Abstract
Introduction
Human papillomaviruses (HPVs), Epstein-Barr virus (EBV), and mouse mammary tumor virus-like virus (MMTV-like virus) can be present and contribute to breast cancer development and progression. However, the role of these oncoviruses and their crosstalk in breast cancer is still unclear.
Methods
We explored the co-presence of high-risk HPVs, EBV, and MMTV-like virus in 74 breast cancer samples from Qatar using PCR.
Results
We found the presence of HPV and EBV in 65% and 49% of our cancer sample cohorts; 47% of the samples are positive for both oncoviruses. The MMTV-like virus alone was detected in 15% of the samples with no significant association with clinicopathological features. The three oncoviruses were co-present in 14% of the cases; no significant association was noted between the co-presence of these viruses and the clinicopathological features.
Conclusion
Despite the presence of the oncoviruses, additional studies are necessary to understand their interactions in human breast carcinogenesis.
Keywords: Human papillomavirus, Epstein-Barr virus, Mouse mammary tumor virus-like virus, Breast cancer, Qatari population
Introduction
Breast cancer is the second most common cancer worldwide, including the Middle East (ME) region and Qatar [1]. In comparison with ME countries, Qatar has the highest incidence of breast cancer, accounting for approximately 38% of all new cancer cases [2, 3]. Intriguingly, in comparison with their counterparts in the West, women in the ME are affected with breast cancer at a relatively younger age (<50 years) [3]. Moreover, women with breast cancer in the ME region usually display advanced-stage disease and a more aggressive phenotype [4]. In addition to genetic and environmental factors, oncogenic viruses, mainly high-risk human papillomaviruses (HPVs), Epstein-Barr virus (EBV), and mouse mammary tumor virus-like virus (MMTV-like virus), are found in various types of human cancers, including breast [5, 6, 7, 8, 9].
High-risk HPVs are double-stranded DNA viruses that can immortalize human mammary epithelial cells. More specifically, E5 and E6/E7 oncoproteins of HPV cooperate in human cancer pathogenesis [10, 11]; in this context, it has been demonstrated that E6/E7 oncoproteins can convert noninvasive and nonmetastatic breast cancer cells into invasive and metastatic phenotypes via their cooperation with other oncogenes [12, 13, 14, 15, 16]. Several studies reported that HPVs could be co-present with other strains of human oncoviruses (EBV and MMTV-like virus) and cooperate with these oncoviruses to initiate and/or enhance carcinogenesis in various types of human cancers, including breast [17, 18, 19, 20, 21, 22, 23, 24].
EBV, a double-stranded DNA gamma-herpes virus, creates a persistent infection in memory B-lymphocytes with latent EBV infection (EBV nuclear antigen 1), and/or latent membrane protein 2 [25]; latent EBV may reactivate and stimulate viral genes (EBNAs, LMP1, and LMP2) inducing epithelial cell growth, proliferation, and angiogenesis while inhibiting apoptosis [26, 27, 28]. Earlier studies reported that EBV is present in several human cancers, including breast [29].
The MMTV-like virus, a part of the Betaretroviridae family, is a nonacutely transforming retrovirus [30]. MMTV-like virus was previously found in several human cancers, including breast [8, 9]; however, the role of MMTV-like virus in the onset of cancer remains unclear.
While several studies have reported the presence/co-presence of high-risk HPVs, EBV, and MMTV-like virus in human breast cancer [21, 22, 24], other investigations could not confirm this [31, 32, 33]. Thus, we herein explored the presence/co-presence of these oncoviruses in breast cancer samples from Qatari women.
Materials and Methods
Breast Cancer Samples
This investigation used formalin-fixed, paraffin-embedded (FFPE) breast cancer samples obtained from patients who received surgical treatment at Hamad General Hospital in Qatar, as described previously [22]. A cohort of 74 FFPE blocks (punch samples of 2 mm thickness) from pathologically confirmed invasive breast cancer patients was used for DNA extraction [22]. None of the patients received neoadjuvant treatment modalities (chemotherapy, radiotherapy, endocrine therapy, immunotherapy, targeted therapy). In addition, we included 14 control samples from another cohort (normal/benign breast tissues) [34]. The study was approved by the Ethical Committees of Qatar University and Hamad Medical Corporation (QU-IBC-2018/22; HMC:24-2-2019, Doha, Qatar).
Oncogenic Viral Detection by PCR
The detection and genotyping of high-risk HPVs (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, and 58) and EBV were performed as previously described [22]. In addition, PCR was performed for MMTV-like virus (forward primer: 5′-GATGGTATGAAGCAGGATGG-3′ and reverse primer: 5′-CCTCTTTTCTCTATATCTATTAGCTGAGGTAATC-3′). For all reactions, GAPDH was used as an internal control. Analyses were completed as illustrated earlier [22]. The Invitrogen Platinum II Hot-Start Green PCR Master Mix (2X) (Thermo Fisher Scientific, Waltham, MA, USA) was used to perform PCR. Briefly, the MMTV-like virus gene was amplified for an initial denaturation at 94°C for 2 min, followed by 40 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 30 s. The samples were finally incubated for 10 min at 72°C for a final extension. HPV and EBV genes were amplified as described previously [22]. The PCR product was resolved using 1.5% agarose gel electrophoresis. For each experiment performed, respective positive and negative controls were used; positive control for HPV included normal oral epithelial cell line expressing E6/E7, for EBV diluted DNA plasmids for EBV nuclear antigen 1 and LMP1, while for MMTV-like virus, we used MDA-MB-231 as the positive control [35]. Nuclease-free water instead of DNA was used for the negative control [15].
Statistical Analysis
Statistical analysis and plotting of graphs were done using GraphPad Prism software (version 8.4.3). To determine a significant association between the presence/co-presence of HPVs, EBV, and MMTV-like virus with clinicopathological data (Nottingham histological grade, tumor stage, and lymph node involvement), we performed chi-square (χ2) test with Yates' correction and Fisher's exact test in addition to Wilcoxon test. Data were calculated as nonparametric files, and statistically significant results were achieved if p values were ≤0.05.
Results
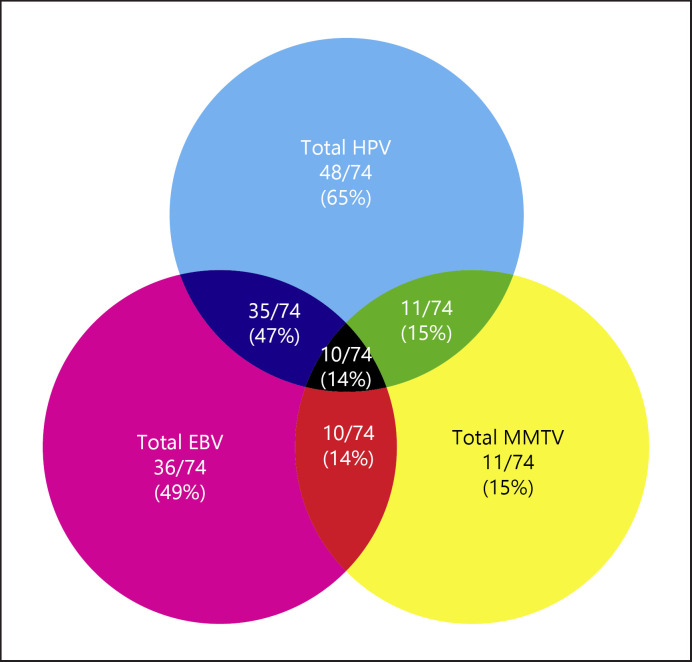
In this study, we investigated the presence of MMTV-like virus in addition to its co-presence with high-risk HPVs and EBV in a cohort of 74 breast cancer samples from the Qatari population and 14 normal/benign breast tissues. We recently revealed that 65% of the samples were positive for high-risk HPVs, while 36/74 (49%) of the samples were positive for EBV [22]; in addition, we found that high-risk HPVs and EBV are co-present in (47%) of breast cancer cases [22].
We found that 11/74 (15%) of breast cancer cases are positive for MMTV-like virus (Fig. 1). In addition, 11/74 (15%) and 10/74 (14%) showed co-presence of HPV and MMTV-like virus as well as EBV and MMTV-like virus, respectively (Fig. 1). More significantly, we report that 10/74 (14%) of the breast cancer samples co-expressed all three oncoviruses (Fig. 1). On the other hand, normal/benign breast samples lacked the co-presence of HPV and MMTV-like virus (p = 0.2), EBV, MMTV and HPV, and EBV and MMTV-like virus in normal breast tissues (p = 0.3, each, respectively).
Fig. 1.
Venn diagram depicting single and multiple infections with HPV, EBV, and/or MMTV-like virus in Qatari breast cancer samples (n = 74).
Concerning the clinicopathological characteristics, in our previous study, we found that the co-presence of high-risk HPVs is associated with tumor grade (p = 0.04) and stage (p = 0.04) in comparison with HPV positive alone and/or EBV positive alone in addition to HPV/EBV negative [22]. In the current study, we did not find any significant associations between the presence of MMTV-like virus alone with patients' age (p = 0.74), tumor grade (p = 0.25), tumor stage (p = 0.12), hormonal receptor (estrogen receptor [ER] and progesterone receptor [PR]) status (p = 0.68 and p = 0.56, respectively), and HER-2 status (p = 0.06) (Table 1). In addition, there was no significant association of the co-presence of high-risk HPVs, EBV, and MMTV-like virus with clinicopathological parameters (patients' age, tumor grade, tumor stage, ER status, PR status, and HER-2 status) (p = 0.93, p = 0.25, p = 0.12, p = 0.79, p = 0.7, and p = 0.21, respectively) (Table 2).
Table 1.
Correlation between clinicopathological characteristics and MMTV-like virus positivity
| Clinicopathological characteristics | MMTV-like virus positive (%) | MMTV-like virus negative (%) | p value |
|---|---|---|---|
| Age | |||
| ≤50 years | 3 (4) | 22 (30) | |
| >50 years | 8 (11) | 41 (55) | 0.74 |
| Total | 11 (15) | 63 (85) | |
| Nottingham histological grade | |||
| Grade | |||
| I | 4 (5.5) | 10 (14) | |
| II | 4 (5.5) | 36 (50) | 0.25 |
| III | 3 (4) | 15 (21) | |
| Total | 11 (15) | 61 (85) | |
| Tumor stage | |||
| Stage | |||
| Early stage (I–I) | 6 (13) | 33 (70) | |
| Advanced stage (III–IV) | 0 (0) | 8 (17) | 0.57 |
| Total | 6 (13) | 41 (87) | |
| ER status | |||
| ER+ | 9 (12) | 50 (70) | |
| ER– | 2 (3) | 11 (15) | 0.68 |
| Total | 11 (15) | 61 (85) | |
| PR status | |||
| PR+ | 7 (10) | 47 (65) | |
| PR– | 4 (5) | 14 (20) | 0.57 |
| Total | 11 (15) | 61 (85) | |
| HER-2 status | |||
| HER-2+ | 5 (7) | 11 (15) | |
| HER-2– | 6 (8) | 50 (70) | 0.06 |
| Total | 11 (15) | 61 (85) |
Comparison was made between presence/absence of HPV (HPV+/HPV−) and clinicopathological characteristics. * Indicates significant p values (p < 0.05).
Table 2.
The correlation between clinicopathological characteristics and HPV/EBV/MMTV-like virus status (the HPV/EBV/MMTV-like virus positive group was compared with other subgroups of breast cancer with various HPV/EBV/MMTV-like virus statuses)
| Clinicopathological characteristics | HPV+/EBV+/MMTV-like virus+ (%) | HPV±/EBV±/MMTV-like virus± (%) | p value |
|---|---|---|---|
| Age | |||
| ≤50 years | 3 (4) | 22 (30) | |
| >50 years | 7 (9) | 42 (57) | 0.93 |
| Total | 10 (13) | 64 (87) | |
| Nottingham histological grade | |||
| Grade | |||
| I | 4 (5.5) | 10 (14) | |
| II | 4 (5.5) | 36 (50) | 0.25 |
| III | 2 (3) | 16 (22) | |
| Total | 10 (14) | 62 (86) | |
| Tumor stage | |||
| Stage | |||
| Early stage (I–II) | 6 (13) | 33 (70) | |
| Advanced stage (III–IV) | 0 (0) | 8 (17) | 0.12 |
| Total | 6 (13) | 41 (87) | |
| ER status | |||
| ER+ | 8 (11) | 51 (71) | |
| ER– | 2 (3) | 11 (15) | 0.79 |
| Total | 10 (14) | 62 (86) | |
| PR status | |||
| PR+ | 7 (10) | 47 (65) | |
| PR– | 3 (4) | 15 (21) | 0.7 |
| Total | 10 (14) | 62 (86) | |
| HER-2 status | |||
| HER-2+ | 4 (6) | 12 (17) | |
| HER-2– | 6 (8) | 50 (69) | 0.21 |
| Total | 10 (14) | 62 (86) |
HPV+/EBV+/MMTV-like virus + denotes a co-presence of HPV, EBV, and MMTV-like virus. HPV±/EBV±/MMTV-like virus±indicates a combination of HPV+/EBV–/MMTV-like virus– (HPV presence/EBV absence/MMTV-like virus absence), HPV–/EBV+/MMTV-like virus– (HPV absence/EBV presence/MMTV-like virus absence), HPV–/EBV–/MMTV-like virus+ (HPV absence/EBV absence/MMTV-like virus presence), and HPV–/EBV–MMTV– (lack of all the three HPV, EBV, and MMTV-like virus). * Indicates significant p values (<0.05).
Discussion
This is the first study on the presence/co-presence of high-risk HPVs, EBV, and MMTV-like virus in human breast cancer samples in the Gulf region. Our data revealed HPV positivity in 65% and 36% of our breast cancer and normal/benign tissue samples, with no significant difference in the HPV positivity between breast cancer and the controls. On the other hand, we found EBV positivity in 49% of breast cancer cases in Qatari women, while normal/benign breast tissue samples lacked EBV presence. In addition, we did not note any significant association of either HPV or EBV positivity with the clinicopathological outcomes in breast cancer cases.
Previous studies reported the presence of the MMTV gene in human breast cancer patients with varying prevalence ranging from 0 to 74% [33, 36, 37, 38, 39, 40, 41, 42] (Table 3). Our study revealed that MMTV-like virus is present in 15% of breast cancer samples in the Qatari cohort, with no presence in normal/benign breast samples (0%). Similar findings were reported in other ME countries [36, 37, 38, 39, 40, 41, 42] (Table 3). Variations in prevalence detection may be attributed to technical and methodological variations among studies, and retroviral etiology needs to be explored further [33]. The presence of MMTV-like virus supports our findings suggesting a plausible role in breast carcinogenesis. Katz et al. [43] demonstrated that proteins expressed by the MMTV-like virus envelope gene could transform normal breast epithelial cells. Moreover, insertion of the MMTV proviral DNA in the vicinity of proto-oncogenes (Wnt-1 and Fgf) enhanced cell growth [44] and the development of mammary tumors [45].
Table 3.
Prevalence of MMTV-like virus in breast cancer in ME populations
| Country | Year | MMTV-like virus (%) |
|---|---|---|
| Egypt | 2021 | Sporadic breast cancer tissues (38/50, 76) Familial breast cancer tissues (21/30, 70) |
|
| ||
| Saudi Arabia | 2018 | Breast cancer tissues (6/101, 6) Noncancerous adjacent tissues (9/93, 10) Control breast tissue obtained from individuals without cancer (0/51, 0) |
|
| ||
| Iran | 2017 | Breast carcinomas (19/59, 32) Nonmalignant breast tissue samples (3/59, 5) |
|
| ||
| Morocco | 2014 | 24/42 (57) |
|
| ||
| Tunisia | 2008 | 17/122 (14) |
|
| ||
| Egypt | 2005 | 3/23 (13) |
|
| ||
| Tunisia | 2004 | 28/38 (74) |
Although the role of viral co-infection in breast cancer is still controversial, it is postulated that MMTV infection can trigger the expression of latent DNA viruses, including HPV or EBV, to initiate the onset and development of cancer. We previously reported that the co-presence of high-risk HPVs and EBV (47% of the breast cancer samples) is associated with advanced tumor stage and grade (p = 0.04) in breast cancer [20]. In the present study, we report the co-infection of HPV and MMTV-like virus in 15% of our cohort, while EBV and MMTV-like virus co-incidence was found in 14% of Qatari breast cancer patients. Moreover, 14% of the samples were positive for all three viruses. Even though the prevalence of co-infection of HPV, EBV, and MMTV-like virus is infrequent, we postulate that a synergistic carcinogenic effect of HPVs, EBV, and MMTV-like virus exists and can play a role in the onset and progression of several human cancers, including breast, via the initiation and/or enhancement of the epithelial-mesenchymal transition event as previously reported by several studies [11, 20, 29, 46, 47]. On the other hand, Rickinson [48] demonstrated that co-infection with these viruses might trigger chronic inflammation due to the release of inflammatory cytokines, which provides a favorable microenvironment for viral infection.
Concordant to other findings, in this study, we did not find any association between the presence of MMTV-like virus and its co-presence with HPV or EBV and clinicopathological characteristics [37, 49, 50, 51]. However, other studies reported a significant association between MMTV-like virus and clinicopathological features [37, 52]. Nevertheless, we believe that further studies are needed to evaluate the role of MMTV-like virus and its crosstalk with other oncoviruses in a larger breast cancer cohort.
The present study has several limitations. First, we had a small sample size; therefore, a larger cohort from Qatar and other ME countries is needed to confirm our findings. Second, an appropriate control group of normal/benign samples from the same patients/population was lacking. Finally, the reported data relied on a single methodology (PCR assay for all three viruses). Nevertheless, our previous studies exploring the presence of HPV and EBV in different types of cancers using PCR and IHC generated comparable results [19, 34, 53, 54, 55].
Conclusion
We herein report for the first time the presence/co-presence of high-risk HPVs, EBV, and MMTV-like virus in breast cancer in the Qatari population without significant correlation with clinicopathological parameters. Finally, the data from the current study indicate that breast cancer incidence and progression could be reduced using available vaccines against oncoviruses and their associated diseases.
Statement of Ethics
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. Patient consent was waived as this study used existing FFPE specimens. The study was approved by the Ethical Committees of Qatar University and Hamad Medical Corporation (QU-IBC-2018/22; HMC:24-2-2019, Doha, Qatar).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The current study was supported by the grants from Qatar University: QUCP-CMED-2021-1 and QUCP-CMED-22/23-529.
Author Contributions
Conceptualization, Halema F. Al-Farsi, Hamda Al-Thawadi, and Ala-Eddin Al Moustafa; methodology, Ishita Gupta; formal analysis, Ishita Gupta and Semir Vranic; resources, Reem Al-Sarraf, Hanan Fargahly, Ali A. Sultan, Halema F. Al-Farsi, and Ala-Eddin Al Moustafa; data curation, Reem Al-Sarraf, Hanan Fargahly, Semir Vranic, and Ishita Gupta; writing − original draft preparation, Ishita Gupta; writing − review and editing, Semir Vranic, Ali A. Sultan, Halema F. Al-Farsi, Hamda Al-Thawadi, and Ala-Eddin Al Moustafa; supervision, Semir Vranic, Halema F. Al-Farsi, Hamda Al-Thawadi, and Ala-Eddin Al Moustafa; funding acquisition, Semir Vranic, Halema F. Al-Farsi, Hamda Al-Thawadi, and Ala-Eddin Al Moustafa. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgment
We would like to thank Mrs. A. Kassab for her critical reading of the manuscript.
Funding Statement
The current study was supported by the grants from Qatar University: QUCP-CMED-2021-1 and QUCP-CMED-22/23-529.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019 Apr 15;144((8)):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Chouchane L, Boussen H, Sastry KS. Breast cancer in Arab populations: molecular characteristics and disease management implications. Lancet Oncol. 2013 Sep;14((10)):e417–e424. doi: 10.1016/S1470-2045(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 3.Parambil J, Najim M, Mahmoud M, Abubeker I, Kartha A, Calaud F, et al. Breast cancer screening practices in a tertiary care center in the state of Qatar: a cross-sectional survey. Breast Cancer. 2021;13:21–30. doi: 10.2147/BCTT.S285210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner LTA, Cheung KL. Correlation between breast cancer and lifestyle within the Gulf Cooperation Council countries: a systematic review. World J Clin Oncol. 2020;11((4)):217–242. doi: 10.5306/wjco.v11.i4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venuti A, Badaracco G, Rizzo C, Mafera B, Rahimi S, Vigili M. Presence of HPV in head and neck tumours: high prevalence in tonsillar localization. J Exp Clin Cancer Res. 2004 Dec;23((4)):561–566. [PubMed] [Google Scholar]
- 6.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007 Oct 15;121((8)):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Peng SL, Yang LF, Chen X, Tao YG, Cao Y. Co-infection of Epstein-Barr virus and human papillomavirus in human tumorigenesis. Chin J Cancer. 2016 Jan 22;35:16. doi: 10.1186/s40880-016-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deligdisch L, Marin T, Lee AT, Etkind P, Holland JF, Melana S, et al. Human mammary tumor virus (HMTV) in endometrial carcinoma. Int J Gynecol Cancer. 2013 Oct;23((8)):1423–1428. doi: 10.1097/IGC.0b013e3182980fc5. [DOI] [PubMed] [Google Scholar]
- 9.Johal H, Faedo M, Faltas J, Lau A, Mousina R, Cozzi P, et al. DNA of mouse mammary tumor virus-like virus is present in human tumors influenced by hormones. J Med Virol. 2010 May;82((6)):1044–1050. doi: 10.1002/jmv.21754. [DOI] [PubMed] [Google Scholar]
- 10.Yeo-Teh NSL, Ito Y, Jha S. High-risk human papillomaviral oncogenes E6 and E7 target key cellular pathways to achieve oncogenesis. Int J Mol Sci. 2018 Jun 8;19((6)):1706. doi: 10.3390/ijms19061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Moustafa AE. E5 and E6/E7 of high-risk HPVs cooperate to enhance cancer progression through EMT initiation. Cell Adh Migr. 2015;9((5)):392–393. doi: 10.1080/19336918.2015.1042197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26((1)):27–39. doi: 10.1615/CritRevEukaryotGeneExpr.v26.i1.40. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira AR, Ramalho AC, Marques M, Ribeiro D. The interplay between antiviral signalling and carcinogenesis in human papillomavirus infections. Cancers. 2020 Mar 10;12((3)):646. doi: 10.3390/cancers12030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimri G, Band H, Band V. Mammary epithelial cell transformation: insights from cell culture and mouse models. Breast Cancer Res. 2005;7((4)):171–179. doi: 10.1186/bcr1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Moustafa AE, Foulkes WD, Benlimame N, Wong A, Yen L, Bergeron J, et al. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene. 2004;23((2)):350–358. doi: 10.1038/sj.onc.1207148. [DOI] [PubMed] [Google Scholar]
- 16.Yasmeen A, Bismar TA, Kandouz M, Foulkes WD, Desprez PY, Al Moustafa AE. E6/E7 of HPV type 16 promotes cell invasion and metastasis of human breast cancer cells. Cell Cycle. 2007 Aug 15;6((16)):2038–2042. doi: 10.4161/cc.6.16.4555. [DOI] [PubMed] [Google Scholar]
- 17.Al Moustafa A-E, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother. 2016;12((7)):1936–1939. doi: 10.1080/21645515.2016.1139255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Thawadi H, Gupta I, Jabeen A, Skenderi F, Aboulkassim T, Yasmeen A, et al. Co-presence of human papillomaviruses and Epstein–Barr virus is linked with advanced tumor stage: a tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020;20((1)):361. doi: 10.1186/s12935-020-01348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Thawadi H, Ghabreau L, Aboulkassim T, Yasmeen A, Vranic S, Batist G, et al. Co-incidence of Epstein-Barr virus and high-risk human papillomaviruses in cervical cancer of Syrian women. Front Oncol. 2018;8:250. doi: 10.3389/fonc.2018.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malki MI, Gupta I, Fernandes Q, Aboulkassim T, Yasmeen A, Vranic S, et al. Co-presence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian colorectal cancer samples. Hum Vaccin Immunother. 2020;16((10)):2403–2407. doi: 10.1080/21645515.2020.1726680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Hamad M, Matalka I, Al Zoubi MS, Armogida I, Khasawneh R, Al-Husaini M, et al. Human mammary tumor virus, human papilloma virus, and Epstein-Barr virus infection are associated with sporadic breast cancer Metastasis. Breast Cancer. 2020;14:1178223420976388. doi: 10.1177/1178223420976388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta I, Jabeen A, Al-Sarraf R, Farghaly H, Vranic S, Sultan AA, et al. The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Hum Vaccin Immunother. 2021 Apr 3;17((4)):982–989. doi: 10.1080/21645515.2020.1802977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawson JS, Günzburg WH, Whitaker NJ. Viruses and human breast cancer. Future Microbiol. 2006;1((1)):33–51. doi: 10.2217/17460913.1.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Lawson JS, Heng B. Viruses and breast cancer. Cancers. 2010;2((2)):752–772. doi: 10.3390/cancers2020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000 Oct;13((4)):497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 26.Shimakage M, Horii K, Tempaku A, Kakudo K, Shirasaka T, Sasagawa T. Association of Epstein-Barr virus with oral cancers. Hum Pathol. 2002 Jun;33((6)):608–614. doi: 10.1053/hupa.2002.129786. [DOI] [PubMed] [Google Scholar]
- 27.Boudreault S, Armero VES, Scott MS, Perreault JP, Bisaillon M. The Epstein-Barr virus EBNA1 protein modulates the alternative splicing of cellular genes. Virol J. 2019 Mar 4;16((1)):29. doi: 10.1186/s12985-019-1137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo AK, Lo KW, Tsao SW, Wong HL, Hui JW, To KF, et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia. 2006 Mar;8((3)):173–180. doi: 10.1593/neo.05625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa AE. Epstein-Barr Virus and human papillomaviruses interactions and their roles in the initiation of epithelial-mesenchymal transition and cancer progression. Front Oncol. 2018;8:111. doi: 10.3389/fonc.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bittner JJ. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science. 1936 Aug 14;84((2172)):162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- 31.de Cremoux P, Thioux M, Lebigot I, Sigal-Zafrani B, Salmon R, Sastre-Garau X. No evidence of human papillomavirus DNA sequences in invasive breast carcinoma. Breast Cancer Res Treat. 2008 May;109((1)):55–58. doi: 10.1007/s10549-007-9626-4. [DOI] [PubMed] [Google Scholar]
- 32.Herrmann K, Niedobitek G. Lack of evidence for an association of Epstein–Barr virus infection with breast carcinoma. Breast Cancer Res. 2002;5((1)):R13–7. doi: 10.1186/bcr561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarante MK, de Sousa Pereira N, Vitiello GAF, Watanabe MAE. Involvement of a mouse mammary tumor virus (MMTV) homologue in human breast cancer: evidence for, against and possible causes of controversies. Microbial Pathogenesis. 2019;130:283–294. doi: 10.1016/j.micpath.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Nagi K, Gupta I, Jurdi N, Jabeen A, Yasmeen A, Batist G, et al. High-risk human papillomaviruses and Epstein–Barr virus in breast cancer in Lebanese women and their association with tumor grade: a molecular and tissue microarray study. Cancer Cell Int. 2021;21((1)):308. doi: 10.1186/s12935-021-02009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May FE, Westley BR. Characterization of sequences related to the mouse mammary tumor virus that are specific to MCF-7 breast cancer cells. Cancer Res. 1989 Jul 15;49((14)):3879–3883. [PubMed] [Google Scholar]
- 36.Shariatpanahi S, Farahani N, Salehi AR, Salehi R. High prevalence of mouse mammary tumor virus-like gene sequences in breast cancer samples of Iranian women. Nucleosides Nucleotides Nucleic Acids. 2017;36((10)):621–630. doi: 10.1080/15257770.2017.1360498. [DOI] [PubMed] [Google Scholar]
- 37.Al Dossary R, Alkharsah KR, Kussaibi H. Prevalence of mouse mammary tumor virus (MMTV)-like sequences in human breast cancer tissues and adjacent normal breast tissues in Saudi Arabia. BMC cancer. 2018;18((1)):170. doi: 10.1186/s12885-018-4074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hachana M, Trimeche M, Ziadi S, Amara K, Gaddas N, Mokni M, et al. Prevalence and characteristics of the MMTV-like associated breast carcinomas in Tunisia. Cancer Lett. 2008 Nov 28;271((2)):222–230. doi: 10.1016/j.canlet.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Kamal SM, Makki RF, Hammoudi DA. Mouse mammary tumor virus (MMTV) like sequence in breast cancer of Egyptian women. Int J Cancer Prevent. 2005;2:109–115. [Google Scholar]
- 40.Levine PH, Pogo BG-T, Klouj A, Coronel S, Woodson K, Melana SM, et al. Increasing evidence for a human breast carcinoma virus with geographic differences. Cancer. 2004;101((4)):721–726. doi: 10.1002/cncr.20436. [DOI] [PubMed] [Google Scholar]
- 41.Loutfy S, Abdallah Z, Shaalan M, Moneer M, Karam A, Moneer M, et al. Prevalence of MMTV-like env sequences and its association with BRCA1/2 genes mutations among Egyptian breast cancer patients. Cancer Manag Res. 2021;13:2835–2848. doi: 10.2147/CMAR.S294584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slaoui M, El Mzibri M, Razine R, Qmichou Z, Attaleb M, Amrani M. Detection of MMTV-like sequences in Moroccan breast cancer cases. Infect Agent Cancer. 2014;9((1)):37. doi: 10.1186/1750-9378-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz E, Lareef MH, Rassa JC, Grande SM, King LB, Russo J, et al. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005 Feb 7;201((3)):431–439. doi: 10.1084/jem.20041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gattelli A, Zimberlin MN, Meiss RP, Castilla LH, Kordon EC. Selection of early-occurring mutations dictates hormone-independent progression in mouse mammary tumor lines. J Virol. 2006 Nov;80((22)):11409–15. doi: 10.1128/JVI.00234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31((1)):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 46.Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One. 2012;7((11)):e48788. doi: 10.1371/journal.pone.0048788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawson JS, Glenn WK. Multiple oncogenic viruses are present in human breast tissues before development of virus associated breast cancer. Infect Agent Cancer. 2017;12:55. doi: 10.1186/s13027-017-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol. 2014;26:99–115. doi: 10.1016/j.semcancer.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Hou J, Shen Q, Yue Y, Xie F, Wang X, et al. Mouse mammary tumor virus-like virus infection and the risk of human breast cancer: a meta-analysis. Am J Transl Res. 2014;6((3)):248–266. [PMC free article] [PubMed] [Google Scholar]
- 50.Mazouni C, Fina F, Romain S, Lh O, Bonnier P, Martin PM. Outcome of Epstein-Barr virus-associated primary breast cancer. Mol Clin Oncol. 2015;3((2)):295–298. doi: 10.3892/mco.2014.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naushad W, Surriya O, Sadia H. Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: a possible etiological role of viruses in breast cancer. Infect Genet Evol. 2017;54:230–237. doi: 10.1016/j.meegid.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 52.de Sousa Pereira N, Akelinghton Freire Vitiello G, Karina Banin-Hirata B, Scantamburlo Alves Fernandes G, José Sparça Salles M, Karine Amarante M, et al. Mouse mammary tumor virus (MMTV)-like env sequence in Brazilian Breast cancer samples: implications in clinicopathological parameters in molecular subtypes. Int J Environ Res Public Health. 2020;17((24)):9496. doi: 10.3390/ijerph17249496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta I, Al Farsi H, Jabeen A, Skenderi F, Al-Thawadi H, AlAhmad YM, et al. High-risk human papillomaviruses and Epstein-Barr virus in colorectal cancer and their association with clinicopathological status. Pathogens. 2020;9((6)):452. doi: 10.3390/pathogens9060452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagi K, Gupta I, Jurdi N, Yasmeen A, Vranic S, Batist G, et al. Copresence of high-risk human papillomaviruses and Epstein-Barr virus in colorectal cancer: a tissue microarray and molecular study from lebanon. Int J Mol Sci. 2021 Jul 29;((15)):22. doi: 10.3390/ijms22158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta I, Ghabreau L, Al-Thawadi H, Yasmeen A, Vranic S, Al Moustafa AE, et al. Co-incidence of human papillomaviruses and Epstein-Barr virus is associated with high to intermediate tumor grade in human head and neck cancer in Syria. Front Oncol. 2020;10:1016. doi: 10.3389/fonc.2020.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.