Abstract
Background
The mechanism of the perivascular adipose tissue (PVAT) anticontractile effect is well characterized in rodent visceral vascular beds; however, little is known about the mechanism of PVAT anticontractile function in subcutaneous vessels. In addition, we have previously shown that PVAT anticontractile function is nitric oxide synthase (NOS) dependent but have not investigated the roles of NOS isoforms.
Objective
Here, we examined PVAT anticontractile function in the mouse gracilis artery, a subcutaneous fat depot, in lean control and obese mice and investigated the mechanism in comparison to a visceral depot.
Method
Using the wire myograph, we generated responses to noradrenaline and electrical field stimulation in the presence of pharmacological tools targeting components of the known PVAT anticontractile mechanism. In addition, we performed ex vivo “fat transplants” in the organ bath.
Results
The mechanism of PVAT anticontractile function is similar between subcutaneous and visceral PVAT depots. Both endothelial and neuronal NOS isoforms mediated the PVAT anticontractile effect. Loss of PVAT anticontractile function in obesity is independent of impaired vasoreactivity, and function can be restored in visceral PVAT by NOS activation.
Conclusions
Targeting NOS isoforms may be useful in restoring PVAT anticontractile function in obesity, ameliorating increased vascular tone, and disease.
Keywords: Adipose tissue, Nitric oxide synthase, Obesity, Resistance arteries, Vascular tone
Introduction
Perivascular adipose tissue (PVAT) is a type of adipose tissue surrounding most blood vessels [1, 2, 3]. We and others have shown that PVAT elicits an anticontractile effect on resistance arteries, which is vital in modulation of vascular tone and therefore blood pressure and nutrient delivery to muscle [4, 5, 6, 7]. In obesity, PVAT anticontractile function is lost, contributing to the development of cardiovascular diseases such as hypertension [8, 9]. How much of this loss of function is due to dysfunctional PVAT versus impaired vasoreactivity of the endothelium or vascular smooth muscle in obesity is unknown.
Adipose tissue is widely considered to be an important endocrine organ and not just an energy store. Adipokines released from adipose tissue play important roles in homeostasis such as immune cell regulation, glucose metabolism, blood pressure regulation, etc., and their dysregulation can contribute to pathogenesis of cardiovascular and metabolic diseases, as well as some cancers (reviewed by [10]). As a consequence of high-calorie diets and low levels of physical activity, adipocytes grow in size and number, leading to hypoxia and chronic low-grade inflammation in obese adipose depots [4, 11, 12]. Adipose tissue distribution determines risk [13]. Increased volumes of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) confer an increased risk of cardiovascular and metabolic diseases, independently of BMI, and become sources of pro-inflammatory cytokines [14]. VAT is strongly associated with hypertension and insulin resistance risk independently of SAT [15, 16], while SAT is strongly associated with lipoedema and several pain pathologies [17]. Paradoxically, increased gluteofemoral adipose tissue, an SAT, has been shown to have a protective metabolic and cardiovascular effect [18, 19].
Whether PVAT is classed as VAT or SAT depends on anatomical location. Mesenteric PVAT, a VAT depot, is commonly used in studies from animal models due to the number of viable arteries present within the mesenteric bed and ease of dissection. Previously, we have investigated the mechanism of the PVAT anticontractile effect in mesenteric vessels [6]. We have shown using electrical field stimulation (EFS) that sympathetic nerves within mesenteric PVAT release noradrenaline (NA). The NA activated adipocyte β3-adrenoceptors, leading to the release of the vasodilator adiponectin. Also, we have shown that organic cation transporter 3 (OCT3) in PVAT sequesters some of the PVAT-derived NA, thereby preventing it from reaching the blood vessel and eliciting contraction. In obese mesenteric PVAT, we have demonstrated that this mechanism is lost and could not be restored by activation of β3-adrenoceptors or application of adiponectin [8]. In another study, we have shown in rat mesenteric PVAT that the anticontractile effect induced by exogenous NA is nitric oxide synthase (NOS) dependent, and the NA-induced effect was absent in endothelial NOS (eNOS) knockout mice, leading us to conclude that the effect must be dependent on eNOS [9]. We have not yet investigated the role of NOS in our EFS-induced anticontractile effect or investigated the role of other NOS isoforms, i.e., neuronal (nNOS) and inducible (iNOS).
Mesenteric PVAT is difficult to access in man, and previously, we have used resistance arteries dissected from gluteal biopsies, a SAT depot [20]. We have demonstrated a role of adiponectin in the gluteal PVAT [4]; however, due to scarcity of human samples, we have not further investigated the mechanism in human tissue. Importantly, a recent study has found that human adipocytes have distinctive subtypes, and these are different in VAT and SAT [21]. Briefly, this study found that white adipocytes could be clearly divided into subtypes hAd1-7. SAT primarily consisted of subtypes hAd1, 3, 4, and 7, whereas VAT exclusively consisted of hAd2 and 6. Importantly, expression of genes important to adipocyte function varied with subtype. For example, hAd3 highly express adiponectin, and hAd5 exhibit highest expression of several insulin signalling components [21]. Surprisingly, the almost exclusively visceral subtype hAd6 expressed genes associated with thermogenesis. Due to the differences in VAT and SAT phenotype and disease risk, it is important to know the validity of animal models and the development of novel therapeutics if these depots will affect vascular function in the same way, or via different mechanisms.
Here, we have studied the mouse gracilis artery and surrounding subcutaneous PVAT to draw comparisons with our previously published mechanism in the visceral mesenteric PVAT. We have taken our studies further by investigating the roles of NOS isoforms. In addition, using an ex vivo “fat transplant” protocol, we investigated whether loss of PVAT anticontractile function in obesity is due to loss of vasoreactivity within the artery. We report that loss of PVAT anticontractile function in obesity is independent of loss of vasoreactivity of the blood vessel. The mechanism of anticontractile function is similar between subcutaneous and visceral depots; however, we add the new finding that PVAT anticontractile function is mediated via both eNOS and nNOS isoforms. In addition, loss of PVAT anticontractile function in mesenteric PVAT can be restored using non-specific NOS activation, but not in gracilis PVAT.
Materials and Methods
Animal Care and Handling
All animal procedures were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 under the appropriate Home Office license (P3A97F3D1), with ethical approval from the University of Manchester Ethics Committee.
Male C57BL/6j mice purchased at 5 weeks old from Charles River Ltd (UK) were housed in a 12 h light:dark cycle and were provided with food and water ad libitum. As NOS is affected by the oestrus cycle, male mice were used to eliminate this potential source of high biological variability. From 8 weeks old, mice were randomly assigned to either control or high-fat feeding groups. For 10–12 weeks, control mice were fed for a standard chow diet (7.2% kcal from fat, cat no. BK001, SDS Diets, UK) and high-fat diet (60% kcal from fat, cat no. 824054, SDS Diets, UK) to induce obesity. We have previously demonstrated that this model exhibits hypertension, hyperglycaemia, and hyperinsulinemia [8]. eNOS knockout mice (eNOS−/−; strain B6.129P2-Nos3tm1Unc/J) were kindly provided by Dr Elizabeth Cottrell. Male adiponectin knockout mice (Adipo−/−; strain Adipoqtm1Chan) were purchased from Jackson Laboratories, USA. At 18–20 weeks old, unfasted mice were sacrificed using CO2 asphyxiation and permanent cessation of circulation by removal of the heart. This was conducted at the same time each day to avoid diurnal variation in hormones or adipokines. Immediately after sacrifice, the mesenteric bed and hind legs were removed and placed into ice-cold physiological salt solution (PSS) (119 mM NaCl, 4.7 mM KCl, 1.17 mM MgSO4, 25 mM NaHCO3, 1.17 mM KH2PO4, 0.03 mM K2EDTA, 5.5 mM glucose and 1.6 mM CaCl2; Thermo Fisher Scientific, UK) until required.
Wire Myography
Second-order mesenteric or gracilis artery segments (<250 µm) with or without PVAT intact were isolated by fine dissection in ice-cold PSS for wire myography. For some experiments, an “exogenous” PVAT preparation was applied. Clean arteries were mounted in the organ bath, with a section of “exogenous” PVAT suspended above using wire (see [6] for an illustration of this preparation). This was necessary for the in vitro “fat transplantation” experiments described below and was also used to allow incubation of PVAT alone with certain pharmacological tools to remove direct effects of certain drugs on the vasculature (see below for more details). This was done when using compounds which affect catecholamines or NO, as these are known to mediate vasoconstriction and vasodilation directly within the blood vessel. Therefore, to isolate their effects specifically within PVAT, the exogenous PVAT preparation was used. Following the incubation period, the exogenous PVAT was briefly rinsed with PSS to avoid transferring the compounds to the blood vessels.
As previously described [6, 8], arteries are mounted onto 40-µm wire in the organ bath of a wire myograph system (Danish MyoTech, Denmark). Arteries were allowed 30 min to equilibrate at 37°C in PSS perfused with 95% air/5% CO2 to maintain pH 7.4. Vessel wall tension was normalized according to a standardized procedure [22] and allowed to equilibrate for a further 30 min before subjecting to a 60 mM high (K+) PSS (KPSS) stimulus (63.7 mM NaCl, 60 mM KCl, 1.17 mM MgSO4, 25 mM NaHCO3, 1.17 mM KH2PO4, 0.03 mM K2EDTA, 5.5 mM glucose, and 1.6 mM CaCl2; Thermo Fisher Scientific, UK). Vessels with responses to KPSS <0.3 mN/mm2 were excluded. Endothelial integrity was tested by pre-constricting with 1 × 10−5 M NA and applying 1 × 10−7 M acetylcholine (both Merck, UK, dissolved in PSS). Vessels with less than a 30% relaxation in response to acetylcholine are considered endothelium-denuded and were excluded.
Traces were continuously generated using LabChart 7 (ADInstruments, UK). Arteries were subjected to increasing frequencies of EFS (0.1–30 Hz, 20 V, 4 s train duration, 0.2 ms pulse duration) using platinum electrodes on either side of the blood vessel (CS4 model, Myopulse software). For some experiments, arteries were instead subjected to increasing doses of NA (1 × 10−9 to 3 × 10−5 M) to generate a cumulative concentration-response curve.
“Fat Transplant” Experiments
To investigate whether loss of PVAT anticontractile function in obesity is due to dysfunction of PVAT, or dysfunction within the blood vessel itself, we conducted a “fat transplant” protocol. Clean mesenteric and gracilis arteries were mounted in the organ bath from either lean control or obese mice side by side. Exogenous PVAT was collected from lean control and obese mice from one branch of the mesentery and was suspended using wire above different arteries to create the 4 following conditions: control artery + control PVAT, control artery + obese PVAT, obese artery + control PVAT, obese artery + obese PVAT. Vessels and their exogenous PVAT were then subjected to EFS.
Pharmacological Assessment
To investigate the PVAT anticontractile mechanism in gracilis arteries for comparison with our previously published data in mesenteric arteries, following the first stimulation with EFS, lean control, obese, or Adipo−/− gracilis arteries were incubated with different pharmacological tools which are summarised in Table 1. Concentrations used are consistent with our previous studies [6, 8]. Drug manufacturers and vehicles can also be found in Table 1. Vehicle controls have previously been performed for all experiments to ensure these had no effect on vascular contractility (data not shown).
Table 1.
Summary of pharmacological tools
| Drug | Target | Concentration | Vehicle | Supplier |
|---|---|---|---|---|
| 6-OHDA | Catecholamine toxin | 2 µM | Sodium | Merck, UK, cat no. H4381 |
| metabisulphate | ||||
| Adiponectin | Adiponectin receptor 1 ligand | 5 µg/mL | N/A | Generon, UK, cat no. cyt-432-50 μg |
| Adiponectin blocking peptide | Adiponectin receptor 1 fragment | 3.5 µg/mL | N/A | Enzo life sciences, UK, cat no. ALX-151-045-C100 |
| CL-316,243 | β3-adrenoceptor agonist | 10 µM | PSS | Bio-techne, UK, cat no. 1499 |
| Corticosterone | OCT-3 inhibitor | 100 µM | Ethanol | Merck, UK, cat. no 27840 |
| Histamine | Non-selective NOS activator | 100 µM | PSS | Merck, UK, cat no. H7250 |
| L-NMMA | Non-selective NOS inhibitor | 100 µM | PSS | Bio-techne, UK, cat. no. 0771 |
| SR59230A | β3-adrenoceptor antagonist | 1 µM | PSS | Bio-techne, UK, cat no. 1151 |
| Vinyl-L-NIO | Selective nNOS inhibitor | 100 µM | PSS | Enzo life sciences, UK, cat no. ALX-270-216-M005 |
In lean control PVAT-intact and PVAT-denuded gracilis arteries, β3-adrenoceptor antagonist SR59230A (1 μM), β3-adrenoceptor agonist CL-316,243 (10 μM), or adiponectin receptor-1 blocking peptide (ABP, 3.5 µg/mL) was added to the bath and incubated for 30 or 45 min, before re-stimulating the vessels. Exogenous globular adiponectin (5 µg/mL) was applied only to PVAT-denuded vessels and allowed 15 min to incubate before re-stimulating. The effects of the β3-adrenoceptor agonist CL-316,243 (10 μM) were also tested in Adipo−/− gracilis arteries.
In lean control gracilis arteries with and without exogenous PVAT, following the first stimulation-response protocol with EFS, PVAT was removed for incubation for 30 min with a rapidly acting and specific catecholamine toxin 6-hydroxydopamine [23, 24, 25, 26] (6-OHDA, 2 µM) or OCT3 inhibitor corticosterone (100 µM), before re-suspending the exogenous PVAT in the bath with its original −PVAT vessel. For PVAT incubated with corticosterone, after a second round of stimulation with EFS, PVAT was again removed for incubation with the combination of SR59230A (1 µM) and corticosterone (100 µM).
In lean control mesenteric and gracilis arteries with and without exogenous PVAT, following the first round of EFS stimulation, exogenous PVAT was removed and incubated for 30 min with non-specific NOS inhibitor L-NG-monomethyl arginine citrate (L-NMMA, 100 µM) or specific NOS inhibitor vinyl-L-NIO (100 nM), before re-suspending the PVAT above its original vessels and repeating the stimulus.
EFS response curves and cumulative-concentration response curves to NA were conducted in mesenteric arteries from eNOS−/− mice with and without PVAT intact. eNOS−/− mesenteric arteries with and without exogenous PVAT were subjected to EFS, before exogenous PVAT was removed for a 30-min incubation with specific nNOS inhibitor vinyl-L-NIO (100 nM), before re-suspending above the arteries and repeating the stimulus.
Obese mesenteric and gracilis arteries with and without exogenous PVAT were subjected to EFS, before exogenous PVAT was removed for 30 min incubation with non-specific NOS activator histamine (100 µM). Following incubation, exogenous PVAT was re-suspended above its original arteries for a second round of EFS. Time controls were conducted alongside all experiments to ensure any change in vascular responsiveness was not a time effect (data not shown).
Statistics
Consistent with previous studies, vessel responses are expressed as a percentage of the initial response to KPSS [6, 8, 27]. EFS responses are calculated as a percentage of the peak of KPSS, and NA responses are calculated as a percentage of the plateau phase of KPSS (See online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000526027, for example traces). Data are expressed as the mean ± SEM. Statistical analysis was performed using GraphPad Prism v7 (GraphPad Software USA). p Values <0.05 were considered statistically significant. Normal distribution was confirmed using the Shapiro-Wilk normality test, supported by Skewness and Kurtosis coefficients (using an acceptable range of ±2). Differences between the frequency-response curves of ±PVAT vessels were tested using a two-way ANOVA, with a Bonferroni post hoc test. Before and after treatments within the same vessel types were compared using a repeated-measures ANOVA, again followed by a Bonferroni post hoc test. Due to expense, an n of 4 was used for exogenous adiponectin and ABP experiments. For all other experiments, a sample number of 7–8 was used.
Results
“Fat Transplant” Experiments Confirm that Loss of PVAT Anticontractile Function in Obesity Is due to Dysfunctional PVAT
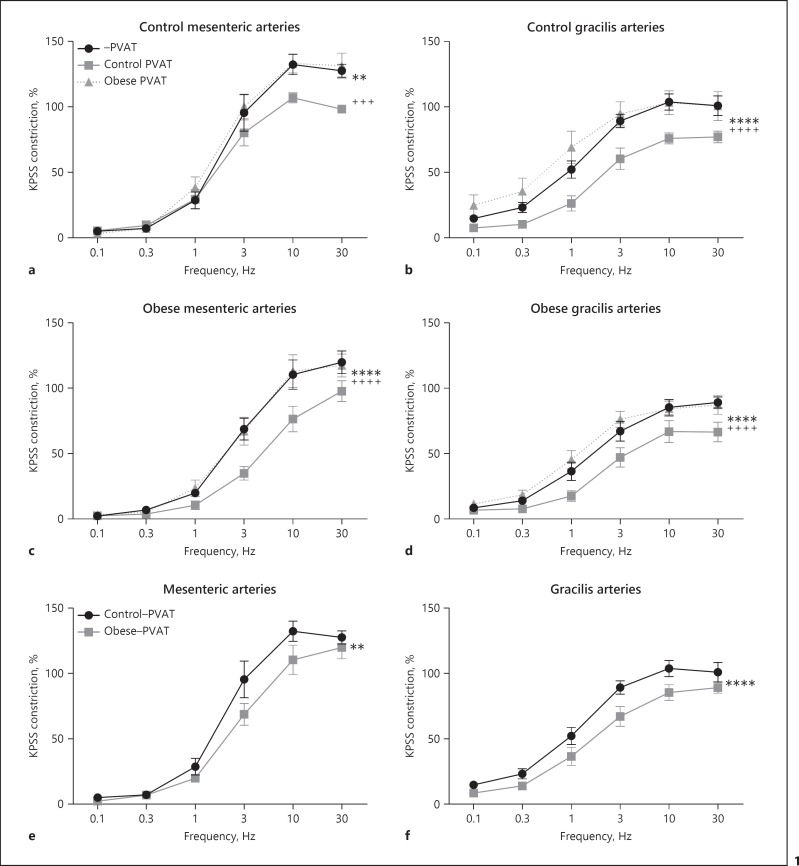
To confirm that the loss of PVAT anticontractile function in obesity is due to dysfunctional PVAT rather than impaired vasodilatory mechanisms in the endothelium and vascular smooth muscle alone, we performed a “fat transplant” experiment. Isolated mesenteric or gracilis lean control and obese −PVAT arteries were stimulated using EFS alone, or with lean control exogenous PVAT, or with obese exogenous PVAT (Fig. 1, n = 7 all groups). When control lean PVAT is suspended above lean control mesenteric or gracilis arteries, it induces an anticontractile effect in response to EFS (mesenteric: Fig. 1a; gracilis: Fig. 1b). When obese PVAT is suspended above lean control mesenteric and gracilis arteries, the obese PVAT does not induce an anticontractile effect (mesenteric: Fig. 1a; gracilis: Fig. 1b). When obese PVAT is suspended above mesenteric and gracilis obese arteries, the obese exogenous PVAT has no anticontractile effect (mesenteric: Fig. 1c; gracilis: Fig. 1d). However, when lean control PVAT is suspended above obese mesenteric and gracilis arteries, the control exogenous PVAT can induce a PVAT anticontractile effect on the obese arteries (mesenteric: Fig. 1c; gracilis: Fig. 1d).
Fig. 1.
Loss of PVAT anticontractile function in obesity is due to dysfunctional PVAT. Mesenteric (a, c) and gracilis (b, d) resistance arteries were isolated from control (a, b) and obese mice (c, d). Arteries were subjected to EFS in the presence and absence of PVAT harvested from either control mice or obese mice, which was suspended above the artery in the organ bath (a −PVAT vs. control PVAT p < 0.01**, −PVAT vs. obese PVAT p > 0.05, control PVAT vs. obese PVAT p < 0.001+++. b −PVAT vs. control PVAT p < 0.0001****, −PVAT vs. obese PVAT p > 0.05, control PVAT vs. obese PVAT p < 0.0001++++. c −PVAT vs. control PVAT p < 0.0001****, −PVAT vs. obese PVAT p > 0.05, control PVAT vs. obese PVAT p < 0.000++++. d −PVAT vs. control PVAT p < 0.0001****, −PVAT vs. obese PVAT p > 0.05, control PVAT vs. obese PVAT p < 0.0001++++). The responses of −PVAT control and obese mesenteric arteries shown in (a, c) are compared in (e) (control −PVAT vs. obese −PVAT p < 0.01**). The responses of −PVAT control and obese gracilis arteries shown in (b, d) are compared in (f) (control −PVAT vs. obese −PVAT p < 0.0001****). Data shown are mean ± SEM (n = 7 all groups) (two-way ANOVAs with Bonferroni post hoc tests).
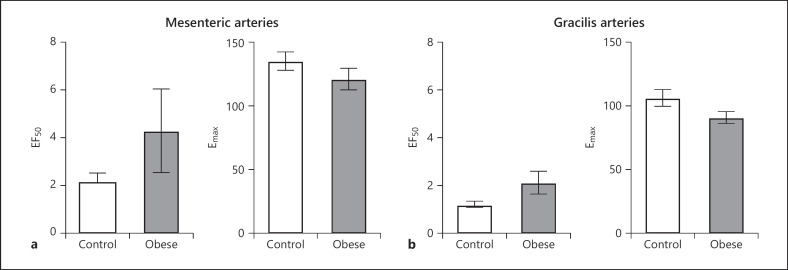
To visualize comparisons of vasoreactivity in −PVAT lean control and obese arteries, the responses of lean and obese mesenteric arteries from Figure 1a and c were superimposed in Figure 1e, and the responses of lean and obese gracilis arteries from Figure 1b and d were superimposed in Figure 1f. The response to EFS in obese mesenteric and gracilis arteries is significantly reduced compared to lean control arteries (mesenteric: Fig. 1e; gracilis: Fig. 1f). EF50 and Emax were calculated for mesenteric and gracilis, control and obese −PVAT arteries, and there was no significant difference (Fig. 2).
Fig. 2.
EF50 and Emax are no different between lean control and obese mesenteric and gracilis arteries. The EF50 and Emax from Figure 1e and f were calculated for mesenteric (a) and gracilis (b) lean control and obese arteries. Data shown are mean ± SEM (n = 7 all groups) (p > 0.05 all comparisons, unpaired t test).
EFS Simulates an Anticontractile Effect in Gracilis PVAT Which Is Mediated via β3-Adrenoceptors, OCT3, and Adiponectin
Previously, we have investigated the mechanism of PVAT anticontractile function in a VAT depot, mesenteric PVAT [6]. We concluded that in healthy mesenteric PVAT, EFS stimulated the release of NA, and from here, the roles of PVAT are two-fold. (1) NA activates adipocyte β3-adrenoceptors, triggering the release of the vasodilator adiponectin. (2) OCT3 “sponges” some of the NA into adipocytes, thereby preventing the PVAT-derived NA from reaching the blood vessels and potentiating contraction. Here, we have investigated whether the same mechanism exists in an SAT depot, gracilis PVAT.
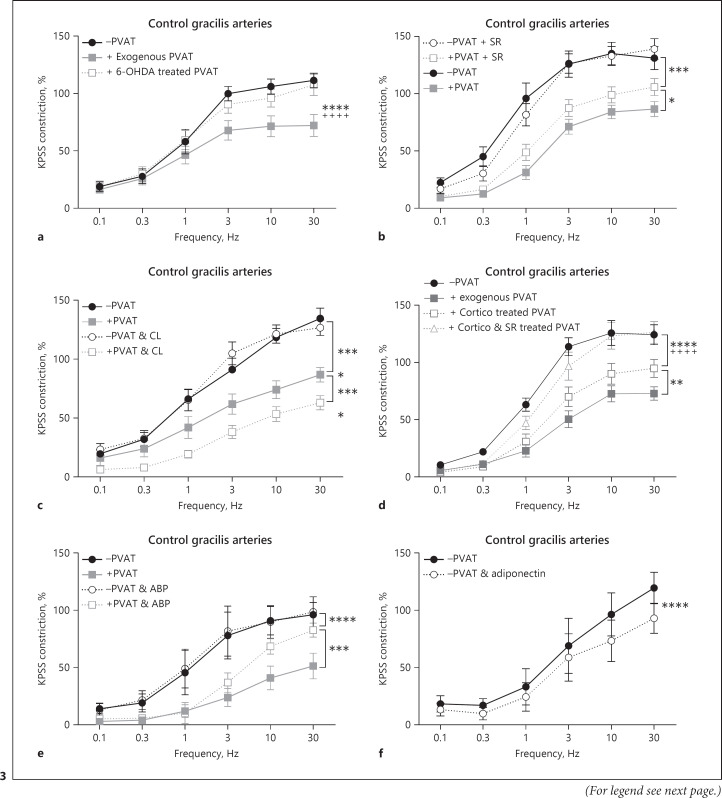
The effects of EFS were tested in −PVAT and +exogenous PVAT gracilis lean control arteries (Fig. 3a, n = 7). In response to EFS, exogenous gracilis PVAT does elicit an anticontractile effect. To determine if the effect in gracilis PVAT is mediated via sympathetic nerves, exogenous PVAT was removed and incubated with the catecholamine toxin, 6-ODHA. 6-OHDA completely abolished the anticontractile effect (Fig. 3a).
Fig. 3.
PVAT anticontractile effect in gracilis arteries is mediated via β3-adrenoceptors and subsequent adiponectin release, and via the NA transporter OCT3. Gracilis arteries were isolated from control mice. a −PVAT vessels with a section of exogenous PVAT suspended above were stimulated with EFS. The exogenous PVAT was removed and incubated for 30 min with 6-OHDA (2 μM), before returning the PVAT to the organ bath and repeating the stimulus (n = 7, −PVAT vs. exogenous PVAT p < 0.0001****, exogenous PVAT vs. 6-OHDA-treated PVAT p < 0.0001++++, −PVAT vs. 6-OHDA-treated PVAT p > 0.05). b Following control responses to EFS ±PVAT vessels were incubated with SR59230A (SR, 1 μM) for 30 min before repeating the EFS stimulus (n = 8, −PVAT vs. +PVAT p < 0.0001, −PVAT vs. −PVAT & SR p > 0.05, +PVAT vs. +PVAT & SR p < 0.0001****, −PVAT vs. +PVAT & SR p < 0.0001****). c Following control responses to EFS ±PVAT vessels were incubated with CL-316,243 (CL, 10 μM) for 30 min before repeating the EFS stimulus (n = 8, −PVAT vs. +PVAT p < 0.0001****, −PVAT vs. −PVAT & CL p > 0.05, +PVAT vs. +PVAT & CL p < 0.0001****, −PVAT vs. +PVAT & CL p < 0.0001). d −PVAT vessels with a section of exogenous PVAT suspended above were stimulated with EFS, before removing the PVAT and first incubating with corticosterone alone (cortico, 100 µM) for 30 min. Following incubation, the PVAT was returned to the organ bath, and the EFS stimulus was repeated. The exogenous PVAT was then removed for the second time and incubated with both corticosterone (cortico, 100 µM) and SR59230A (SR, 1 μM) for 30 min. The PVAT was then returned to the organ bath again to repeat the EFS stimulus for the 3rd time (n = 8, −PVAT vs. exogenous PVAT p < 0.0001, exogenous PVAT vs. cortico-treated PVAT p < 0.01**, −PVAT vs. cortico-treated PVAT p < 0.0001****, cortico-treated PVAT vs. cortico & SR-treated PVAT p < 0.0001++++, exogenous PVAT vs. cortico & SR-treated PVAT p < 0.0001, −PVAT vs. cortico & SR-treated PVAT p > 0.05). e Following control responses to EFS ±PVAT, vessels were incubated with a blocking peptide for adiponectin receptor 1 (ABP, 3.5 μg/mL) for 45 min before repeating the EFS stimulus (n = 4, −PVAT vs. +PVAT p < 0.0001, −PVAT vs. −PVAT & ABP p > 0.05, +PVAT vs. +PVAT & ABP p < 0.001***, −PVAT vs. +PVAT & ABP p < 0.0001****). f Following the control EFS protocol, PVAT-denuded vessels were allowed 15 min to recover before incubating with recombinant globular mouse adiponectin (5 μg/mL) and repeating the stimulus (n = 4, p < 0.0001****). Data shown are mean ± SEM (−PVAT vs. +PVAT vessels were tested using two-way ANOVA. Before and after drugs within the same vessel type, e.g., −PVAT before drug vs. −PVAT after drug were tested using repeated-measures ANOVA. Both followed by Bonferroni post hoc tests).
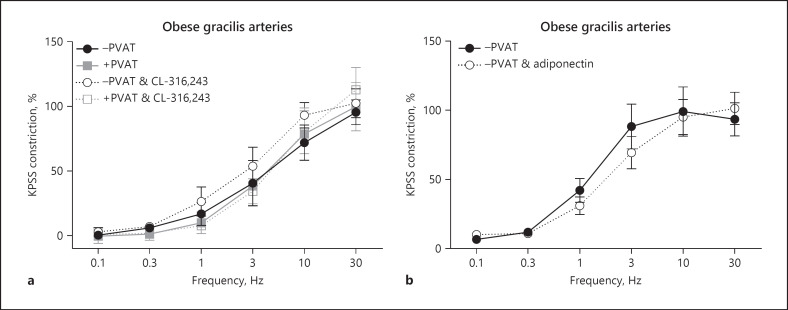
To determine the role of β3-adrenoceptors in lean control gracilis PVAT, a β3-adrenoceptor antagonist, SR59230A, and a β3-adrenoceptor agonist, CL-316,243, were used (Fig. 3b, c, respectively, n = 8 both). Using PVAT-intact and PVAT-removed preparation, arteries were subjected to EFS before incubating for 30 min with the antagonist or agonist. β3-Adrenoceptor antagonist SR59230A had no direct effect on -PVAT arteries (Fig. 3b); however, SR59230A did reduce the PVAT anticontractile effect. β3-Adrenoceptor agonist CL-316,243 had no direct effect on -PVAT arteries (Fig. 3c); however, CL-316,243 did enhance the PVAT anticontractile effect.
To investigate whether subcutaneous gracilis PVAT also acts as a reservoir for NA, OCT3 inhibitor corticosterone was used (Fig. 3d, n = 8). Following control stimulation with EFS, exogenous PVAT was removed and incubated with corticosterone for 30 min, before re-suspending the PVAT and repeating the stimulus. Corticosterone significantly reduced the PVAT anticontractile effect. The exogenous PVAT was then removed for a second time and incubated with a combination of corticosterone and β3-adrenoceptor antagonist SR59230A for 30 min before re-suspending the PVAT and repeating the EFS stimulus for a 3rd time (Fig. 3d). The combination of both corticosterone and SR59230A completely abolished the PVAT anticontractile effect.
To determine the role of adiponectin in gracilis PVAT anticontractile function, an ABP and exogenous globular adiponectin were used. In PVAT-intact and PVAT-removed gracilis arteries, following control stimulation with EFS, vessels were incubated with ABP for 45 min (Fig. 3e, n = 4). ABP significantly reduced the PVAT anticontractile effect and had no effect on −PVAT vessels. In PVAT-denuded vessels, following control stimulation with EFS, gracilis arteries were incubated with exogenous adiponectin for 15 min before repeating the stimulus (Fig. 3f, n = 4). Exogenous adiponectin significantly reduced the responses of −PVAT arteries.
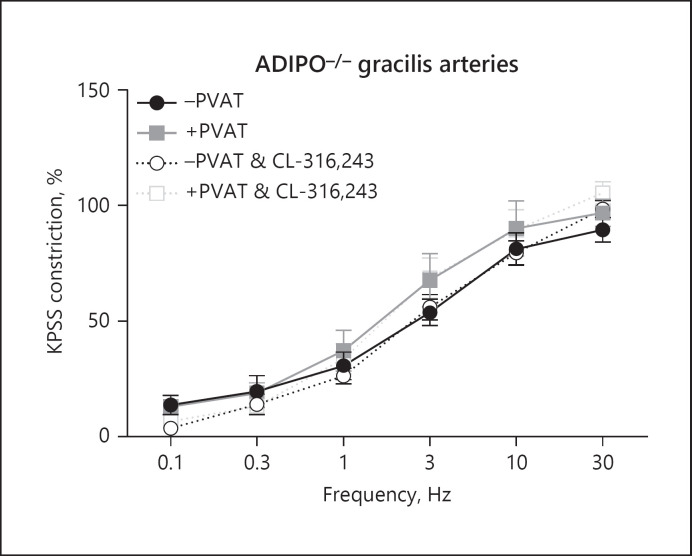
To reinforce the role of adiponectin in PVAT anticontractile function, gracilis arteries from the Adipo−/− mice were isolated (Fig. 4, n = 8). EFS did not induce an anticontractile effect in these arteries. To confirm that our enhancement of PVAT anticontractile function by the β3-adrenoceptor agonist is mediated via adiponectin, Adipo−/− vessels were incubated with the β3-adrenoceptor agonist, CL-316, 243 for 30 min, and this had no effect.
Fig. 4.
PVAT anticontractile effect is absent in gracilis arteries from Adipo−/− mice. Gracilis arteries from Adipo−/− mice with and without PVAT were subjected to EFS, before incubating with CL-316,243 for 30 min and repeating the stimulus. Data shown are mean ± SEM (n = 8, p > 0.05 all comparisons). PVAT versus. +PVAT vessels were tested using two-way ANOVA. Before and after drugs within the same vessel type, e.g., −PVAT before drug versus. −PVAT after drug were tested using repeated-measures ANOVA. Both followed by Bonferroni post hoc tests.
Loss of PVAT Anticontractile Function in Gracilis Arteries in Obesity Cannot Be Restored by β3-Adrenoceptor Activation or Application of Adiponectin
In Figure 1, we demonstrated that PVAT anticontractile function in obesity is lost in both mesenteric and gracilis arteries. Previously, we have shown in obese mesenteric arteries that β3-adrenoceptor activation or application of adiponectin could not restore function [8]. Here, we repeat these experiments in our subcutaneous gracilis PVAT depot.
PVAT-intact and PVAT-removed gracilis arteries were isolated from obese mice and subjected to EFS (Fig. 5a, n = 7). Intact PVAT did not elicit an anticontractile response. Arteries were incubated with β3-adrenoceptor agonist, CL-316,243 for 30 min before repeating the EFS stimulus. The agonist had no effect on either −PVAT or +PVAT vessels.
Fig. 5.
β3-adrenoceptor function in gracilis PVAT is lost in obesity, and the vasodilator function of adiponectin is lost. a Gracilis arteries from obese mice with and without PVAT were subjected to EFS, before incubating with CL-316,243 for 30 min and repeating the stimulus (n = 7, p > 0.05 all comparisons). b Following the control EFS protocol, PVAT-denuded vessels were allowed 15 min to recover before incubating with recombinant globular mouse adiponectin (5 μg/mL) and repeating the stimulus (n = 4, p > 0.05). Data shown are mean ± SEM (−PVAT vs. +PVAT vessels were tested using two-way ANOVA. Before and after drugs within the same vessel type, e.g., −PVAT before drug vs. −PVAT after drug were tested using repeated-measures ANOVA. Both followed by Bonferroni post hoc tests).
The effects of exogenous globular adiponectin were tested on −PVAT gracilis arteries from obese mice (Fig. 5b, n = 4). After control stimulations to EFS, −PVAT arteries were incubated with exogenous adiponectin for 15 min, and this had no effect.
The EFS-Induced Anticontractile Effect Is NOS Dependent
We have previously shown that the NA-induced PVAT anticontractile in NOS dependent using the non-specific NOS inhibitor L-NMMA [9]. Here, we investigated whether the EFS-induced anticontractile effect is also NOS dependent in both mesenteric and gracilis arteries.
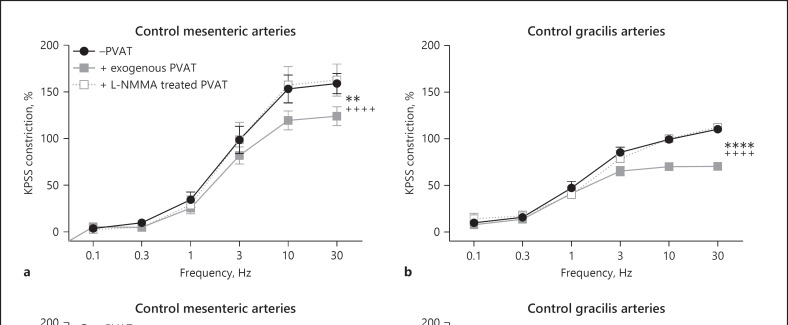
Control responses to EFS in mesenteric (Fig. 6a) and gracilis (Fig. 6b) with and without exogenous PVAT (both n = 8). Exogenous PVAT was removed and incubated with NOS inhibitor L-NMMA for 30 min, before re-suspending the exogenous PVAT above its original vessel and repeating the stimulus. Incubation with the non-specific NOS inhibitor abolished the EFS-induced anticontractile effect in both mesenteric and gracilis arteries.
Fig. 6.
EFS-induced PVAT anticontractile effect is NOS dependent and can be reduced by inhibition of nNOS. Mesenteric (a) and gracilis (b) −PVAT arteries from control mice with a section of exogenous PVAT suspended above were stimulated with EFS. The exogenous PVAT was removed and incubated for 30 min with L-NMMA (100 µM), before returning the PVAT to the organ bath and repeating the stimulus (n = 8 both groups. a −PVAT vs. exogenous PVAT p < 0.01**, exogenous PVAT vs. L-NMMA-treated PVAT p < 0.0001++++, −PVAT vs. L-NMMA-treated PVAT p > 0.05. b −PVAT vs. exogenous PVAT p < 0.0001****, exogenous PVAT vs. L-NMMA-treated PVAT p < 0.0001++++, −PVAT vs. L-NMMA-treated PVAT p > 0.05). Mesenteric (c) and gracilis (d) −PVAT arteries from control mice with a section of exogenous PVAT suspended above were stimulated with EFS. The exogenous PVAT was removed and incubated for 30 min with vinyl-L-NIO (100 nM), before returning the PVAT to the organ bath and repeating the stimulus (n = 8 both groups. c −PVAT vs. exogenous PVAT p < 0.0001, exogenous PVAT vs. vinyl-L-NIO-treated PVAT p < 0.0001****, −PVAT vs. vinyl-L-NIO-treated PVAT p < 0.01**. d −PVAT vs. exogenous PVAT p < 0.0001, exogenous PVAT vs. vinyl-L-NIO-treated PVAT p < 0.0001****, −PVAT vs. vinyl-L-NIO-treated PVAT p < 0.0001****). Data shown are mean ± SEM (−PVAT vs. +PVAT vessels were tested using two-way ANOVA. Before and after drugs within the same vessel type, e.g., +exogenous PVAT before drug vs. +exogenous PVAT after drug were tested using repeated-measures ANOVA. Both followed by Bonferroni post hoc tests).
Inhibition of nNOS Reduces the EFS-Induced Anticontractile Effect
While there are no specific iNOS and eNOS inhibitors, at 100 nM, vinyl-L-NIO has been shown to be nNOS specific [28]. Here, we tested the effects of vinyl-L-NIO on the EFS-induced anticontractile effect in both mesenteric and gracilis arteries.
Control responses to EFS in mesenteric (Fig. 6c) and gracilis (Fig. 6d) with and without exogenous PVAT (both n = 8). Exogenous PVAT was removed and incubated with nNOS inhibitor vinyl-L-NIO for 30 min, before re-suspending the exogenous PVAT above its original vessel and repeating the stimulus. Incubation with the specific nNOS inhibitor reduced the EFS-induced anticontractile effect in both mesenteric and gracilis arteries.
EFS Induces a PVAT Anticontractile Effect in eNOS−/− Mice, but NA Does Not
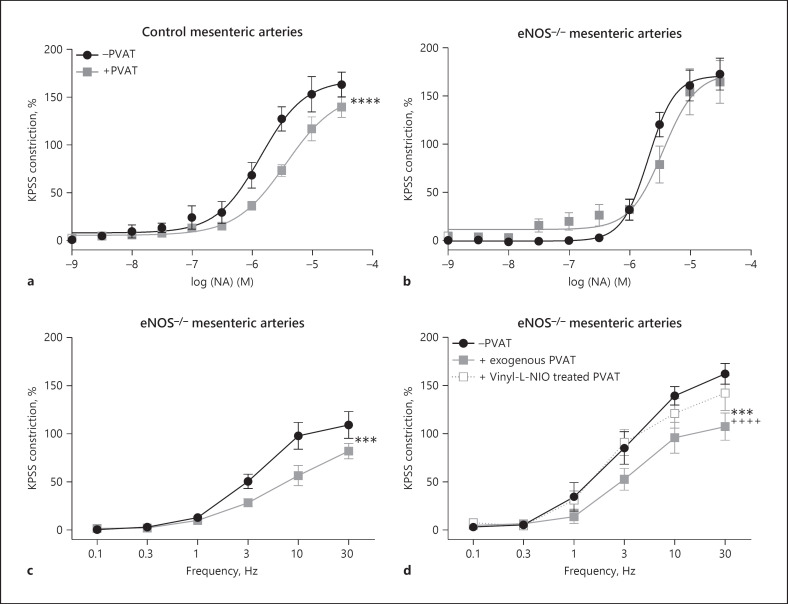
We and others have shown that NA induces a PVAT anticontractile effect in control rats, and this effect is absent in eNOS−/− mice, which has previously led us to conclude that eNOS must be the isoform responsible for the PVAT anticontractile effect [9]. These experiments are repeated in Figure 7a (control mice) and b, respectively (eNOS−/− mice). Here, we subjected mesenteric arteries from eNOS−/− mice ±PVAT and found that EFS was able to induce a PVAT anticontractile effect (Fig. 7c).
Fig. 7.
EFS-induced PVAT anticontractile effect is modulated by eNOS and nNOS isoforms. ±PVAT mesenteric resistance arteries from control (a) and eNOS−/− (b) mice were subjected to a NA concentration-response protocol (n = 8 both groups. a −PVAT vs. +PVAT p < 0.0001****. b −PVAT vs. +PVAT p > 0.05). c Mesenteric resistance arteries from eNOS−/− were stimulated with EFS. d −PVAT mesenteric arteries from eNOS−/− mice with a section of exogenous PVAT suspended above were stimulated with EFS. The exogenous PVAT was removed and incubated for 30 min with vinyl-L-NIO (100 nM), before returning the PVAT to the organ bath and repeating the stimulus (n = 8, −PVAT vs. exogenous PVAT p < 0.001***, exogenous PVAT vs. vinyl-L-NIO-treated PVAT p < 0.0001++++, −PVAT vs. vinyl-L-NIO-treated PVAT p > 0.05). Data shown are mean ± SEM (−PVAT vs. +PVAT vessels were tested using two-way ANOVA. Before and after drugs within the same vessel type, e.g., +exogenous PVAT before drug vs. +exogenous PVAT after drug were tested using repeated-measures ANOVA. Both followed by Bonferroni post hoc tests).
The EFS-Induced Anticontractile Effect in eNOS−/− Mice Is Abolished by nNOS Inhibition
Since EFS evokes a neural response, we tested the effects of the nNOS inhibitor on the EFS-induced PVAT anticontractile effect in eNOS−/− mice. Following control stimulation with EFS, exogenous PVAT was removed and incubated for 30 min with vinyl-L-NIO before repeating the stimulus (Fig. 7d, n = 8). Incubation with the nNOS inhibitor abolished the EFS-induced PVAT anticontractile effect in eNOS−/− mice.
Activation of NOS in Obesity Restores PVAT Anticontractile Function in Subcutaneous but Not Visceral PVAT
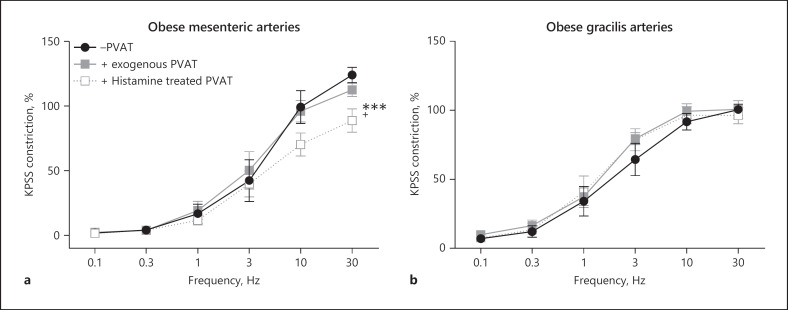
To determine if activation of NOS may be useful in restoring PVAT anticontractile function, we tested the effects of the non-specific NOS activator histamine in mesenteric and gracilis arteries from obese mice. Following control responses to EFS, exogenous PVAT was removed and incubated for 30 min with histamine, before returning the PVAT to the organ bath and repeating the stimulus. In obese mesenteric arteries, incubation of PVAT with histamine restored PVAT anticontractile function (Fig. 8a), whereas incubation of gracilis PVAT did not restore function (Fig. 8b, n = 8).
Fig. 8.
Activation of NOS restores PVAT function in obesity in mesenteric arteries but not in the gracilis artery. −PVAT mesenteric (a) and gracilis (b) arteries from obese mice with a section of exogenous PVAT suspended above were stimulated with EFS. The exogenous PVAT was removed and incubated for 30 min with histamine (100 μM), before returning the PVAT to the organ bath and repeating the stimulus (n = 8, a −PVAT vs. exogenous PVAT p > 0.05, exogenous PVAT vs. histamine-treated PVAT p < 0.001***, −PVAT vs. histamine-treated PVAT p < 0.05+. b p > 0.5 all comparisons). Data shown are mean ± SEM (−PVAT vs. +PVAT vessels were tested using two-way ANOVA. Before and after drugs within the same vessel type, e.g., +exogenous PVAT before drug vs. +exogenous PVAT after drug were tested using repeated-measures ANOVA. Both followed by Bonferroni post hoc tests).
Discussion
For the first time, we have performed a direct comparison of the mechanism of PVAT anticontractile function in subcutaneous and visceral depots and have further investigated the roles of NOS isoforms. The main findings of this study are (1) while vasoreactivity of obese arteries is impaired, healthy fat “transplanted” onto obese arteries is able to induce an anticontractile effect, (2) the mechanism of PVAT anticontractile effect in a subcutaneous PVAT depot is similar to that of visceral − the effect is mediated via β3-adrenoceptor, adiponectin, and OCT3, (3) the sympathetic nerve-evoked PVAT anticontractile effect is mediated via contributions from both eNOS and nNOS isoforms, (4) non-specific activation of NOS in VAT restores PVAT anticontractile function, but not in subcutaneous PVAT.
Here, we have shown for the first time that loss of PVAT anticontractile function in obesity is independent of impaired vasoreactivity of vascular smooth muscle and endothelium in obesity (Fig. 1). When obese exogenous adipose tissue is applied to either lean control or obese arteries, it cannot induce an anticontractile effect. However, exogenous lean control PVAT can induce an anticontractile effect on both lean control and obese arteries, therefore loss of PVAT anticontractile function is likely due to a change in the secretion profile of obese PVAT, and indeed we have previously shown that adiponectin secretion is reduced from obese mesenteric PVAT [8].
Surprisingly, superimposing the responses of lean control and obese −PVAT arteries shows a slight reduction in the baseline responses to EFS, i.e., they constrict less (Fig. 1). However, there were no significant differences in the Emax or EF50 (Fig. 2). This occurred in both mesenteric and gracilis arteries. Similarly, another study examining arteries from epididymal fat in the pressure myograph found that the vasoconstrictor response to NA was reduced following a high-fat diet, despite no change in adrenoceptor expression, or in the mechanical properties of the arteries, suggesting that it may be adrenoceptor sensitivity which is affected [29]. In obesity, the autonomic nervous system becomes pathologically overactive [30, 31], and some studies have reported an increase in α-adrenergic constriction in obesity [32, 33, 34]. In heart failure, autonomic overactivity results in a desensitization of β-adrenoceptors [35]. A similar mechanism could be occurring here in the vascular smooth muscle, and there are some reports of decreased β-adrenoceptor responsiveness in obesity [36]. Reductions in the vasodilator capacity of arteries in obesity are well characterized [37, 38]. An increase in endothelial-dependent vasoconstrictors and a decrease in endothelial-dependent vasodilators have long been linked to development of obesity-related diseases, whereas this paradoxical decrease in vasoconstrictor capacity of arteries in obesity has not been well studied.
Here, we have confirmed that the mechanism of the PVAT anticontractile effect in two different adipose depots; visceral/mesenteric [6], and subcutaneous/gracilis, are similar. Here, we show that stimulation of sympathetic nerves in gracilis arteries with EFS stimulates a PVAT anticontractile effect. The roles of PVAT are two-fold: (1) nerve-derived NA activates adipocyte β3-adrenoceptors, subsequently releasing the vasodilator adiponectin, (2) PVAT sequesters some NA via OCT3, thereby preventing it from reaching the vascular smooth muscle and potentiating contraction. These findings confirm the validity of using either visceral or subcutaneous depots to study the PVAT anticontractile effect, which is vital due to the difficulty in collecting human tissue.
For some experiments, compounds were added directly to the organ bath, whereas in others, the exogenous PVAT preparation was used. This was necessary in instances where we know that the compound will have a direct effect on the blood vessel, and we need to avoid this to isolate the specific effects of PVAT. For example, in Figure 3, we applied SR59230A, CL-316,243, and ABP directly on blood vessels, with and without PVAT. Each of these drugs have no effect on the −PVAT vessels, and only effect vessels with +PVAT, which tells us that their effects are mediated via receptors in the adipocytes. Therefore, we can add them directly to the blood vessels with and without PVAT, being safe in the knowledge that the effects we see must be mediated via PVAT. For drugs affecting catecholamines and NO (e.g., 6-OHDA, corticosterone, L-NMMA, histamine, and vinyl-L-NIO), it is well documented these neurotransmitters/messengers mediate vasoconstriction and vasodilation directly in the blood vessel. Therefore, to isolate their roles in PVAT, it is necessary to only add these drugs to the exogenous PVAT, incubate them separately, then add the exogenous PVAT into the organ bath with the blood vessel. We believe the compounds will stay in the tissues. For example, in Figure 3a, we have used 6-OHDA in exogenous PVAT. 6-OHDA abolished the anticontractile effect, and the response to EFS is greater. If 6-OHDA were being transferred from the PVAT into the organ bath, the vasoconstriction would decrease. Nonetheless, to minimize transference of compounds from the exogenous PVAT into the organ bath with the clean blood vessel, the exogenous PVAT was briefly rinsed with PSS. In addition, it is worth noting that the EFS protocol is conducted quickly and is complete within approximately 10 min of the exogenous PVAT being returned to the organ bath. Therefore, if minimal traces of compound are transferred to the organ bath, it is unlikely they would have the time to take effect.
Leaving PVAT intact or using the exogenous PVAT preparation makes no difference to the anticontractile effect, as we have previously demonstrated that anticontractile function is mediated via the release of a transferable anticontractile factor and via sequestering NA released from sympathetic nerves, rather than PVAT simply acting as a barrier [6]. In our previous study, using a solution transfer protocol, we confirmed that PVAT released a transferable factor into the solution which could be blocked by β3-adrenoceptor inhibition. However, when the β3-adrenoceptor antagonist is added to the myograph bath (both here, and in our previous publication), the β3-adrenoceptor antagonist only reduced the anticontractile effect, it does not abolish it, which suggests there is more to PVAT than release of an anticontractile factor. When NA transport via OCT3 is inhibited, this also reduces the anticontractile effect, and when combined with β3-adrenoceptor antagonism, the effect is completely abolished. As discussed above, this indicates PVAT is not a barrier, it releases a vasodilator upon β3-adrenoceptor activation (adiponectin), and sequesters NA. Based on the differences in the shapes of the responses to EFS in exogenous PVAT experiments versus PVAT-intact experiments (e.g., in Fig. 3a vs. b), there appears to be less of a difference between −PVAT and +PVAT at lower frequencies, particularly at 0.1 Hz and 0.3 Hz. This may suggest that at these very low frequencies, when PVAT is intact, it may have a slight barrier effect; however, there is no statistically significant difference in the responses of −PVAT versus +PVAT arteries at these frequencies. Therefore, based on our previous studies described above, we are confident that PVAT is more than a barrier, and the use of the exogenous PVAT preparation is valid.
Previously, we have concluded that the PVAT anticontractile effect was entirely dependent on the eNOS isoform, as when arteries from eNOS−/− mice were stimulated with exogenous NA, there was no PVAT anticontractile effect [9] (Fig. 7). However, we have now conducted studies in these mice using EFS and found that unlike exogenous NA, EFS was able to induce a PVAT anticontractile effect. This must be due to electrical activation of nerves and the release of the nNOS isoform. Indeed, when an nNOS isoform inhibitor was used on eNOS−/− mouse arteries, the EFS-induced anticontractile effect was abolished (Fig. 7). In lean control mouse arteries, the anticontractile effect induced by EFS was only reduced by the nNOS inhibitor but could be abolished by the non-specific NOS inhibitor. These results confirm that both nNOS and eNOS isoforms are responsible for the nerve-evoked PVAT anticontractile effect, which is more physiologically relevant than our previous data using exogenous NA.
Like in mesenteric arteries [8], we have shown that activation of β3-adrenoceptors using an agonist could not restore gracilis PVAT anticontractile function in obesity (Fig. 5), suggesting downregulation of these receptors as previously reported by ourselves [8] and others [39]. Moreover, application of exogenous adiponectin did not induce a vasodilator effect in gracilis arteries isolated from obese mice (Fig. 5). Here, for the first time, we have tested activation of NOS as a method of restoring PVAT anticontractile function. Surprisingly, non-specific activation of NOS using histamine had no effect on obese gracilis arteries but was able to restore anticontractile function in obese mesenteric PVAT (Fig. 8). This could highlight differences between VAT and SAT NOS isoform expression in health and obesity; however, due to the low protein content of adipose tissue and limited amount of gracilis PVAT, we have been unable to quantify this so far. Another possible explanation could involve the third NOS isoform, iNOS. iNOS activity is increased during an immune response. During obesity, PVAT becomes chronically inflamed, and we and others have described roles of immune cells (e.g., eosinophils, macrophages, dendritic cells, T cells, and neutrophils) in both homeostatic and pathological PVAT function [9, 40, 41, 42]. As discussed in the introduction, both VAT and SAT become inflamed during obesity, but it is possible that there are differences in iNOS activation in obesity between the two depots. In addition, these immune cell populations in PVAT could be stimulated directly by histamine (reviewed by [43]). To the best of our knowledge, no one has performed direct comparisons of any NOS isoforms between VAT and SAT in obesity. As VAT is associated with a higher risk of hypertension and diabetes than SAT, studies into the differences in NOS activation between these depots are vital.
To conclude, the mechanism of PVAT anticontractile function in the subcutaneous gracilis PVAT depot is similar to that of our previously published mechanism in visceral mesenteric PVAT, which confirms the validity of using subcutaneous human biopsies to study PVAT anticontractile function in place of more difficult to access visceral depots. Loss of PVAT anticontractile function in obesity is independent of impaired vascular contractility, and investigation of NOS isoforms in PVAT may reveal use of therapeutic targets in obesity-related vascular diseases.
Limitations and Future Direction
In order to fully investigate the potential for NOS activation in restoring PVAT function in obesity, protein and gene expression studies must be conducted on VAT and SAT tissue depots. In addition, we did not investigate the diminished response to EFS in mesenteric or gracilis arteries taken from obese mice. In future studies, we will measure adrenoceptor expression in the vascular smooth muscle and measure arterial wall diameters to look for signs of remodelling.
Statement of Ethics
All animal experiments were approved by and conducted with permission from the UK Home Office and the University of Manchester.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the British Heart Foundation (PG/16/52/32229).
Author Contributions
Sophie N. Saxton, Sarah B. Withers, and Anthony M. Heagerty designed research studies. Sophie N. Saxton conducted experiments; acquired and analysed data; drafted, edited, and revised the manuscript. All authors approved the final manuscript.
Data Availability Statement
Data is available from the authors upon reasonable request.
Supplementary Material
Supplementary data
Acknowledgements
We thank the Biological Services Facility at the University of Manchester for their assistance with animal work and Dr. Elizabeth Cartwright for allowing us to conduct these studies under her Home Office license. We are very grateful for the kind donation of eNOS−/− mice by Dr. Elizabeth Cottrell.
Funding Statement
This work was supported by the British Heart Foundation (PG/16/52/32229).
References
- 1.Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med. 2012;44((Suppl 1)):S74–84. doi: 10.3109/07853890.2012.663928. [DOI] [PubMed] [Google Scholar]
- 2.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13((2)):277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 3.Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol. 2012;165((3)):633–642. doi: 10.1111/j.1476-5381.2011.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119((12)):1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 5.Yudkin JS, Eringa E, Stehouwer CDA. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365((9473)):1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 6.Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM. Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2018;38((4)):880–891. doi: 10.1161/ATVBAHA.118.310777. [DOI] [PubMed] [Google Scholar]
- 7.Torok J, Zemancikova A, Kocianova Z. Interaction of perivascular adipose tissue and sympathetic nerves in arteries from normotensive and hypertensive rats. Physiol Res. 2016;65((Suppl 3)):S391–9. doi: 10.33549/physiolres.933434. [DOI] [PubMed] [Google Scholar]
- 8.Saxton SN, Toms LK, Aldous RG, Withers SB, Ohanian J, Heagerty AM. Restoring perivascular adipose tissue function in obesity using exercise. Cardiovasc Drugs Ther. 2021 Dec;35((6)):1291–1304. doi: 10.1007/s10557-020-07136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussey CE, Withers SB, Aldous RG, Edwards G, Heagerty AM. Obesity-related perivascular adipose tissue damage is reversed by sustained weight loss in the rat. Arterioscler Thromb Vasc Biol. 2016;36((7)):1377–1385. doi: 10.1161/ATVBAHA.116.307210. [DOI] [PubMed] [Google Scholar]
- 10.Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol Rev. 2019;99((4)):1701–1763. doi: 10.1152/physrev.00034.2018. [DOI] [PubMed] [Google Scholar]
- 11.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100((2)):274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54((6)):945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 13.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128((7)):951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62((10)):921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116((1)):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 16.Scheuer SH, Færch K, Philipsen A, Jorgensen ME, Johansen NB, Carstensen B, et al. Abdominal fat distribution and cardiovascular risk in men and women with different levels of glucose tolerance. J Clin Endocrinol Metab. 2015;100((9)):3340–3347. doi: 10.1210/JC.2014-4479. [DOI] [PubMed] [Google Scholar]
- 17.Herbst KL. Subcutaneous adipose tissue diseases: dercum disease, lipedema, familial multiple lipomatosis, and Madelung disease. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. Endotext. South Dartmouth (MA): MDText.com . [Google Scholar]
- 18.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34((6)):949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 19.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74((3)):315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 20.Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62((2)):128–135. doi: 10.1016/j.jacc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emont MP, Jacobs C, Essene AL, Pant D, Tenen D, Colleluori G, et al. A single-cell atlas of human and mouse white adipose tissue. Nature. 2022;603((7903)):926–933. doi: 10.1038/s41586-022-04518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41((1)):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 23.Evetts KD, Uretsky NJ, Iversen LL, Iversen SD. Effects of 6-hydroxydopamine on CNS catecholamines, spontaneous motor activity and amphetamine induced hyperactivity in rats. Nature. 1970;225((5236)):961–962. doi: 10.1038/225961a0. [DOI] [PubMed] [Google Scholar]
- 24.Berretta N, Freestone PS, Guatteo E, de Castro D, Geracitano R, Bernardi G, et al. Acute effects of 6-hydroxydopamine on dopaminergic neurons of the rat substantia nigra pars compacta in vitro. Neurotoxicology. 2005;26((5)):869–881. doi: 10.1016/j.neuro.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Kim-Han JS, Harmon S, Sakiyama-Elbert SE, O'Malley KL. The Parkinsonian mimetic, 6-OHDA, impairs axonal transport in dopaminergic axons. Mol Neurodegener. 2014;9:17. doi: 10.1186/1750-1326-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sastre E, Caracuel L, Xavier FE, Balfagon G, Blanco-Rivero J. Opposite effect of mast cell stabilizers ketotifen and tranilast on the vasoconstrictor response to electrical field stimulation in rat mesenteric artery. PLoS One. 2013;8((8)):e73232. doi: 10.1371/journal.pone.0073232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxton SN, Whitley AS, Potter RJ, Withers SB, Grencis R, Heagerty AM. Interleukin-33 rescues perivascular adipose tissue anticontractile function in obesity. Am J Physiol Heart Circ Physiol. 2020;319((6)):H1387–h1397. doi: 10.1152/ajpheart.00491.2020. [DOI] [PubMed] [Google Scholar]
- 28.Babu BR, Griffith OW. N5-(1-Imino-3-butenyl)-L-ornithine. A neuronal isoform selective mechanism-based inactivator of nitric oxide synthase. J Biol Chem. 1998;273((15)):8882–8889. doi: 10.1074/jbc.273.15.8882. [DOI] [PubMed] [Google Scholar]
- 29.Hazra S, Henson GD, Bramwell RC, Donato AJ, Lesniewski LA. Impact of high-fat diet on vasoconstrictor reactivity of white and brown adipose tissue resistance arteries. Am J Physiol Heart Circ Physiol. 2019;316((3)):H485–94. doi: 10.1152/ajpheart.00278.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590((8)):1787–1801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manolis AJ, Poulimenos LE, Kallistratos MS, Gavras I, Gavras H. Sympathetic overactivity in hypertension and cardiovascular disease. Curr Vasc Pharmacol. 2014;12((1)):4–15. doi: 10.2174/15701611113119990140. [DOI] [PubMed] [Google Scholar]
- 32.Frisbee JC. Enhanced arteriolar alpha-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 19852004;97((2)):764–772. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- 33.Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;282((3)):H816–20. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- 34.Limberg JK, Morgan BJ, Sebranek JJ, Proctor LT, Eldridge MW, Schrage WG. Neural control of blood flow during exercise in human metabolic syndrome. Exp Physiol. 2014;99((9)):1191–1202. doi: 10.1113/expphysiol.2014.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Post SR, Hammond HK, Insel PA. Beta-adrenergic receptors and receptor signaling in heart failure. Annu Rev Pharmacol Toxicol. 1999;39:343–360. doi: 10.1146/annurev.pharmtox.39.1.343. [DOI] [PubMed] [Google Scholar]
- 36.Masuo K, Lambert GW. Relationships of adrenoceptor polymorphisms with obesity. J Obes. 2011;2011:609485. doi: 10.1155/2011/609485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauricio MD, Aldasoro M, Ortega J, Vila JM. Endothelial dysfunction in morbid obesity. Curr Pharm Des. 2013;19((32)):5718–5729. doi: 10.2174/1381612811319320007. [DOI] [PubMed] [Google Scholar]
- 38.Barton M, Baretella O, Meyer MR. Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br J Pharmacol. 2012;165((3)):591–602. doi: 10.1111/j.1476-5381.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins S, Daniel KW, Rohlfs EM. Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int J Obes Relat Metab Disord. 1999;23((7)):669–677. doi: 10.1038/sj.ijo.0800894. [DOI] [PubMed] [Google Scholar]
- 40.Withers SB, Forman R, Meza-Perez S, Sorobetea D, Sitnik K, Hopwood T, et al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep. 2017;7:44571. doi: 10.1038/srep44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saxton SN, Heagerty AM, Withers SB. Perivascular adipose tissue: an immune cell metropolis. Exp Physiol. 2020;105((9)):1440–1443. doi: 10.1113/EP087872. [DOI] [PubMed] [Google Scholar]
- 42.Bolus WR, Kennedy AJ, Hasty AH. Obesity-induced reduction of adipose eosinophils is reversed with low-calorie dietary intervention. Physiol Rep. 2018;6((22)):e13919. doi: 10.14814/phy2.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:9524075. doi: 10.1155/2018/9524075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
Data is available from the authors upon reasonable request.