combined with 3 mg/kg cisplatinum, with treatment for 2 weeks.
Results: Cisplatinum at 6 mg/kg significantly inhibited breast osteo-sarcoma growth compared with the untreated control and mice treated with 3 mg/kg cisplatinum (p=0.01 and 0.009, respectively). There was no significant difference in tumor growth between mice treated with cisplatinum at 3 mg/kg and the control (p=0.16). Combination therapy with cisplatinum at 3 mg/kg and twice daily o-rMETase regressed the osteosarcoma of the mammary gland (p=0.009), similar to the inhibition by cisplatinum at 6 mg/kg alone. Cisplatinum at 6 mg/kg caused a significant loss of mouse body weight, compared to the control (p=0.02). There was no significant body-weight loss with the combination therapy of o-rMETase and cisplatinum at 3 mg/kg, compared to the untreated control.
Conclusion: o-rMETase halved the effective dose of cisplatinum, thereby eliminating cisplatinum toxicity, demonstrating a future clinical strategy for therapy of osteosarcoma of the breast.
Keywords: Breast osteosarcoma, PDOX, combination therapy, cisplatinum, efficacy, methioninase, oral administration, methionine addiction, Hoffman effect, nude mice
Primary osteosarcoma of the mammary gland is a rare and recalcitrant disease. Fewer than 1% of breast tumors have been diagnosed as primary osteosarcoma of the mammary gland (1-3). First-line therapy for osteosarcoma of the mammary gland has not been established. The 5-year survival rate for this disease is only 38% (1). Previously we showed cisplatinum and eribulin to be effective in a patient-derived orthotopic xenograft (PDOX) model of primary osteosarcoma of the mammary gland (4).
Nephrotoxicity is an adverse effect of cisplatinum, which directly damages the renal tubules and is dose-dependent (5-7). In clinical practice, water loading, and diuretics are used to reduce the concentration of cisplatinum in the kidney (8).
Methionine addiction is a basic and general feature of cancer known as the Hoffman effect (9-11). The methionine requirement for cancer cells is very high compared to normal cells (9,10). The methionine-degrading enzyme recombinant methioninease (rMETase) effectively targets methionine addiction and arrests cancer cells in the late S/G2 phase of the cell cycle (12).
We have previously shown in mouse models that the combination of oral rMETase (o-rMETase) and cisplatinum has a synergistic effect on colon and bladder cancer (13,14). In the present study, we investigated the efficacy of this combination in a PDOX model of primary osteosarcoma of the breast.
Materials and Methods
Mice. Athymic (nu/nu) nude female mice, 4-6 weeks old (AntiCancer, Inc., San Diego, CA, USA) were used under an AntiCancer, Inc. Institutional. Animal Care and Use Committee protocol. This was specifically approved for the present study, which followed the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals, Assurance No. A3873-1.
Patient-derived tumor. A PDOX model of primary osteosarcoma of the mammary gland was previously established from a surgical specimen (15).
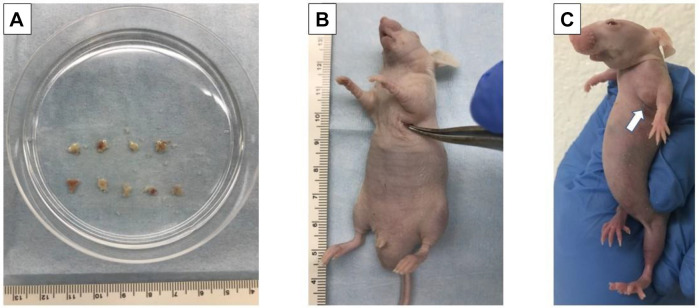
Establishment of a PDOX nude-mouse model of breast osteosarcoma. Tumors from mice subcutaneously implanted with patient-derived primary osteosarcoma of the breast were minced into approximately 5 mm3 fragments and prepared for transplantation (Figure 1A) (15). Mice were implanted with both tumor and surrounding tissue using the method of Hozumi (16). Mice were anesthetized with a ketamine mixture. A 1 cm skin incision was then made over the left mammary gland of nude mice. Using surgical scissors, the skin was peeled away from the dermis to create a pocket (Figure 1B). Fragments of breast osteosarcoma were inserted into the pocket along with surrounding normal tissue. The wound was closed with 5-0 PDS-II sutures. The implanted tumor grew to 70 mm3 in approximately 3 weeks (Figure 1C).
Figure 1. Establishment of mammary osteosarcoma of the breast in a patient-derived orthotopic xenograft model. A: Fragmentation of breast osteosarcoma tumor tissue. B: Insertion of tumor fragment into a pocket on the left mammary gland. C: White arrow indicates primary osteosarcoma of the breast growing in the left mammary gland of a nude mouse after 2 weeks.
rMETase production. Procedures for generating rMETase from recombinant Escherichia coli have been reported previously and include fermentation, heat treatment, polyethylene glycol precipitation, and DEAE-Sepharose column chromatography (17).
Treatment scheme. In treatment scheme A, three weeks after orthotopic transplantation, a 2-week treatment was initiated when the tumor volume reached 70-100 mm3. First, in treatment scheme A, PDOX mouse models of mammary primary osteosarcoma were randomly assigned to each of three treatment groups as follows (n=8 or n=9): non-treated control; or weekly intraperitoneal (i.p.) injection of 3.0 mg/kg or 6.0 mg/kg cisplatinum.
In treatment scheme B, PDOX mouse models were randomly assigned to four groups with seven mice per group as follows: phosphate-buffered saline (PBS)-treated control (0.2 ml/day, orally twice daily); cisplatinum (weekly i.p. injection of 6.0 mg/kg); 50 units rMETase (orally twice daily); combination of rMETase (50 units, orally twice daily) and cisplatinum (3.0 mg/kg, weekly i.p. injection).
The short and long axes of the tumors were measured using calipers. The mice were weighed once a week. Tumor volume was determined as short axis2×long axis/2. All mice were sacrificed on day 15 after administration. Tumors were then resected for histological evaluation.
Hematoxylin and eosin staining. Tumor tissue was minced to 7 mm pieces and fixed with 10% formalin at room temperature for 48 h. The tissue was washed with 70% alcohol to remove formalin from the tissue followed by dehydration by ascending alcohol (70% alcohol, 80% alcohol, 90% alcohol, 100% alcohol twice exchanged, 1 h each) and xylene twice exchanged (1 h each). The tissue samples were then placed three times in high-grade paraffin wax, 1 h each. After implantation, each paraffin block was sliced to a thickness of 4 μm using a sliding microtome. Warm water at 37˚C was used to expand the sections. Tissue sections were mounted on glass slides and dried overnight on a slide warmer at 60˚C. Specimens were stained according to the standard hematoxylin-eosin protocol for histopathological evaluation and observed under a BH-2 model microscope (Olympus Corporation, Tokyo, Japan) for the degree of necrosis (18).
Statistical analyses. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface of R (The R Foundation for Statistical Computing, Vienna, Austria) (19). The Tukey-Kramer test was used for parametric tests of between-group comparisons. The Kruskal-Wallis test was used for nonparametric tests using the Steel-Dwass method to test between each group. Graphs represent means and error bars represent standard error of means. A value of p≤0.05 was defined as indicating a statistically significant difference.
Results
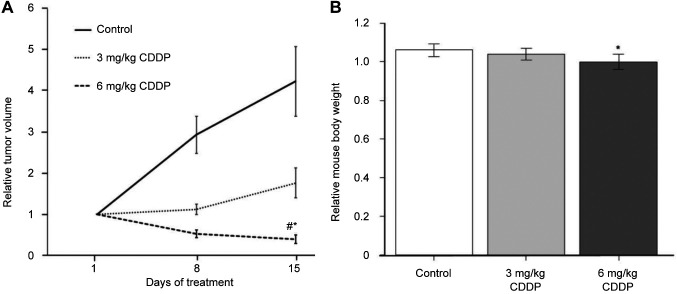
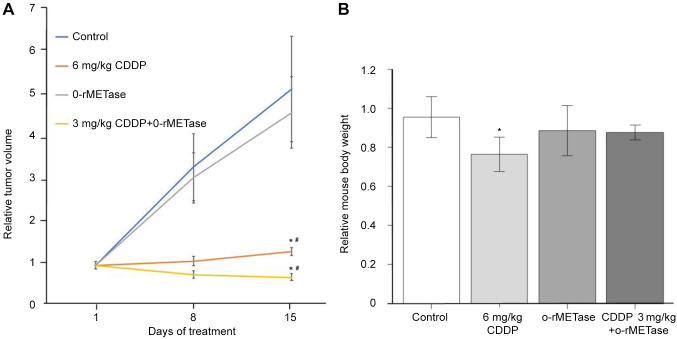
Treatment efficacy in the breast osteosarcoma PDOX. In treatment scheme A, 6.0 mg/kg cisplatinum led to a significant decrease in tumor volume compared to the untreated control and mice treated with cisplatinum at 3.0 mg/kg (p=0.01 and 0.009, respectively). However, there was no significant decrease in tumor volume of mice treated with 3.0 mg/kg cisplatinum compared with the control (p=0.16) (Figure 2A). In treatment scheme B, cisplatinum at 6.0 mg/kg significantly reduced the tumor volume compared to the control (p=0.009). The combination of o-rMETase and 3.0 mg/kg cisplatinum led to tumor regression compared to the control (p=0.009) and similar to 6.0 mg/kg cisplatinum alone, which was significantly different from the control (p=0.02), and significantly different from o-rMETase alone (p=0.009) (Figure 3A). However, there was no significant decrease in tumor volume in o-rMETase-alone-treated mice compared to the control (p=0.99) (Figure 3A).
Figure 2. Efficacy of treatment scheme A, cisplatinum (CDDP), on breast osteosarcoma in a patient-derived orthotopic nude-mouse xenograft model. A: Tumor volume at the indicated times relative to that at the start of treatment. B: Mouse body weight at day 15 relative to that at day 1 of treatment. Data are the mean±standard error. Significantly different at p<0.05 from: *control; #3 mg/kg CDDP.
Figure 3. Efficacy of treatment scheme B, cisplatinum (CDDP) and oral recombinant methioninase (o-rMETase), alone and in combination with 3 mg/kg cisplatinum, on osteosarcoma of the breast in a patient-derived orthotopic xenograft nude-mouse model. A: Tumor volume at the indicated times relative to that at the start of treatment. B: Mouse body weight at day 15 relative to that at day 1 of treatment. Data are the mean±standard error. Significantly different at p<0.05 from: *control; #o-rMETase alone.
Toxicity of treatment drugs. In treatment scheme A, 6.0 mg/kg cisplatinum caused a significant decrease in mouse body weight compared to the control (p=0.02) (Figure 2B). In treatment scheme B, 6.0 mg/kg cisplatinum caused a significant loss of mouse body weight compared to the control (p=0.009). However, o-rMETase alone and in combination with 3.0 mg/kg cisplatinum did not cause a significant loss in mouse body weight compared to the PBS-treated control (Figure 3B).
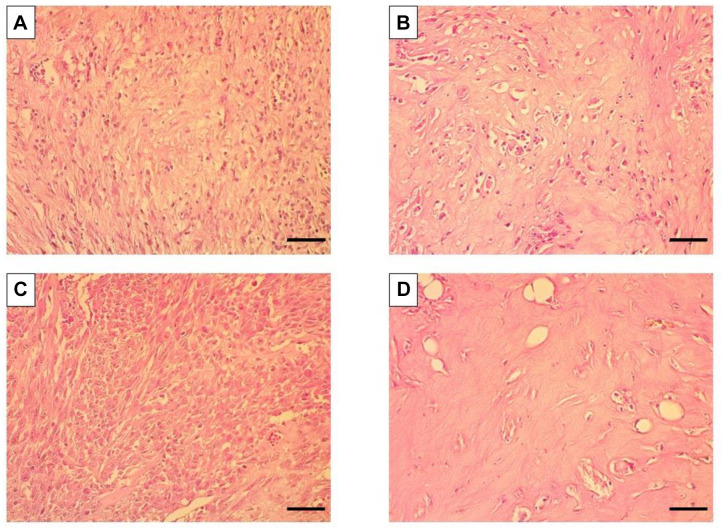
Histology of breast osteosarcoma-PDOX. PDOX tissue in the control group consisted of dense spindle-shaped cancer cells (Figure 4A). Treatment with o-rMETase alone did not affect the histological phenotype of the breast osteosarcoma, which was similar to that of the control mice (Figure 4C). Treatment with 6 mg/kg cisplatinum alone reduced the cell density of the tumor (Figure 4B). The combination of 3 mg/kg cisplatinum and o-rMETase severely reduced the cancer-cell density of the tumor (Figure 4D).
Figure 4. Representative photomicrographs of hematoxylin and eosin-stained tissue sections from breast-osteosarcoma in a patient-derived orthotopic xenograft nude-mouse model. A: Untreated control. B: Cisplatinum-treated (6 mg/kg). C: Oral recombinant methioninase (o-rMETase)-treated. D: Combination treatment with cisplatinum at 3 mg/kg and o-rMETase. Magnification: 200×. Scale bar: 50 μm.
Discussion
Primary osteosarcoma of the mammary gland is histologically similar to osteosarcoma of the bone. Primary osteosarcoma of the mammary gland may arise from mesenchymal cells within the mammary gland and may be related to fibroadenomas and phyllodes tumors (20). We have previously shown that cisplatinum and eribulin are effective for primary osteosarcoma of the mammary gland for which there is no established first-line therapy (4).
In treatment scheme A, the higher concentration of cisplatinum (6 mg/kg) was more effective than the lower concentration (3 mg/kg). However, at the higher dose, the mice lost weight significantly. The combination of the 3 mg/kg dose of cisplatinum and o-rMETase had similar efficacy to the 6 mg/kg dose of cisplatinum alone. This combination of o-rMETase and 3 mg/kg CDDP did not result in significant mouse-body-weight loss, unlike cisplatinum alone at 6 mg/kg. Thus, o-rMETase appears to reduce the effective dose of cisplatinum by 50% without apparent toxicity.
Methionine dependence in cancer cells was first reported by Sugimura et al. in 1959 (21). Methionine dependence is due to methionine addiction of cancer cells (9-11). Methionine addiction is caused by excessive use of methionine in transmethylation reactions (22) that deplete endogenous free methionine and S-adenosylmethionine under methionine restriction (10).
rMETase selectively traps cancer cells in the S/G2 phase of the cell cycle, where they are most sensitive to cytotoxic chemotherapy and can be successfully eradicated (12,23,24), which is what apparently occurred in the present study.
The present study showed that o-rMETase allowed the effective dose of CDDP to be reduced by 50%, avoiding body weight loss while maintaining efficacy. In the future, we plan to evaluate the efficacy of o-rMETase in various cancer types to allow reduction of the effective dose of various toxic chemotherapy drugs in the clinic.
o-rMETase has shown clinical promise as therapy for advanced prostate cancer, stage IV pancreatic cancer and rectal cancer (25-29). Future studies will determine efficacy of rMETase in clinical breast cancer.
We originally discovered (30) that methionine-restriction and chemotherapy are synergistic (the Hoffman protocol). The present study further emphasizes the potential of combining chemotherapy and methionine restriction to target methionine addiction which is a fundamental characteristic of cancer (9,10,31,32,33).
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study. AntiCancer Inc. uses PDOX models for contract research.
Authors’ Contributions
N.M. and N.F.W. conceived the study. J.W. provided the original surgical tumor specimen. Q.H. supplied rMETase, N.M., C.S., C.H., K.O., Y.K., and Y.A. performed the experiments and J.M. and R.M.H. provided scientific advice. N.M. wrote the paper and R.M.H. revised the paper.
Acknowledgements
This article is dedicated to the memory of A. R. Moossa, M.D., Sun Lee, M.D., Professor Li Jiaxi, Masaki Kitajima, M.D., Shigeo Yagi, Ph.D. and Jack Geller, M.D. The Robert M. Hoffman Foundation for Cancer Research provided funds for the study.
References
- 1.Silver SA, Tavassoli FA. Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998;22(8):925–933. doi: 10.1097/00000478-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Pollard SG, Marks PV, Temple LN, Thompson HH. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer. 1990;66(5):941–944. doi: 10.1002/1097-0142(19900901)66:5<941::aid-cncr2820660522>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy T, Biggart JD. Sarcoma of the breast. Br J Cancer. 1967;21(4):635–644. doi: 10.1038/bjc.1967.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masaki N, Wu NF, Aoki Y, Yamamoto J, Miyazaki J, Hoffman RM. Osteosarcoma of the breast in a patient derived orthotopic xenograft (PDOX) mouse model is arrested by both cisplatinum and eribulin. In Vivo. 2021;35(6):3107–3110. doi: 10.21873/invivo.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basak D, Arrighi S, Darwiche Y, Deb S. Comparison of anticancer drug toxicities: paradigm shift in adverse effect profile. Life (Basel) 2021;12(1):48. doi: 10.3390/life12010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi N, Kobayashi M, Itagaki S, Hirano T, Takekuma Y, Sugawara M, Iseki K. [Basic investigation for classification of anticancer drugs by pharmacological effects] Yakugaku Zasshi. 2012;132(6):777–783. doi: 10.1248/yakushi.132.777. [DOI] [PubMed] [Google Scholar]
- 7.Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32(8):1469–1486. doi: 10.1021/acs.chemrestox.9b00204. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi T, Uoi M, Omura F, Tsumagari K, Maesaki S, Yokota C. Risk factors for cisplatin-induced nephrotoxicity: a multicenter retrospective study. Oncology. 2021;99(2):105–113. doi: 10.1159/000510384. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman RM, Erbe RW. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci U.S.A. 1976;73(5):1523–1527. doi: 10.1073/pnas.73.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coalson DW, Mecham JO, Stern PH, Hoffman RM. Reduced availability of endogenously synthesized methionine for S-adenosylmethionine formation in methionine-dependent cancer cells. Proc Natl Acad Sci U.S.A. 1982;79(14):4248–4251. doi: 10.1073/pnas.79.14.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser P. Methionine dependence of cancer. Biomolecules. 2020;10(4):568. doi: 10.3390/biom10040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc Natl Acad Sci USA. 1980;77(12):7306–7310. doi: 10.1073/pnas.77.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Y, Sun X, Xu M, Tan X, Sasson A, Rashidi B, Han Q, Tan X, Wang X, An Z, Sun FX, Hoffman RM. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clin Cancer Res. 1999;5(8):2157–2163. [PubMed] [Google Scholar]
- 14.Sun YU, Nishino H, Sugisawa N, Yamamoto J, Hamada K, Zhu G, Lim HI, Hoffman RM. Oral recombinant methioninase sensitizes a bladder cancer orthotopic xenograft mouse model to low-dose cisplatinum and prevents metastasis. Anticancer Res. 2020;40(11):6083–6091. doi: 10.21873/anticanres.14629. [DOI] [PubMed] [Google Scholar]
- 15.Wu NF, Wu J, Yamamoto J, Aoki Y, Hozumi C, Bouvet M, Hoffman RM. The first mouse model of primary osteosarcoma of the breast. In Vivo. 2021;35(4):1979–1983. doi: 10.21873/invivo.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murata T, Hozumi C, Hiroshima Y, Shimoya K, Hongo A, Inubushi S, Tanino H, Hoffman RM. Co-implantation of tumor and extensive surrounding tissue improved the establishment rate of surgical specimens of human-patient cancer in nude mice: toward the goal of universal individualized cancer therapy. In Vivo. 2020;34(6):3241–3245. doi: 10.21873/invivo.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y, Xu M, Tan X, Tan X, Wang X, Saikawa Y, Nagahama T, Sun X, Lenz M, Hoffman RM. Overexpression and large-scale production of recombinant L-methionine-alpha-deamino-gamma-mercaptomethane-lyase for novel anticancer therapy. Protein Expr Purif. 1997;9(2):233–245. doi: 10.1006/prep.1996.0700. [DOI] [PubMed] [Google Scholar]
- 18.Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014(6):655–658. doi: 10.1101/zpdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remadi S, Doussis-Anagnostopoulu I, Mac Gee W. Primary osteosarcoma of the breast. Pathol Res Pract. 1995;191(5):471–4. doi: 10.1016/S0344-0338(11)80737-5. discussion 475-7. [DOI] [PubMed] [Google Scholar]
- 21.Sugimura T, Birnbaum SM, Winitz M, Greenstein JP. Quantitative nutritional studies with water-soluble, chemically defined diets. VIII. The forced feeding of diets each lacking in one essential amino acid. Arch Biochem Biophys. 1959;81(2):448–455. doi: 10.1016/0003-9861(59)90225-5. [DOI] [PubMed] [Google Scholar]
- 22.Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro. 1984;20(8):663–670. doi: 10.1007/BF02619617. [DOI] [PubMed] [Google Scholar]
- 23.Yano S, Li S, Han Q, Tan Y, Bouvet M, Fujiwara T, Hoffman RM. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget. 2014;5(18):8729–8736. doi: 10.18632/oncotarget.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman RM. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: a 40-year odyssey. Expert Opin Biol Ther. 2015;15(1):21–31. doi: 10.1517/14712598.2015.963050. [DOI] [PubMed] [Google Scholar]
- 25.Han Q, Hoffman RM. Chronic treatment of an advanced prostate-cancer patient with oral methioninase resulted in long-term stabilization of rapidly rising PSA levels. In Vivo. 2021;35(4):2171–2176. doi: 10.21873/invivo.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota Y, Han Q, Hozumi C, Masaki N, Yamamoto J, Aoki Y, Tsunoda T, Hoffman RM. Stage IV pancreatic cancer patient treated with FOLFIRINOX combined with oral methioninase: a highly-rare case with long-term stable disease. Anticancer Res. 2022;42(5):2567–2572. doi: 10.21873/anticanres.15734. [DOI] [PubMed] [Google Scholar]
- 27.Han Q, Tan Y, Hoffman RM. Oral dosing of recombinant methioninase is associated with a 70% drop in PSA in a patient with bone-metastatic prostate cancer and 50% reduction in circulating methionine in a high-stage ovarian cancer patient. Anticancer Res. 2020;40(5):2813–2819. doi: 10.21873/anticanres.14254. [DOI] [PubMed] [Google Scholar]
- 28.Han Q, Hoffman RM. Lowering and stabilizing PSA levels in advanced-prostate cancer patients with oral methioninase. Anticancer Res. 2021;41(4):1921–1926. doi: 10.21873/anticanres.14958. [DOI] [PubMed] [Google Scholar]
- 29.Kubota Y, Han Q, Hamada K, Aoki Y, Masaki N, Obara K, Tsunoda T, Hoffman RM. Long-term Stable Disease in a Rectal-cancer Patient Treated by Methionine Restriction With Oral Recombinant Methioninase and a Low-methionine Diet. Anticancer Res. 2022;42(8):3857–3861. doi: 10.21873/anticanres.15877. [DOI] [PubMed] [Google Scholar]
- 30.Stern PH, Hoffman RM. Enhanced in vitro selective toxicity of chemotherapeutic agents for human cancer cells based on a metabolic defect. J Natl Cancer Inst. 1986;76(4):629–639. doi: 10.1093/jnci/76.4.629. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto J, Han Q, Inubushi S, Sugisawa N, Hamada K, Nishino H, Miyake K, Kumamoto T, Matsuyama R, Bouvet M, Endo I, Hoffman RM. Histone methylation status of H3K4me3 and H3K9me3 under methionine restriction is unstable in methionine-addicted cancer cells, but stable in normal cells. Biochem Biophys Res Commun. 2020;533(4):1034–1038. doi: 10.1016/j.bbrc.2020.09.108. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto J, Inubushi S, Han Q, Tashiro Y, Sugisawa N, Hamada K, Aoki Y, Miyake K, Matsuyama R, Bouvet M, Clarke SG, Endo I, Hoffman RM. Linkage of methionine addiction, histone lysine hypermethylation, and malignancy. iScience. 2022;25(4):104162. doi: 10.1016/j.isci.2022.104162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto J, Aoki Y, Inubushi S, Han Q, Hamada K, Tashiro Y, Miyake K, Matsuyama R, Bouvet M, Clarke SG, Endo I, Hoffman RM. Extent and instability of trimethylation of histone H3 lysine increases with degree of malignancy and methionine addiction. Cancer Genomics Proteomics. 2022;19(1):12–18. doi: 10.21873/cgp.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]