Abstract
Background
The effect of supplemental oxygen on sleep has not been studied in preterm infants.
Methods
We studied 18 stable late-preterm infants with observed periodic breathing at a median gestational age of 36 weeks. Polysomnography was performed on room air and on 25% oxygen-enriched ambient air.
Results
Supplemental oxygen did not affect sleep stage distribution, sleep efficiency, the frequency of sleep stage transitions, the appearance of rapid-eye movement (REM) sleep periods, or the high number of spontaneous arousals. The percentage in periodic breathing out of total sleep time decreased from 10% (interquartile range [IQR] 5–9%) on room air to 1% (IQR 0–3%) (p < 0.001) on supplemental oxygen. Also, the number of central apneas decreased from 48 (IQR 32–68) to 23 (IRQ 15–32) per hour (p < 0.001), and the number of oxygen desaturations of a minimum 3% from 38 (IQR 29–74) to 10 (IQR 5–24) per hour (p < 0.001). On room air in non-REM sleep, the median end-tidal carbon dioxide values were systematically lower during periodic breathing at 5.1 (IQR 4.6–6.4) kPa than during stable breathing at 5.5 (4.9–5.9) kPa (p < 0.0001).
Conclusions
In late-preterm infants, supplemental oxygen effectively reduces periodic breathing and the number of oxygen desaturations while having no significant effect on sleep. The results support the importance of carotid body over-reactivity on the genesis of periodic breathing in preterm infants.
Keywords: Periodic breathing, Supplemental oxygen, Apnea, Preterm infant, Polysomnography
Introduction
Supplemental oxygen is used to treat or prevent hypoxia and is one of the most used drugs in neonatal intensive care units. Hypoxia and especially intermittent hypoxia have adverse effects on cognitive and language development [1], while the main detrimental effect of supplemental oxygen is an increased risk of retinopathy of prematurity [2]. The detrimental effects of oxygen may be related to hyperoxia itself [3, 4] or to an increase in oxidative stress with a higher amount of fluctuation in the blood and tissue oxygen levels with high pulse oximeter oxyhemoglobin saturation (SpO2) targets [5].
In late-preterm infants, periodic breathing is one cause of intermittent hypoxia [6, 7]. The appearance of periodic breathing decreases with increasing gestational age (GA) [8, 9]. Supplemental oxygen [7, 10, 11, 12, 13, 14, 15, 16] and methylxanthines, such as caffeine [17, 18], reduce the amount of periodic breathing and hypoxia.
Previous studies on the effect of supplemental oxygen have been performed on polygraphic settings without sleep monitoring [10, 11, 12, 13, 14, 15, 16]. The effect of supplemental oxygen on sleep has not been studied in preterm periods. Simakarjonboon and associates [19] studied the effect of supplement oxygen on sleep quality in 23 premature infants at a post-conceptual age of 38 weeks. On oxygen, these infants showed reduced amounts of rapid-eye movement (REM) sleep. During the preterm period, REM sleep and dreaming provide holistic and important endogenous stimulation to developing neural networks of the brain [20]. In preterm rat pup studies, the reduction of REM sleep and REM sleep deprivation has been shown systematically to have a high and broad negative impact on neurodevelopment [20, 21]. We compared the effect of 25% oxygen-enriched air and room air (oxygen 21%) on sleep and breathing in 18 late-preterm infants with polysomnography (PSG).
Materials and Methods
We studied 18 preterm infants with observed periodic breathing or apneas who were at stable condition without caffeine treatment, ventilatory support, or supplemental oxygen before the study. The infants underwent a full PSG study with Siesta PSG equipment (Compumedics, Abbotsford, Australia) on room air and during supplemental oxygen (fraction of inhaled oxygen [FiO2] 25%) (Fig. 1). The recordings comprised four electroencephalogram (EEG) channels (C4-M1, Cz-Fz, Cz-O2, and O2-M1), two electro-oculogram channels (right and left), chin and diaphragm electromyogram (EMG), measurement of nasal airflow (pressure sensor), an abdominal band for respiratory movement detection, SpO2 with a 4-s averaging interval, end-tidal carbon dioxide (EtCO2) monitoring, and an electrocardiogram. We administered the supplemental oxygen with a special-purpose transparent headbox instead of nasal cannulas to avoid disruption of nasal breathing, sleep, and measurement of EtCO2. We continuously monitored the FiO2 (HandiTM+ Oxygen Analyzer; Maxtec, Salt Lake City, UT, USA).
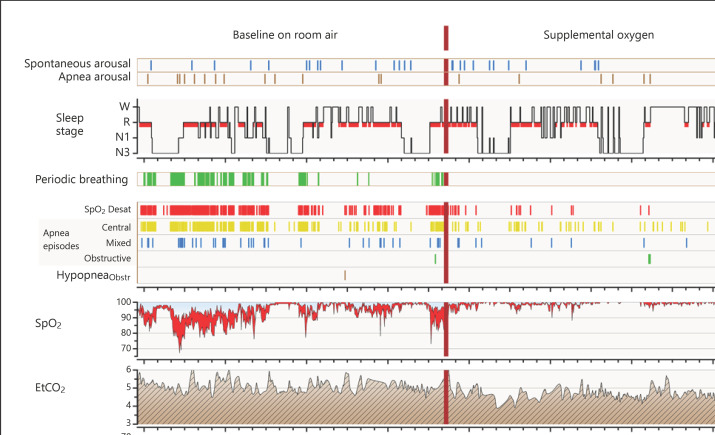
Fig. 1.
Study protocol and a graphic summary of PSG results. PSG reports summary of one study infant with a high tendency for periodic breathing and related oxygen desaturations. The figure demonstrates the study protocol and an example of high treatment responses. The PSG was performed at baseline on room air and on 25% oxygen content of ambient air. One event bar, one tick mark, represents one event. EtCO2, end-tidal carbon dioxide content of exhaled air; HypopneaObstr, obstructive hypopnea episodes; Respfreq, breathing frequency; SpO2, pulse oximeter oxyhemoglobin saturation; SpO2 Desat, SpO2 drops of 3% or more.
PSG recording analyses and visual scoring were done with Embla® RemLogicTM PSG software (Natus Medical Inc., Pleasanton, CA, USA), together with additional special-purpose software allowing detailed event analysis and analysis of SpO2 and EtCO2 signals. One experienced scorer (TK) performed the PSG scoring. For sleep staging, we followed the current AASM infant sleep stage criteria and the recommendations of Grigg-Dammberger and associates [22, 23] with some modifications. We did not use the transitional stage classification and scored corresponding epochs as either REM or non-REM based on chin EMG measurements. The definitions of apneas, periodic breathing, and arousals are presented in Table 1.
Table 1.
Apnea and arousal definitions used in the study
| Definition | Duration | |
|---|---|---|
| Apnea | ||
|
| ||
| Central apnea | Apnea with no breathing movements and no airflow | Pause in breathing lasting: >2 breathing cycles and ≥4 s |
|
| ||
| Obstructive apnea | Apnea with breathing movements but no airflow | All obstructive pauses of breathing |
|
| ||
| Mixed apnea | Apnea with both central and obstructive components | All mixed pauses of breathing |
|
| ||
| Obstructive hypopnea | Resistive airflow flattening pattern during inspiration with >50% drop in airflow rate combined with a sudden interruption of an event by respiratory arousal | |
|
| ||
| AOP | Apnea with: heart rate decreases to <100 beats per minute, or drop in SpO2 to <80%, or apnea length of >20 s | |
|
| ||
| Arousal | ||
|
| ||
| Arousal | A sustained increase in chin and diaphragm EMG from baseline recording excluding sucking of the dummy, or gross body movements causing artifacts in ECG, EEG, respiratory signal | ≥3 s |
|
| ||
| Apnea arousal | An arousal appearing during an apnea or within 5 s after the end of an apnea episode | ≥3 s |
|
| ||
| Awakening | Same as arousal | ≥15 s |
EMG, electromyogram; ECG, electrocardiogram; EEG, electroencephalogram.
We used the nonparametric Wilcoxon signed-rank test for pairwise comparison. The level of significance was set at p < 0.05.
The Helsinki University Ethics Committee (174/13/03/03/2012) and the Children's Hospital Institutional Review Board (Project #1025) approved the study protocol. Parents provided written consent forms and did not receive any monetary compensation for participation.
Results
Infant demographics are outlined in Table 2. The infants were born at a median 31.4 (interquartile range [IQR] 28.3–33.7) weeks of GA, with a birth weight of 1,695 (IQR 1,133–2,139) g. They were studied at a median post-conceptual age of 35.6 (IQR 35.0–36.2) weeks. Of the 18 infants, 12 had previous caffeine treatment that was discontinued a median of 8.5 (IQR 7.0–11.0) days before the study. The infants exhibited central, mixed, and apnea of prematurity (AOP) defined apneas, periodic breathing, and recurrent desaturations.
Table 2.
Demographic data
| Infants, n | 18 |
| Female, n (%) | 10 (55.6) |
| GA at birth, weeks | 31.4 (28.3–33.7) |
| Weight at birth, g | 1,695 (1,133–2,139) |
| BPD, n (%) | 5 (27.8) |
| GMV-IVH, n (%) | 3 (16.7) |
| Grade 1 to 2 | 2 (11.1) |
| Grade 4 | 1 (5.6) |
| Apgar | |
| 1 min | 6.5 (5.0–7.0) |
| 5 min | 7.0 (6.0–8.0) |
| Age at study, weeks | 4.7 (2.0–6.8) |
| GA at study, weeks | 35.6 (35.0–36.2) |
| Weight at study, kg | 2,203 (1,919–2,533) |
| Infants with caffeine previously, n (%) | 12 (66.7) |
| Caffeine-free period before study, days | 8.5 (7.0–11.0) |
Results presented as median (IQR) or n (%). BPD, bronchopulmonary dysplasia; GMH-IVH, germinal matrix or intraventricular hemorrhage.
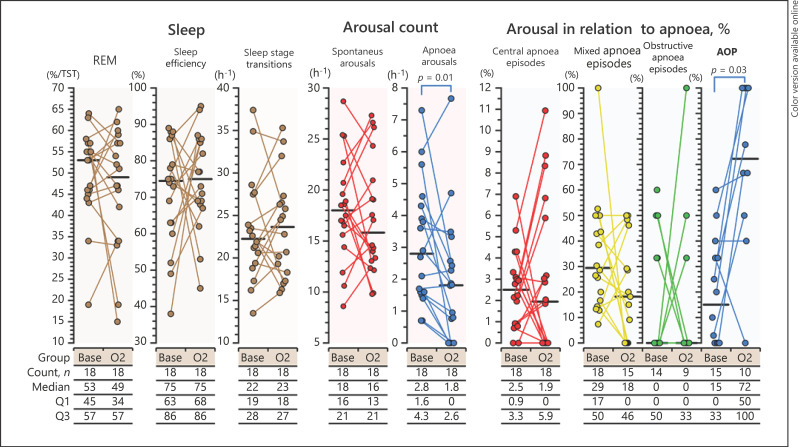
Supplemental oxygen had only minor effects on sleep (Fig. 2). In line with the decrease in the number of apneas, the number of apnea-related arousals decreased (p = 0.01). Supplemental oxygen did not affect sleep stage distribution, sleep efficiency, frequency of sleep stage transitions, appearance of REM periods, or the high number of spontaneous arousals (Fig. 2). Spontaneous arousals were more frequent in REM than in non-REM sleep both during baseline recordings on room air and during supplemental oxygen (p < 0.001). Arousal percentile to AOPs increased from 20% (IQR 0–40) on room air to 72% (IQR 48–100) (p = 0.03) during supplemental oxygen.
Fig. 2.
Effect of supplemental oxygen on sleep. Apnea-related arousals were reduced with supplemental oxygen, while basic sleep architecture remained unaffected. Long apneas (AOP) ended more often with arousal on supplemental oxygen. AOP, apnea of prematurity; REM, rapid-eye movement sleep; Base, baseline PSG results on room air; O2, PSG results during supplemental oxygen; TST, total sleep time; Q1, 1st quartile; Q3, 3rd quartile.
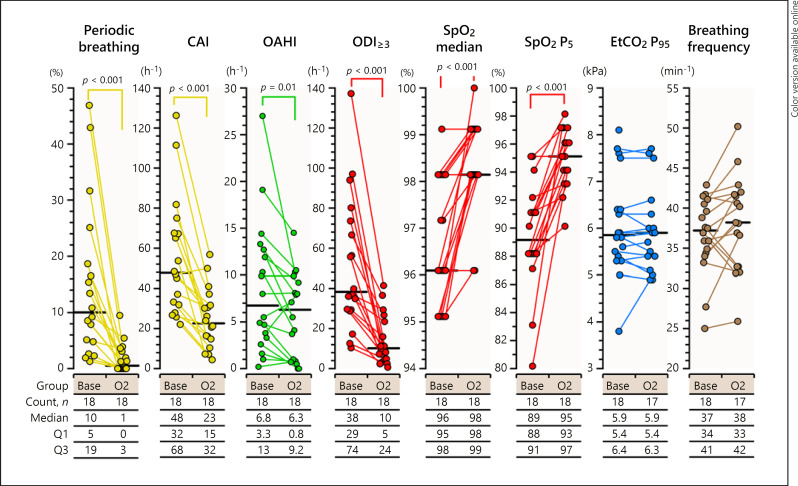
Supplemental oxygen reduced periodic breathing and related oxygen desaturations effectively (Fig. 1, 3). The percentage of periodic breathing decreased from 10% (IQR 5–19) of sleep time at baseline on room air to 1% (IQR 0–3) (p < 0.001) on supplemental oxygen, the number of central apneas decreased from 48 (IQR 32–68) to 23 (IRQ 15–32) per hour (p < 0.001), and the number of oxygen desaturations of a minimum 3% from 38 (IQR 29–74) to 10 (IQR 5–24) per hour (p < 0.001). Simultaneously, the baseline SpO2 level and the low fifth percentile levels of SpO2 increased (p < 0.001).
Fig. 3.
The effect of supplemental oxygen on breathing. Supplemental oxygen effectively reduced the number of central apneas and periodic breathing. Base, baseline PSG results on room air; CAI, central apnea index; EtCO2 P95; 95th percentile level of EtCO2 during sleep; nPB, periods without periodic breathing in nREM; nREM, non-rapid-eye movement sleep; O2, PSG results on supplemental oxygen; OAHI, obstructive apnea and hypopnea index; ODI≥3, pulse oximeter oxyhemoglobin desaturation of 3% or more; PB, periodic breathing episodes in non-REM sleep; SpO2, pulse oximeter oxyhemoglobin saturation; SpO2 P5, 5th percentile level of SpO2 during sleep.
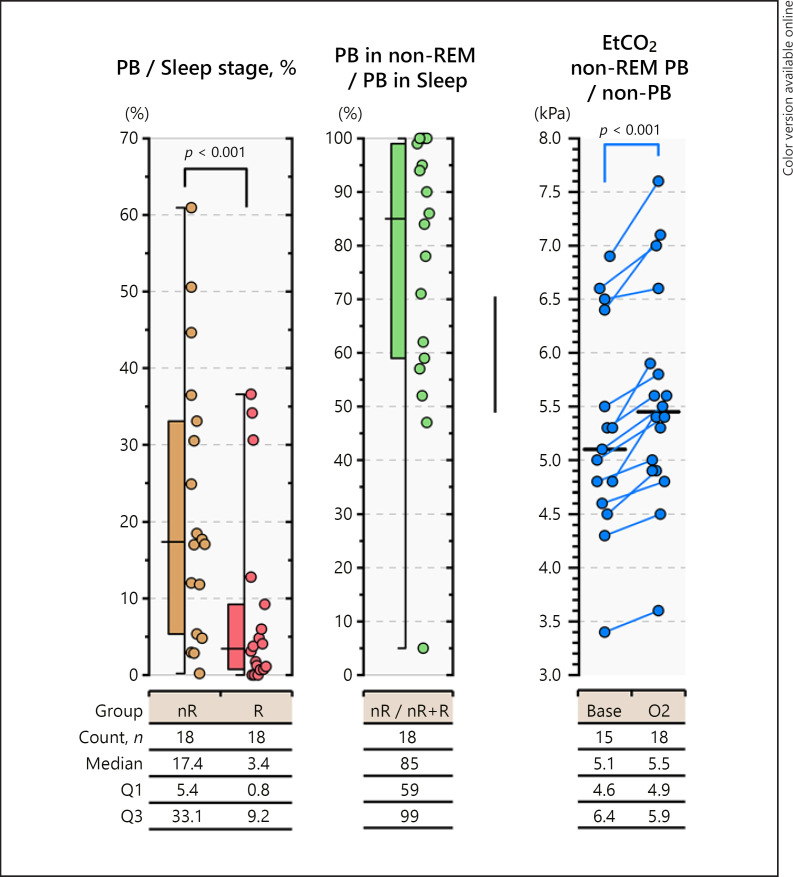
Periodic breathing appeared primarily in non-REM sleep (p < 0.001) as a state of mild hyperventilation on PSG (Fig. 4). At baseline recordings on room air, median EtCO2 values in non-REM sleep were systematically lower during periodic breathing at 5.1 (IQR 4.6–6.4) kPa than during stable breathing at 5.5 (4.9–5.9) kPa (p < 0.0001). EtCO2 95th percentile values were similar during periods of breathing room air and on supplemental oxygen. The online supplementary holds three figures presenting the number of apneas at baseline, the number of SpO2 desaturations, and the effect of pulse oximeter signal-averaging time window on the detection of SpO2 desaturations during periodic breathing.
Fig. 4.
The appearance and characteristics of periodic breathing during baseline recording on room air in non-REM and REM sleep. Most periodic breathing episodes occurred during non-REM sleep and represented a state of increased ventilation compared with non-REM periods of stable breathing. The median EtCO2 was systematically lower in non-REM sleep during periodic breathing than during stable breathing. Base, baseline PSG results on room air; EtCO2, end-tidal carbon dioxide content of exhaled air; nR, non-REM sleep; O2, PSG results on supplemental oxygen; PB, periodic breathing; R, REM sleep.
Discussion
Our study shows that supplemental oxygen has no clear short-term effects on sleep in late-preterm infants. Supplemental oxygen effectively stabilizes sleep disordered breathing by removing periodic breathing and related central apneas. Subsequently, oxygen also reduces the number of apnea-related arousals. Supplemental oxygen did not affect ventilation or breathing frequency.
Our sleep results contradict the previous study by Simakajorboon and associates [19]. They found that supplemental oxygen increased quiet sleep and decreased active sleep while having no effect on sleep efficacy in healthy preterm infants at 38 weeks of post-conceptual age. The difference between our studies was the way the supplemental oxygen was given. We used headbox to avoid any discomfort or additional blockage of the nose which may occur while giving the oxygen by additional low-flow nasal cannula.
Other studies concerning sleep and supplemental oxygen on preterm infants have all been performed at an older age in infants with bronchopulmonary dysplasia (BPD) and a clear manifestation of pulmonary complications. In an early study by Harris and associates [24], seven infants with BPD showed a clear improvement in sleep quality and the ability to sleep REM sleep when treated with supplemental oxygen. The same research group was not able to confirm the finding in a larger infant cohort [25]. Our study included five infants with a diagnosis of mild BPD. In them, sleep stage distribution, sleep efficiency, number of spontaneous arousals, and EtCO2 levels remained similarly unaltered during supplemental oxygen as in infants without a diagnosis of BPD. However, the number of apnea-related arousals did not decrease during supplemental oxygen as it did in infants without diagnosis of BPD.
The pathophysiology of periodic breathing is not fully understood [26]. Most animal and human data suggest that periodic breathing is likely caused by increased ventilation chemodetection mediated by either carotid body hyperactivity or increased central chemoreception [27, 28, 29]. These responses lead to a hyperventilation state during metabolic control of breathing in non-REM sleep in which periods of hyperpnea are counterbalanced by periods of central apnea or hypopnea. The effect of supplemental oxygen is most likely mediated via suppression of the function of the carotid body [28]. Our study supports the hypothesis that periodic breathing in preterm infants is related to a high carotid body reactivity because periodic breathing appeared as a state of mild hyperventilation and was stabilized by suppression of the carotid body function by supplemental oxygen.
Higher oxygen saturation targets increase the incidence of retinopathy of prematurity [2, 30]. In addition, preterm infants with a higher incidence of intermittent hypoxia present with more severe retinopathy [31]. In the current study, supplemental oxygen effectively reduced the number and degree of oxygen desaturations. Thus, the low level of supplemental oxygen used (FiO2 25%) may have differing effects on develpment of retinopathy that higher fractions of inspired O2.
Clinical Significance of Periodic Breathing
The clinical significance of periodic breathing and exposure to mild intermittent hypoxia in neonates is not well established [1, 32]. In animal models, mild intermittent hypoxia has shown both negative and positive effects on neurodevelopment and cardiovascular function [32, 33]. However, in general, intermittent hypoxia is considered detrimental for neurodevelopment [1, 34]. In the current study, the observed amount of mild intermittent hypoxia was high, and although apneas related to periodic breathing were short, they were frequently followed by SpO2 desaturations (Fig. 1, online suppl. Fig. 1, 2; see www.karger.com/doi/10.1159/000525196 for all online suppl. material). The amount and extent of intermittent hypoxia related to periodic breathing are easily vastly underestimated, as the length of apneas and desaturations is commonly short (online suppl. Fig. 2). In addition, the long signal-averaging times used with pulse oximeters effectively filter out the actual SpO2 desaturations (online suppl. Fig. 3). Nevertheless, we believe that the majority of the beneficial effects of caffeine in preterm infants are relayed through its effect of decreasing central apneas and periodic breathing [35].
Limitations
The study was not carried out in cross-over setup, but baseline recordings on room air were performed first, followed by recordings with supplemental oxygen. We do not expect that this would have had significant effect on the results.
There is no consensus on how sleep and arousals in preterm infants should be scored. We applied the established definitions for one- to 6-month-old infants [22, 31]. However, due to the immaturity of EEG, the EEG signals did not have the same impact on sleep stage scoring as in older infants and, in addition, we did not separate cortical and subcortical arousals.
Conclusions
In late-preterm infants, supplemental oxygen does not affect sleep, but it effectively stabilizes breathing in non-REM sleep and reduces the number of oxygen desaturations. Periodic breathing in preterm infants is a mild hyperventilation state. These results support the importance of carotid body over-reactivity as an important etiology of periodic breathing in preterm infants.
Statement of Ethics
The Helsinki University Ethics Committee reviewed and approved the study protocol, approval number 174/13/03/03/2012. The Children's Hospital Institutional Review Board of the Helsinki University Hospital approved execution of the study, Project #1025.
Parents provided written consent forms and did not receive any monetary compensation for participation.
Conflict of Interest Statement
None of the authors have any conflicts of interest to declare.
Funding Sources
This work was supported by grants from the Foundation for Pediatric Research in Finland as personal Grants to Maija Seppä-Moilanen (#140029, #160031, #170056, #200160, #210192) and as group Grants to Turkka Kirjavainen (#160268, #190140, #200184).
Author Contributions
All authors, Maija Seppä-Moilanen, Sture Andersson, and Turkka Kirjavainen, had substantial contributions to the design of the work and interpretation of data, drafting, revising, and having a final approval of the manuscript. Maija Seppä-Moilanen and Turkka Kirjavainen performed polysomnography studies and data and statistical analysis. Turkka Kirjavainen was responsible for the software development needed of the detailed data analysis and the scoring of polysomnography recordings.
Data Availability Statement
Inquiries of the data presented in this study can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgments
We thank the study infants and their families for taking part in our study. We thank the staff of the Helsinki University Hospital neonatal wards for assistance in recruiting the study infants and providing aid during the PSG recordings, and the staff at the Helsinki University Hospital Children's neurophysiology department for aid and assistance. We thank the Finnish Foundation for Pediatric Research for financial support.
Funding Statement
This work was supported by grants from the Foundation for Pediatric Research in Finland as personal Grants to Maija Seppä-Moilanen (#140029, #160031, #170056, #200160, #210192) and as group Grants to Turkka Kirjavainen (#160268, #190140, #200184).
References
- 1.Poets CF. Intermittent hypoxia and long-term neurological outcome: how are they related? Semin Fetal Neonatal Med. 2020 Apr;25((2)):101072. doi: 10.1016/j.siny.2019.101072. [DOI] [PubMed] [Google Scholar]
- 2.Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA. 2018 Jun 5;319((21)):2190–2201. doi: 10.1001/jama.2018.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Jiang P, Du M, Chen K, Chen A, Wang Y, et al. Hyperoxia-induced immature brain injury through the TLR4 signaling pathway in newborn mice. Brain Res. 2015 Jun 12;1610:51–60. doi: 10.1016/j.brainres.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Du M, Tan Y, Liu G, Liu L, Cao F, Liu J, et al. Effects of the Notch signalling pathway on hyperoxia-induced immature brain damage in newborn mice. Neurosci Lett. 2017 Jul 13;653:220–227. doi: 10.1016/j.neulet.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 5.Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015 Jan;122((1)):200–210. doi: 10.1016/j.ophtha.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Fiore JM, Martin RJ, Gauda EB. Apnea of prematurity: perfect storm. Respir Physiol Neurobiol. 2013;189((2)):213–222. doi: 10.1016/j.resp.2013.05.026. Published by The Netherlands: Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 7.Seppä-Moilanen M, Andersson S, Rantakari K, Mikkola K, Kirjavainen T. Caffeine and supplemental oxygen effectively suppress periodic breathing with only minor effects during long episodes of apnoea in preterm infants. Acta Paediatr. 2019 Aug 17;108((3)):443–451. doi: 10.1111/apa.14541. [DOI] [PubMed] [Google Scholar]
- 8.Henderson-Smart DJ. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J. 1981 Dec;17((4)):273–276. doi: 10.1111/j.1440-1754.1981.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira AJ, Nunes ML, Fojo-Olmos A, Reis FM, da Costa JC. Clinical correlates of periodic breathing in neonatal polysomnography. Clin Neurophysiol. 2004;115:2247–2251. doi: 10.1016/j.clinph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Wilson J, Long S, Howard P. Respiration of premature infants. Am J Dis Child. 1942;63((6)):1080–1085. [Google Scholar]
- 11.Graham BD, Reardon HS, Wilson JL, Tsao MW, Baumann ML. Physiologic and chemical response of premature infants to oxygen-enriched atmosphere. Pediatrics. 1950 Jul;6((1)):55–71. [PubMed] [Google Scholar]
- 12.Cross KW, Oppe TE. The effect of inhalation of high and low concentrations of oxygen on the respiration of the premature infant. J Physiol. 1952 May;117((1)):38–55. [PMC free article] [PubMed] [Google Scholar]
- 13.Miller HC. Effect of high concentrations of carbon dioxide and oxygen on the respiration of fullterm infants. Pediatrics. 1954 Aug;14((2)):104–113. [PubMed] [Google Scholar]
- 14.Rigatto H, Brady JP. Periodic breathing and apnea in preterm infants. I. Evidence for hypoventilation possibly due to central respiratory depression. Pediatrics. 1972 Aug;50((2)):202–218. [PubMed] [Google Scholar]
- 15.Fenner A, Schalk U, Hoenicke H, Wendenburg A, Roehling T. Periodic breathing in premature and neonatal babies: incidence, breathing pattern, respiratory gas tensions, response to changes in the composition of ambient air. Pediatr Res. 1973 Apr;7((4)):174–183. doi: 10.1203/00006450-197304000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub Z, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Effects of inhaled oxygen (up to 40%) on periodic breathing and apnea in preterm infants. J Appl Physiol. 1992 Jan;72((1)):116–120. doi: 10.1152/jappl.1992.72.1.116. [DOI] [PubMed] [Google Scholar]
- 17.Henderson-Smart DJ, De Paoli AG. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev. 2010;((12)):CD000140. doi: 10.1002/14651858.CD000140.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Seppä-Moilanen M, Andersson S, Kirjavainen T. Spontaneous and apnea arousals from sleep in preterm infants. Pediatr Res. 2021 Apr;89((5)):1261–1267. doi: 10.1038/s41390-020-1068-2. [DOI] [PubMed] [Google Scholar]
- 19.Simakajornboon N, Beckerman RC, Mack C, Sharon D, Gozal D. Effect of supplemental oxygen on sleep architecture and cardiorespiratory events in preterm infants. Pediatrics. 2002 Nov;110((5)):884–888. doi: 10.1542/peds.110.5.884. [DOI] [PubMed] [Google Scholar]
- 20.Graven S. Sleep and brain development. Clin Perinatol. 2006;33((3)):693–706. doi: 10.1016/j.clp.2006.06.009. vii. [DOI] [PubMed] [Google Scholar]
- 21.Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7((4)):321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 22.Grigg-Damberger MM. The visual scoring of sleep in infants 0 to 2 months of age. J Clin Sleep Med. 2016 Mar;12((3)):429–445. doi: 10.5664/jcsm.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry RB, Quan SF, Abreu AR, Bibbs ML, DelRosso L. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 24.Harris MA, Sullivan CE. Sleep pattern and supplementary oxygen requirements in infants with chronic neonatal lung disease. Lancet. 1995 Apr 1;345((8953)):831–832. doi: 10.1016/s0140-6736(95)92966-5. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald D, Van Asperen P, Leslie G, Arnold J, Sullivan C. Higher SaO2 in chronic neonatal lung disease: does it improve sleep? Pediatr Pulmonol. 1998;26((4)):235–240. doi: 10.1002/(sici)1099-0496(199810)26:4<235::aid-ppul1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Edwards BA, Sands SA, Berger PJ. Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. Respir Physiol Neurobiol. 2013 Jan 1;185((1)):144–155. doi: 10.1016/j.resp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox I, Grunstein RR, Collins FL, Berthon-Jones M, Kelly DT, Sullivan CE. The role of central chemosensitivity in central apnea of heart failure. Sleep. 1993 Dec;16((8 Suppl l)):S37–8. doi: 10.1093/sleep/16.suppl_8.s37. [DOI] [PubMed] [Google Scholar]
- 28.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol. 2013 Jan;3((1)):141–163. doi: 10.1002/cphy.c110057. [DOI] [PubMed] [Google Scholar]
- 29.Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body chemoreflex in the pathophysiology of heart failure: a perspective from animal studies. Adv Exp Med Biol. 2015;860:167–185. doi: 10.1007/978-3-319-18440-1_19. [DOI] [PubMed] [Google Scholar]
- 30.Higgins RD. Oxygen saturation and retinopathy of prematurity. Clin Perinatol. 2019 Sep;46((3)):593–599. doi: 10.1016/j.clp.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010 Jul;157((1)):69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Fiore JM, MacFarlane PM, Martin RJ. Intermittent hypoxemia in preterm infants. Clin Perinatol. 2019 Sep;46((3)):553–565. doi: 10.1016/j.clp.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belaidi E, Khouri C, Harki O, Baillieul S, Faury G, Briançon-Marjollet A, et al. Cardiac consequences of intermittent hypoxia: a matter of dose? A systematic review and meta-analysis in rodents. Eur Respir Rev. 2022 Jun;30((164)):31. doi: 10.1183/16000617.0269-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004 Sep;114((3)):805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354((20)):2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Inquiries of the data presented in this study can be directed to the corresponding author.