Abstract
Background/Aim: To prospectively evaluate the efficacy and safety of the BNT162b2 vaccine in solid cancer patients undergoing systemic chemotherapy (n=63).
Patients and Methods: COVID-19 anti-spike protein antibody levels were measured before the first BNT162b2 vaccination, just before the second BNT162b2 vaccination, one month after the second BNT162b2 vaccination, and 3 months after the second BNT162b2 vaccination. Anti-spike protein antibody seropositivity was set at ≥0.8 U/ml.
Results: Colorectal cancer was the most commonly observed primary disease (36.5%). ECOG-PS 0 was observed in the majority (52.4%) of patients. The overall response rate and the median (range) anti-spike protein antibody levels in the whole cohort at 3 months after the second BNT162b2 vaccination were 98.4% (62/63) and 206 (0.4-3,813) U/ml. None of the patients required postponement or discontinuation of systemic chemotherapy because of an adverse reaction.
Conclusion: The BNT162b vaccine in solid cancer patients undergoing systemic chemotherapy is effective and safe.
Keywords: BNT162b2 vaccine, advanced cancer, systemic chemotherapy, efficacy, safety
The COVID-19 pandemic is still ongoing, and antibody acquisition against COVD-19 through vaccination is necessary for infection control (1,2). Currently, several COVID-19 vaccines are under development worldwide (3,4). Three types of COVID-19 vaccines [Pfizer (BNT162b2), Moderna (mRNA-1273), and AstraZeneca (ChAdOx1 nCov19)] are currently approved in Japan, all of which have shown high efficacy (94-95%) in preventing the onset of symptomatic COVID-19 (5-7).
The Pfizer BNT162b2 vaccine is a lipid nanoparticle-formulated, nucleoside-modified RNA (mRNA) vaccine encoding a prefusion-stabilized, membrane-anchored severe acute respiratory syndrome coronavirus 2 full-length spike protein (8). In Japan, the BNT162b2 mRNA vaccine was approved by the regulatory authorities in February 2021, with priority vaccination for healthcare workers. Priority vaccination for the elderly and patients with underlying diseases had also been initiated in April 2021. Reports have begun to emerge from both Japan and overseas on the rate of antibody acquisition and reduction of COVID-19 infection rates through vaccination for COVID-19 in healthy subjects (8-11). On the other hand, cancer patients have a significantly higher incidence, severity, and mortality rate of COVID-19 infection, and patients with solid tumors are also priority candidates for vaccination. Early vaccination of cancer patients is also recommended in the NCCN guidelines (12), but evidence supporting the safety and efficacy of vaccines for cancer patients, especially those undergoing systemic chemotherapy, is scarce (13,14). In an overseas phase III study that examined the effectiveness of BNT162b2, 80% of the participants were healthy individuals with no underlying disease, and the percentage of participants with malignant disease in the BNT162b2 vaccine group was as low as 3.9%, but these details are not available (9). An observational study of approximately 29,000 cancer patients who received the BNT162b2 vaccine has been published (15). This study showed that two doses of the BNT162b2 vaccine reduced the risk of infection, even in cancer patients, and the vaccine was 58% effective in reducing the risk of infection. The risk reduction effect of the BNT162b2 vaccine in non-cancer patients was more than 90%, suggesting that the vaccine may be less effective in cancer patients (15). However, data for cancer patients with systemic chemotherapy are not available in that study (15). Therefore, it would be highly significant to conduct an observational study to investigate the efficacy and safety of BNT162b2.
Based on this background, in this prospective study, we sought to evaluate the efficacy and safety of the BNT162b2 vaccine in solid cancer patients undergoing systemic chemotherapy.
Patients and Methods
Study design. The study subjects were solid tumor patients undergoing systemic chemotherapy at the Outpatient Chemotherapy Center and the Breast Surgery Outpatient Clinic in the Osaka Medical and Pharmaceutical University Hospital between May 2021 and November 2021, who agreed to participate in our prospective study. Cases meeting any of the following conditions were excluded: 1) patients aged less than 20 years, 2) patients not expected to survive longer than 6 months, 3) patients receiving intensive corticosteroid therapy or immunosuppressive therapy except for cases of prophylactic corticosteroid therapy for chemotherapy-induced nausea and vomiting, 4) patients with hematological disease, and 5) patients receiving intensive systemic chemotherapy with the long-term persistent myelosuppressive condition.
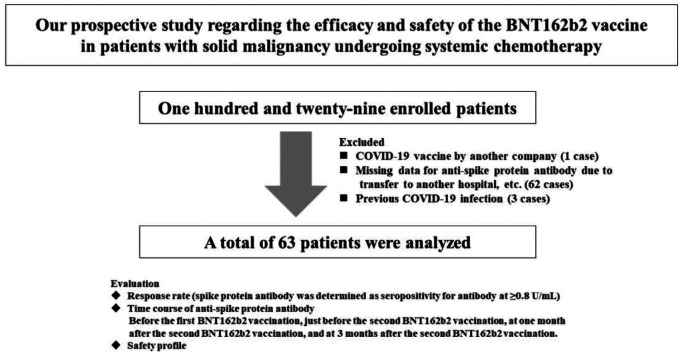
COVID-19 anti-spike protein antibody levels were measured before the first BNT162b2 vaccination, just before the second BNT162b2 vaccination (patients received two BNT162b2 vaccine doses, 21 days apart) (Time A), at one month after the second BNT162b2 vaccination (Time B), and at 3 months after the second BNT162b2 vaccination (Time C). Anti-spike protein antibody was measured using Elecsys® Anti-SARS-Cov-2 S RUO (Roche diagnostics K.K., Tokyo, Japan). Anti-spike protein antibody seropositivity was determined at ≥0.8 U/ml (16). A total of 129 patients were enrolled. One case with COVID-19 vaccine by another company, 62 cases in which anti-spike protein antibody measurements could not be performed before and after BNT162b2 vaccination due to transfer to another hospital, and 3 cases confirmed as previously infected with COVID-19 were excluded from the analysis, leaving 63 cases for the final analysis (Figure 1).
Figure 1. Study design.
Response rates for the BNT162b2 vaccine (percentage of seropositivity for anti-spike protein antibody), the time course of anti-spike protein antibody levels, and safety profiles were evaluated for all cases and compared according to sex, age, ECOG-PS, body mass index (BMI), serum albumin level, total lymphocyte count, corticosteroid use and immune checkpoint inhibitor (ICI) therapy. The safety profile was evaluated using the questionnaire of adverse reactions for all patients at the first and second BNT162b2 vaccination. The Ethics Committee of our hospital provided ethical approval (approval number, 2021-010). Written informed consent was obtained from all included patients.
Statistics. In the analysis of continuous variables, the appropriate choice in paired t-test, unpaired t-test, and Mann-Whitney U-test was made to compare 2 groups. In the data presentation, median (range) was used. A p=0.05 was set at the significant level by the JMP ver. 16 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics. Baseline characteristics in this study [n=63, 29 males, median (range) age=70 (35-87) years] are shown in Table I. In terms of ECOG-PS, ECOG-PS 0 was seen in the majority (n=33, 52.4%) of patients. In terms of primary disease, colorectal cancer was the disease observed in the majority of cases (n=23, 36.5%), followed by gastric cancer (n=15, 23.8%). Regarding disease clinical-stage, stage IV was found in the majority of cases (n=52, 82.5%). All analyzed patients received standard chemotherapeutic regimens based on current guidelines. Corticosteroid therapy with the aim of symptom control was followed in 36 cases (57.1%). ICI therapy was performed in 7 cases (11.1%). Sixty-one patients received two doses of the BNT162b2 vaccine during systemic chemotherapy, and two received one dose of the BNT162b2 vaccine during systemic chemotherapy.
Table I. Baseline characteristics (n=63).
ICI: Immune checkpoint inhibitor.
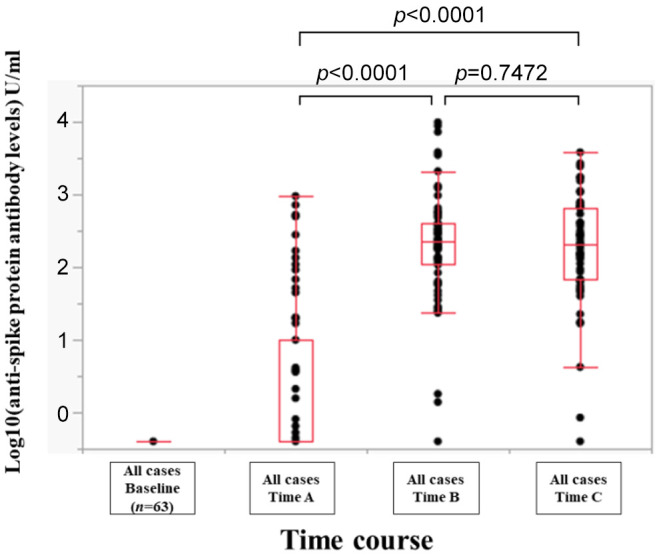
Efficacy of BNT162b2 vaccine for all cases. The overall response rate in the whole cohort at the point of Time A (just before the second BNT162b2 vaccination), Time B (1 month after the second BNT162b2 vaccination) and Time C (3 months after the second BNT162b2 vaccination) was 34.9% (22/63), 96.8% (61/63) and 98.4% (62/63). The median (range) anti-spike protein antibody levels at the Time point A, B, and C were 0.4 (0.4-950) U/ml, 230 (0.4-9,871) U/ml, and 206 (0.4-3,813) U/ml (Time point A vs. Time point B, p<0.0001; Time point A vs. Time point C, p<0.0001; Time point B vs. Time point C, p=0.7472) (Figure 2).
Figure 2. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A (just before the second BNT162b2 vaccination), Time point B (1 month after the second NT162b2 vaccination), and Time point C (3 months after the second BNT162b2 vaccination) for all cases.
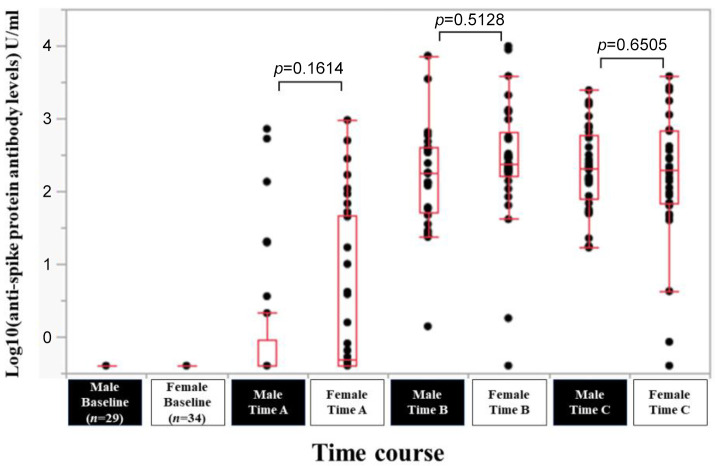
Efficacy of BNT162b2 vaccine according to sex. In male (n=29) and female (n=34) patients, the response rate at the Time point A, B, and C was 24.1% (7/29), 100% (29/29) and 100% (29/29) in male patients, and 44.1% (15/34), 94.1% (32/34) and 97.1% (33/34) in female patients. The median (range) anti-spike protein antibody levels in male vs. female patients at the Time point A, B, and C was 0.4 (0.4-718) U/ml vs. 0.49 (0.4-950) U/ml at Time point A (p=0.1614), 182 (1.39-7,260) U/ml vs. 236.5 (0.4-9,871) U/ml at Time point B (p=0.5128), and 206 (16.8-2,436) U/ml vs. 196 (0.4-3,813) U/ml at Time point C (p=0.6505) (Figure 3).
Figure 3. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to sex.
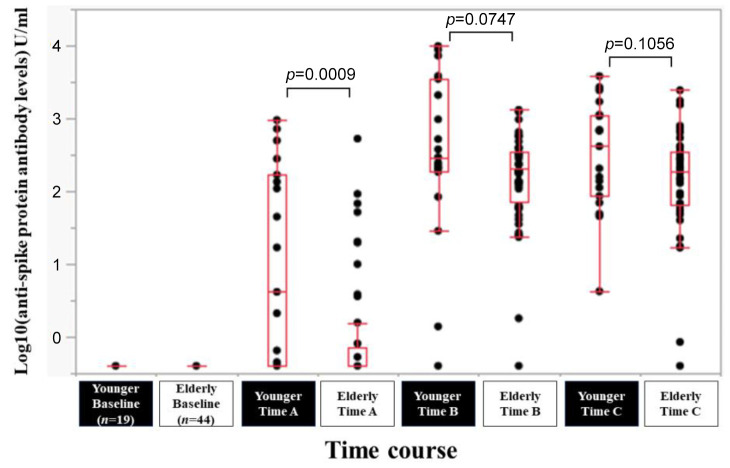
Efficacy of BNT162b2 vaccine according to age. In younger (less than 65 years, n=19) and elderly (65 years or more, n=44) patients, the response rate at the Time point A, B, and C was 57.9% (11/19), 94.7% (18/19) and 100% (19/19) in younger patients, and 25.0% (11/44), 97.7% (43/44) and 97.7% (43/44) in elderly patients. The median (range) anti-spike protein antibody levels in younger vs. elderly patients at the Time point A, B, and C were 4.2 (0.4-950) U/ml vs. 0.4 (0.4-527) U/ml at Time point A (p=0.0009), 294 (0.4-9,871) U/ml vs. 211 (0.4-1,310) U/ml at Time point B (p=0.0747), and 414 (4.23-3,813) U/ml vs. 191 (0.4-2,436) U/ml at Time point C (p=0.1056) (Figure 4).
Figure 4. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to age (cutoff=65 years).
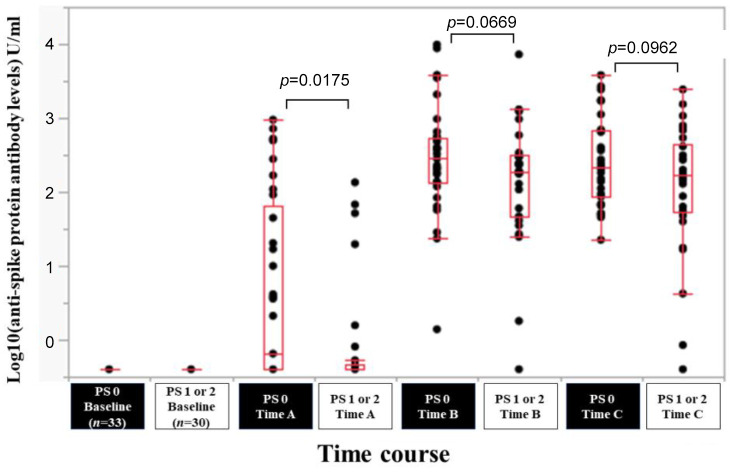
Efficacy of BNT162b2 vaccine according to ECOG-PS. In patients with ECOG-PS 0 (n=33) and ECOG-PS 1 or 2 (n=30), the response rate at the Time point A, B, and C was 48.5% (16/33), 100% (33/33) and 100% (33/33) in patients with ECOG-PS 0, and 20.0% (6/30), 93.3% (28/30) and 96.7% (29/30) in patients with ECOG-PS 1 or 2. The median (range) anti-spike protein antibody levels in patients with ECOG-PS 0 vs. ECOG-PS 1 or 2 at the Time point A, B, and C were 0.4 (0.4-950) U/ml vs. 0.4 (0.4-135) U/ml at Time point A (p=0.0175), 287 (1.39-9,871) U/ml vs. 189.5 (0.4-7,260) U/ml at Time point B (p=0.0669), and 219 (22.6-3,813) U/ml vs. 168.5 (0.4-2,436) U/ml at Time point C (p=0.0962) (Figure 5).
Figure 5. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to ECOG-PS.
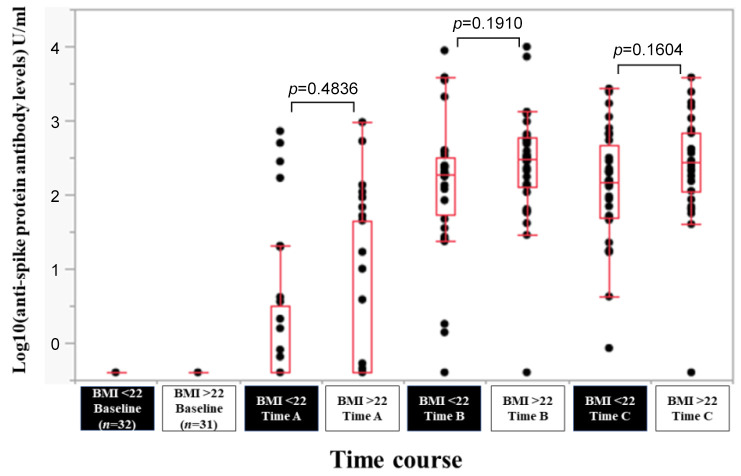
Efficacy of BNT162b2 vaccine according to BMI. In patients with BMI >22 kg/m2 (n=31) and BMI <22 kg/m2 (n=32), the response rate at the Time point A, B, and C was 35.5% (11/31), 96.8% (30/31) and 96.8% (30/31) in patients with BMI >22 kg/m2, and 34.4% (11/32), 96.9% (31/32) and 100% (32/32) in patients with BMI <22 kg/m2. The median (range) anti-spike protein antibody levels in patients with BMI >22 kg/m2 vs. BMI <22 kg/m2 at the Time point A, B, and C were 0.4 (0.4-950) U/ml vs. 0.4 (0.4-718) U/ml at Time point A (p=0.4,836), 305 (0.4-9,871) U/ml vs. 189.5 (0.4-8,773) U/ml at Time point B (p=0.1910), and 276 (0.4-3,813) U/ml vs. 144.5 (0.85-2,682) U/ml at Time point C (p=0.1604) (Figure 6).
Figure 6. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to body mass index (BMI).
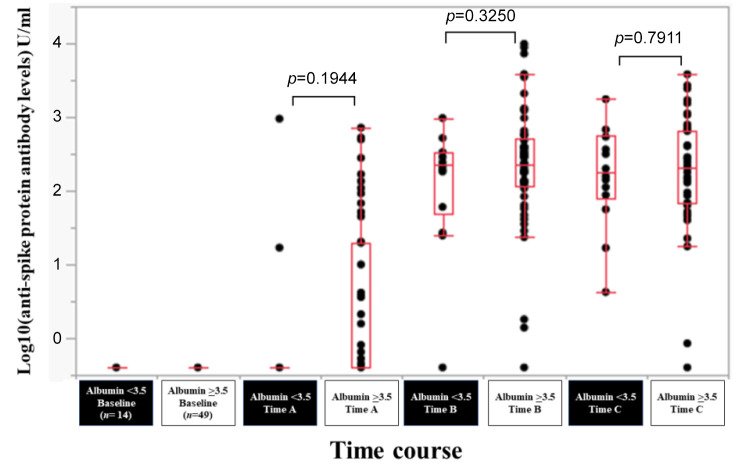
Efficacy of BNT162b2 vaccine according to serum albumin level. In patients with serum albumin <3.5 g/dl (n=14) and serum albumin ≥3.5 g/dl (n=49), the response rate at the Time point A, B, and C was 14.3% (2/14), 92.9% (13/14) and 100% (14/14) in patients with serum albumin <3.5 g/dl, and 40.8% (20/49), 98.0% (48/49) and 98.0% (48/49) in patients with serum albumin ≥3.5 g/dl. The median (range) anti-spike protein antibody levels in patients with serum albumin <3.5 g/dl vs. serum albumin ≥3.5 g/dl at the Time point A, B, and C were 0.4 (0.4-950) U/ml vs. 0.4 (0.4-718) U/ml at Time point A (p=0.1944), 227 (0.4-970) U/ml vs. 230 (0.4-9,871) U/ml at Time point B (p=0.3250), and 179 (4.23-1,749) U/ml vs. 210 (0.4-3813) U/ml at Time point C (p=0.7911) (Figure 7).
Figure 7. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to serum albumin level.
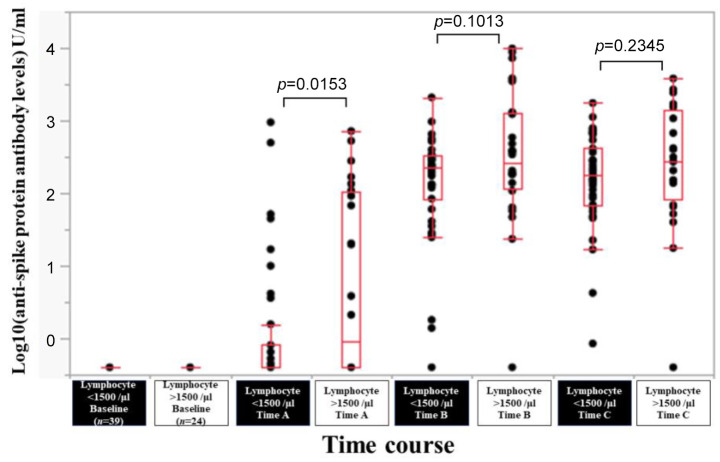
Efficacy of BNT162b2 vaccine according to total lymphocyte count. In patients with total lymphocyte count <1,500/μl (n=39) and total lymphocyte count >1,500/μl (n=24), the response rate at the Time point A, B, and C was 25.6% (10/39), 97.4% (38/39) and 100% (39/39) in patients with total lymphocyte count <1,500/μl, and 50.0% (12/24), 95.8% (23/24) and 95.8% (23/24) in patients with total lymphocyte count >1,500/μl. The median (range) anti-spike protein antibody levels in patients with total lymphocyte count <1,500/μl vs. total lymphocyte count >1,500/μl at the Time point A, B, and C were 0.4 (0.4-950) U/ml vs. 1.255 (0.4-718) U/ml at Time point A (p=0.0153), 230 (0.4-2,090) U/ml vs. 273.5 (0.4-9,871) U/ml at Time point B (p=0.1013), and 179 (0.85-1,749) U/ml vs. 274 (0.4-3,813) U/ml at Time point C (p=0.2345) (Figure 8).
Figure 8. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to total lymphocyte count.
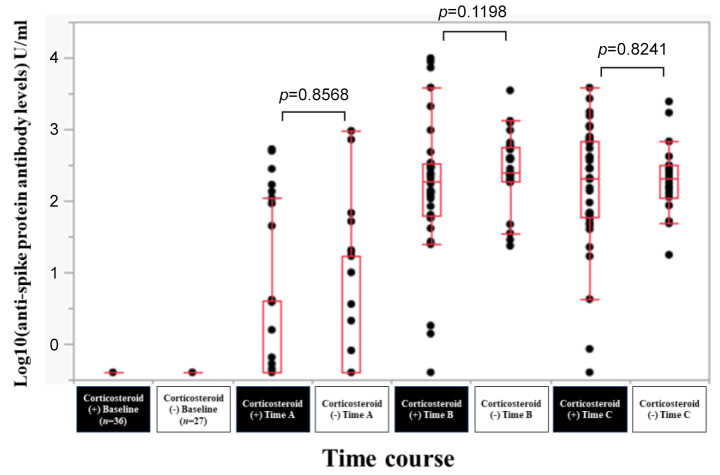
Efficacy of BNT162b2 vaccine according to corticosteroid therapy. In patients with corticosteroid therapy (n=36) and without corticosteroid therapy (n=27), the response rate at the Time point A, B, and C was 30.6% (11/36), 94.4% (34/36) and 97.2% (35/36) in patients with corticosteroid therapy, and 40.7% (11/27), 100% (27/27) and 100% (27/27) in patients without corticosteroid therapy. The median (range) anti-spike protein antibody levels in patients with corticosteroid therapy vs. without corticosteroid therapy at the Time point A, B, and C were 0.4 (0.4-527) U/ml vs. 0.4 (0.4-950) U/ml at Time point A (p=0.8568), 190.5 (94-9,871) U/ml vs. 247 (23.5-3,489) U/ml at Time point B (p=0.1198), and 205 (0.4-3,813) U/ml vs. 206 (17.6-2,436) U/ml at Time point C (p=0.8241) (Figure 9).
Figure 9. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to corticosteroid therapy.
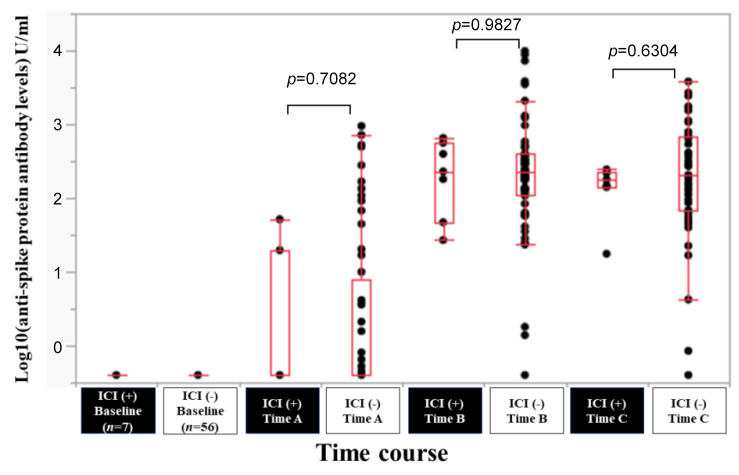
Efficacy of BNT162b2 vaccine according to ICI therapy. In patients with ICI therapy (n=7) and without ICI (n=56), the response rate at the Time point A, B, and C was 28.6% (2/7), 100% (7/7) and 100% (7/7) in patients with ICI therapy, and 35.7% (20/56), 96.4% (54/56) and 98.2% (55/56) in patients without ICI therapy. The median (range) anti-spike protein antibody levels in patients with ICI vs. without ICI at the Time point A, B, and C were 0.4 (0.4-51.7) U/ml vs. 0.4 (0.4-950) U/ml at Time point A (p=0.7082), 230 (27-654) U/ml vs. 231 (0.4-9,871) U/ml at Time point B (p=0.9827), and 179 (17.6-245) U/ml vs. 208 (0.4-3,813) U/ml at Time point C (p=0.6304) (Figure 10).
Figure 10. Log10(anti-spike protein antibody levels) U/ml at baseline, Time point A, Time point B, and Time point C according to immune checkpoint inhibitor (ICI) therapy.
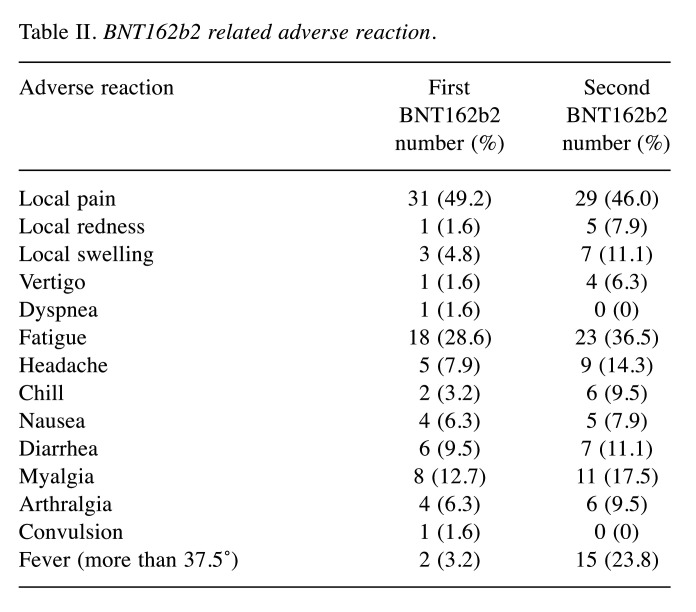
Safety profile. At the initial BNT162b2 vaccination, adverse reactions that occurred with a frequency of 10% or greater were local pain (31 cases, 49.2%), fatigue (18 cases, 28.6%), and myalgia (8 cases, 12.7%) (Table II). All adverse reactions improved with symptomatic treatment, and none of the patients required postponement or discontinuation of systemic chemotherapy because of an adverse reaction.
Table II. BNT162b2 related adverse reaction.
At the second BNT162b2 vaccination, adverse reactions that occurred with a frequency of 10% or greater were local pain (29 cases, 46.0%), fatigue (23 cases, 36.5%), fever (more than 37.5℃) (15 cases, 23.8%), myalgia (11 cases, 17.5%), headache (9 cases, 14.3%), local swelling (7 cases, 11.1%) and diarrhea (7 cases, 11.1%) (Table II). All adverse reactions improved with symptomatic treatment, and none of the patients required postponement or discontinuation of systemic chemotherapy because of an adverse reaction.
Discussion
In Japan, during the COVID-19 pandemic, the first clinical introduction of the BNT162b2 mRNA vaccine was launched under pressure of time (17,18). Despite evidence supported by large-scale clinical trials (8,9), speculation about its safety has been rife and has confused even medical professionals. Under these circumstances, it is essential to disseminate solid evidence on vaccination against COVID-19 infection. Cancer patients are at higher risk of COVID-19 severity and mortality (19,20). Although Japanese and foreign administrative agencies list cancer patients as a priority group for vaccination, it is unclear whether data from healthy individuals can be extrapolated. On the other hand, it is ethically unacceptable to conduct a randomized controlled trial to test for the efficacy and safety of the COVID-19 vaccine in cancer patients only during the COVID-19 pandemic. In the current study, we focused on immunogenicity. We confirmed the effectiveness of the BNT162b2 vaccine in cancer patients undergoing systemic chemotherapy by following the course of anti-spike protein antibody levels, which are easily measured. As mentioned earlier, sufficient evidence supporting the safety and efficacy of vaccines for cancer patients undergoing systemic chemotherapy is currently lacking (13,14). Thus, we believe that the current data are worthy of reporting.
The present study was conducted in patients with solid tumors undergoing systemic chemotherapy, and it showed a high response rate (our response rate: 98.4% (62/63) at 3 months after the second BNT162b2 vaccination), which is similar to a previous report on healthy adults (21). In the univariate analyses according to sex, age, ECOG-PS, BMI, serum albumin level, total lymphocyte count, corticosteroid use, and ICI therapy, no significant difference in anti-spike protein antibody levels was found between any two groups at 3 months after the second BNT162b2 vaccination, which indicates the consistent efficacy of BNT162b2 vaccine independent of patient status and tumor status. Although the anti-spike protein antibody levels were lower than those previously reported in healthy adults (10), they were sufficiently high to be effective against COVID-19 (the median anti-spike protein antibody levels in our data: 230 U/ml and 206 U/ml at 1 and 3 months after the second BNT162b2 vaccination). The only patient who did not respond to the BNT162b2 vaccine in this study was a 70-year-old female with ascending colon cancer, a case with no immunological abnormalities or other risk factors. It may be difficult to predict the response to the BNT162b2 vaccine in cancer patients.
It was difficult to obtain sufficient antibody titers in patients with hematologic malignancies undergoing systemic chemotherapy (13,22,23). Herishanu et al. reported that in 167 patients with chronic lymphocytic leukemia, the antibody response rate of the BNT162b vaccine was 39.5% (22). Perry et al. reported that the response rates of the BNT162b vaccine were 49% in patients with B-cell non-Hodgkin lymphoma vs. 98.5% in 65 healthy controls (23). This study’s results show a higher response rate for patients with solid tumors undergoing chemotherapy than patients with hematological malignancies undergoing multidisciplinary chemotherapy. This may be partly due to the reduced ability to produce anti-spike protein antibodies specific to hematological malignancies and the intensity of chemotherapy (24).
The safety profile of BNT162b in cancer patients undergoing chemotherapy is comparable to previously reported results on healthy adults (8,9), indicating that BNT162b is effective and safe in Japanese cancer patients undergoing systemic chemotherapy. COVID-19 has not yet subsided, and data on the safety and efficacy of additional vaccinations are essential. We will continue to accumulate data on the BNT162b vaccine in solid cancer patients receiving systemic chemotherapy.
We acknowledge several limitations to the study. First, this is a single-center study. Second, patient backgrounds are highly heterogeneous, involving various kinds of solid malignancies and chemotherapeutic regimens. Third, many patients were excluded from the analysis due to missing data of anti-spike protein antibodies, which also led to bias. Fourth, it is not clear whether antibody responses in the present study are protective towards COVID-19. Fifth, because the control group (i.e., healthy volunteer) is missing in this study, it is difficult to evaluate whether chemotherapy affected antibody responses. Thus, further studies will be needed to confirm these results.
In conclusion, the BNT162b vaccine in solid cancer patients undergoing systemic chemotherapy is effective and safe. Even in solid cancer patients undergoing systemic chemotherapy, aggressive BNT162b vaccination can be recommended.
Funding
Nihon Kayaku Corporation funded a part of this study.
Conflicts of Interest
None of the Authors has any conflicts of interest to declare.
Authors’ Contributions
Data curation: Eiki Yamasaki, Fukutaro Shimamoto, Hiroki Nishikawa, Mitsuhiko Iwamoto, Kosei Kimura, Naofumi Oosaka, Tetsuji Terazawa, Toshifumi Yamaguchi, Ken Asaishi and Takako Ikegami; Formal analysis: Hiroki Nishikawa and Toshifumi Yamaguchi; Funding acquisition: Fukutaro Shimamoto and Masahiro Goto; Methodology: Fukutaro Shimamoto; Project administration: Fukutaro Shimamoto and Akira Ukimura; Supervision: Masahiro Goto, Akira Ukimura, Fumihito Ono, Kazuhisa Uchiyama, Shiro Nakamura and Kazuhide Higuchi; Writing – original draft: Eiki Yamasaki and Hiroki Nishikawa; Writing – review & editing: Kohei Taniguchi and Toshifumi Yamaguchi.
Acknowledgements
The Authors gratefully thank all medical staff in our Department for their help with data collection.
References
- 1.Motamedi H, Ari MM, Dashtbin S, Fathollahi M, Hossainpour H, Alvandi A, Moradi J, Abiri R. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int Immunopharmacol. 2021;96:107763. doi: 10.1016/j.intimp.2021.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eroglu B, Nuwarda RF, Ramzan I, Kayser V. A narrative review of COVID-19 vaccines. Vaccines (Basel) 2021;10(1):62. doi: 10.3390/vaccines10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awadasseid A, Wu Y, Tanaka Y, Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int J Biol Sci. 2021;17(1):8–19. doi: 10.7150/ijbs.52569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Iwagami M, Ishiguro C, Fujii D, Yamamoto N, Narisawa M, Tsuboi T, Umeda H, Kinoshita N, Iguchi T, Noda T, Tsuruta S, Oka A, Morio T, Nakai K, Hayashi S. Safety monitoring of COVID-19 vaccines in Japan. Lancet Reg Health West Pac. 2022;23:100442. doi: 10.1016/j.lanwpc.2022.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamb YN. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs. 2021;81(4):495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 7.Doroftei B, Ciobica A, Ilie OD, Maftei R, Ilea C. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics (Basel) 2021;11(4):579. doi: 10.3390/diagnostics11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU, C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kageyama T, Ikeda K, Tanaka S, Taniguchi T, Igari H, Onouchi Y, Kaneda A, Matsushita K, Hanaoka H, Nakada TA, Ohtori S, Yoshino I, Matsubara H, Nakayama T, Yokote K, Nakajima H. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27(12):1861.e1–1861.e5. doi: 10.1016/j.cmi.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajema KL, Dahl RM, Evener SL, Prill MM, Rodriguez-Barradas MC, Marconi VC, Beenhouwer DO, Holodniy M, Lucero-Obusan C, Brown ST, Tremarelli M, Epperson M, Mills L, Park SH, Rivera-Dominguez G, Morones RG, Ahmadi-Izadi G, Deovic R, Mendoza C, Jeong C, Schrag SJ, Meites E, Hall AJ, Kobayashi M, McMorrow M, Verani JR, Thornburg NJ, Surie D, SUPERNOVA COVID-19 , Surveillance Group , Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans - Five Veterans Affairs medical centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700–1705. doi: 10.15585/mmwr.mm7049a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCCN COVID-19 vaccination guide for people with cancer. Available at: https://www.nccn.org/docs/default-source/covid-19/covid-vaccine-and-cancer-05. [Last accessed on August 31, 2022]
- 13.Tadmor T, Benjamini O, Braester A, Rahav G, Rokach L. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2727–2730. doi: 10.1038/s41375-021-01380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funakoshi Y, Yakushijin K, Ohji G, Hojo W, Sakai H, Takai R, Nose T, Ohata S, Nagatani Y, Koyama T, Kitao A, Nishimura M, Imamura Y, Kiyota N, Harada K, Tanaka Y, Mori Y, Minami H. Safety and immunogenicity of the COVID-19 vaccine BNT162b2 in patients undergoing chemotherapy for solid cancer. J Infect Chemother. 2022;28(4):516–520. doi: 10.1016/j.jiac.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JT, La J, Branch-Elliman W, Huhmann LB, Han SS, Parmigiani G, Tuck DP, Brophy MT, Do NV, Lin AY, Munshi NC, Fillmore NR. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: A US Nationwide Veterans Affairs study. JAMA Oncol. 2022;8(2):281–286. doi: 10.1001/jamaoncol.2021.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Internal R&D data on file. Roche Diagnostics GmbH, Penzberg, Germany. Available at: https://www.roche.com/innovation/structure/rnd-locations/dia-penzberg. [Last accessed on September 9, 2022]
- 17.Maeda H. Japan’s special approval for emergency system during the COVID-19 pandemic. Clin Pharmacol Ther. 2022;111(3):551–558. doi: 10.1002/cpt.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilevbare SI, McPherson G. Understanding COVID-19: A hybrid threat and its impact on sport mega-events. A focus on Japan and the Tokyo 2020 Olympic Games. Front Sports Act Living. 2022;4:720591. doi: 10.3389/fspor.2022.720591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep. 2020;22(5):53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuderer NM, Hill JA, Carpenter PA, Lyman GH. Challenges and opportunities for COVID-19 vaccines in patients with cancer. Cancer Invest. 2021;39(3):205–213. doi: 10.1080/07357907.2021.1885596. [DOI] [PubMed] [Google Scholar]
- 21.Nam M, Seo JD, Moon HW, Kim H, Hur M, Yun YM. Evaluation of humoral immune response after SARS-CoV-2 vaccination using two binding antibody assays and a neutralizing antibody assay. Microbiol Spectr. 2021;9(3):e0120221. doi: 10.1128/Spectrum.01202-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, Morales M, Ziv T, Shorer Arbel Y, Scarfò L, Joffe E, Perry C, Ghia P. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, Tabib Y, Cohen YC, Benyamini N, Beyar-Katz O, Neaman M, Vitkon R, Keren-Khadmy N, Levin M, Herishanu Y, Avivi I. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riccardi N, Falcone M, Yahav D. Vaccination for SARS-CoV-2 in hematological patients. Acta Haematol. 2022;145(3):257–266. doi: 10.1159/000523753. [DOI] [PMC free article] [PubMed] [Google Scholar]