Abstract
The field of neurodegenerative diseases is a major challenge faced by public health and is still in need of robust preventive measures and disease-modifying treatments. Population-based studies can offer the framework in the context of primary and secondary prevention of neurodegenerative diseases. The epidemiology of neurodegenerative disorders in the last decades has focused on descriptive studies mainly based on the use of clinical criteria. However, clinical definition is basically insufficient both to well-characterize different phenotypes and to make an early diagnosis. Descriptive epidemiology needs a new framework to update the area of neurodegenerative research, based on the advancement of both clinical and biological diagnostic criteria and the urgency for an early diagnosis of the disease. In here, we address the present and future of population-based studies in neurodegenerative disorders and discuss the shift of paradigms in the diagnosis of disease and disease definition. We further debate the changes in biomarker implementation models and type of biomarkers used in population-based studies. Descriptive epidemiology of neurodegenerative disorders is rapidly evolving. These implementations will improve the future design and outcome of population-based studies and policy-making in public health intervention.
Keywords: Aging-related diseases, Alzheimer's disease, Biomarkers, Dementia, Epidemiology, Parkinson's disease, Population-based studies, Public health
Introduction
Descriptive epidemiology is the basis of epidemiological research focusing on measuring disease occurrence (incidence and prevalence) and risk factor distribution. In this context, the definition of disease is central to make the basic counts possible (subjects with disease of interest in the numerator). There are two main challenges as follows:
1. the distinction of normal from a specific phenotype of interest (subjects with disease vs. subjects without disease);
2. the distinction across different phenotypes that often, at least partially, overlap.
The area of neurodegenerative diseases is particularly challenging because many distinctions are subtle, the domains of clinical assessment are several (three main domains: cognition, behavior, motor), and the evolution of the clinical features may have different speeds across individuals and syndromes. Neurodegenerative diseases are characterized by the progressive deposition of specific proteins within distinct areas of the brain. There are seven basic types of neuropathological deposition or changes (alpha-synuclein inclusions, tau neurofibrillary tangles, TDP-43 inclusions, amyloid plaques, neuronal loss, angiopathy, and gliosis) [1].
The deposition of many proteins is present at the same time and is not specific. The simultaneous deposition of several proteins is present in subjects with neurodegenerative diseases and healthy individuals as well [2]. Different proteins interact with different mechanisms, often accelerating the deposition process [3]. This cross-entity approach has also been applied to epidemiology, with an age-at-onset-related pattern supporting the view of neurodegeneration as a unified rather than a fragmentary phenomenon [4]. All these processes are less specific and more complex as age advances, especially in the very old [5, 6]. The clinical definition of disease is basically insufficient both to well-characterize different phenotypes and to make an early diagnosis, possible in most cases only late in the natural history of disease. Early diagnosis is a key and emergent goal needed in the research for an effective therapy. Descriptive epidemiology needs a new framework to update the area of neurodegenerative research, based on advancement of diagnostic criteria (clinical + biomarkers) and the urgency for an early diagnosis. In this new context, there are some questions and issues that need to be addressed: (1) What is disease? (2) What disease definition should we use for projections? (3) When does disease start? (4) Phenotyping: clinical-pathological or system biology model? and (5) Neuropathology as the gold standard in epidemiology research: pros and cons. In this manuscript, we attempt to answer those questions in the most common late-life neurodegenerative disorders, Alzheimer's disease (AD) and Parkinson's disease (PD), in a time when clinical and basic research has developed new classification systems and novel understanding of the pathogenic mechanisms of disease.
Diagnosis of Disease: The Example of Dementia (What Is Disease?)
The classification of the etiology of dementia is changing rapidly. Large population-based studies, conducted mainly in the USA and North America, have established that AD is the most common form of dementia. Almost all the epidemiological evidence on frequencies and exposure are based on studies with a clinical classification of dementia and AD. The clinical classification, used in epidemiological studies, is based essentially on different versions of DSM and the NINDS-NIH criteria [7, 8].
Many subjects classified as AD do not have AD neuropathology, while many subjects with AD pathology do not have amnestic presentation of typical AD. These discrepancies and the need for earlier diagnosis have prompted new classification systems based on clinical symptoms and signs plus in vivo biomarkers to classify living subjects with evidence of AD pathology [9, 10]. The new criteria have included a preclinical entity, preclinical AD, with the diagnosis based only on AD-positive biomarkers, with a possible classification in three preclinical stages [11]. These criteria were established with a proper classification based on biomarkers, unifying all possible stages of diseases, based on the categorical classification of three types of biomarkers in a unique ATN system [11]. The new modified research NIH diagnostic criteria completely change the approach in the epidemiological setting. In population-based studies, we need to know (1) what is the prevalence of AD based on new NIA criteria and (2) what is the prevalence of preclinical AD.
To our knowledge, there is only one large epidemiological study addressing all these complex questions: the Mayo Clinical Study on Aging (MCAS) [12]. In this study, the biomarkers were assessed using PET imaging for amyloid and tau in about fifteen hundred subjects out of five thousand participants of the MCAS, aged 60–89 years. The prevalence of AD and clinically defined AD was very low at age 70 years but increased constantly with age, and at age 80, the prevalence of AD assessed through biomarkers was about three times the prevalence of clinically defined probable AD. The conclusions of this study are of paramount importance. The prevalence and incidence of clinically defined AD in various stages indicate the area of intervention of clinicians and health planners. The subjects with clinical symptoms are a small percentage (16% of participants: 640 MCI and 94 dementia) compared to normal individuals (84% of participants: 3,926 cognitively unimpaired). The biologically defined AD indicates the area of secondary prevention based on new criteria; this is for intervention that should delay the onset of clinical disease (MCI and dementia). This is of course key to identify subjects who are candidates for the new disease-modifying therapies. In the MCAS study, the interesting result is that up to age 85 years considering all the clinical spectrum (both MCI and dementia), the large majority of subjects with biologically defined AD are without clinical symptoms. In a study conducted in Goteborg, Sweden [13], among those with a CDR score of 0 (normal individuals), in a limited sample of about three hundred subjects, the prevalence of amyloid pathology was 22.8%, of t-tau pathology was 33.2%, and of p-tau pathology was 6.9%. AD pathology therefore was observed in almost one-half of the 70-year-old with a CDR score of 0. Two population-based studies show therefore similar results: amyloid and tau pathologies are relatively common in normal older adults [14, 15]. There are therefore a high number of subjects over the age of 65 years who, despite not having symptoms yet, already have evidence of the characteristic neuropathological accumulation of AD.
Population-based studies have also shed light on potentially modifiable risk factors for dementias. Excessive alcohol consumption, traumatic brain injury, and air pollution (especially exposure to PM 2.5 [16]) have been recently incorporated in the updated 12 risk factor life-course model of dementia prevention (less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and low social contact). Interestingly, these modifiable risk factors may account for around 40% of worldwide dementias, which consequently could theoretically be prevented or delayed [17].
What Disease Definition Should We Use for Projections?
The identification of subjects with preclinical disease is the basis for early intervention aiming to delay the clinical onset of disease. The subjects with preclinical disease are the optimal target to prevent further brain damage. In a recent study [18], using the analyses merging the results of 13 cohort studies [19], the estimated numbers of subjects with clinical AD in different stages in the USA in 2017 were about six million, while the subjects with preclinical AD were 47 million (22 million with amyloidosis, 8 million with neurodegeneration, 16 million with both amyloidosis and neurodegeneration) [18]. The ratio between clinical and preclinical subjects with AD is about 1:8; most people with evidence of biological AD are in the preclinical phase. These projections indicate that epidemiological studies should be including a biomarker-based definition of disease in the future. Based on these data, we also need in this context a new definition of prevention. Hence, primary prevention strategies should begin prior to amyloid deposition in the brain and be aimed at decreasing amyloid, tau, and subsequent neurodegeneration.
The introduction of the new classification criteria has, in fact, pushed toward new study designs with the identification of early targets for conducting preventive trials on at-risk or prodromal presymptomatic or even in preclinical stages of AD. This will lead to a shift in the timeline, significantly anticipating the timing of primary prevention. It will be possible in population-based setting to use the new diagnostic criteria to identify subjects in the preclinical phase of the disease (before the onset of the first clinical symptom) and to determine the staging of the natural history of AD.
After several classifications of AD that have emerged in recent years, it is important to underline that at the moment, there are two main independent definitions and guidelines predominant for the diagnosis of asymptomatic AD, the International Working Group [10] and the joint effort of the National Institute of Aging and the Alzheimer's Association (NIA-AA) [11]. Clearly, the step change is to recognize that AD can be diagnosed before clinical symptoms appear, shifting the target from a clinical diagnosis to a biological diagnosis.
Both classification criteria depend on the in vivo evidence of the brain pathology characteristic of AD, with demonstration of amyloid deposition, but a key difference between these two guidelines is that there are two different interpretations of biomarker positivity in the absence of symptoms:
IWG considers asymptomatic individuals with evidence of AD pathology biomarkers as at risk for AD.
NIA-AA identifies criteria for a preclinical diagnosis of AD.
Based on the two published classification systems, the prevention trials are secondary if they prevent the onset of clinical symptoms in subjects with evidence (through biomarkers) of AD and primary if they delay or prevent the deposition of amyloid plaques. Primary prevention trials will be targeting lifestyle components like diet, exercise, and social connection and medical conditions increasing the risk of AD such as hypertension and diabetes [20]. The progression in understanding AD and the AD stages, and the consequent different approach for therapies, therefore shift the horizon of the epidemiological research.
Plasma biomarkers are able to identify status and to predict progression in cognitive disorders. In the Northern Manhattan study [21], a community study in a multiethnic community, the plasma biomarker concentrations of phosphorylated tau, p-tau217, were strongly associated with autopsy-confirmed AD. Among individuals who were considered normal, the abeta 42/40 ratio and higher concentration of p-tau217 and p-tau181 were predictive of conversion to clinical AD. Another study [14] comparing three different blood-based techniques measuring plasma amyloid-β and p-tau181, in dementia-free members of Insight 46, a substudy of the population-based British 1946 birth cohort, shows that liquid chromatography-mass spectrometry methods for measuring plasma amyloid-β1-42/amyloid-β1-40 and a plasma composite outperform the single-molecule arrays (Simoa) measure of amyloid-β42/amyloid-β40 and p-tau181 in their ability to discern amyloid PET status. While preclinical AD can be considered a robust concept, the identification of individuals with dementia with Lewy body (DLB) and frontotemporal dementia (FTD) in their prodromal stages is hindered by a less explicit clinical definition. This is also part of the workup needed to identify subjects with true AD. Recently, recommendations for the identification of individuals with DLB and FTD have been published [22, 23]. However, those clinical criteria are intended for use in research and not in clinical settings. The challenge in identifying prodromal FTD and DLB is due to heterogeneity of clinical manifestations of these neurodegenerative disorders, where the three clinical domains (cognition, behavior, motor) are more entangled and above all for the lack of an accurate antemortem biological definition of the disease state. Several novel in vivo biomarker candidates are under development, and probably population-based studies in the near future could also rely on the use of precise definition of preclinical or early clinical stages in non-AD dementias. Both DLB and FTD are rare neurodegenerative diseases. The epidemiological collection of data should be based on the reconstructed cohort design, an approach that takes advantage of clinical collection of data within a large population of residents in a well-defined geographic area [24, 25]. This approach could also be useful to identify proper biomarkers for the clinical diagnosis of these conditions [25].
When Does Disease Start? The Example of PD
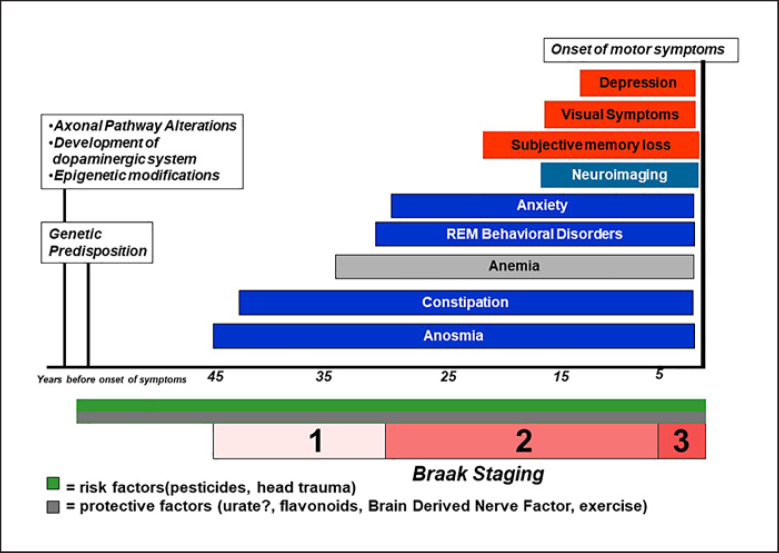
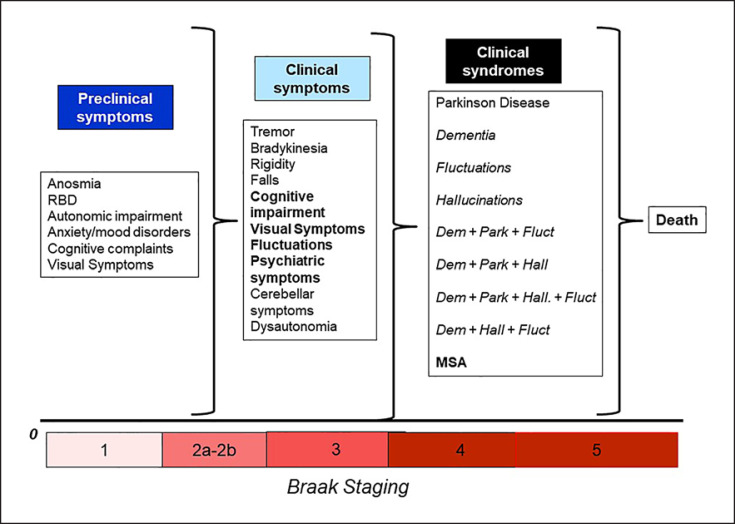
PD has been considered the second most common degenerative disease [26] and classically has been described as a condition affecting the basal ganglia and, subsequently, as clinical features, primarily changing the movement pattern. However, the advent of the Braak theory has modified the dogmatic ideas around PD [27]. The pattern of neurodegeneration and alpha-synuclein accumulation may follow the theoretical framework of the Braak theory: the degenerative cascade of PD would begin in the gut and/or in the olfactory bulb years before the motor onset and then, with an ascending pattern would involve the brainstem, the basal ganglia, and the cortex. The strength of this theory is the matching of the preclinical symptoms with the stages of Braak hypothesis [28]. Constipation, anosmia, REM dream enactment behavior disorders are considered early manifestations of PD that may occur decades before the motor onset [29], and their presence helps the clinician to support the diagnosis and potentially the disease progression. Also, manifestations such as REM dream enactment behavior disorders have been recently used as possible predictors of synucleinopathies (PD but also DLB) [30] and given the high specificity [31], a possible early surrogate marker of disease that can be targeted for early protective interventions [32]. Such information has been supported by a number of epidemiological studies that confirm the hypothesis [33, 34]. On the other hand, the “ascending” pattern of degeneration in PD does not always occur in a discrete and predictable manner, and other possible modalities of degeneration have been considered such as “top-down” or “patchy” [35, 36]. The endophenotypic difference of the degeneration in the early manifestations of PD is definitively complicating the diagnosis but also the understanding of the disease [37]. The concomitant presence of risk and protective factors are active throughout our entire life span and may modify substantially the phenotype of the disease; in addition, genetic and metabolic individual factors are active as well synergistically and additively through the years to further impact the presentation and progression of PD (Fig. 1). Indeed, the number of possible permutations is finite and is compiled by a combination of risk/protective factors, genetic predisposition, early manifestations, clinical manifestations, syndromic definition (Fig. 2). In the next future, epidemiology descriptive studies will have the power and the ability to identify such combinations of symptoms, leading to an individual understanding on when and where PD started in the individual patients. Clearly, large population-based studies and consortia need to come together to improve the quality of the data that need to be representative of the constantly changing population.
Fig. 1.
Preclinical epidemiology timeline of alpha-synucleinopathies.
Fig. 2.
Natural history and spectrum of alpha-synucleinopathies.
Currently, it is clear that there are macrodifferences that have been identified and supported already by multiple investigators: sex difference, young versus late-onset PD, tremor versus akinetic rigid subtype [38]. Indeed, men are twice more affected by PD than women [39], and women seem to have a better prognosis, progression [40], and different risk factors than men [41]. Likewise, young-onset PD can be considered as a different disease from an epidemiological and biological standpoint. Young-onset cases seem to be pure “nigropathies” [42] and quite different in the incidence and survival compared to late-onset PD [43]. Finally, clinical phenotypes of the disease (tremor vs. akinetic rigid) may impact the progression and the survival as well in PD [40].
A New Model for Phenotyping: Clinical-Pathological or System Biology Model? The Example of PD
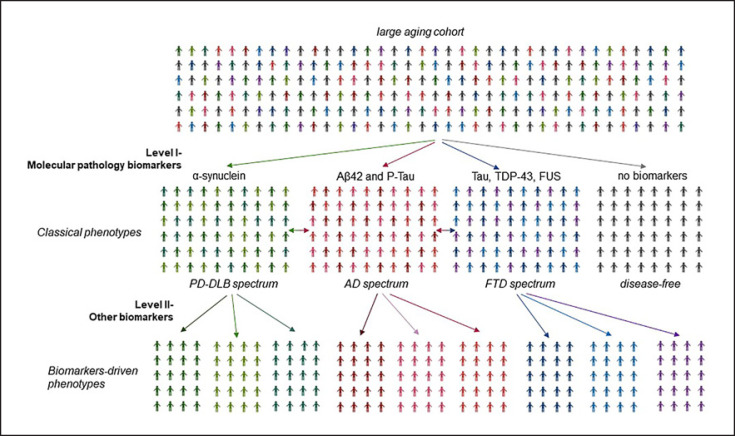
PD is very clinically heterogenous, and unlike AD, the race for in vivo biomarkers is slowed down by the intrinsic differences in the constellation of patients' clinical manifestations, which are combinations of motor and nonmotor features, some of which associate predictably and others less so. The phenotype-driven biomarker development program has been driven by the assumed existence of biological/molecular underpinnings for each diagnostic entity and corresponding prodromal state, anchored to the reductionist model of PD as a single but heterogeneous disease [44], whose complexity converges into the presence of Lewy bodies. However, this approach yielded no robust results and left unanswered the question of which biomarkers are good measures of a “real” biological state in PD and might potentially assist in individualizing molecular therapy in a precision medicine future. Furthermore, molecular elements traditionally considered a domain of other neurodegenerative disorders explains only some of the variability in PD [45]. An alternative could be the systems biology approach to guide nosology, biomarkers identification, descriptive epidemiology, and eventually disease-modifying treatment in PD. From this perspective, PD can be considered a group of disorders characterized by nigrostriatal dopaminergic denervation and the presence of Lewy body but exhibiting a unique genetic, biological, and molecular “signature,” which probably responds differentially to a given therapeutic approach. Under this model, only biomarker-defined, homogenous subtypes of PD are likely to respond optimally to therapies proven to affect the biological processes within each subtype [46]. The ideal biomarker discovery program is based on deeply phenotyped large population-based aging cohorts with and without a neurological diagnosis at baseline (Fig. 3).
Fig. 3.
Development of biomarker-validated phenotypes using in aging populations. The first level of biomarkers development relies on molecular-biology biomarkers which could identify individuals belonging to the disease spectrum in large, aging populations with and without a neurological diagnosis at baseline. The second level in biomarkers development will emerge from exploratory, unbiased, nonhypothesis-driven approach.
Neuropathology as the Gold Standard in Epidemiology Research: Pros and Cons
The search for a surrogate marker of PD that can diagnose PD with certainty in vivo has been a long and difficult road. Finding a minimally invasive, reliable, rapid, and easy reproducible biomarker of PD can be considered the holy grail of the current times in PD. As of today, neuropathology needs to be considered the gold standard for diagnosis; however, such gold standard impacts profoundly the descriptive epidemiological studies in PD. Indeed, most of the studies are based on clinical criteria without pathology confirmation; some population-based studies have also pathology data to be used to confirm the diagnosis [47]. These studies as the population-based conducted in the Olmsted County (Minnesota), named Rochester Epidemiologic Project, on synucleinopathies reveal a good agreement of neuropathology with clinical diagnosis in an advanced setting with low number of discrepancies (about 13%) [48]. On the other hand, usually, the pathology data are available only in portion of cases, providing only a partial confirmation of the full population. Thus, many studies adopted large datasets based upon different sources without any pathology confirmation but also, at times, without clinical information, using only ICD-10 codes or analog electronic information. Despite the large numbers of such datasets, the clinical confirmation is crucial, and the possibility of generative false positives is a well-known problem of using only codes for diagnosis in PD, although it is usually ignored or overlooked by many researchers. Overall, the need of a pathological, or if not possible, clinical research is needed and required in descriptive epidemiology until a different and reliable biomarker is not available. However, new concepts have arisen regarding pathology in PD and the need to reshape the concept of synuclein degeneration. Indeed, PD can be considered a final outcome of different biological mechanisms that impact the basal ganglia leading to similar disease [49]; thus, a mere pathology confirmation may not be representing the “true” and entire spectrum of disorders in PD. In the future, such a theoretical approach needs to be explored and investigated with descriptive studies assessing the discrepancy in pathology confirmation in larger population.
The Evolution of Clinical Biomarkers in Epidemiology
Advanced studies, both in clinical and population-based settings, mainly on dementia and AD, were based on PET assessment of neuropathology in vivo and measurement of amyloid, t-tau, and p-tau in the CSF. However, PET is a very expensive procedure, and lumbar puncture needed to extract CSF is perceived in most settings as invasive. The recent developments of AD blood-based biomarkers may help to change the field of neuropathology assessment in vivo and finally implement an approach to reach a valid diagnosis in population-based research.
Plasma biomarkers are able to identify status and predict progression of cognitive disorders. However, considering the fact that false positive will be expected using screening tests for AD in population-based settings, any use of plasma biomarker-based screening will require robust protocols with dedicated effort to develop proper communication and counseling of plasma tests results [50]; a positive result is likely to require confirmation with another more definitive modality (PET or CSF). The advancement of this research is slower in parkinsonism (mainly synucleinopathies) and rare dementias.
In the near future, plasma biomarkers will be the first choice for future diagnostic approaches in the area of cognitive decline in epidemiological studies due to their greater accessibility than CSF markers and lower costs compared to nuclear medicine brain imaging. The numbers projected for the future are huge both for clinical and preclinical AD. However, the optimal combination of sensitivity and specificity for plasma biomarkers may not be the most useful in every possible setting (i.e., clinical trials, diagnostic settings in tertiary centers, or population-based observational study). The follow-up of the subjects with normal cognition and amyloid positivity will be key. The evidence from cohort studies shows that the presence of clinical symptoms (MCI) significantly changes the probability of transition to successive stages of disease [18].
Conclusions
We propose a different consideration in ideating and designing neurodegenerative disease studies that will take in consideration the biological and phenotypical subtypes of disease and also, when available, a deep biomarker endophenotype that can identify subgroups having precise biological characteristic in a large population. Also, it is crucial, especially in cohort of neurodegenerative studies, to understand the possible discrepancies between clinical diagnosis and biological outcomes that can, at times, impact the diagnostic criteria and the natural history of neurodegenerative diseases.
We have transition models that are based on few epidemiological studies. We need more population-based studies assessing the rate of transition from preclinical to clinical AD, with assessment of individual risk. The distribution of positive and negative risk factors (risk factors prevalence) is key in determining the individual and the group risk both for disease in normal and for disease progression in subjects with neurodegenerative diseases [51]. In this framework, it is also important to address behaviors and factor associated to healthy lifestyle. Healthy lifestyle and connected risk and life expectancy estimates might help healthcare administrators to plan intervention to improve brain health in large populations. The prevention strategy based on large intervention is needed to improve the expected growing numbers. We also need to expand all of these strategies to the other neurodegenerative diseases, including those rarer forms, such as FTD.
In this new research scenario (Table 1), future challenges for epidemiological research in this area are the following:
Table 1.
Present and future of descriptive epidemiology and population-based studies in neurodegenerative diseases
| Descriptive epidemiology issues | Present | Future |
|---|---|---|
| Diagnosis of disease | Mainly based on clinical criteria | Based on clinical and biological criteria |
|
| ||
| Disease definition in projections | Clinical diagnosis of disease (AD, PD, FTD, DLB) | Preclinical diagnosis of disease assisted by the use of biomarkers. Prodromal AD is a robust concept and has been already used in population-based studies. The definition of prodromal PD, DLB, and FTD is currently under development |
|
| ||
| Biomarker implementation models | Clinical-pathological model: clinical phenotypes are established, and then biomarkers are validated | Systems biology model: biomarkers are identified using larger, phenotype-agnostic studies of aging |
|
| ||
| Type of biomarkers used in population-based studies | Expensive PET imaging; CSF-based biomarkers perceived as invasive | Blood-based biomarkers, advanced radiotracer-free neuroimaging biomarkers with greater accessibility and low cost. A multimodal approach would add accuracy |
|
| ||
| Gold standard for disease diagnostic validity | Mainly clinical. In some cases, pathological confirmation | Deeply phenotyped large population-based aging cohorts will facilitate analyses anchored on outlier biological signals |
|
| ||
| Intervention strategies | Based on the concept of disease as a single biological entity. Amyloid reduction in AD, synuclein reduction in PD | Tailored on different disease phenotypes which share a common biological mechanism. Precision medicine approach |
1. move from a clinical definition to a biological definition of neurodegenerative diseases in epidemiological studies;
2. estimate prevalence and incidence of neurodegenerative diseases that are biologically based;
3. target population-based intervention in the new context aiming primary prevention of biological neurodegenerative diseases (following the track already established in AD);
4. working with low-cost and easy-to-use biomarkers such as plasma biomarkers;
5. more epidemiological studies focusing on prevention are needed with the identification of the right strategy of possible intervention.
Epidemiological studies show that the increase of neurodegenerative diseases will be mostly in low- and medium-income countries in Asia, sub-Saharan Africa, the Middle East, and South America [52, 53]. We need to consider the cost and the availability of new diagnostic approaches based on biology in settings with poor resources and no advanced technologies. This is therefore an area where the contribution of epidemiological research in large populations will be of paramount relevance in the next future.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work has been partially supported with the funding from Regione Puglia and CNR for Tecnopolo per la Medicina di Precisione. D.G.R. n. 2117 of November 21, 2018 (B84I18000540002).
Author Contributions
G.L., D.U., and R.S. conceptualized, wrote, and revised the article.
Acknowledgments
We thank Marco Musio, Center for Neurodegenerative Diseases and the Aging Brain, for the technical assistance in the preparation of the manuscript. This paper is published in celebration of the 40th anniversary of the inception of Neuroepidemiology, 1982–2022.
Funding Statement
This work has been partially supported with the funding from Regione Puglia and CNR for Tecnopolo per la Medicina di Precisione. D.G.R. n. 2117 of November 21, 2018 (B84I18000540002).
References
- 1.Cornblath EJ, Robinson JL, Irwin DJ, Lee EB, Lee VM, Trojanowski JQ, et al. Defining and predicting transdiagnostic categories of neurodegenerative disease. Nat Biomed Eng. 2020;4((8)):787–800. doi: 10.1038/s41551-020-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellinger KA. Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med. 2012;16((6)):1166–1183. doi: 10.1111/j.1582-4934.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30((21)):7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Pedro-Cuesta J, Rábano A, Martínez-Martín P, Ruiz-Tovar M, Alcalde-Cabero E, Almazán-Isla J, et al. Comparative incidence of conformational, neurodegenerative disorders. PLoS One. 2015;10((9)):e0137342. doi: 10.1371/journal.pone.0137342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128((6)):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142((6)):1503–1527. doi: 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of health and human services task force on Alzheimer's disease. Neurology. 1984;34((7)):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and statistical manual of mental disorders: DSM-5 . American Psychiatric Association, American Psychiatric Association DSMTF. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 9.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7((3)):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13((6)):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14((4)):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Therneau TM, Weigand SD, Wiste HJ, Knopman DS, Vemuri P, et al. Prevalence of biologically versus clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer's Association research framework. JAMA Neurol. 2019;76((10)):1174–1183. doi: 10.1001/jamaneurol.2019.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern S, Zetterberg H, Kern J, Zettergren A, Waern M, Höglund K, et al. Prevalence of preclinical Alzheimer disease: comparison of current classification systems. Neurology. 2018;90((19)):e1682–e91. doi: 10.1212/WNL.0000000000005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keshavan A, Pannee J, Karikari TK, Rodriguez JL, Ashton NJ, Nicholas JM, et al. Population-based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain. 2021;144((2)):434–449. doi: 10.1093/brain/awaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12((10)):957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air pollution and dementia: a systematic review. J Alzheimers Dis. 2019;70((s1)):S145–s63. doi: 10.3233/JAD-180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396((10248)):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement. 2018;14((2)):121–129. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vos SJB, Verhey F, Frölich L, Kornhuber J, Wiltfang J, Maier W, et al. Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain. 2015;138((Pt 5)):1327–1338. doi: 10.1093/brain/awv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu D, Marshall GA. Primary and secondary prevention trials in Alzheimer disease: looking back, moving forward. Curr Alzheimer Res. 2017;14((4)):426–440. doi: 10.2174/1567205013666160930112125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17((8)):1353–1364. doi: 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benussi A, Alberici A, Samra K, Russell LL, Greaves CV, Bocchetta M, et al. Conceptual framework for the definition of preclinical and prodromal frontotemporal dementia. Alzheimers Dement. 2021 doi: 10.1002/alz.12485. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- 23.McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94((17)):743–755. doi: 10.1212/WNL.0000000000009323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logroscino G, Kurth T, Piccininni M. The reconstructed cohort design: a method to study rare neurodegenerative diseases in population-based settings. Neuroepidemiology. 2020;54((2)):114–122. doi: 10.1159/000502863. [DOI] [PubMed] [Google Scholar]
- 25.Borroni B, Graff C, Hardiman O, Ludolph AC, Moreno F, Otto M, et al. FRONTotemporal dementia incidence European research study-frontiers: rationale and design. Alzheimers Dement. 2022 Mar;18((3)):498–506. doi: 10.1002/alz.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5((6)):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Del Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24((2)):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 28.Savica R, Boeve BF, Mielke MM. When do α-Synucleinopathies start? An epidemiological timeline: a review. JAMA Neurol. 2018;75((4)):503–509. doi: 10.1001/jamaneurol.2017.4243. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5((3)):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 30.Stang CD, Mullan AF, Hajeb M, Camerucci E, Turcano P, Martin P, et al. Timeline of rapid eye movement sleep behavior disorder in overt alpha-synucleinopathies. Ann Neurol. 2021;89((2)):293–303. doi: 10.1002/ana.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142((3)):744–759. doi: 10.1093/brain/awz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130((Pt 11)):2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 33.Boot BP, Boeve BF, Roberts RO, Ferman TJ, Geda YE, Pankratz VS, et al. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71((1)):49–56. doi: 10.1002/ana.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16((6)):653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 35.Foffani G, Obeso JA. A cortical pathogenic theory of Parkinson's disease. Neuron. 2018;99((6)):1116–1128. doi: 10.1016/j.neuron.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJA, Kraneveld AD. Exploring Braak's hypothesis of Parkinson's disease. Front Neurol. 2017;8:37. doi: 10.3389/fneur.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol. 2010;67((7)):798–801. doi: 10.1001/archneurol.2010.135. [DOI] [PubMed] [Google Scholar]
- 38.Marras C, Lang A. Parkinson's disease subtypes: lost in translation? J Neurol Neurosurg Psychiatry. 2013;84((4)):409–415. doi: 10.1136/jnnp-2012-303455. [DOI] [PubMed] [Google Scholar]
- 39.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to Parkinsonism. JAMA Neurol. 2013;70((7)):859–866. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savica R, Turcano P, Bower JH, Ahlskog JE, Mielke MM. Survival and progression in synucleinopathy phenotypes with Parkinsonism: a population-based study. Mayo Clin Proc. 2019;94((9)):1825–1831. doi: 10.1016/j.mayocp.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Risk factors for Parkinson's disease may differ in men and women: an exploratory study. Horm Behav. 2013;63((2)):308–314. doi: 10.1016/j.yhbeh.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahlskog JE. Parkin and PINK1 parkinsonism may represent nigral mitochondrial cytopathies distinct from Lewy body Parkinson's disease. Parkinsonism Relat Disord. 2009;15((10)):721–727. doi: 10.1016/j.parkreldis.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camerucci E, Stang CD, Hajeb M, Turcano P, Mullan AF, Martin P, et al. Early-onset Parkinsonism and early-onset Parkinson's disease: a population-based study (2010–2015) J Parkinsons Dis. 2021;11((3)):1197–1207. doi: 10.3233/JPD-202464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espay AJ, Schwarzschild MA, Tanner CM, Fernandez HH, Simon DK, Leverenz JB, et al. Biomarker-driven phenotyping in Parkinson's disease: a translational missing link in disease-modifying clinical trials. Mov Disord. 2017;32((3)):319–324. doi: 10.1002/mds.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMillan CT, Wolk DA. Presence of cerebral amyloid modulates phenotype and pattern of neurodegeneration in early Parkinson's disease. J Neurol Neurosurg Psychiatry. 2016;87((10)):1112–1122. doi: 10.1136/jnnp-2015-312690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espay AJ, Lang AE. Parkinson diseases in the 2020s and beyond: replacing clinico-pathologic convergence with systems biology divergence. J Parkinsons Dis. 2018;8((s1)):S59–s64. doi: 10.3233/JPD-181465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turcano P, Mielke MM, Josephs KA, Bower JH, Parisi JE, Boeve BF, et al. Clinicopathologic discrepancies in a population-based incidence study of parkinsonism in olmsted county: 1991–2010. Mov Disord. 2017;32((10)):1439–1446. doi: 10.1002/mds.27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52((6)):1214. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 49.Espay AJ, Vizcarra JA, Marsili L, Lang AE, Simon DK, Merola A, et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology. 2019;92((7)):329–337. doi: 10.1212/WNL.0000000000006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harkins K, Sankar P, Sperling R, Grill JD, Green RC, Johnson KA, et al. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7((1)):26. doi: 10.1186/s13195-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhana K, Franco OH, Ritz EM, Ford CN, Desai P, Krueger KR, et al. Healthy lifestyle and life expectancy with and without Alzheimer's dementia: population based cohort study. BMJ. 2022;377:e068390. doi: 10.1136/bmj-2021-068390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7((2)):e105–e25. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.GBD 2019 Collaborators Global mortality from dementia: application of a new method and results from the global burden of disease study 2019. Alzheimers Dement. 2021;7((1)):e12200. doi: 10.1002/trc2.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]