Abstract
Background/Aim: The skin protects the body from ultraviolet rays and other external factors. Various studies have been conducted to identify methods to prevent skin aging and damage. To investigate the protective effects of methylsulfonylmethane (MSM), in this study, a hairless mouse model was used.
Patients and Methods: Mice divided into Groups B, C, and D were subjected to UVB irradiation for six weeks, and Group A was considered the control. Retinoic acid is a substance that has been proven to have anti-aging properties. Group C was injected with MSM, group D was injected with retinoic acid, and groups A and B were injected with saline. At the end of the experiment, the degree of senescence was confirmed through visual evaluation, histopathological analysis, immunohistochemistry, and elasticity measurement using SEM.
Results: After the end of the experiment, the wrinkle score was 0.4, 2.5, 1.8, 1.5 for Groups A, B, C, and D, respectively. Epidermal thickness was 40 μm, 70 μm, 60 μm, 55 μm in Groups A, B, C, and D, respectively. Group C showed less collagen confirmation loss and more angiogenesis and elastin precursor production. Elastic fiber linearity was 0.901±0.02, 0.551±0.04, 0.751±0.04, 0.822±0.03 for Groups A, B, C, and D, respectively.
Conclusion: Injection of MSM in mice subjected to UVB-induced skin damage reduces the wrinkle score and protects against photoaging.
Keywords: Methylsulfonylmethane, UVB, antiaging, retinoic acid
Aging occurs due to a combination of various processes (1). DNA damage due to environmental factors such as ultraviolet (UV) (B) rays can cause intracellular changes in genetically programmed pathways such as telomere shortening that may result in aging (1-3). According to previous studies, UV-induced skin aging accounts for 80% of overall aging. UV rays stimulate the production of reactive oxygen species (ROS) that destroy DNA and activate a skin aging pathway, leading to photoaging (4-6).
Many studies are being conducted to identify ways to eliminate ROS and to prevent skin aging and damage caused by DNA destruction (7). Consequently, various antioxidants such as Vitamin C and E, carotenoid, and flavonoid were discovered. These substances play a significant role in the prevention of free radical damage (8-10).
Methylsulfonylmethane (MSM) has been commonly used as an anti-inflammatory agent. Recently, its antioxidant capacity to reduce oxidative stress was identified. Hence, it became popular in aging-related research (11,12). Recent research reported that when MSM was administered orally or topically, there was a statistically significant improvement in skin condition along with anti-aging effects (13). However, research on MSM is limited to oral administration and application. Therefore, we focused on how effectively this substance can achieve a protective effect against UV rays in terms of non-wrinkling and protect against photoaging when injected.
This study was designed to evaluate the effects of MSM on photoaging. Using an animal model, photoaging was induced using UVB. The anti-aging effects of MSM on photoaging was evaluated through visual assessment over a period of time, a histopathology test, by monitoring the changes in the expression levels of CD31, tropoelastin, and fibrillin-1, and by evaluating skin elasticity using a scanning electron microscope (SEM).
Materials and Methods
Materials.
Hairless mouse (SKH-1). Twenty male hairless mice (SKH-1) aged six weeks were obtained from Orient Experimental Animal located in Yeongnam and kept in Experimental Animal Centre Re-entry Area of Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF). The following conditions were maintained and monitored: temperature of 22±3˚C, relative humidity of 50±20%, number of air changes amounting to 10-15 times/h, and light-dark cycle of 12 h/day (08:00-20:00).
The animal experiments were performed after receiving a review and approval from the Institutional Animal Care and Use Committee of Daegu-Gyeongbuk Medical Innovation Foundation (Approval No. DGMIF-19082701-00). After the end of the experiment, the animals used in the experiments were euthanized using a carbon dioxide chamber, and then tissues were collected.
MSM, retinoic acid. The experimental substance used was 10% MSM (Sigma-Aldrich, St Louis, MO, USA). The positive control group was treated with 0.05% retinoic acid (Sigma). Retinoic acid is a material that has been shown to have anti-aging effects when injected or applied. Therefore, it was used as a positive control group.
Dermashine Balance®. Dermashine Balance® (HUMEDIX, Seongnam-si, Republic of Korea) was used to inject the accurate amount of experimental substance at an appropriate depth. The nine pin (1 cm × 1 cm) was used to ensure the substance was equally distributed across the target site.
Experimental group. A photoaging animal model was designed by targeting SKH-1 (20.2-26.3 g, average 23.1 g). Experimental animals were divided into a total of four groups: normal group (Group A), UV control group (Group B), UV/MSM group (Group C), and UV/retinoic acid group (Group D). Using the spine of the experimental animal as the reference, 0.1 cc of the experimental substance was administered into the left and right sides with the use of Dermashine Balance®. From the first to the fourth week of the experiment, the substance was administered four times in total, thereby a total of 0.4 cc was injected into each side.
Group A (normal group): no UVB radiation. Saline injection, n=5;
Group B (UV control group): UVB radiation. Saline injection, n=5;
Group C (UV/MSM group): UVB radiation. MSM injection, n=5;
Group D (UV/Retinoic acid group): UVB radiation. Retinoic acid injection, n=5.
UV irradiation. The measurement tool for UVB irradiation was installed 30 cm away from the back of SKH-1. To obtain an accurate measurement of the amount of irradiation, a UV meter (YK35UV, LUTRON) was used. Four UVB lamps (Philips, TL20W, wavelength 290-320 mm) were attached to the cage to ensure the direct irradiation of UVB rays on the back. Minimal erythema dose (MED, approximately 75 mJ/cm2) was measured in advance. To promote the formation of photoaging-induced wrinkles, Groups B, C, and D were irradiated with 120 MED of UVB for six weeks.
Methods.
Visual assessment, micro-computed tomography (Micro-CT). To examine the anti-aging effects, the backs of the SKH-1 were recorded using a digital camera [Leica M80 Stereo Microscope® (Leica Microsystems, Wetzlar, Germany)]. A micro-CT scan was used for high quality visualization of the wrinkles. The scoring system developed by Bissett et al. was used every week to follow-up the average wrinkle score of each group. Wrinkle scoring: Grade 0: no coarse wrinkles; Grade 1: a few shallow coarse wrinkles; Grade 2: some coarse wrinkles; Grade 3: several deep wrinkles.
Histopathology tests. Hematoxylin and eosin (H&E) staining was used to identify histopathological changes, and Masson’s trichrome staining to identify the collagen content in the dermis. Images of the stained tissue were obtained using Nikon electric drill fluorescence microscope (Nikon, Tokyo, Japan), and NIS-Elements Software was used to analyze the images.
Immunohistochemistry: CD 31, Tropoelastin, Fibrillin-1. Immunohisto-chemistry was performed to selectively identify antigens in a tissue section with the use of antibodies (such as CD 31, Tropoelastin, Fibrillin-1) binding specifically to antigens, using standard protocols. CD 31 immunohistochemistry was performed to identify angiogenesis. Tropoelastin and fibrillin-1 immunohistochemistry was performed to identify precursors of elastin fiber. Images of the stained tissue were obtained using Nikon electric drill fluorescence microscope (Nikon), and NIS-Elements Software was used to analyze the images.
Elasticity evaluation (scanning electron microscope; SEM). After obtaining the tissue sample, it was cut to the size of 1×1×4 mm. The tissue was fixed using 0.5% glutaraldehyde and 0.5% paraformaldehyde and rinsed with 0.1 M phosphoric acid buffer. Then, it was fixed in 1% osmium tetroxide solution for 2 h and rinsed with the same buffer again. After submerging the rinsed tissue in 25% dimethylsulfoxide (DMSO) solution for 30 min and in 50% DMSO solution for 30 min, it was frozen using liquid nitrogen to be cut into smaller pieces. After melting the smaller pieces using 50% DMSO solution and rinsing them using the same buffer, they were post-fixated in 1% osmium tetroxide solution for 2 h, dehydrated using ethanol, and infiltrated using t-butyl alcohol. Thereafter, the tissue sample underwent a freeze-drying process using a freeze dryer (Hitachi ES-2030). After attaching the dry sample to a sample plate, it was vapor-deposited into Pt-Pd alloy using Ion sputter (Hitachi E-1030). Then, the sample was inspected using Hitachi S-4200 SEM. Elastic fiber linearity was examined based on the images obtained using SEM Elastic fiber linearity, which allowed the comparison of the changes in the degree of elasticity of SKH-1 after exposure to UV irradiation.
Elastic fiber linearity. The smallest rectangle that can contain a single elastic fiber fragment was automatically created by the computer and the length and width of the rectangle were defined as B and C, respectively. The area occupied by the elastic fibers was defined as A. Elastic fiber linearity of each elastic fiber fragment was defined as A/(B×C). For example, the elastic fiber linearity of a linear elastic fiber fragment is one. However, when UV irradiation causes the elastic fiber to bend, the area occupied by the elastic fiber within the smallest rectangle reduces, thus the linearity decreases below one.
Statistical analysis. All collected data were subjected to statistical analysis using SPSS ver. 12.0 (SPSS, Chicago, IL, USA). One-way ANOVA was used to compare the average score from each group and to verify the statistical significance in the experimental group with a significance level <5% (p<0.05).
Results
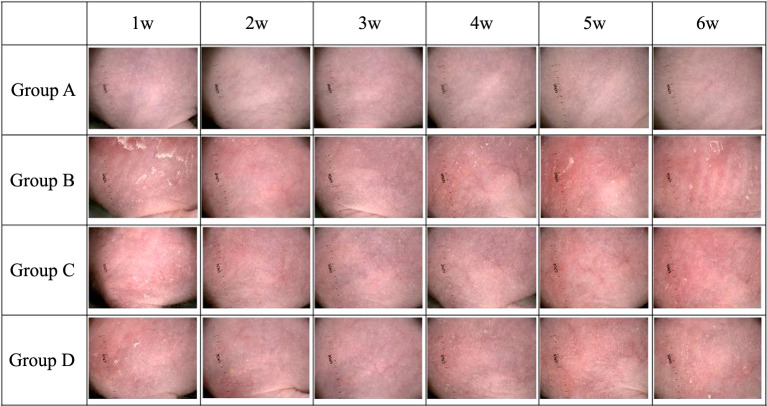
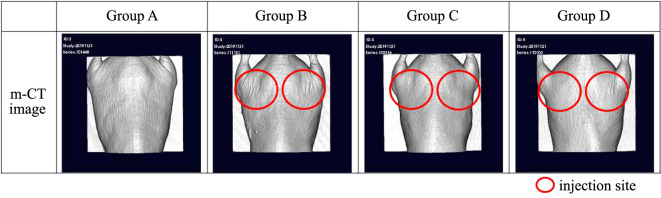
During the UVB irradiation period, Group B mice showed an increase in the number and depth of skin wrinkles based on visual inspection (Figure 1). Micro-CT results revealed that Groups C and D mice had fewer wrinkles and were in better condition than Group B mice (Figure 2 and Figure 3). After three weeks of exposure to UVB, the wrinkle score of Group B rapidly increased. After the end of the experiment (Week 6), the average wrinkle score increased up to 2.5.
Figure 1. The dorsal side of the mouse was closely photographed with a digital camera (Leica M80 Stereo Microscope) to determine the severity of wrinkles. Group A: normal control; Group B: UV control; Group C: UV/methylsulfonylmethane; Group D: UV/Retinoic acid.
Figure 2. Micro-CT was used to analyze the wrinkles more precisely. The material was injected into the circled area. The various groups were treated as follows: Group A: normal control (non-treated); Group B: UV control; Group C: UV/methylsulfonylmethane; Group D: UV/Retinoic acid.
Figure 3. At the end of the experiment (week 6), a section of the UV-irradiated area was collected, and micro-CT was used for a closer observation. The various groups were treated as follows: Group A: normal control (non-treated); Group B: UV control; Group C: UV/methylsulfonylmethane; Group D: UV/Retinoic acid.
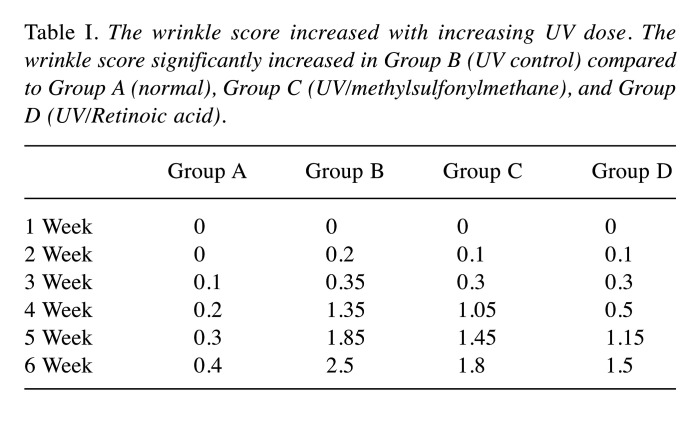
Similar to Group B, the wrinkle score of Group C also gradually increased. Four weeks after exposure to UVB, the wrinkle score began to rapidly increase, but not more than that of Group B. At the end of the experiment (Week 6), the average wrinkle scores increased up to 0.4 for Group A, 1.8 for Group C, and 1.5 for Group D (Table I).
Table I. The wrinkle score increased with increasing UV dose. The wrinkle score significantly increased in Group B (UV control) compared to Group A (normal), Group C (UV/methylsulfonylmethane), and Group D (UV/Retinoic acid).
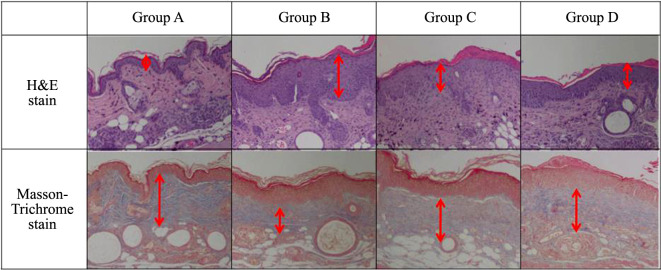
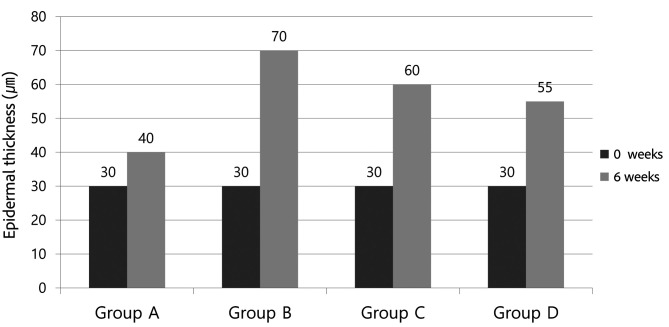
Generally, with UV damage, the epidermal thickness becomes thicker. The H&E stain showed thickening of the epidermis and infiltration of inflammatory cells in the dermis of Group B mice (Figure 4). The average epidermal thickness of SKH-1 before the experiment was 30 mm, whereas the epidermal thickness after the experiment was 40 mm for Group A, 70 mm for Group B, 60 mm for Group C, and 55 mm for Group D (Figure 5).
Figure 4. Hematoxylin and eosin (H&E) and Masson’s trichrome staining. The UV/methylsulfonylmethane (MSM) group (Group C) and the UV/Retinoic acid group (Group D) were able to prevent the epidermal thickness from significantly increasing compared with that in the UV control group (Group B). There was a loss of staining in the upper dermal layer in the UV control group (Group B), but little damage of the dermal layer in the UV/MSM group (Group C) and the UV/Retinoic acid group (Group D) (original magnification ×100).
Figure 5. Epidermal thickness after the experiment. Group B (UV control) showed a marked increase in epidermal thickness compared to Group A (normal; non-treated), Group C (UV/methylsulfonylmethane), and Group D (UV/Retinoic acid).
The Masson-Trichrome stain was used to identify the collagen content in the dermis. Generally, the collagen layer of the dermis is destroyed during UV damage. No staining was observed in the upper dermal layer of Group B mice and almost no damage of the dermis of Groups C and D mice (Figure 4).
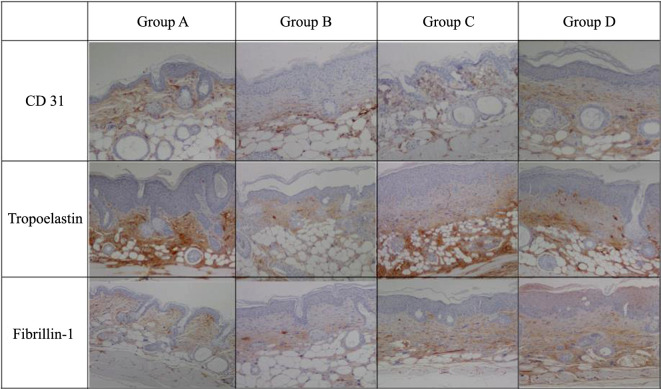
Results of CD 31 immunohistochemical analysis showed significantly reduced angiogenesis in Group B (Figure 6). Tropoelastin and fibrillin-1 immunohistochemistry results showed reduced expression of precursors of elastic fiber in Group B (Figure 6).
Figure 6. CD 31, Tropoelastin, and Fibrillin-1 immunohistochemistry. CD 31 immunohistochemistry was performed to confirm angiogenesis. Tropoelastin and fibrillin-1 immunohistochemistry was performed to confirm the presence of the elastin fiber precursor. Group A: normal control; Group B: UV control; Group C: UV/methylsulfonylmethane; Group D UV/Retinoic acid.
Generally, during UV damage, curling occurs in elastic fibers and the degree of distortion increases. This results in poor elastic fiber linearity. The SEM analysis showed that the elastic fiber linearity in Group A (0.901±0.02) was close to 1 (Figure 7). However, elastic fiber linearity in Groups B, C, and D that were exposed to UVB significantly decreased below 1. Elastic fiber linearity in Groups B, C, and D were 0.551±0.04, 0.751±0.04, and 0.822±0.03, respectively. The elastic fiber linearity in Group B was significantly lower than that in other groups. There was a statistically significant difference in elastic fiber linearity among Groups A, B, and C (p<0.05) (Table II).
Figure 7. The tissues were observed with a Hitachi S-4200 type scanning electron microscope. (20.0 kV, X9.00K, 2.00 μm) Group A: normal control; Group B: UV control; Group C: UV/methylsulfonylmethane; Group D: UV/Retinoic acid.
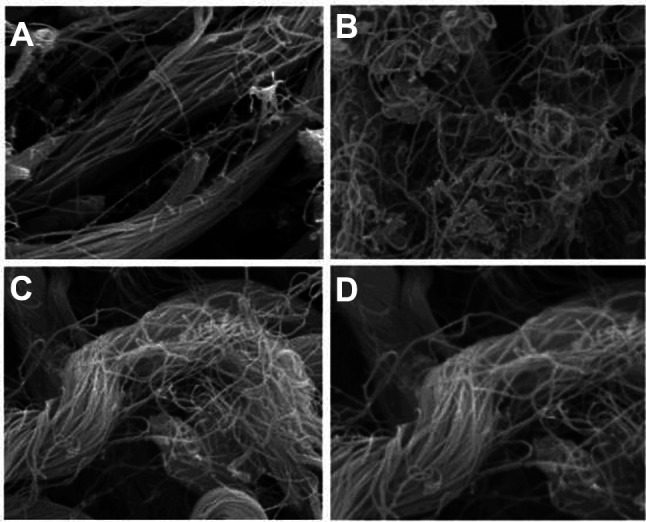
Table II. In Group A, elastic fiber linearity (0.901±0.02) was found to be close to 1. Elastic fiber linearity of Group B was the lowest. Group A: normal control; Group B: UV control; Group C: UV/methylsulfonylmethane; Group D: UV/Retinoic acid.
Discussion
MSM is a dietary sulfur that promotes sulfur-sulfur combination within cellular proteins. Since the effects of MSM in improving pain related to osteoarthritis was established, additional studies have been conducted. Consequently, MSM has been considered safe for the management of pain and inflammation (11,12).
In a previous cosmetic and aging-related study, MSM was administered orally or topically at local sites (13). However, there are no studies on the effect of MSM local injection. If the injection method is effective, we expect that it will be widely used in the field of plastic surgery in the future. In this study, an appropriate amount of MSM was injected, and the skin was continuously exposed to UVB. Several procedures were used to monitor how MSM provided protection against UVB and how the skin recovered from the damages.
According to the visual assessment using a digital camera and micro-CT, the severity of wrinkles was significantly lower in Group C than that in Group B (Figure 1, Figure 2, Figure 3). In Table I, there is no significant difference in wrinkle score between groups, which suggests that the natural anti-aging protective mechanism of mouse skin against UVB exposure was sustained until week 3. However, the widening difference in the severity of wrinkles after week 3 suggests that the skin protection system failed to prevent aging after experiencing serious damage. There were differences between Groups B and C. There was less damage to the skin protection system in Group C because of the effects of MSM. After week four, the rate of skin aging accelerated in Group C. This shows that MSM should be injected again after a certain period of time following the initial injection.
According to the H&E staining, Groups C and D were able to prevent the thickening of the epidermis layer compared to Group B. Based on findings using Masson-Trichrome staining, a decrease in collagen degradation was identified in Groups C and D (Figure 4). It can be inferred that the collagen layer was protected and regenerated against UV damage in the group injected with MSM and retinoic acid.
CD 31 immunohistochemistry examined the degree of angiogenesis in response to UVB damage. Group B had a low degree of angiogenesis. It was verified that angiogenesis was better activated in Groups C and D after experiencing UVB damage (Figure 6). It can be inferred that the group injected with MSM, and retinoic acid has a strong ability to regenerate against UV damage.
Tropoelastin and fibrillin-1 immunohistochemistry showed the reduced expression of the precursor of elastic fiber (Figure 6). When UVB damage was induced in Groups C and D, there was an increase in the number of elastin fiber precursors, such as tropoelastin and fibrillin-1. Thus, the production of elastic fiber is enhanced as a defense mechanism to maintain the elasticity despite the UVB damage (14). In regard to skin aging, the elasticity of the skin is very important. In order for the elastin fiber to remain strong, the precursor must be sufficiently activated to regenerate the elastin fiber. In this respect, it can be suggested that the regenerating force of the elastin fiber was strong against UV damage in the group injected with MSM and retinoic acid.
According to the SEM images, curling and/or distortion in Group B was found to be greater than those of Groups C and D (Figure 7). Elastin is responsible for matrix formation from the boundary between the epidermis and dermis to the lower dermis and for maintenance of skin elasticity (15). Elastic fiber linearity can be used to examine curling and/or distortion. Although Groups C and D had UVB-induced photoaging, they had less distribution of elastic fiber. It is assumed that MSM played a role in preventing the denaturation of elastin in this study (15,16).
Further studies are necessary to determine the exact mechanism underlying UVB-induced skin aging and the protective role of MSM against this type of damage. Since MSM is a natural ingredient, it is relatively safe. There are no known side effects yet. However, it has not been found to be stable for long-term use. Furthermore, there is a possibility of causing flushing (sudden warmth, redness, or tingly feeling) when used as a high-dose injection. This study found that proper use of MSM in treating UVB-induced skin aging can prevent epidermal and dermal photoaging and promote anti-aging (11).
Numerous studies and attempts on the use of MSM in pain management, inhibition of ROS, and treatment of many skin diseases such as allergy, acne, burns, and scars are currently in progress (11). MSM is currently being actively used as a drug to be applied or eaten, and there were no special side effects. Additionally, in this experiment, it was confirmed that there were no special side effects even when used as an injection. Clinically, cosmetics that apply MSM can be made, and for a more dramatic effect, MSM solution is used as an injection at the clinic and can be used as a medicine to prevent photoaging and improve the skin like botox or fillers. If so, the area of plastic surgery can be expanded more easily by using this product in the clinic. This study examined the protective mechanism of MSM against UVB damage; however, the underlying mechanisms are yet to be explored further. For more accurate and further research, we should perform molecular biology studies such as real time-polymerase chain reaction to evaluate the real effect of this product, in comparison to the others and to the control on the DNA and on pro- and anti-inflammatory markers, on type I, III and IV collagen and on all markers of skin and dermal regeneration. If these additional studies are performed in the future, it will help us to better understand the mechanisms of aging and the anti-inflammatory and antioxidant functions of MSM. The use of as a new antiaging product should be verified in other randomized studies. If the local, topical application or injection technique of MSM improves in the cosmetic and/or aging field, production of anti-photoaging products using MSM can be expected (12).
Conclusion
This study confirmed the protective effects of MSM against UVB damage when injected into the back of mice. The decrease in wrinkle formation was not only visible based on inspection, but histopathological, elasticity, and immunohisto-chemical analyses also confirmed the protective effects of MSM against photoaging. The specific mechanism underlying this protective system could not be identified in the present study, and further cellular biological research is needed (17-20). Similar to retinoic acid, a verified anti-aging material, the results of this study indicate that MSM is an anti-aging compound that can protect against photoaging (21-24).
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
All Authors contributed to the study conception and design. Material preparation and data collection were performed by J.Y., Y.J., and J.S. Sample processing and data analysis were performed by K.Y., H.Y. Preparation and stylistic revision of the manuscript were performed by B.C. The first draft of the manuscript was written by S.G., and J.D. provided revisions to the scientific content of the manuscript.
Acknowledgements
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2019), Daegu, Republic of Korea.
References
- 1.Tsukahara K, Nakagawa H, Moriwaki S, Takema Y, Fujimura T, Imokawa G. Inhibition of ultraviolet-B-induced wrinkle formation by an elastase-inhibiting herbal extract: implication for the mechanism underlying elastase-associated wrinkles. Int J Dermatol. 2006;45(4):460–468. doi: 10.1111/j.1365-4632.2006.02557.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 3.Cho HS, Lee MH, Lee JW, No KO, Park SK, Lee HS, Kang S, Cho WG, Park HJ, Oh KW, Hong JT. Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation. Photodermatol Photoimmunol Photomed. 2007;23(5):155–162. doi: 10.1111/j.1600-0781.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Chen X, Chen H, Wang L, Liang J, Luo D, Liu Y, Yang H, Li Y, Xie J, Su Z. Anti-inflammatory activity of β-patchoulene isolated from patchouli oil in mice. Eur J Pharmacol. 2016;781:229–238. doi: 10.1016/j.ejphar.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Wang XF, Huang YF, Wang L, Xu LQ, Yu XT, Liu YH, Li CL, Zhan JY, Su ZR, Chen JN, Zeng HF. Photo-protective activity of pogostone against UV-induced skin premature aging in mice. Exp Gerontol. 2016;77:76–86. doi: 10.1016/j.exger.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Lin RF, Feng XX, Li CW, Zhang XJ, Yu XT, Zhou JY, Zhang X, Xie YL, Su ZR, Zhan JY. Prevention of UV radiation-induced cutaneous photoaging in mice by topical administration of patchouli oil. J Ethnopharmacol. 2014;154(2):408–418. doi: 10.1016/j.jep.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Ros M, Carrascosa JM. Current nutritional and pharmacological anti-aging interventions. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165612. doi: 10.1016/j.bbadis.2019.165612. [DOI] [PubMed] [Google Scholar]
- 8.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today. 2007;12(5-6):218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Wang Z. Which is the most reasonable anti-aging strategy: meta-analysis. Adv Exp Med Biol. 2018;1086:267–282. doi: 10.1007/978-981-13-1117-8_17. [DOI] [PubMed] [Google Scholar]
- 10.Kong R, Cui Y, Fisher GJ, Wang X, Chen Y, Schneider LM, Majmudar G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J Cosmet Dermatol. 2016;15(1):49–57. doi: 10.1111/jocd.12193. [DOI] [PubMed] [Google Scholar]
- 11.Ebisuzaki K. Aspirin and methylsulfonylmethane (MSM): a search for common mechanisms, with implications for cancer prevention. Anticancer Res. 2003;23(1A):453–458. [PubMed] [Google Scholar]
- 12.Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane: applications and safety of a novel dietary supplement. Nutrients. 2017;9(3):290. doi: 10.3390/nu9030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrager E, Veltmann JR Jr, Schauss AG, Schiller RN. A multicentered, open-label trial on the safety and efficacy of methylsulfonylmethane in the treatment of seasonal allergic rhinitis. J Altern Complement Med. 2002;8(2):167–173. doi: 10.1089/107555302317371451. [DOI] [PubMed] [Google Scholar]
- 14.Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105(2):285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 15.Song SH, Roach MR. A morphological comparison of aortic elastin from five species as seen with the scanning electron microscope. Acta Anat (Basel) 1985;123(1):45–50. doi: 10.1159/000146037. [DOI] [PubMed] [Google Scholar]
- 16.Lavker RM, Zheng PS, Dong G. Aged skin: a study by light, transmission electron, and scanning electron microscopy. J Invest Dermatol. 1987;88(3 Suppl):44s–51s. doi: 10.1111/1523-1747.ep12468934. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, Tavakkol A, Yi JY, Griffiths CE, Elder JT. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105(4):549–556. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- 19.Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of Retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14(1):40–46. doi: 10.1111/jocd.12131. [DOI] [PubMed] [Google Scholar]
- 20.El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 21.Kambayashi H, Yamashita M, Odake Y, Takada K, Funasaka Y, Ichihashi M. Epidermal changes caused by chronic low-dose UV irradiation induce wrinkle formation in hairless mouse. J Dermatol Sci. 2001;27 Suppl 1:S19–S25. doi: 10.1016/s0923-1811(01)00113-x. [DOI] [PubMed] [Google Scholar]
- 22.Meningaud JP, SidAhmed-Mezi M, Billon R, Rem K, La Padula S, Hersant B. Clinical benefit of using a multifractional Er:YAG laser combined with a spatially modulated ablative (SMA) module for the treatment of striae distensae: A prospective pilot study in 20 patients. Lasers Surg Med. 2019;51(3):230–238. doi: 10.1002/lsm.23042. [DOI] [PubMed] [Google Scholar]
- 23.Hersant B, La Padula S, SidAhmed-Mezi M, Rodriguez AM, Meningaud JP. Use of platelet-rich plasma (PRP) in microsurgery. J Stomatol Oral Maxillofac Surg. 2017;118(4):236–237. doi: 10.1016/j.jormas.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 24.La Padula S, Hersant B, SidAhmed M, Niddam J, Meningaud JP. Objective estimation of patient age through a new composite scale for facial aging assessment: The face - Objective assessment scale. J Craniomaxillofac Surg. 2016;44(7):775–782. doi: 10.1016/j.jcms.2016.01.022. [DOI] [PubMed] [Google Scholar]