Abstract
Background/Aim: Carbohydrate antigen 19-9 (CA19-9) levels may aid in the determination of subsequent treatment in patients with unresectable locally-advanced pancreatic cancer (LAPC) treated with chemotherapy. However, the relationship between the timing and magnitude of CA19-9 changes and clinical outcomes remains unclear. This study was conducted to identify the timing and magnitude of CA19-9 changes, which are most strongly associated with outcomes in LAPC patients.
Patients and Methods: We retrospectively analyzed consecutive LAPC patients treated with gemcitabine plus nab-paclitaxel (GnP) or modified FOLFIRINOX (mFFX) as the first-line chemotherapy between March 2014 and December 2018 in our hospital. Multivariate analyses were performed to identify prognostic factors of chemotherapy in LAPC.
Results: Ninety-four patients were included (GnP/mFFX: 72/22). The median overall survival was 20.3 months, and the median progression-free survival was 8.8 months. CA19-9 values before treatment did not affect prognosis. However, CA19-9 <100 U/ml or more than a 70% reduction in CA19-9 four months after commencing treatment was associated with a good prognosis (hazard ratio=0.17; 95% confidence interval=0.09-0.33; p<0.01).
Conclusion: CA19-9 values 4 months after commencing treatment are a significant prognostic factor in LAPC patients undergoing chemotherapy.
Keywords: Locally-advanced pancreatic cancer, CA19-9, gemcitabine with nab-paclitaxel, FOLFIRINOX, prognostic factor
Pancreatic cancer (PC) is the fourth leading cause of cancer-related death in the United States and Japan (1,2). PC has a poor prognosis as early detection is difficult, with approximately 80% presenting with locally-advanced or metastatic disease (3). Locally-advanced pancreatic cancer (LAPC) refers to PC without distant metastases but with blood vessel involvement, which makes it difficult to achieve a margin-free resection (4,5).
Various treatment modalities have been reported for LAPC, including chemotherapy, radiation, and chemoradiation. However, no standard treatment has been established due to the lack of data from randomized controlled trials. Recently, gemcitabine plus nab-paclitaxel (GnP) and FOLFIRINOX (FFX) have been widely used as standard first-line treatment for metastatic PC (6,7). Data on the superiority of one over the other remains inconclusive (8). The efficacies of these combination chemotherapy regimens have also been reported in LAPC (9-12).
Several reports refer to CA19-9 as a prognostic factor for PC without distant metastasis, but most analyses focus on borderline resectable PC or treatment with chemoradiotherapy (13-17). Reports on outcomes of chemotherapy alone for LAPC are scarce. Although the National Comprehensive Cancer Network (NCCN) guidelines suggest that a decrease in CA19-9 aids in the determination of subsequent treatment, the relationship between the timing and the magnitude of CA19-9 changes and outcomes remains unclear (18). Previously, we reported the results of chemotherapy based on tumor location and first-line regimen in LAPC patients (9). In this study, we conducted a more detailed analysis to determine the association between the timing and the magnitude of CA19-9 reductions and prognosis in LAPC treated with chemotherapy as first-line treatment.
Patients and Methods
Patients. We retrospectively analyzed consecutive patients extracted from prospectively registered databases of patients with LAPC diagnosed pathologically and treated with GnP or modified FFX (mFFX) as first-line chemotherapy between March 2014 and December 2018. Patients who did not start chemotherapy at our hospital and those with pre-treatment CA19-9 levels of less than 2 U/ml (defined as CA19-9 non-secretors) were excluded. All patients underwent computed tomography (CT) for tumor staging. The diagnosis of LAPC was based on the 7th edition of the Japanese classification of pancreatic cancer (5), which is defined as one of the following cases, with no identifiable metastatic lesions: 1) abutment or invasion of more than 180 degrees of the circumference or occlusion of the superior mesenteric vein (SMV) or portal vein (PV), which extends beyond the inferior border of the duodenum, 2) abutment or invasion of more than 180 degrees of the superior mesenteric artery (SMA) or celiac artery (CA) circumference and abutment or invasion of the proper hepatic artery (PHA) or CA, or 3) abutment or invasion of the aorta. This study was approved by our institutional review board (Institutional Review Board number: 2020-1122).
Treatment. The GnP regimen consisted of 125 mg/m2 nab-paclitaxel administered for 30 min, followed by 1,000 mg/m2 gemcitabine administered for 30 min, on days 1, 8, and 15. Treatment cycles were repeated every four weeks. The mFFX regimen consisted of 85 mg/m2 oxaliplatin administered for 2 h, followed by 200 mg/m2 L-leucovorin administered for 2 h and 150 mg/m2 irinotecan for 90 min. This treatment was followed by 2,400 mg/m2 fluorouracil, administered as a 46-h continuous infusion (19). Treatment cycles were repeated every two weeks. In patients displaying 1) homozygosity for uridine diphosphate glucuronosyltransferase (UGT) 1A1 *6 or *28 or 2) compound heterozygosity for UGT1A1 *6 and *28, irinotecan was administered at a reduced dose (100 mg/m2). No prophylactic granulocyte-colony stimulating factor (G-CSF) was used. If severe adverse events (AEs) occurred, the dose was reduced and/or delayed at the discretion of the physician. Chemotherapy was continued until tumor progression, intolerable toxicity, or patient refusal. Patients with tumor shrinkage on CT suggesting a high likelihood of curative resection after chemotherapy for more than eight months (20) underwent conversion surgery if approved at a multidisciplinary conference of surgeons, oncologists, and radiologists. Second-line treatment was introduced at the oncologist’s discretion, taking each patient’s general condition into account.
Evaluations. To assess the efficacy of treatment, CT was performed every 8-12 weeks. Magnetic resonance imaging and/or fluorodeoxyglucose positron emission tomography were performed as needed. CT findings were assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (21). CA19-9 was measured every month and was evaluated at two months (6-10 weeks), four months (14-18 weeks), 6 months (22-26 weeks), and eight months (30-34 weeks) after treatment. The CA19-9 reduction rate of X months after treatment was defined as [(CA19-9 level at X months after treatment) – (CA19-9 level before treatment)]/(CA19-9 level before treatment). AE categories and grading were evaluated according to the Common Terminology Criteria for Adverse Events, version 4.0 (22). Follow-up data were confirmed up to August 31, 2020.
Statistical analysis. Continuous variables are presented as medians (ranges) and were compared using the Mann-Whitney U-test. Categorical variables are presented as absolute numbers (proportions) and were analyzed using the chi-squared or Fisher’s exact test. A p-value <0.05 was considered statistically significant. Progression-free survival (PFS) was defined as the period from the first day of chemotherapy to disease progression or the last follow-up. Overall survival (OS) was defined as the period from the first day of chemotherapy to the time of death or the last follow-up. Recurrence-free survival (RFS) was defined as the period from the day of surgery to recurrence or the last follow-up. PFS, OS and RFS were analyzed using the Kaplan-Meier method and the log-rank test. Univariate analysis was performed using the Cox proportional hazard model, and variables with p-values <0.20 in the univariate analysis were evaluated in multivariate analysis. Hazard ratio for OS was evaluated for the percentage of CA19-9 decrease and cut-off value of CA19-9 decrease. The combination of percentage and cut-off value of CA19-9 decrease, which had the lowest hazard ratio (HR) were selected for multivariable analysis.
All statistical analyses were performed using EZR ver. 1.40 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (23). All procedures were performed in accordance with the Declaration of Helsinki. Written informed consent for chemotherapy was obtained from all patients.
Results
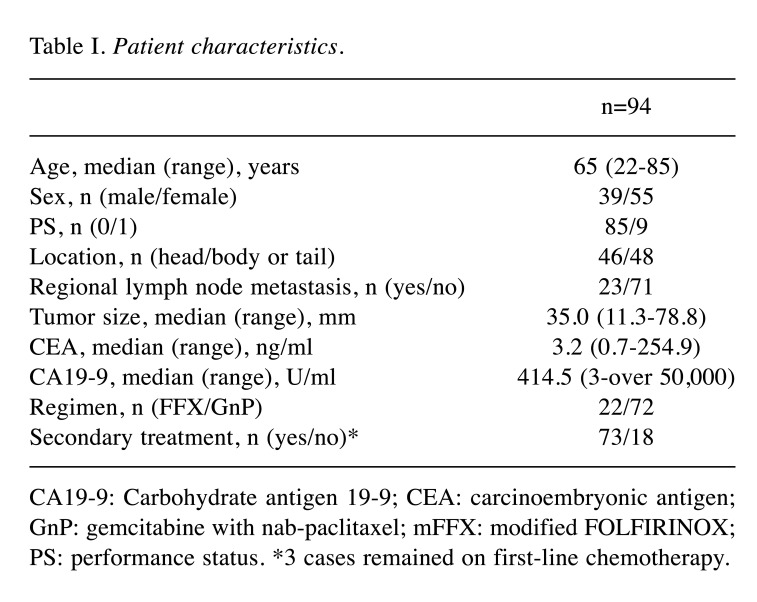
Patient characteristics. One hundred twenty-four consecutive patients with LAPC were treated with first-line GnP or mFFX chemotherapy between March 2014 and December 2018. Eighteen patients who started chemotherapy at another hospital and twelve CA19-9 non-secretors were excluded. As a result, 94 patients were included in this analysis. Patient characteristics are shown in Table I. The median age was 65 years (range=22-85 years). Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0 in 85 patients and 1 in nine patients. Twenty-two patients received mFFX and 72 patients received GnP as first-line chemotherapy. Seventy-three patients went on to receive second-line therapy (conversion surgery in seven patients, chemotherapy in 52 patients, chemoradiation in 12 patients, and radiation in two patients). One patient underwent conversion surgery after second-line treatment.
Table I. Patient characteristics.
CA19-9: Carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; GnP: gemcitabine with nab-paclitaxel; mFFX: modified FOLFIRINOX; PS: performance status. *3 cases remained on first-line chemotherapy.
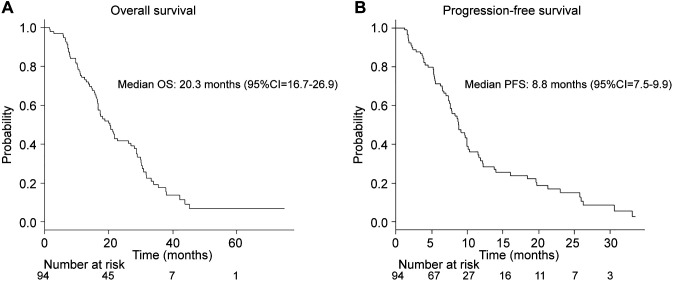
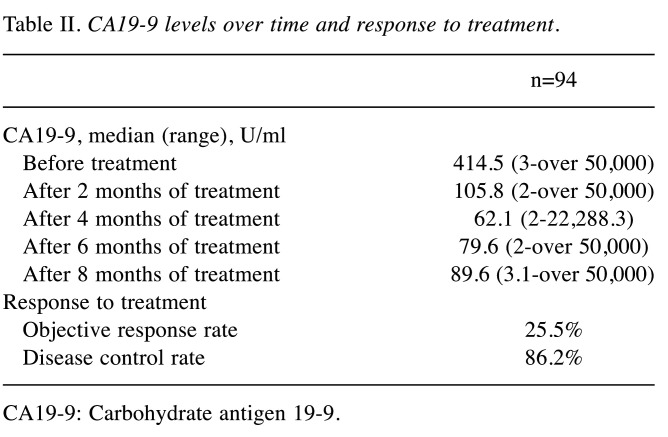
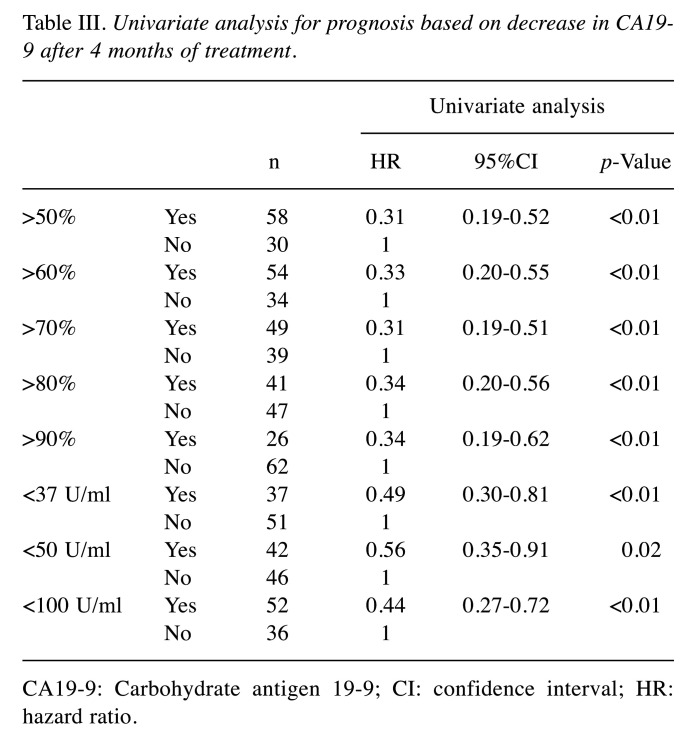
Treatment outcomes and CA19-9 trends. Median OS was 20.3 months (95% confidence interval (95%CI)=16.7-26.9 months) (Figure 1A). Median PFS was 8.8 months (95%CI=7.5-9.9 months) (Figure 1B). The median CA19-9 level before treatment and after 2, 4, 6, and 8 months of treatment was 414.5, 105.8, 62.1, 79.6, and 89.6 U/ml, respectively. The objective response rate was 25.5% and the disease control rate was 86.2%. The lowest level of CA19-9 before median PFS was observed after 4 months of treatment (Table II). Next, HRs for OS based on various degrees of reductions in CA19-9 at four months after treatment were calculated (Table III). Reduction rates were examined in 10% increments starting at reductions of more than 50%. HRs for OS based on raw CA19-9 values below the upper limit of normal (37 U/ml), below 50 U/ml, and below 100 U/ml at four months were also calculated. The lowest HRs were achieved with cutoffs at a reduction of 70% from the base level and at an absolute cutoff of 100 U/ml.
Figure 1. Overall survival (A) and progression-free survival (B) analysis for all patients. CI: Confidence interval; OS: overall survival; PFS: progression-free survival.
Table II. CA19-9 levels over time and response to treatment.
CA19-9: Carbohydrate antigen 19-9.
Table III. Univariate analysis for prognosis based on decrease in CA19-9 after 4 months of treatment.
CA19-9: Carbohydrate antigen 19-9; CI: confidence interval; HR: hazard ratio.
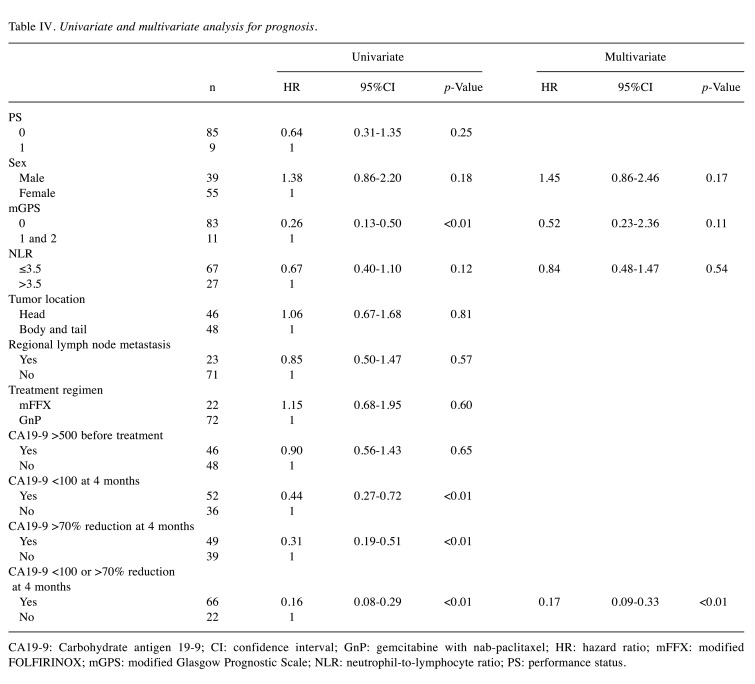
Prognostic factors. In the univariate Cox proportional hazard model, modified Glasgow Prognostic Scale (mGPS) of 0 (HR=0.26; 95%CI=0.13-0.50; p<0.01), CA19-9 below 100 U/ml at four months (HR=0.44; 95%CI=0.27-0.72; p<0.01), CA19-9 reduction of more than 70% from baseline at four months (HR=0.31; 95% CI=0.19-0.51; p<0.01), and CA19-9 below 100 U/ml or CA19-9 reduction of more than 70% from baseline at four months (HR=0.16; 95%CI=0.08-0.29; p<0.01) were significant factors predicting a favorable prognosis. Female sex and low neutrophil-to-lymphocyte ratio (NLR) also had a tendency to predict favorable prognoses, with p<0.2.
To avoid multicollinearity issues, CA19-9 below 100 U/ml or CA19-9 reduction of more than 70% from baseline at four months was selected as the sole factor to be used in multivariate analysis relating to this tumor marker, as the lowest HR was observed under these conditions. Multivariate analysis showed that less than 100 U/ml or more than 70% reduction of CA19-9 at four months (HR=0.17; 95%CI=0.09-0.33; p<0.01) was an independent predictor of a favorable prognosis (Table IV).
Table IV. Univariate and multivariate analysis for prognosis.
CA19-9: Carbohydrate antigen 19-9; CI: confidence interval; GnP: gemcitabine with nab-paclitaxel; HR: hazard ratio; mFFX: modified FOLFIRINOX; mGPS: modified Glasgow Prognostic Scale; NLR: neutrophil-to-lymphocyte ratio; PS: performance status.
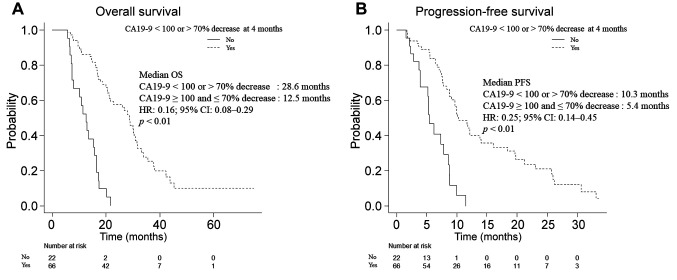
Median OS of patients with CA19-9 below 100 U/ml or CA19-9 reduction of more than 70% from baseline at four months (the Reduction group) was 28.6 months, which was significantly longer than that of patients without such reductions in CA19-9 (12.5 months in the Non-reduction group; HR=0.16; 95%CI=0.08-0.29; p<0.01) (Figure 2A). Median PFS was 10.3 months in the Reduction group and 5.4 months in the Non-reduction group (HR=0.25; 95%CI=0.14-0.45; p<0.01) (Figure 2B). Of the 76 patients who remained on first-line chemotherapy at four months, 58 were in the Reduction group. The rate of patients who were progression-free and remained on first-line treatment was significantly higher in the Reduction group (74.1% in the Reduction group vs. 33.3% in the Non-reduction group, p<0.01).
Figure 2. Overall survival (A) and progression-free survival (B) analysis according to CA19-9 reduction level. CA19-9: Carbohydrate antigen 19-9; CI: confidence interval; HR: hazard ratio; OS: overall survival; PFS: progression-free survival.
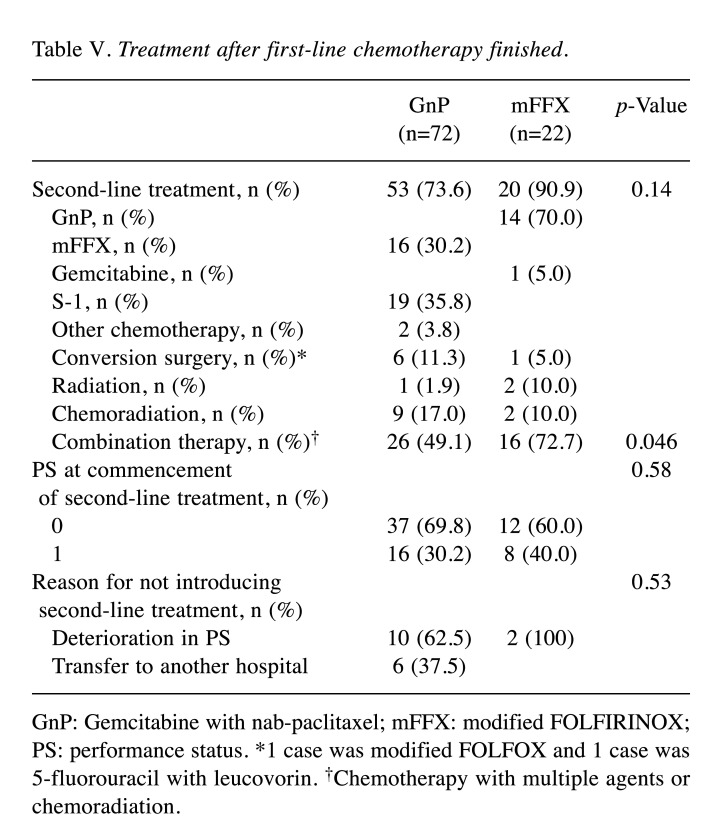
Secondary treatment. Secondary treatment was introduced in 77% of patients (of which 53 received first-line GnP therapy and 20 received first-line mFFX therapy) (Table V). Patients receiving first-line mFFX therapy tended to be more likely to go on to receive second-line treatment, but no significant difference was observed (73.6% in the GnP group and 90.9% in the mFFX group, p=0.14). Secondary combination therapy (chemotherapy with multiple agents or chemoradiation) was significantly more common in the mFFX group (p=0.046).
Table V. Treatment after first-line chemotherapy finished.
GnP: Gemcitabine with nab-paclitaxel; mFFX: modified FOLFIRINOX; PS: performance status. *1 case was modified FOLFOX and 1 case was 5-fluorouracil with leucovorin. †Chemotherapy with multiple agents or chemoradiation.
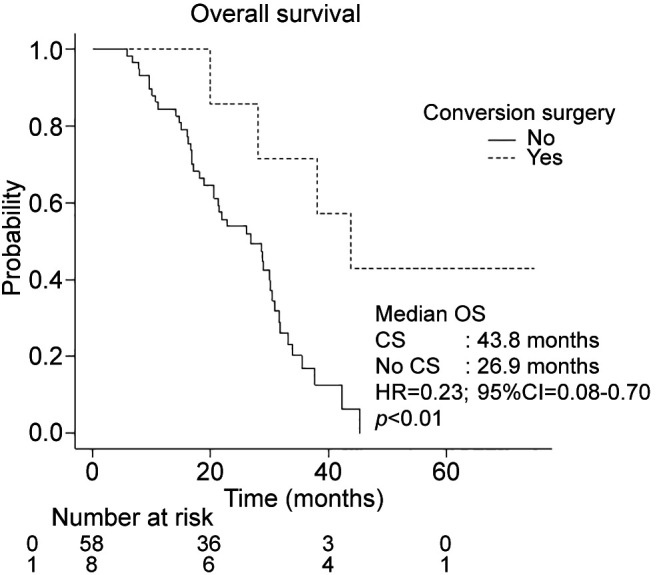
Eight patients underwent conversion surgery after chemotherapy. Seven underwent conversion surgery after first-line chemotherapy (six after GnP and one after mFFX), and one after second-line chemotherapy (GnP as first-line treatment, followed by mFFX as second-line treatment). Postoperative recurrence was observed in five cases, of which three had pulmonary metastasis, two had local recurrence, and one had peritoneal dissemination. Median RFS of the eight patients was 24.1 months (95%CI=3.4–not available). All patients who underwent conversion surgery were in the Reduction group. Within the Reduction group, those who underwent conversion surgery achieved longer median OS than those who did not (43.8 vs. 26.9 months, p<0.01) (Figure 3).
Figure 3. Overall survival in the CA19-9 reduction group with or without conversion surgery. CI: Confidence interval; CS: conversion surgery; HR: hazard ratio; OS: overall survival; PFS: progression-free survival.
Adverse events. There was a trend for more severe (Grade 3 or higher) anorexia in the mFFX group than in the GnP group, but no other difference was observed between the two groups. There was no difference in febrile neutropenia between the two groups (1.4 % in the GnP group vs. 9.1% in the mFFX group, p=0.14). No chemotherapy-related deaths were observed.
Discussion
In this study, we found that CA19-9 values four months after commencing treatment is a significant prognostic factor in LAPC patients undergoing chemotherapy. Median OS was 20.3 months, with some cases achieving long-term survival.
CA19-9 is a well-known tumor marker in patients with PC, with reported sensitivity and specificity of 41-86% and 33-100%, respectively (24). Also known as sialyl Lewis antigen A, CA19-9 is not secreted in the 5-10% of patients with negative Lewis blood group antigen (25). Pre-treatment CA19-9 levels and decreases in CA19-9 after treatment have been reported as prognostic factors (13-17,26,27). Various reductions in CA19-9 have been reported as positive prognostic factors, ranging from 15-94.4% (14,17,27,28). One report suggests that a decrease in CA19-9 could be an earlier marker for chemotherapeutic efficacy than CT findings based on the RECIST guideline (27). In this study, the lowest median CA19-9 level was at four months after treatment, but the median PFS was 8.8 months. Thus, a rebound in CA19-9 levels may be observed before signs of disease progression can be observed on diagnostic imaging.
Past reports did not clarify whether the percentage decrease in CA19-9 or the trough CA19-9 level is more important as a prognostic factor. While both can be affected by pre-treatment values, we found that pre-treatment CA19-9 was not a prognostic factor in LAPC. Our analyses point to CA19-9 below 100 U/ml or CA19-9 reduction of more than 70% from baseline at four months as the most relevant prognostic indicator, suggesting that both the percentage decrease and the absolute CA19-9 levels play a role in predicting outcomes in LAPC. The Reduction group achieved a favorable median OS of 28.6 months, while median OS in the Non-reduction group was 12.5 months. In the Non-reduction group, median PFS was only 5.4 months. Thus, patients not achieving two-digit CA19-9 levels or a 70% reduction in CA19-9 at four months may be likely to experience disease progression on their next follow-up CT.
The NCCN guidelines recommend referral for potential surgery despite radiographic stability if there is marked clinical improvement or decline in CA19-9 (18). However, the timing and extent of the decrease warranting such referral is not stated. Reni and colleagues (28) reported that median time to CA19-9 nadir was four months. In their study, longer time to CA19-9 nadir was associated with longer OS (1<2<4<6 months) in patients with Stage II and III PC. They also found that patients with a CA19-9 decrease of over 50% at nadir had longer OS than those with a smaller decrease. Our analysis further investigated the precise cut-off point for CA19-9 reduction at four months to predict OS, concluding that a CA19-9 decrease of at least 70% or to less than 100 U/ml at four months was the most statistically significant decrease for predicting prognosis in LAPC. Our study simplifies the use of CA19-9 as a prognostic factor, allowing all patients to be evaluated after four months. This avoids the need to wait until CA19-9 nadir is confirmed, which is only possible after a subsequent rebound in CA19-9 is observed.
In this study, eight patients underwent conversion surgery, all of whom were in the Reduction group. Thus, a marked decrease in CA19-9 at four months may be associated with the likelihood of becoming a candidate for conversion surgery. Chemotherapy for more than eight months has been reported as a favorable prognostic factor in conversion surgery (20), and whether first-line chemotherapy can be continued up to eight months was an important factor in considering conversion surgery. In the Reduction group, 74.1% of patients were able to continue first-line chemotherapy without progression for at least eight months. Median OS of patients undergoing conversion surgery was significantly better than those who did not (43.8 months vs. 26.9 months, p<0.01). Median recurrence-free survival after conversion surgery was over 2 years, which provided patients with time away from chemotherapy. Further studies are required to evaluate the impact of conversion surgery on OS.
This study has several limitations. First, it was a retrospective cohort study in a single center. Second, outcomes in CA19-9 non-secretors could not be evaluated. Third, CA19-9 as a prognostic factor was assessed only after four months of treatment. Fourth, the study was limited to patients who received GnP or mFFX as first-line therapy.
In conclusion, CA19-9 of less than 100 U/ml or a decrease of more than 70% from baseline at four months was a positive prognostic factor for chemotherapy in LAPC. Patients meeting these criteria should be followed closely, with consideration given to possible conversion surgery in the future.
Conflicts of Interest
The Authors have no relevant financial or non-financial interests to disclose.
Authors’ Contributions
Study concept and design: T.M., M.O., N.S. Acquisition of data: T.M., T.T., Y.U., C.M., T.F., Y.Y., A.K., M.M., T.S. Analysis of data: T.M., M.O., T.T., N.S. Study supervision: M.O., Y.I., Y.T., N.S.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. 2022. Available at: https://ganjoho.jp/public/qa_links/report/statistics/2022_en.html. [Last accessed on August 23, 2022]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146(1):291–304.e1. doi: 10.1053/j.gastro.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Isaji S. Revised 7th edition of the General Rules for the Study of Pancreatic Cancer by Japan Pancreas Society -revised concepts and updated points. Nihon Shokakibyo Gakkai Zasshi. 2017;114(4):617–626. doi: 10.11405/nisshoshi.114.617. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of Unicancer , PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mie T, Sasaki T, Takeda T, Fukuda K, Furukawa T, Yamada Y, Kasuga A, Matsuyama M, Ozaka M, Sasahira N. Comparison of treatment outcomes between gemcitabine with nab-paclitaxel and modified FOLFIRINOX for first-line chemotherapy in metastatic and recurrent pancreatic cancer: propensity score matching. Pancreas. 2021;50(4):595–601. doi: 10.1097/MPA.0000000000001801. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, Sasaki T, Mie T, Furukawa T, Yamada Y, Kasuga A, Matsuyama M, Ozaka M, Sasahira N. The prognostic impact of tumour location and first-line chemotherapy regimen in locally advanced pancreatic cancer. Jpn J Clin Oncol. 2021;51(5):728–736. doi: 10.1093/jjco/hyab014. [DOI] [PubMed] [Google Scholar]
- 10.Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, El-Rayes BF, Wang-Gillam A, Lacy J, Hosein PJ, Moorcraft SY, Conroy T, Hohla F, Allen P, Taieb J, Hong TS, Shridhar R, Chau I, van Eijck CH, Koerkamp BG. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano F, Formisano L, Giardino A, Girelli R, Servetto A, Santaniello A, Foschini F, Marciano R, Mozzillo E, Carratù AC, Cascetta P, De Placido P, De Placido S, Bianco R. Neoadjuvant treatment in locally advanced pancreatic cancer (LAPC) patients with FOLFIRINOX or gemcitabine nabpaclitaxel: a single-center experience and a literature review. Cancers (Basel) 2019;11(7):981. doi: 10.3390/cancers11070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perri G, Prakash L, Qiao W, Varadhachary GR, Wolff R, Fogelman D, Overman M, Pant S, Javle M, Koay EJ, Herman J, Kim M, Ikoma N, Tzeng CW, Lee JE, Katz MHG. Response and survival associated with first-line FOLFIRINOX vs. gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 2020;155(9):832–839. doi: 10.1001/jamasurg.2020.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi YH, Lee SH, You MS, Shin BS, Paik WH, Ryu JK, Kim YT, Kwon W, Jang JY, Kim SW. Prognostic factors for patients with borderline resectable or locally advanced pancreatic cancer receiving neoadjuvant FOLFIRINOX. Gut Liver. 2021;15(2):315–323. doi: 10.5009/gnl19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YJ, Koh HK, Chie EK, Oh DY, Bang YJ, Nam EM, Kim K. Change in carbohydrate antigen 19-9 level as a prognostic marker of overall survival in locally advanced pancreatic cancer treated with concurrent chemoradiotherapy. Int J Clin Oncol. 2017;22(6):1069–1075. doi: 10.1007/s10147-017-1129-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee W, Park Y, Kwon JW, Jun E, Song KB, Lee JH, Hwang DW, Yoo C, Kim KP, Jeong JH, Chang HM, Ryoo BY, Park SY, Kim SC. Reduced and normalized carbohydrate antigen 19-9 concentrations after neoadjuvant chemotherapy have comparable prognostic performance in patients with borderline resectable and locally advanced pancreatic cancer. J Clin Med. 2020;9(5):1477. doi: 10.3390/jcm9051477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JL, Kadera BE, Nguyen AH, Muthusamy VR, Wainberg ZA, Hines OJ, Reber HA, Donahue TR. CA19-9 normalization during pre-operative treatment predicts longer survival for patients with locally progressed pancreatic cancer. J Gastrointest Surg. 2016;20(7):1331–1342. doi: 10.1007/s11605-016-3149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent L, Sefrioui D, Bignon AL, Parzy A, Sidali S, Hassine M, Gangloff A, Galais MP, Bouhier-Leporrier K, Michel P, Di Fiore F. CA19.9 decrease >15% is a predictor of favourable outcome in patients treated for advanced pancreatic carcinoma: analysis of two independent cohorts. HPB (Oxford) 2019;21(5):582–588. doi: 10.1016/j.hpb.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology Pancreatic Adenocarcinoma. Version 1. 2021, October 23, 2020. Available at: https://www.nccn.org/guidelines/guidelines-process/transparencyprocess-and-recommendations/GetFileFromFileManager?fileManagerId=11052. [Last accessed on September 20, 2020] [DOI] [PubMed]
- 19.Ozaka M, Ishii H, Sato T, Ueno M, Ikeda M, Uesugi K, Sata N, Miyashita K, Mizuno N, Tsuji K, Okusaka T, Furuse J. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81(6):1017–1023. doi: 10.1007/s00280-018-3577-9. [DOI] [PubMed] [Google Scholar]
- 20.Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, Kwon AH, Hirano S, Kinoshita T, Nakao A, Nagano H, Nakajima Y, Sano K, Miyazaki M, Takada T. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20(6):590–600. doi: 10.1007/s00534-013-0616-0. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 4th ed, June 14, 2010. Available at: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Last accessed on September 20, 2020]
- 23.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70(4):255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 25.Guo M, Luo G, Lu R, Shi W, Cheng H, Lu Y, Jin K, Yang C, Wang Z, Long J, Xu J, Ni Q, Liu C, Yu X. Distribution of Lewis and Secretor polymorphisms and corresponding CA19-9 antigen expression in a Chinese population. FEBS Open Bio. 2017;7(11):1660–1671. doi: 10.1002/2211-5463.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnier J, Ewald J, Marchese U, Gilabert M, Launay S, Moureau-Zabotto L, Poizat F, Giovannini M, Delpero JR, Turrini O. Outcomes of patients with initially locally advanced pancreatic adenocarcinoma who did not benefit from resection: a prospective cohort study. BMC Cancer. 2020;20(1):203. doi: 10.1186/s12885-020-6690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiorean EG, Von Hoff DD, Reni M, Arena FP, Infante JR, Bathini VG, Wood TE, Mainwaring PN, Muldoon RT, Clingan PR, Kunzmann V, Ramanathan RK, Tabernero J, Goldstein D, McGovern D, Lu B, Ko A. CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol. 2016;27(4):654–660. doi: 10.1093/annonc/mdw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reni M, Peretti U, Zanon S, Macchini M, Balzano G, Mazza E, Tamburrino D, Orsi G, Arcidiacono PG, Falconi M, Gianni L. Time to CA19-9 nadir: a clue for defining optimal treatment duration in patients with resectable pancreatic ductal adenocarcinoma. Cancer Chemother Pharmacol. 2020;85(4):641–650. doi: 10.1007/s00280-020-04047-7. [DOI] [PubMed] [Google Scholar]