Abstract
Background
Tinea versicolor is a common superficial fungal infection of the skin with various clinical manifestations. This review aims to familiarize physicians with the clinical features, diagnosis and management of tinea versicolor.
Methods
A search was conducted in July 2022 in PubMed Clinical Queries using the key terms “tinea versicolor” OR “pityriasis versicolor”. The search strategy included all clinical trials, observational studies and reviews published within the past 10 years.
Results
Tinea versicolor is caused by Malassezia species, notably M. globosa, M. furfur and M. sympodialis. The condition is characterized by scaly hypopigmented or hyperpigmented macules/patches, primarily located on the upper trunk, neck and upper arms. The diagnosis is usually based on characteristic clinical features. If necessary, a potassium hydroxide preparation test can be performed to reveal numerous short, stubby hyphae intermixed with clusters of spores. Most patients with tinea versicolor respond to topical antifungal therapy, which has a better safety profile (fewer adverse events, fewer drug interactions) and lower cost compared to systemic treatment and is therefore the treatment of choice. Oral antifungal therapy is typically reserved for patients with extensive disease, frequent recurrences or disease that is refractory to topical therapy. Advantages of oral antifungal therapy include increased patient compliance, shorter duration of treatment, increased convenience, less time involved with therapy and reduced recurrence rates. On the other hand, oral antifungal therapy is associated with higher cost, greater adverse events and potential drug–drug interactions and is therefore not the first-line treatment for tinea versicolor. Long-term intermittent prophylactic therapy should be considered for patients with frequent recurrence of the disease.
Conclusion
Selection of antifungal agents depends on several factors, including efficacy, safety, local availability, ease of administration, likelihood of compliance and potential drug interactions of the antifungal agent.
Keywords: evoked scale sign, fluconazole, itraconazole, ketoconazole, Malassezia species, pityriasis versicolor, selenium sulfide, terbinafine, zinc pyrithione
Introduction
Tinea versicolor (also known as pityriasis versicolor) is a common superficial fungal infection of the skin. Patients with tinea versicolor typically present with asymptomatic hypopigmented or hyperpigmented, finely scaled, oval or round macules/patches on the trunk and upper arms.1 Patients occasionally report pruritus, particularly when the condition is more extensive. The term ‘versicolor’ refers to the variable colours of the skin lesions that may occur in this disorder. Clinical manifestations of tinea versicolor are myriad, and the differential diagnoses are broad. This review aims to familiarize readers with the various clinical manifestations of tinea versicolor to avoid misdiagnosis, unnecessary investigations and mismanagement of the disease and will also highlight the correct management of this disease.
Methods
A search was conducted in July 2022 in PubMed Clinical Queries using the key terms “tinea versicolor” OR “pityriasis versicolor”. The search strategy included all clinical trials (including open trials, non-randomized controlled trials and randomized controlled trials), observational studies and reviews (including narrative reviews and meta-analyses) published within the past 10 years. Only papers published in the English literature were included in this review. The information retrieved from the search was used in the compilation of this article.
Review
Aetiopathogenesis
Tinea versicolor is caused by dimorphic lipophilic and lipid-dependent yeasts in the genus Malassezia (formerly known as Pityrosporum) species, notably Malassezia globosa (M. globose), M. furfur and M. sympodialis.2–12 Other species that have been implicated include M. restricta, M. obtuse, M. slooffiae, M. pachydermatis and M. japonica.13–16 These yeasts are normal commensals on the skin surface.17,18 Skin colonization increases with age; 25% of children and almost 100% of adults are affected.19 Tinea versicolor occurs when the saprophytic yeast or budding form of the organism converts to the pathogenic hyphal or mycelial form. The fungal infection is localized to the stratum corneum. Predisposing factors for the conversion include a hot and humid environment, hyperhidrosis, application of oily lotion or cream to the skin, wearing of masks, excessive lipid-containing sebaceous secretions, malnutrition, poor general health, use of oral contraceptives, pregnancy, diabetes mellitus, use of topical or systemic corticosteroids, Cushing disease, Helicobacter pylori infection, immunodeficiency and genetic predisposition.20–36 A recent study showed oxidative stress has no role in the pathogenesis of tinea versicolor.29
Hypopigmented lesions (more commonly noted in darker skin tones) seen in tinea versicolor are thought to result from damage to melanocytes and inhibition of tyrosinase by azelaic acid (a dicarboxylic acid) produced by the Malassezia species involved, small melanosomes and accumulation of lipid-like material in the stratum corneum blocking ultraviolet light.37–40 On the other hand, hyperpigmented lesions (more commonly noted in lighter skin tones) may result from a hyperaemic inflammatory response elicited by Malassezia species, more tonofilaments in the granulosum, a thicker stratum corneum and abnormally large melanosomes.8,38,41,42 Keratinase, produced by the Malassezia species, causes loosening of the stratum corneum with subsequent scale formation.43,44
Prevalence
Tinea versicolor occurs worldwide. The prevalence is very high in hot and humid climates.35 In some tropical countries, the prevalence is as high as 50% whereas, in Sweden, the prevalence is as low as 0.5%.17,45,46 Tinea versicolor is most common amongst adolescents and young adults, presumably because of increased sebum production in individuals of these age groups.47–55 Although uncommon, the condition can occur in young children and elderly individuals.47–55 Rarely, tinea versicolor has been reported in infants including neonates.56–60 Tinea versicolor is slightly more common in men than in women presumably due to increased sebaceous activity in men.8,47,54 A positive family history of tinea versicolor is present in approximately 17% of affected individuals.61,62 The incidence of tinea versicolor appears to be the same in all races, though the change in skin pigmentation is more visually apparent in dark-skinned individuals.8
Histopathology
Histological findings include parakeratosis, hyperkeratosis, slight acanthosis and a mild superficial, perivascular infiltrate in the upper dermis.63 Haematoxylin-eosin, methenamine silver or periodic acid–Schiff staining reveal the presence of yeast in a ‘spaghetti and meatballs’ pattern in the stratum corneum.63–65 Hyperpigmented lesions tend to contain more hyphae and spores than hypopigmented lesions.62,65,66 In hypopigmented lesions, the horny layer tends to be slightly hyperkeratotic and there may be a decrease in melanosomes in the stratum spinosum.39,45
Clinical manifestations
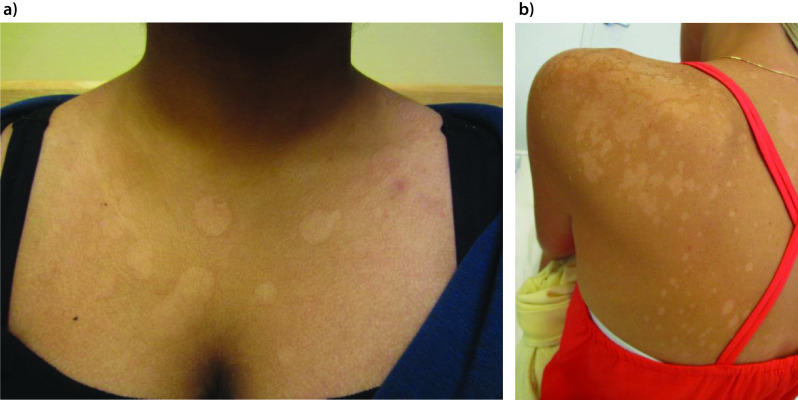
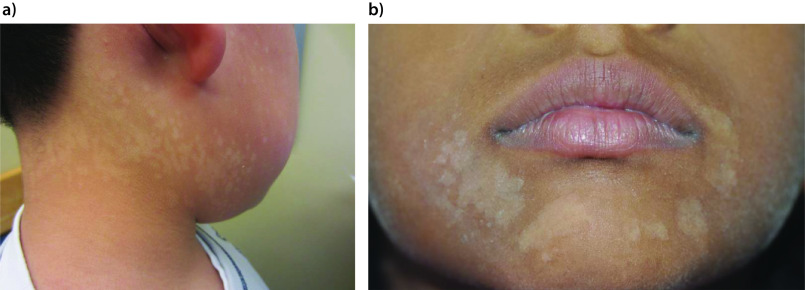
Tinea versicolor is characterized by mildly scaly hypopigmented or hyperpigmented macules/patches, most commonly affecting areas of skin that are rich in sebum production such as the trunk (especially the upper part), neck, shoulders and upper arms (Figures 1–3A).19,21,24,67–69 Facial involvement is less common in adults. On the other hand, facial involvement is common in children and may be the only site involved (Figure 3B).19,70 The forehead is the usual site of facial involvement.71–74 Other sites of involvement, such as forearms and thighs, are less common (Figure 4).75 Unusual sites of involvement include the scalp, eyelid, axilla, areola, periareolar area, antecubital fossae, popliteal fossa, pubis, groin, perineum, penile shaft and vulva.75–86
Figure 1.
Tinea versicolor presenting as multiple hypopigmented macules and patches on the chest (part a) and left shoulder and upper back (part b).
Figure 3.
Multiple hypopigmented macules and patches on the neck and face of a 5-year-old child (part a) and on the face of a 10-year-old child (part b).
Figure 4.
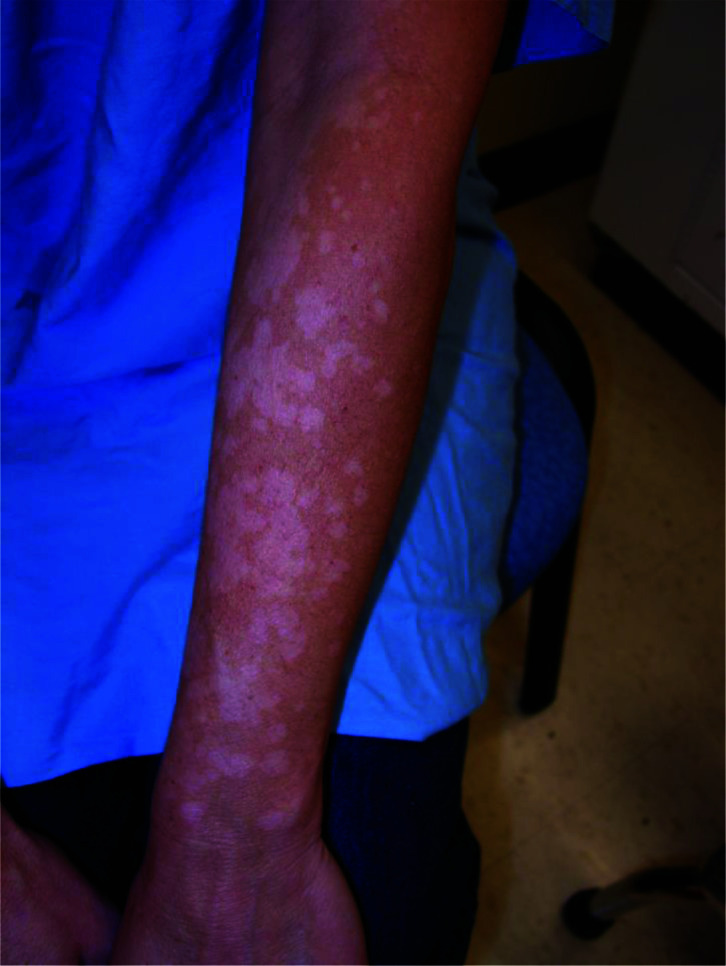
Multiple hypopigmented macules and patches on the left forearm.
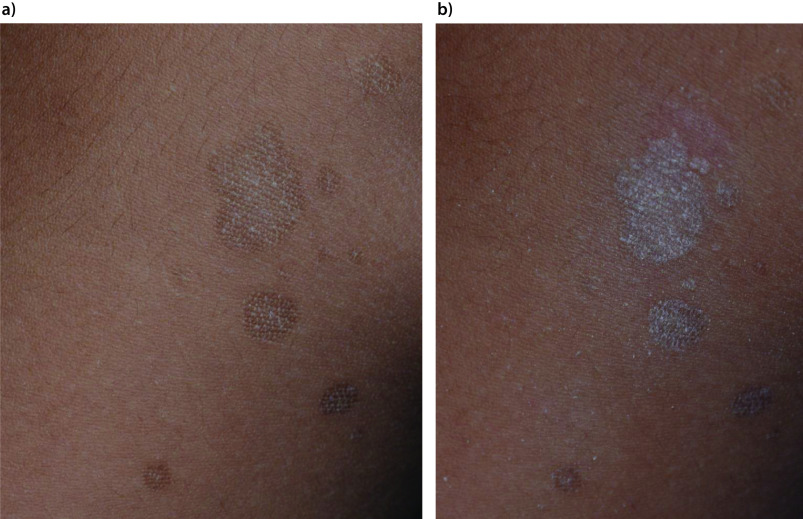
Typically, lesions arise as multiple, small, oval or round, well-demarcated, round or oval macules.8 Smaller macules may have a powdery appearance because of flaking.87 Over time, the macules enlarge radially and coalesce into patches or very superficial plaques.8 The lesions are covered with a fine scale, which is often difficult to appreciate upon clinical examination. On the other hand, the scale becomes more apparent when the lesion is stretched or scraped (the ‘evoked scale sign’) (Figure 5A,B).1,44,88,89 It should be noted that burned-out or treated lesions usually lack scale.90 In patients with tinea versicolor, when the affected skin is wiped with a piece of wet cloth and scraped, it yields a considerable amount of dirty brown keratin. Tinea versicolor lesions are typically asymptomatic, although some patients complain of mild pruritus,45 which may become worse in hot and humid conditions.
Figure 5.
Hyperpigmented macules with minimal desquamation on the upper back (part a) and with prominent desquamation on the upper back after the skin lesion was scrapped (the evoked scale sign, part b).
The eruption varies in colour from individual to individual, but each individual usually has lesions of a single hue. Lesions are usually evenly pigmented. In general, hyperpigmented lesions tend to occur in fair-skinned patients whereas hypopigmented lesions tend to occur in dark-skinned individuals.8,91 When hyperpigmented lesions occur in dark-skinned individuals, they are often grey-black, dark brown or black whereas these are often tan, light brown, red or pink in fair-skinned individuals.1,8,40 Lesions may become more apparent following exposure to the sun and are thus more noticeable during the summer months. Mixed hyperpigmented and hypopigmented lesions may be found, especially in the axilla and groin.8,41,91
Several morphological variants have been described. An inverse form of tinea versicolor has been described, especially in patients who are immunocompromised.92 Some authors favour labelling this variant as ‘tinea InVersicolor’.92 In this variant, lesions tend to localize in the flexural areas (axilla, elbow, popliteal fossa and groin) and isolated areas of the extremities (Figure 6).92–94 Atrophying tinea versicolor typically presents with numerous, hypopigmented or erythematous/violaceous, round to oval, scaly lesions with a typically depressed appearance.95–98 Lesions tend to cluster and are generally uniform in size (a few millimetres to several centimetres) on the same patient and may have a wrinkled surface.99 The atrophy is limited to the area affected by tinea versicolor.100 The exact aetiology of the atrophy is not known. It is postulated that the atrophy may result from prolonged use of topical corticosteroids or delayed type IV hypersensitivity to epicutaneous antigens from the Malassezia species.100–104 Folliculocentric tinea versicolor involves the hair follicle and presents with asymptomatic hypopigmented or hyperpigmented macules located exclusively around the follicles105,106; these macules may merge into patches.106 The disorder is typically localized to the chest and back.106,107 A Wood lamp examination reveals folliculocentric fluorescence in hypopigmented areas.107 Papular tinea versicolor presents with multiple, asymptomatic, monomorphic, red-brown papules (2–3 mm), which may or may not show the fine overlying scale.105 They are usually found on the trunk.105 Confetti-like tinea versicolor presents with asymptomatic confetti-like spots with slightly scaly surfaces (Figure 7). The spots are usually bilateral and symmetrically distributed. The formation of scaly, guttate and coalescent hypopigmented patches or plaques is unique in African Americans; this variant is colloquially known as acid skin.108
Figure 6.
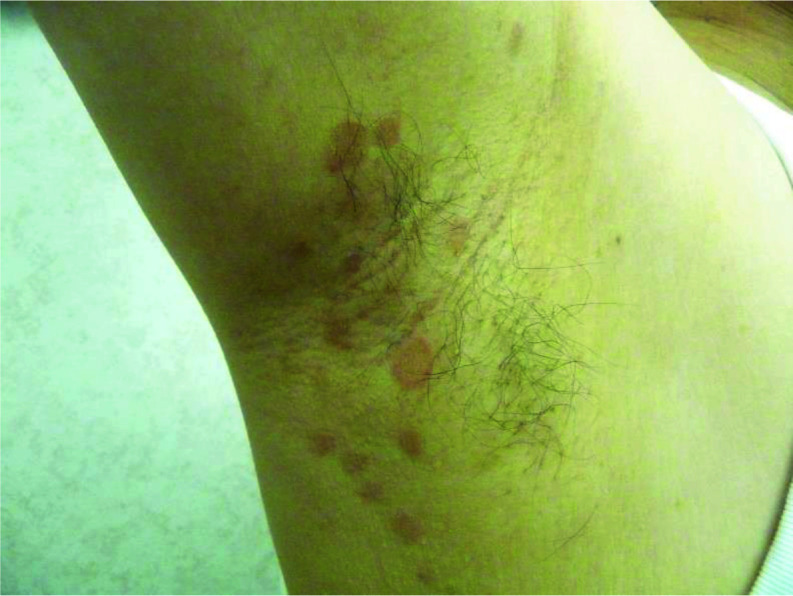
Hyperpigmented macules in the axillary region of a 17-year-old male with tinea InVersicolor.
Figure 7.
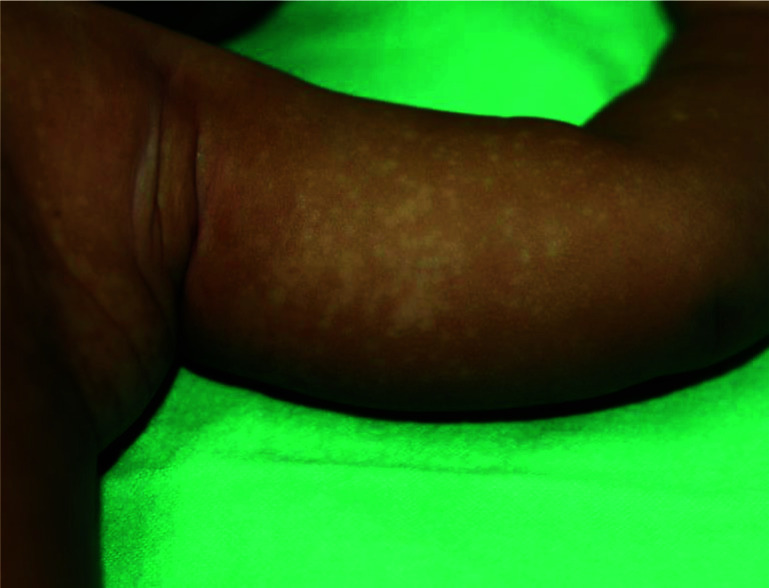
Confetti-like spots on the left arm, left lateral upper chest and armpit of a child with confetti-like tinea versicolor.
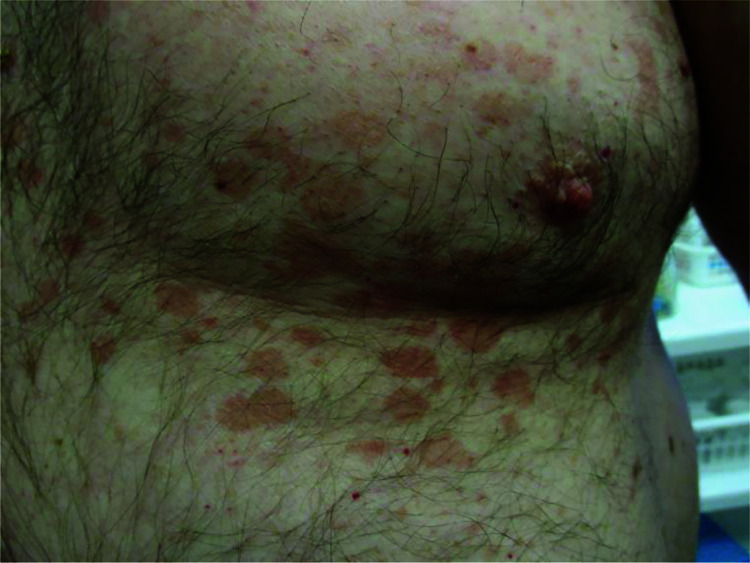
Figure 2.
Multiple erythematous macules and patches on the upper abdomen and chest.
Diagnosis
The diagnosis is usually clinical, based on the characteristic features (multiple hypopigmented or hyperpigmented, centrally coalescing, oval to round, finely scaling macules or patches and the ‘evoked scale sign’).35 However, the varied presentations of tinea versicolor may be confusing to an inexperienced physician. Examination of the lesion with a Wood lamp (filtered ultraviolet light with a peak of 365 nm) may show gold-yellow, yellowish-green or coppery-orange fluorescence, although some lesions do not fluoresce.17,56 The fluorescence may include areas surrounding clinically visible lesions, suggesting that the fungal infection is spreading.17
Dermoscopy is a useful ancillary tool for the diagnosis of tinea versicolor.109–114 Typical dermoscopic findings include alteration in the background pigmentation, a ‘contrast halo’ sign (a ring of hypopigmentation surrounding the primary lesion of increased pigmentary network in a hyperpigmented lesion or a ring of increased pigmentation surrounding the primary lesion of decreased pigmentary network in hypopigmented lesion), fine scale on the involved skin, folliculocentricity and hypopigmentation of the invaded hair follicle.110–114
If necessary, a potassium hydroxide (KOH) preparation test can be performed; examination of scrapings from the border of the lesion soaked with 10–15% KOH reveals numerous short, stubby hyphae intermixed with clusters of spores (the so-called ‘spaghetti and meatballs’ appearance) (Figure 8).51 KOH helps to dissolve the keratin and debris so that the hyphae and spores can be readily visible by microscopy. The border of the lesion contains the highest number of fungi. Because the standard KOH mount does not show a colour contrast, ink blue, Parker blue-black ink, methylene blue, chlorazol black E, Swartz–Lamkin, Swartz–Medrik or Chicago Sky Blue (CSB) 6B staining may be added for better visualization of the causative organism.35,115,116 Thus far, the contrast stain containing 1% CSB 6B has the greatest specificity and sensitivity.115
Figure 8.
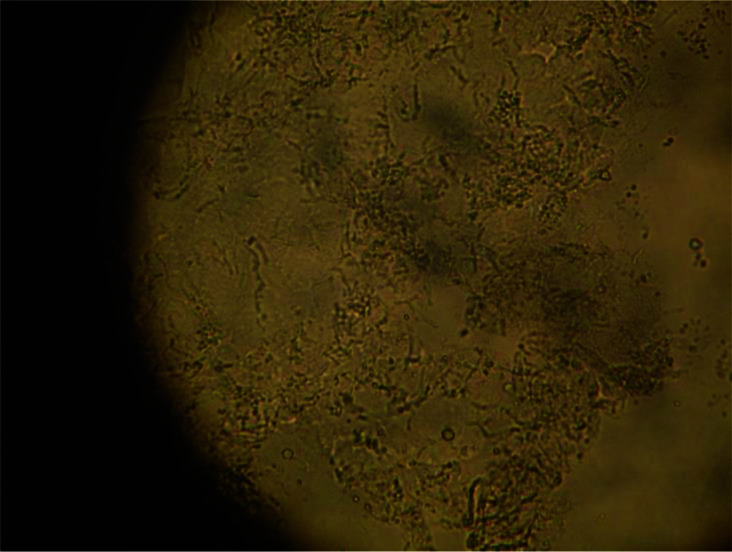
KOH preparation of tinea versicolor showing numerous short, stubby, hyphae intermixed with clusters of spores (the so-called ‘spaghetti and meatballs’ appearance).
Differential diagnosis
The differential diagnoses are broad, especially those with unusual presentations. The differential diagnosis of hypopigmented lesions of tinea versicolor includes pityriasis alba, nevus anemicus, nevus depigmentosus, idiopathic guttate hypomelanosis, eruptive hypomelanosis, progressive macular hypomelanosis, leukoderma punctate, hypomelanosis of Ito, vitiligo, ash-leaf spot in tuberous sclerosis, corticosteroid-induced hypopigmentation, arsenicosis, leprosy, hypopigmented mycosis fungoides and post-inflammatory hypopigmentation.117–124 On the other hand, hyperpigmented lesions of tinea versicolor should be differentiated from tinea corporis, pityriasis rosea, pityriasis rotunda, tinea imbricata, acanthosis nigricans, terra firma-forme dermatosis, café au lait macules, ephelides, solar lentigines, melasma, erythrasma, guttate psoriasis, nummular eczema, seborrheic dermatitis, contact dermatitis, post-inflammatory hyperpigmentation, type 1 (classic adult) pityriasis rubra pilaris, secondary syphilis, confluent and reticulated papillomatosis (also known as Gougerot–Carteaud syndrome), and epidermodysplasia verruciformis (also known as Lewandowsky–Lutz dysplasia).125–138 The distinctive features of many of these conditions help to differentiate them from tinea versicolor.
Complications
Skin discolouration can be cosmetically unsightly and socially embarrassing especially if it occurs in exposed areas of the body. This may have an adverse effect on quality of life. The discolouration of the skin (without overlying scaling) may persist for weeks to months even after completion of successful therapy. The disappearance of the scale is evidence that the hyphal yeast has been eradicated.44 A preliminary study showed that topical application of cycloserine, a transaminase 1 inhibitor, to the hyperpigmented lesions of tinea versicolor twice a day for 5 days resulted in complete clearing of the hyperpigmentation.139 Well-designed, large-scale, multicentre, randomized, placebo-controlled trials are needed to confirm or refute this finding.
Hair thinning and/or hair loss within the tinea versicolor lesions has been reported.140 In a study of 39 patients with tinea versicolor, hair thinning and/or hair loss within the tinea versicolor lesions occurred in 24 (61.5%) patients.140 Hair thinning and/or hair loss occurred most commonly on the forearms, abdomen, neck and, in men, the beard area.
Prognosis
The prognosis is good. Mycological cure is usually achieved soon after antifungal treatment. Tinea versicolor tends to persist for years if left untreated.141–143 The disorder has a high recurrence rate, especially in patients with a positive family history of tinea versicolor.51,63,144,145 Framil et al. followed 102 patients with clinical and laboratory diagnosis of tinea versicolor for one year.145 After appropriate treatment, 33 (33.35%) patients did not have any relapsing episodes, 54 (52.94%) patients had one to four relapsing episodes and 15 (14.7%) patients had more than four relapsing episodes.145 Patients with a positive family history of tinea versicolor also have a longer duration of the disease.51 Relapse rates as high as 80% following treatment have been reported.146
Management
Most patients with tinea versicolor respond to topical antifungal therapy (Box 1). Additionally, topical antifungal therapy has a better safety profile (fewer adverse events, fewer drug interactions) and lower cost compared to systemic treatment and is therefore the treatment of choice. Oral antifungal therapy is typically reserved for patients with extensive disease, frequent recurrences or disease that is refractory to topical therapy (Box 1). For resistant or stubborn cases, combining oral and topical therapies may be considered. Alternative and complementary therapies are still used in many parts of the world.141,142
Box 1. Treatment options for tinea versicolor.
-
Topical antifungals
Azoles (for example, ketoconazole, econazole, eberconazole, efinaconazole, bifonazole, luliconazole, clotrimazole, miconazole, sertaconazole, sulconazole, oxiconazole, fenticonazole, tioconazole, fluconazole and dapaconazole)
Terbinafine
Naftifine
Butenafine
Ciclopirox olamine
Non-specific topical antifungal agents (for example, selenium sulfide, zinc pyrithione, propylene glycol, Whitfield ointment, sulfur plus salicylic acid and benzoyl peroxide)
-
Oral antifungals
Itraconazole
Fluconazole
Laser and photodynamic therapies
Alternative therapies
Topical antifungals
Many topical antifungals have proved to be effective in the treatment of tinea versicolor. Various antifungal preparations are available, including shampoos, foams, gels, lotions and creams and are usually applied once to twice daily.1 Shampoo is preferred when a large percentage of the body surface area is involved. Treatment regimens range from a few days to 4 weeks.1,147 A systematic review of 93 controlled trials (n=8327) showed that most topical antifungal medications used to treat tinea versicolor are effective compared to placebo, with numbers needed to treat of 1–3.147 Additionally, greater cure rates can be achieved with higher concentrations of active ingredients in the topical antifungal medications and longer duration of treatment.147,148 The most common side-effects of topical antifungal agents are skin irritation and contact allergy.35
Azoles
Topical azole drugs (for example, ketoconazole, econazole, eberconazole, efinaconazole, bifonazole, luliconazole, clotrimazole, miconazole, sertaconazole, sulconazole, oxiconazole, fenticonazole, tioconazole, fluconazole and dapaconazole) have become an important treatment class for tinea versicolor.149–165 This group of antifungal agents are fungistatic and works by inhibiting the P-450-dependent enzyme lanosterol 14-α-demethylase, which is involved in the biosynthesis of ergosterol.166,167 Ergosterol is an important structural component of fungal cell membranes.62,166 Impairment of biosynthesis of ergosterol may limit cell function and cell growth.62,166 Randomized clinical trials have supported the efficacy of various topical azole antifungal agents.149–165 Of the topical azole antifungal agents, ketoconazole has been most studied for the treatment of tinea versicolor. In addition to its ability to block the synthesis of ergosterol, ketoconazole also has sebum-lowering effects by inhibiting androgenesis, anti-inflammatory effects by inhibiting 5-lipoxygenase and barrier-restoring effects by inhibiting hyperproliferation of keratinocytes.166,168–170 As ketoconazole is highly lipophilic, it concentrates at sites of tinea versicolor, thereby further increasing its efficacy.166 A 2019 systematic view of 40 randomized controlled trials (n=4566) focusing on the use of topical ketoconazole for the treatment of Malassezia-related conditions, such as tinea versicolor, showed that the efficacy rate of topical ketoconazole for the treatment of tinea versicolor was 71–89%.166 Topical azole drugs have a favourable safety. Adverse events are usually mild and uncommon and include skin dryness, irritation, pruritus, burning sensation and erythema.147 Rarely, allergic contact dermatitis may occur.166
Studies have shown that combination therapies using 2% ketoconazole cream and 1% adapalene gel are more efficacious in the treatment of tinea versicolor than ketoconazole cream alone.171,172 To further improve the efficacy of topical ketoconazole, future drug development should focus on improving topical delivery to allow better permeation of drugs into skin by using nanostructured lipid carriers, nanoparticles, microemulsions, copolymeric micelles, niosomes and microemulsions.156,173–176 In this regard, the development of topical gels containing fluconazole-loaded solid lipid nanoparticles allows fluconazole to be used topically as the product exhibits skin penetration as a result of large particle surface area and film formation, enhancing contact between fluconazole and skin.156 Recently, it has been shown that itraconazole-loaded aspasomal cream has a higher efficacy in the treatment of tinea versicolor than non-formulated itraconazole cream alone.177
Terbinafine
Terbinafine, an allylamine antifungal, works by inhibiting squalene epoxidase, thereby blocking the biosynthesis of ergosterol.87,163,178 The accumulation of squalene accounts for its fungicidal activity whilst ergosterol deficiency accounts for its fungistatic activity.163 The effectiveness of topical terbinafine in the treatment of tinea versicolor has been demonstrated in many double- blind, randomized, placebo-controlled studies.178–181
There are very few good quality studies comparing the efficacy of topical terbinafine with topical azole drugs for the treatment of tinea versicolor. Most trials are underpowered to detect clinically meaningful differences. In an open clinical trial, 60 adults with tinea versicolor were randomized to receive either eberconazole 1% cream or terbinafine 1% cream once daily for 2 weeks.163 At the end of the treatment period, there was a significant improvement in all the clinical parameters in both groups. Clinical cure was present in 80% of patients in the eberconazole group versus 63% of patients in the terbinafine group. Mycological cure was achieved in 100% of patients in the eberconazole group versus 97% of patients in the terbinafine group. No adverse events were noted. No relapse was seen in patients treated with eberconazole but one patient treated with terbinafine had a relapse at the end of 8 weeks. The authors concluded that both terbinafine and eberconazole were efficacious and safe in the treatment of tinea versicolor but better response was observed in patients treated with eberconazole. In another study, 110 patients (≥14 years of age) with a clinical diagnosis of tinea versicolor confirmed by KOH microscopy were randomized to receive either terbinafine 1% cream (n=55) or ketoconazole 2% cream (n=55) twice daily.182 Patients with negative mycological examination either with clearance of skin lesions or presence of mild residual skin lesions were considered cure. Cure rates at the end of the second, fourth and eighth weeks of treatment were 72% and 64.3%, 81.2% and 69%, and 70.8% and 61.9% for the terbinafine group and the ketoconazole groups, respectively. The authors concluded that there were no significant statistical differences between the terbinafine group and the ketoconazole group with regard to the cure and recurrence rates. However, the numbers of patients were higher and the recurrent cases were lower in those treated with topical terbinafine. Chopra et al. randomized 50 patients with tinea versicolor confirmed by KOH microscopy to receive either terbinafine 1% cream (n=25) or ketoconazole 2% cream (n=25) once daily for 2 weeks.183 At the end of treatment, the clinical and mycological cure rate was 96% for patients treated with topical terbinafine and 88% for patients treated with topical ketoconazole; no adverse events were reported. At 3 months of follow-up, the relapse rate was 8.33% in patients treated with topical terbinafine and 13.53% in patients treated with topical ketoconazole.
Naftifine
Naftifine is a synthetic allylamine derivative with broad-spectrum antifungal activity.184 The medication works by blocking the biosynthesis of ergosterol via inhibition of squalene epoxidase, with resulting accumulation of squalene, increase in fungal cell membrane fragility and permeability, and inhibition of fungal cell growth.184 As naftifine is highly lipophilic, it can penetrate efficiently into the epidermis when applied topically.184 Open studies have shown that topical naftifine is safe and efficacious in the treatment of tinea versicolor.185–187 Well-designed, large-scale, randomized, double-blind and placebo-controlled studies are necessary to further elucidate its clinical efficacy and safety.
Butenafine
Butenafine, a synthetic benzylamine antifungal agent with fungicidal activity, has also been used topically for the treatment of tinea versicolor.188 The medication inhibits squalene epoxidation with resultant blockage of ergosterol biosynthesis. Small randomized controlled trials have shown the clinical efficacy of topical butenafine in the treatment of tinea versicolor.189–190 In a randomized, double-blind, parallel-group trial, the rate of mycological cure in patients with tinea versicolor treated with topical butenafine and topical bifonazole was 87.5% and 83.3%, respectively, after 2 weeks of treatment.189 The rate of effective clinical response in patients with tinea versicolor treated with butenafine and bifonazole was 91.7% and 83.3%, respectively. There was no significant statistical difference in terms of mycological cure and effective clinical response between treatment with topical butenafine and topical bifonazole. Well-designed, large-scale, randomized, double-blind and placebo-controlled studies are necessary to determine the safety and efficacy of topical butenafine in treating tinea versicolor.
Ciclopirox olamine
Ciclopirox olamine is a hydroxypyridone with broad-spectrum antifungal activity.62,191 The medication works by inhibiting the transport of essential elements, which is required for the synthesis of the fungal cell membrane.63 Ciclopirox olamine also interferes with the synthesis of DNA, RNA and protein. Ciclopirox olamine has been shown to be safe and effective for the treatment of tinea versicolor in several studies.191–193 The safety and efficacy of topical ciclopirox olamine in the treatment of tinea versicolor need to be substantiated by well-designed, large-scale, randomized, double-blind and placebo-controlled studies.
Non-specific topical antifungal agents
Non-specific topical antifungal agents for the treatment of tinea versicolor include selenium sulfide, zinc pyrithione, propylene glycol, Whitfield ointment, sulfur plus salicylic acid and benzoyl peroxide. These topical agents do not act specifically against Malassezia species. Their mode of action is to remove dead, infected stratum corneum either physically and/or chemically.194
Selenium sulfide, available as a shampoo, lotion and cream in 1–2.5% concentrations, is safe and effective in the treatment of tinea versicolor.147,195–197 Studies have shown that the success rate of selenium sulfide shampoo in the treatment of tinea versicolor is comparable to that of topical bifonazole and econazole.198,199 Advantages of selenium sulfide include over-the-counter availability, low cost and convenient application. Disadvantages include irritation of the skin, unpleasant odour, staining of clothes and bedding, and a high relapse rate.195,196
The efficacy of zinc pyrithione 1% shampoo versus its vehicle in the treatment of tinea versicolor has been shown in an open trial200 as well as in a double-blind controlled trial.201 In the latter, 20 patients with tinea versicolor were treated with either zinc pyrithione 1% shampoo or the shampoo base for 5 minutes per day for 2 weeks.201 At the end of the study, all 20 patients treated with zinc pyrithione 1% shampoo had clearing of the tinea versicolor lesions compared to none of the patients in the shampoo base group.
Propylene glycol is a keratolytic agent. Faergemann et al. treated 20 patients with tinea versicolor with propylene glycol 50% in water daily for 2 weeks.202 At the end of treatment, all 20 patients were cured.
Whitfield ointment consists of 3% salicylic acid and 6% benzoic acid in an emulsifying ointment.153,203 Salicylic acid is keratolytic whereas benzoic acid is fungistatic.17 Whitfield ointment has been shown to be effective in the treatment of tinea versicolor in a limited number of studies.153,203
A combination of sulfur and salicylic acid has been shown to be effective in the treatment of tinea versicolor in a small number of studies.204,205 The combination can be in the form of 2% micropulverized sulfur and 2% salicylic acid in a shampoo base.204,205 The formulation is cosmetically pleasant and safe.204
Benzoyl peroxide has been used with success in the treatment of tinea versicolor.206–208 In three studies, the vehicle for benzoyl peroxide was propylene glycol, which by itself is effective in the treatment of tinea versicolor.206–208 As such, it is not certain whether the beneficial effect is due to benzoyl peroxide or propylene glycol. It is possible that benzoyl peroxide and propylene glycol may have a synergistic effect in the treatment of tinea versicolor.
Oral antifungals
Oral antifungals are usually reserved for treating severe, widespread, recalcitrant or recurrent tinea versicolor.17 Advantages of oral antifungal therapy include increased patient compliance, shorter duration of treatment, increased convenience, less time involved with treatment and reduced recurrence rates.17,209 On the other hand, oral antifungal therapy is associated with higher cost, greater adverse events, and potential drug–drug interactions and is therefore not the first-line treatment of tinea versicolor, especially in children.1 Oral azole antifungals, such as itraconazole and fluconazole, are the preferred systemic agents.210,211 Adverse events associated with the use of oral antifungals include fatigue, malaise, headache, cutaneous eruption, pruritus, dyspepsia, nausea, vomiting, abdominal pain, diarrhoea, hypertension, congestive cardiac failure, thrombocytopenia, hypokalaemia, albuminuria, hypertriglyceridemia and abnormal liver function.147,212,213
Oral itraconazole
Oral itraconazole, a triazole derivative with strong keratophilic and lipophilic properties, is highly effective for the treatment of tinea versicolor143,210,214; the absorption of itraconazole is enhanced by food.17,215 The recommended dose is 200 mg per day for 5–7 days.1,63,141,142 Adverse events are uncommon.216 Rarely, congestive heart failure and hepatotoxicity have been reported.35 Oral itraconazole should therefore be avoided in patients with a history of congestive heart failure or in patients with active hepatic disease.35 As itraconazole inhibits the enzyme cytochrome P450-dependent system, the medication may cause drug–drug interactions. As such, oral itraconazole should not be given to patients who are on astemizole or cisapride for fear of cardiovascular adverse events.35
Oral fluconazole
Oral fluconazole, a triazole antifungal, is also highly effective for the treatment of tinea versicolor through the inhibition of cytochrome P450-dependent ergosterol synthesis.217–219 When administered orally, fluconazole can persist in the stratum corneum for approximately 2 weeks following administration.219 The recommended dose is 300 mg once weekly for 2–4 weeks.1,63,211 Because fluconazole has little affinity for mammalian cytochromes, the antifungal has low toxicity. Serious adverse events are rare.35 As fluconazole inhibits the cytochrome P450- dependent system, the medication should likewise be avoided in patients treated with astemizole or cisapride for fear of cardiovascular adverse events.35
Oral ketoconazole
Oral ketoconazole at a dose of 200 mg daily for 10 days is also effective for the treatment of tinea versicolor.147 The risk of hepatotoxic adverse events associated with oral ketoconazole is about 1 in 500 and therefore outweighs its potential benefits.220,221 Because of the risk of hepatotoxicity, adrenal insufficiency and multiple drug–drug interactions, oral ketoconazole should no longer be prescribed.1,35,87
Oral terbinafine
Oral terbinafine is not effective in the treatment of tinea versicolor.87 Terbinafine is not excreted in sweat and fungicidal levels of terbinafine cannot be achieved in the stratum corneum with oral administration of the medication.87
Oral griseofulvin
Oral griseofulvin is not effective for the treatment of tinea versicolor.17,43
Laser and photodynamic therapies
A limited number of studies reported the successful treatment of tinea versicolor with 308-nm excimer laser, narrow-band ultraviolet (UV)-B phototherapy, 5- aminolevulinic acid photodynamic therapy and methylene blue photodynamic therapy.222–225 Well-designed, large-scale, multicentre, randomized, placebo-controlled trials are needed to confirm or refute these findings.
Alternative therapies
A wide variety of alternative medicines have been shown to have some therapeutic effects on tinea versicolor. In some cultures, alternative therapies are popular for the treatment of tinea versicolor. These include topical application of beeswax and honey,226 essential oils of Cymbopogon citratus,227 quince seed mucilage hydrogel decorated with essential oils of Nigella sativa, Citrus sinensis and Cinnamon verum,228 polyherbal Unani formulation,229 Pentas longiflora leaf extract,230 Acalypha wilkesiana leaf extract,231 Artemisia sieberi shrub extract,182 nitric oxide-liberating cream232 and irradiated human amniotic membrane in combination with tea tree oil.233 None of these treatments have yet been subjected to rigorous studies nor randomized clinical trials.
Prophylactic treatment
The relapse rate is high because Malassezia species are normal commensals on the skin surface.87 Good personal hygiene may limit recurrences to a certain extent. Long-term intermittent prophylactic therapy should be considered for patients with frequent recurrence of the disease who desire treatment, especially during the warmer months of the year.87 Unfortunately, research studies evaluating the efficacy of prophylactic antifungal treatment are scarce. Prophylactic administration of topical ketoconazole 2%, clotrimazole 1% or selenium sulfide 2.5% shampoo applied to the whole body for 10 minutes once a month may lead to decreased relapse rate of tinea versicolor.166 An alternative is to prophylactically administer an oral antifungal agent such as itraconazole, especially if topical antifungal prophylaxis is not successful. Oral itraconazole is easy to administer and less time-consuming and thereby has better compliance.63 The recommended dose of itraconazole for prophylaxis is 200 mg twice a day once per month.1,216
Conclusion
Tinea versicolor is a common superficial fungal infection of the skin caused by Malassezia species. Because the clinical manifestations of tinea versicolor are myriad, clinical acumen is essential to make the correct diagnosis. As tinea versicolor is often a chronic and recurrent disease, repetitive treatment courses are often necessary. A wide range of antifungal agents are effective in the treatment of tinea versicolor. In general, topical antifungal agents are the first-line treatment of tinea versicolor as there are fewer adverse events associated with their use. Oral antifungal agents are usually reserved for severe, widespread, recalcitrant or recurrent disease. Apart from considering the severity and extensiveness of tinea versicolor, patient age, and patient and physician preferences, the selection of antifungal agents depends on a number of factors, including the efficacy, safety, local availability, ease of administration, likelihood of compliance and potential drug interactions of the antifungal agent. In clinical practice, it is often the patient preferences and the physician experience that dictate the selected treatment.
Acknowledgements
None.
Footnotes
Contributions: AKCL is the principal author. BB, JML, KFL and KLH are coauthors who contributed and helped with the drafting of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work and have given their approval for this version to be published. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: AKCL and KLH are associate editors of Drugs in Context and confirm that this article has no other conflicts of interest otherwise. This manuscript was sent out for independent peer review. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/10/dic.2022-9-2-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Leung AKC, Barankin B, Lam JM, Leong KF, Hon KL. https://doi.org/10.7573/dic.2022-9-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Goldstein BG, Goldstein AO. Tinea versicolor (pityriasis versicolor) In: Dellavalle RP, Levy ML, Rosen T, editors. Waltham, MA: UpToDate; [Accessed July 26, 2022]. https://www.uptodate.com/contents/tinea-versicolor-pityriasis-versicolor . [Google Scholar]
- 2.Aghaei Gharehbolagh S, Mafakher L, Salehi Z, et al. Unveiling the structure of GPI-anchored protein of Malassezia globosa and its pathogenic role in pityriasis versicolor. J Mol Model. 2021;27(9):246. doi: 10.1007/s00894-021-04853-7. [DOI] [PubMed] [Google Scholar]
- 3.Archana BR, Beena PM, Kumar S. Study of the distribution of Malassezia Species in patients with pityriasis versicolor in Kolar Region, Karnataka. Indian J Dermatol. 2015;60(3):321. doi: 10.4103/0019-5154.156436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad AK, Al-Ezzy AIA, Jameel GH. Phenotypic identification and molecular characterization of Malassezia Spp. isolated from pityriasis versicolor patients with special emphasis to risk factors in Diyala Province, Iraq. Open Access Maced J Med Sci. 2019;7(5):707–714. doi: 10.3889/oamjms.2019.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diongue K, Kébé O, Faye MD, et al. MALDI-TOF MS identification of Malassezia species isolated from patients with pityriasis versicolor at the seafarers’ medical service in Dakar, Senegal. J Mycol Med. 2018;28(4):590–593. doi: 10.1016/j.mycmed.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Framil VM, Melhem MS, Szeszs MW, Corneta EC, Zaitz C. Pityriasis versicolor circinata: isolation of Malassezia sympodialis - Case report. An Bras Dermatol. 2010;85(2):227–228. doi: 10.1590/s0365-05962010000200015. [DOI] [PubMed] [Google Scholar]
- 7.Hamdino M, Saudy AA, El-Shahed LH, Taha M. Identification of Malassezia species isolated from some Malassezia associated skin diseases. J Mycol Med. 2022;32(4):101301. doi: 10.1016/j.mycmed.2022.101301. [DOI] [PubMed] [Google Scholar]
- 8.Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137–141. doi: 10.1111/ijd.12345. [DOI] [PubMed] [Google Scholar]
- 9.Lyakhovitsky A, Shemer A, Amichai B. Molecular analysis of Malassezia species isolated from Israeli patients with pityriasis versicolor. Int J Dermatol. 2013;52(2):231–233. doi: 10.1111/j.1365-4632.2012.05595.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedrosa AF, Lisboa C, Faria-Ramos I, et al. Epidemiology and susceptibility profile to classic antifungals and over-the-counter products of Malassezia clinical isolates from a Portuguese University Hospital: a prospective study. J Med Microbiol. 2019;68(5):778–784. doi: 10.1099/jmm.0.000966. [DOI] [PubMed] [Google Scholar]
- 11.Vest BE, Krauland K. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; [Accessed October 13, 2022]. Malassezia furfur. https://www.ncbi.nlm.nih.gov/books/NBK553091/ [Google Scholar]
- 12.Wang K, Cheng L, Li W, et al. Susceptibilities of Malassezia strains from pityriasis versicolor, Malassezia folliculitis and seborrheic dermatitis to antifungal drugs. Heliyon. 2020;6(6):e04203. doi: 10.1016/j.heliyon.2020.e04203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duy Nguyen B, Thi Thanh Vo H, Dinh Thi Thanh M, et al. Epidemiological characterization of pityriasis versicolor and distribution of Malassezia species among students in Hai Phong city, Vietnam. Curr Med Mycol. 2020;6(2):11–17. doi: 10.18502/CMM.6.2.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogunbiyi AO, George AO. Pityriasis versicolor: current concepts in aetiology and management. Niger Postgrad Med J. 2005;12(3):183–188. [PubMed] [Google Scholar]
- 15.Petry V, Tanhausen F, Weiss L, Milan T, Mezzari A, Weber MB. Identification of Malassezia yeast species isolated from patients with pityriasis versicolor. An Bras Dermatol. 2011;86(4):803–806. doi: 10.1590/s0365-05962011000400032. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Sandoval K, Costa AA, Teixeira Sousa MG, et al. Recurrent and disseminated pityriasis versicolor: a novel clinical form consequent to Malassezia-host interaction? Med Hypotheses. 2017;109:139–144. doi: 10.1016/j.mehy.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Bluhm R, Summerbell R. Pityriasis versicolor. J Eur Acad Dermatol Venereol. 2002;16(1):19–33. doi: 10.1046/j.1468-3083.2002.00378.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurniadi I, Hendra Wijaya W, Timotius KH. Malassezia virulence factors and their role in dermatological disorders. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31(2):65–70. [PubMed] [Google Scholar]
- 19.Leung AKC. Pityriasis versicolor. In: Lang F, editor. The Encyclopedia of Molecular Mechanisms of Disease. Berlin: Springer-Verlag; 2009. pp. 1652–1654. [Google Scholar]
- 20.Aghaei Gharehbolagh S, Kordbacheh P, Hashemi SJ, et al. MGL_3741 gene contributes to pathogenicity of Malassezia globosa in pityriasis versicolor. Mycoses. 2018;61(12):938–944. doi: 10.1111/myc.12840. [DOI] [PubMed] [Google Scholar]
- 21.Alam HS, Ward JM, Davis LS. Generalized tinea versicolor following initiation of ixekizumab therapy. JAAD Case Rep. 2021;18:54–56. doi: 10.1016/j.jdcr.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balestri R, Rech G, Piraccini BM, et al. Pityriasis versicolor during anti-TNF-α monoclonal antibody therapy: therapeutic considerations. Mycoses. 2012;55(5):444–446. doi: 10.1111/j.1439-0507.2012.02170.x. [DOI] [PubMed] [Google Scholar]
- 23.Borelli D, Jacobs PH, Nall L. Tinea versicolor: epidemiologic, clinical, and therapeutic aspects. J Am Acad Dermatol. 1991;25(2 Pt 1):300–305. doi: 10.1016/0190-9622(91)70198-b. [DOI] [PubMed] [Google Scholar]
- 24.Brandi N, Starace M, Alessandrini A, Piraccini BM. Tinea versicolor of the neck as side effect of topical steroids for alopecia areata. J Dermatolog Treat. 2019;30(8):757–759. doi: 10.1080/09546634.2019.1573308. [DOI] [PubMed] [Google Scholar]
- 25.da Fraga CM, de Cássia Birschiner R, Naseri AP, Diniz LM. Influence of systemic corticotherapy on the triggering of pityriasis versicolor. Mycoses. 2014;57(9):565–571. doi: 10.1111/myc.12200. [DOI] [PubMed] [Google Scholar]
- 26.Elmets CA. Management of common superficial fungal infections in patients with AIDS. J Am Acad Dermatol. 1994;31(3 Pt 2):S60–S63. doi: 10.1016/s0190-9622(08)81270-4. [DOI] [PubMed] [Google Scholar]
- 27.Güleç AT, Demirbilek M, Seçkin D, et al. Superficial fungal infections in 102 renal transplant recipients: a case-control study. J Am Acad Dermatol. 2003;49(2):187–192. doi: 10.1067/s0190-9622(03)00861-2. [DOI] [PubMed] [Google Scholar]
- 28.Hafez M, el-Shamy S. Genetic susceptibility in pityriasis versicolor. Dermatologica. 1985;171(2):86–88. doi: 10.1159/000249397. [DOI] [PubMed] [Google Scholar]
- 29.Kilinc F, Akbas A, Sener S, Ergin M, Baran P, Metin A. The effect of tinea versicolor on thiol/disulphide homeostasis. Postepy Dermatol Alergol. 2018;35(3):299–303. doi: 10.5114/ada.2018.76227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaliyadan F, Ashique KT, Jayasree P. Increased incidence of facial pityriasis versicolor in children during the COVID-19 pandemic-A consequence of mask usage? Pediatr Dermatol. 2022;39(5):834–835. doi: 10.1111/pde.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kutlu Ö, Doğan Z, Ekşioğlu HM, Kekilli M. Relationship between helicobacter pylori infection and pityriasis versicolor: can helicobacter pylori infection be a new etiologic factor for pityriasis versicolor? Turk J Med Sci. 2020;50(4):771–775. doi: 10.3906/sag-1910-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy MS, Polsky D, Davidson A, Strober BE. Tinea versicolor associated with etanercept therapy. J Am Acad Dermatol. 2008;58(5 Suppl 1):S99–S100. doi: 10.1016/j.jaad.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Mendez-Tovar LJ. Pathogenesis of dermatophytosis and tinea versicolor. Clin Dermatol. 2010;28(2):185–189. doi: 10.1016/j.clindermatol.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Miotto IZ, De Oliveira WRP. Epidermodysplasia verruciformis: report of two patients with autosomal dominant inheritance. Dermatol Online J. 2021;27(2) 13030/qt53t469nn. [PubMed] [Google Scholar]
- 35.Renati S, Cukras A, Bigby M. Pityriasis versicolor. BMJ. 2015;350:h1394. doi: 10.1136/bmj.h1394. [DOI] [PubMed] [Google Scholar]
- 36.Schechtman RC, Midgley G, Hay RJ. HIV disease and Malassezia yeasts: a quantitative study of patients presenting with seborrhoeic dermatitis. Br J Dermatol. 1995;133(5):694–698. doi: 10.1111/j.1365-2133.1995.tb02740.x. [DOI] [PubMed] [Google Scholar]
- 37.Charles CR, Sire DJ, Johnson BL, Beidler JG. Hypopigmentation in tinea versicolor: a histochemical and electronmicroscopic study. Int J Dermatol. 1973;12(1):48–58. doi: 10.1111/j.1365-4362.1973.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 38.Galadari I, el Komy M, Mousa A, Hashimoto K, Mehregan AH. Tinea versicolor: histologic and ultrastructural investigation of pigmentary changes. Int J Dermatol. 1992;31(4):253–256. doi: 10.1111/j.1365-4362.1992.tb03565.x. [DOI] [PubMed] [Google Scholar]
- 39.Karaoui R, Bou-Resli M, Al-Zaid NS, Mousa A. Tinea versicolor: ultrastructural studies on hypopigmented and hyperpigmented skin. Dermatologica. 1981;162(2):69–85. doi: 10.1159/000250253. [DOI] [PubMed] [Google Scholar]
- 40.Nazzaro-Porro M, Passi S. Identification of tyrosinase inhibitors in cultures of Pityrosporum. J Invest Dermatol. 1978;71(3):205–208. doi: 10.1111/1523-1747.ep12547184. [DOI] [PubMed] [Google Scholar]
- 41.Aljabre SH, Alzayir AA, Abdulghani M, Osman OO. Pigmentary changes of tinea versicolor in dark-skinned patients. Int J Dermatol. 2001;40(4):273–275. doi: 10.1046/j.1365-4362.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 42.Dotz WI, Henrikson DM, Yu GS, Galey CI. Tinea versicolor: a light and electron microscopic study of hyperpigmented skin. J Am Acad Dermatol. 1985;12(1 Pt 1):37–44. doi: 10.1016/s0190-9622(85)70006-0. [DOI] [PubMed] [Google Scholar]
- 43.Han A, Calcara DA, Stoecker WV, Daly J, Siegel DM, Shell A. Evoked scale sign of tinea versicolor. Arch Dermatol. 2009;145(9):1078. doi: 10.1001/archdermatol.2009.203. [DOI] [PubMed] [Google Scholar]
- 44.Rivard SC. Pityriasis versicolor: avoiding pitfalls in disease diagnosis and therapy. Mil Med. 2013;178(8):904–906. doi: 10.7205/MILMED-D-13-00057. [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Batra R, Bluhm R, Faergemann J. Pityriasis versicolor. Dermatol Clin. 2003;21(3):413–429. v–vi. doi: 10.1016/s0733-8635(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 46.Hellgren L, Vincent J. The incidence of tinea versicolor in central Sweden. J Med Microbiol. 1983;16(4):501–502. doi: 10.1099/00222615-16-4-501. [DOI] [PubMed] [Google Scholar]
- 47.Bélec L, Testa J, Bouree P. Pityriasis versicolor in the Central African Republic: a randomized study of 144 cases. J Med Vet Mycol. 1991;29(5):323–329. doi: 10.1080/02681219180000491. [DOI] [PubMed] [Google Scholar]
- 48.Faergemann J, Fredriksson T. Tinea versicolor with regard to seborrheic dermatitis. An epidemiological investigation. Arch Dermatol. 1979;115(8):966–968. doi: 10.1001/archderm.1979.04010080030017. [DOI] [PubMed] [Google Scholar]
- 49.Ghosh SK, Dey SK, Saha I, Barbhuiya JN, Ghosh A, Roy AK. Pityriasis versicolor: a clinicomycological and epidemiological study from a tertiary care hospital. Indian J Dermatol. 2008;53(4):182–185. doi: 10.4103/0019-5154.44791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He SM, Du WD, Yang S, et al. The genetic epidemiology of tinea versicolor in China. Mycoses. 2008;51(1):55–62. doi: 10.1111/j.1439-0507.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 51.Jena DK, Sengupta S, Dwari BC, Ram MK. Pityriasis versicolor in the pediatric age group. Indian J Dermatol Venereol Leprol. 2005;71(4):259–261. doi: 10.4103/0378-6323.16618. [DOI] [PubMed] [Google Scholar]
- 52.Karakaş M, Turaç-Biçer A, Ilkit M, Durdu M, Seydaoğlu G. Epidemiology of pityriasis versicolor in Adana, Turkey. J Dermatol. 2009;36(7):377–382. doi: 10.1111/j.1346-8138.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 53.Leung AKC, Barankin B. Tinea versicolor in a 69-year-old man: an uncommon finding. Sch J Med Case Rep. 2015;3(10A):993–994. [Google Scholar]
- 54.Rao GS, Kuruvilla M, Kumar P, Vinod V. Clinico-epidermiological studies on tinea versicolor. Indian J Dermatol Venereol Leprol. 2002;68(4):208–209. [PubMed] [Google Scholar]
- 55.Schmidt A. Malassezia furfur: a fungus belonging to the physiological skin flora and its relevance in skin disorders. Cutis. 1997;59(1):21–24. [PubMed] [Google Scholar]
- 56.Abdollahimajd F, Niknezhad N, Niknejad N, Nikvar M. Infantile hypopigmented pityriasis versicolor: two uncommon cases. Turk Pediatri Ars. 2019;54(4):277–280. doi: 10.14744/TurkPediatriArs.2018.62134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Congly H. Pityriasis versicolor in a 3-month-old boy. Can Med Assoc J. 1984;130(7):844–845. [PMC free article] [PubMed] [Google Scholar]
- 58.Jubert E, Martín-Santiago A, Bernardino M, Bauzá A. Neonatal pityriasis versicolor. Pediatr Infect Dis J. 2015;34(3):329–330. doi: 10.1097/INF.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 59.Nanda A, Kaur S, Bhakoo ON, Kaur I, Vaishnavi C. Pityriasis (tinea) versicolor in infancy. Pediatr Dermatol. 1988;5(4):260–262. doi: 10.1111/j.1525-1470.1988.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 60.Wyre HW, Jr, Johnson WT. Neonatal pityriasis versicolor. Arch Dermatol. 1981;117(11):752–753. doi: 10.1001/archderm.117.11.752. [DOI] [PubMed] [Google Scholar]
- 61.Burke RC. Tinea versicolor: susceptibility factors and experimental infection in human beings. J Invest Dermatol. 1961;36:389–402. doi: 10.1038/jid.1961.60. [DOI] [PubMed] [Google Scholar]
- 62.Gupta AK, Kogan N, Batra R. Pityriasis versicolor: a review of pharmacological treatment options. Expert Opin Pharmacother. 2005;6(2):165–178. doi: 10.1517/14656566.6.2.165. [DOI] [PubMed] [Google Scholar]
- 63.Karray M, McKinney WP. StatPearls [Internet] Treasure Island, FL: StatPearls Publishing; [Accessed October 13, 2022]. Tinea versicolor. https://www.ncbi.nlm.nih.gov/books/NBK482500/ [PubMed] [Google Scholar]
- 64.Holliday A, Grider D. Images in clinical medicine. Tinea versicolor. N Engl J Med. 2016;374(10):e11. doi: 10.1056/NEJMicm1501201. [DOI] [PubMed] [Google Scholar]
- 65.Tosti A, Villardita S, Fazzini ML. The parasitic colonization of the horny layer in tinea versicolor. J Invest Dermatol. 1972;59(3):233–237. doi: 10.1111/1523-1747.ep12627257. [DOI] [PubMed] [Google Scholar]
- 66.Hattori M, Ogawa H, Takamori K, Gritiyaranson P, Kotarajaras R. De-(hypo)pigmentation mechanisms of the affected area of pityriasis versicolor. J Dermatol. 1984;11(1):63–66. doi: 10.1111/j.1346-8138.1984.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 67.Chebil W, Haouas N, Chaâbane-Banaoues R, et al. Epidemiology of pityriasis versicolor in Tunisia: clinical features and characterization of Malassezia species. J Mycol Med. 2022;32(2):101246. doi: 10.1016/j.mycmed.2022.101246. [DOI] [PubMed] [Google Scholar]
- 68.Cohen L, Seminario-Vidal L, Lockey RF. Dermatologic problems commonly seen by the allergist/immunologist. J Allergy Clin Immunol Pract. 2020;8(1):102–112. doi: 10.1016/j.jaip.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 69.Tan C, Zhu WY, Min ZS. Blaschkoid pityriasis versicolor. Mycoses. 2010;53(4):366–368. doi: 10.1111/j.1439-0507.2009.01721.x. [DOI] [PubMed] [Google Scholar]
- 70.Terragni L, Lasagni A, Oriani A, Gelmetti C. Pityriasis versicolor in the pediatric age. Pediatr Dermatol. 1991;8(1):9–12. doi: 10.1111/j.1525-1470.1991.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 71.Glazer SD, Zugerman C. Tinea versicolor of the face. Cutis. 1980;26(1):87. [PubMed] [Google Scholar]
- 72.Pontasch MJ, Kyanko ME, Brodell RT. Tinea versicolor of the face in black children in a temperate region. Cutis. 1989;43(1):81–84. [PubMed] [Google Scholar]
- 73.Sandhu K, Kanwar AJ. Extensive pityriasis versicolor of the face. J Dermatol. 2004;31(3):258–259. doi: 10.1111/j.1346-8138.2004.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 74.Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34(7–8):345–347. doi: 10.1111/j.1439-0507.1991.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 75.Romano C, Feci L, Mancianti F, Fimiani M. Perineal and genital pityriasis versicolor due to Malassezia globosa. J Eur Acad Dermatol Venereol. 2015;29(9):1857–1858. doi: 10.1111/jdv.12547. [DOI] [PubMed] [Google Scholar]
- 76.Aljabre SH. Intertriginous lesions in pityriasis versicolor. J Eur Acad Dermatol Venereol. 2003;17(6):659–662. doi: 10.1046/j.1468-3083.2003.00727.x. [DOI] [PubMed] [Google Scholar]
- 77.Aste N, Pau M, Aste N. Pityriasis versicolor on the groin mimicking erythrasma. Mycoses. 2004;47(5–6):249–251. doi: 10.1111/j.1439-0507.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 78.Cinelli E, Vastarella M, Fabbrocini G, Gallo L. Erythrasmoid pityriasis versicolor with exclusive involvement of the pubis and inguinal region. Ital J Dermatol Venerol. 2021;156(Suppl 1 to 6):11–12. doi: 10.23736/S2784-8671.18.06208-9. [DOI] [PubMed] [Google Scholar]
- 79.Day T, Scurry J. Vulvar pityriasis versicolor in an immunocompetent woman. J Low Genit Tract Dis. 2014;18(3):e71–e73. doi: 10.1097/LGT.0b013e3182a874bb. [DOI] [PubMed] [Google Scholar]
- 80.Greco V, Megna M, Luciano MA, Fabbrocini G. Pityriasis versicolor with uncommon localizations. G Ital Dermatol Venereol. 2020;155(6):786–787. doi: 10.23736/S0392-0488.18.06120-5. [DOI] [PubMed] [Google Scholar]
- 81.Huang WW, Tharp MD. A case of tinea versicolor of the eyelids. Pediatr Dermatol. 2013;30(6):e242–e243. doi: 10.1111/j.1525-1470.2012.01753.x. [DOI] [PubMed] [Google Scholar]
- 82.Khaddar RK, Cherif F, Ben Hadid R, Mokni M, Ben Osman A. Penile shaft involvement in pityriasis versicolor. Acta Dermatovenerol Alp Pannonica Adriat. 2008;17(2):86–89. [PubMed] [Google Scholar]
- 83.Nevas J. Tinea versicolor: understanding effective treatment options. Nurse Pract. 2012;37(1):11–13. doi: 10.1097/01.NPR.0000409912.87769.f0. [DOI] [PubMed] [Google Scholar]
- 84.Ryu HW, Cho JW, Lee KS. Pityriasis versicolor on penile shaft in a renal transplant recipient. Ann Dermatol. 2012;24(3):345–347. doi: 10.5021/ad.2012.24.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sárdy M, Korting HC, Ruzicka T, Wolff H. Bilateral areolar and periareolar pityriasis versicolor. J Dtsch Dermatol Ges. 2010;8(8):617–618. doi: 10.1111/j.1610-0387.2010.07348.x. [DOI] [PubMed] [Google Scholar]
- 86.Smith BL, Koestenblatt EK, Weinberg JM. Areolar and periareolar pityriasis versicolor. J Eur Acad Dermatol Venereol. 2004;18(6):740–741. doi: 10.1111/j.1468-3083.2004.01040.x. [DOI] [PubMed] [Google Scholar]
- 87.Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel) 2015;1(1):13–29. doi: 10.3390/jof1010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hudacek KD, Haque MS, Hochberg AL, Cusack CA, Chung CL. An unusual variant of confluent and reticulated papillomatosis masquerading as tinea versicolor. Arch Dermatol. 2012;148(4):505–508. doi: 10.1001/archdermatol.2011.2812. [DOI] [PubMed] [Google Scholar]
- 89.Shi VS, Lio PA. Diagnosis of pityriasis versicolor in paediatrics: the evoked scale sign. Arch Dis Child. 2011;96(4):392–393. doi: 10.1136/adc.2010.200238. [DOI] [PubMed] [Google Scholar]
- 90.McNally B, McGraw T. Picture this... Tinea versicolor. J Spec Oper Med. 2010;10(1):107–110. [PubMed] [Google Scholar]
- 91.Bonifaz A, Gómez-Daza F, Paredes V, Ponce RM. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28(2):140–145. doi: 10.1016/j.clindermatol.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 92.Ferry M, Shedlofsky L, Newman A, Mengesha Y, Blumetti B. Tinea in versicolor: a rare distribution of a common eruption. Cureus. 2020;12(1):e6689. doi: 10.7759/cureus.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rudolph RI, Holzwanger JM. Letter: inverse tinea versicolor. Arch Dermatol. 1975;111(9):1213. doi: 10.1001/archderm.111.9.1213c. [DOI] [PubMed] [Google Scholar]
- 94.Varada S, Dabade T, Loo DS. Uncommon presentations of tinea versicolor. Dermatol Pract Concept. 2014;4(3):93–96. doi: 10.5826/dpc.0403a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haiduk J, Treudler R, Ziemer M. Atrophying tinea versicolor with epidermal atrophy. J Dtsch Dermatol Ges. 2016;14(7):740–743. doi: 10.1111/ddg.12894. [DOI] [PubMed] [Google Scholar]
- 96.Moon SY, Lee WJ, Lee SJ, Kim DW, Jang YH. Pityriasis versicolor atrophicans: is it true atrophy or pseudoatrophy? J Cutan Pathol. 2016;43(2):187–189. doi: 10.1111/cup.12596. [DOI] [PubMed] [Google Scholar]
- 97.Romano C, Maritati E, Ghilardi A, Miracco C, Mancianti F. A case of pityriasis versicolor atrophicans. Mycoses. 2005;48(6):439–441. doi: 10.1111/j.1439-0507.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 98.Tellechea O, Cravo M, Brinca A, Robalo-Cordeiro M. Pityriasis versicolor atrophicans. Eur J Dermatol. 2012;22(2):287–288. doi: 10.1684/ejd.2012.1661. [DOI] [PubMed] [Google Scholar]
- 99.Allegue F, Fachal C, González-Vilas D, Zulaica A. Atrophying pityriasis versicolor. Actas Dermosifiliogr. 2018;109(5):455–457. doi: 10.1016/j.ad.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 100.Cullingham K, Hull PR. Atrophying pityriasis versicolor. CMAJ. 2014;186(10):776. doi: 10.1503/cmaj.131846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crowson AN, Magro CM. Atrophying tinea versicolor: a clinical and histological study of 12 patients. Int J Dermatol. 2003;42(12):928–932. doi: 10.1111/j.1365-4632.2003.02110.x. [DOI] [PubMed] [Google Scholar]
- 102.Levy JM, Magro C. Atrophying pityriasis versicolor as an idiosyncratic T cell-mediated response to Malassezia: a case series. J Am Acad Dermatol. 2017;76(4):730–735. doi: 10.1016/j.jaad.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 103.Marinello E, Piaserico S, Alaibac M. Atrophic pityriasis versicolor occurring in a patient with Sjögren’s syndrome. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218108. bcr2016218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang YS, Shin MK, Haw CR. Atrophying pityriasis versicolor: is this a new variant of pityriasis versicolor? Ann Dermatol. 2010;22(4):456–469. doi: 10.5021/ad.2010.22.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baz K, Kokturk A, Kaya TI, Ikizoglu G, Yazici AC, Bocekli E. Confetti-like pityriasis versicolor. J Eur Acad Dermatol Venereol. 2004;18(2):240–241. doi: 10.1111/j.1468-3083.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 106.Nabatian AS, Millett CR, Heymann WR. What is your diagnosis? Folliculocentric tinea versicolor. Cutis. 2012;90(3):113, 117–118. [PubMed] [Google Scholar]
- 107.Hudson A, Carroll B, Kim SJ. Folliculocentric tinea versicolor. Dermatol Online J. 2017;23(2) doi: 10.5070/D3232033966. [DOI] [PubMed] [Google Scholar]
- 108.VanDersarl JV, Arnold WL. “Acid skin” in black patients. Arch Dermatol. 1983;119(8):627. doi: 10.1001/archderm.119.8.627b. [DOI] [PubMed] [Google Scholar]
- 109.Al-Refu K. Dermoscopy is a new diagnostic tool in diagnosis of common hypopigmented macular disease: a descriptive study. Dermatol Reports. 2018;11(1):7916. doi: 10.4081/dr.2018.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kapadia F, Kharkar V, Vishwanath T. Dermoscopy to the rescue in an annular enigma: a rare case of annular pityriasis versicolor presenting in an unusual location. Dermatol Pract Concept. 2022;12(2):e2022057. doi: 10.5826/dpc.1202a57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaur I, Jakhar D, Singal A. Dermoscopy in the evaluation of pityriasis versicolor: a cross sectional study. Indian Dermatol Online J. 2019;10(6):682–685. doi: 10.4103/idoj.IDOJ_502_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mathur M, Acharya P, Karki A, Kc N, Shah J. Dermoscopic pattern of pityriasis versicolor. Clin Cosmet Investig Dermatol. 2019;12:303–309. doi: 10.2147/CCID.S195166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas N, Malakar S. Dermoscopy: an easy way to solve the diagnostic puzzle in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2019;85(6):664–665. doi: 10.4103/ijdvl.IJDVL_816_16. [DOI] [PubMed] [Google Scholar]
- 114.Zhou H, Tang XH, De Han J, Chen MK. Dermoscopy as an ancillary tool for the diagnosis of pityriasis versicolor. J Am Acad Dermatol. 2015;73(6):e205–e206. doi: 10.1016/j.jaad.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 115.Lim SL, Lim CS. New contrast stain for the rapid diagnosis of pityriasis versicolor. Arch Dermatol. 2008;144(8):1058–1059. doi: 10.1001/archderm.144.8.1058. [DOI] [PubMed] [Google Scholar]
- 116.Lodha N, Poojary SA. A novel contrast stain for the rapid diagnosis of pityriasis versicolor: a comparison of Chicago Sky Blue 6B Stain, potassium hydroxide mount and culture. Indian J Dermatol. 2015;60(4):340–344. doi: 10.4103/0019-5154.160476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58(6):e125–e126. doi: 10.1111/ijd.14439. [DOI] [PubMed] [Google Scholar]
- 118.Jadotte YT, Janniger CK. Pityriasis alba revisited: perspectives on an enigmatic disorder of childhood. Cutis. 2011;87(2):66–72. [PubMed] [Google Scholar]
- 119.Leung AK, Robson WL. Tuberous sclerosis complex: a review. J Pediatr Health Care. 2007;21(2):108–114. doi: 10.1016/j.pedhc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 120.Leung AKC, Lam JM, Leong KF, Hon KL. Vitiligo: an updated narrative review. Curr Pediatr Rev. 2021;17(2):76–91. doi: 10.2174/1573396316666201210125858. [DOI] [PubMed] [Google Scholar]
- 121.Massone C, Cavalchini A, Clapasson A, Nunzi E. Hypopigmented macules: leprosy, atopy or pityriasis versicolor? G Ital Dermatol Venereol. 2010;145(6):779–782. [PubMed] [Google Scholar]
- 122.Qualia CM, Brown MR, Leung AK, et al. Index of suspicion. Pediatr Rev. 2007;28(5):193–198. doi: 10.1542/pir.28-5-193. [DOI] [PubMed] [Google Scholar]
- 123.Saleem MD, Oussedik E, Picardo M, Schoch JJ. Acquired disorders with hypopigmentation: a clinical approach to diagnosis and treatment. J Am Acad Dermatol. 2019;80(5):1233–1250.e10. doi: 10.1016/j.jaad.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 124.Yang S, Makredes M, O’Donnell P, Levin NA. A case of Hansen disease presenting as tinea versicolor. Dermatol Online J. 2013;19(4):18176. doi: 10.5070/D365H88318. [DOI] [PubMed] [Google Scholar]
- 125.Aste N, Pau M, Aste N, Biggio P. Case report. Pityriasis versicolor mimicking pityriasis rotunda. Mycoses. 2002;45(3–4):126–128. doi: 10.1046/j.1439-0507.2002.00735.x. [DOI] [PubMed] [Google Scholar]
- 126.Berry M, Khachemoune A. Extensive tinea versicolor mimicking Pityriasis rubra pilaris. J Drugs Dermatol. 2009;8(5):490–491. [PubMed] [Google Scholar]
- 127.Bossini B, Mazzolai M, Berti I, Barbi E. Hyperpigmented pityriasis versicolor misdiagnosed as acanthosis nigricans. Arch Dis Child. 2022;107(1):92. doi: 10.1136/archdischild-2021-322030. [DOI] [PubMed] [Google Scholar]
- 128.Darling MJ, Lambiase MC, Young RJ. Tinea versicolor mimicking pityriasis rubra pilaris. Cutis. 2005;75(5):265–267. [PubMed] [Google Scholar]
- 129.Ena P, Siddi GM. Pityriasis versicolor resembling pityriasis rotunda. J Eur Acad Dermatol Venereol. 2002;16(1):85–87. doi: 10.1046/j.1468-3083.2002.373_5.x. [DOI] [PubMed] [Google Scholar]
- 130.Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12(9):e10733. doi: 10.7759/cureus.10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leung AKC, Barankin B, Lam JM. Terra firma-forme dermatosis. J Pediatr. 2018;195:302–303. doi: 10.1016/j.jpeds.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 132.Leung AKC, Leong KF, Lam JM. Tinea imbricata. J Pediatr. 2018;200:285–285.e1. doi: 10.1016/j.jpeds.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 133.Leung AKC, Leong KF, Lam JM. Tinea imbricata: an overview. Curr Pediatr Rev. 2019;15(3):170–174. doi: 10.2174/1573396315666190207151941. [DOI] [PubMed] [Google Scholar]
- 134.Leung AK, Lam JM, Leong KF, Hon KL. Tinea corporis: an updated review. Drugs Context. 2020;9 doi: 10.7573/dic.2020-5-6. 2020-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leung AKC, Lam JM, Leong KF, Hon KL. Vitiligo: an updated narrative review. Curr Pediatr Rev. 2021;17(2):76–91. doi: 10.2174/1573396316666201210125858. [DOI] [PubMed] [Google Scholar]
- 136.Leung AKC, Lam JM, Leong KF, Hon KL. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17(3):201–211. doi: 10.2174/1573396316666200923161330. [DOI] [PubMed] [Google Scholar]
- 137.Plensdorf S, Livieratos M, Dada N. Pigmentation disorders: diagnosis and management. Am Fam Physician. 2017;96(12):797–804. [PubMed] [Google Scholar]
- 138.Zhang B, Xing H, Rui H, Song L, Ma L. Epidermodysplasia verruciformis mimicking pityriasis versicolor. Pediatr Investig. 2021;5(4):325–326. doi: 10.1002/ped4.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mayser P, Rieche I. Rapid reversal of hyperpigmentation in pityriasis versicolor upon short-term topical cycloserine application. Mycoses. 2009;52(6):541–543. doi: 10.1111/j.1439-0507.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 140.Mostafa WZ, Assaf MI, Ameen IA, El Safoury OS, Al Sulh SA. Hair loss in pityriasis versicolor lesions: a descriptive clinicopathological study. J Am Acad Dermatol. 2013;69(1):e19–e23. doi: 10.1016/j.jaad.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 141.Gupta AK, Lyons DC. Pityriasis versicolor: an update on pharmacological treatment options. Expert Opin Pharmacother. 2014;15(12):1707–1713. doi: 10.1517/14656566.2014.931373. [DOI] [PubMed] [Google Scholar]
- 142.Gupta AK, Lane D, Paquet M. Systematic review of systemic treatments for tinea versicolor and evidence-based dosing regimen recommendations. J Cutan Med Surg. 2014;18(2):79–90. doi: 10.2310/7750.2013.13062. [DOI] [PubMed] [Google Scholar]
- 143.Pantazidou A, Tebruegge M. Recurrent tinea versicolor: treatment with itraconazole or fluconazole? Arch Dis Child. 2007;92(11):1040–1042. doi: 10.1136/adc.2007.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dyląg M, Leniak E, Gnat S, Szepietowski JC, Kozubowski L. A case of anti-pityriasis versicolor therapy that preserves healthy mycobiome. BMC Dermatol. 2020;20:9. doi: 10.1186/s12895-020-00106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Framil VM, Melhem MS, Szeszs MW, Zaitz C. New aspects in the clinical course of pityriasis versicolor. An Bras Dermatol. 2011;86(6):1135–1140. doi: 10.1590/s0365-05962011000600011. [DOI] [PubMed] [Google Scholar]
- 146.Faergemann J. Pityrosporum species as a cause of allergy and infection. Allergy. 1999;54(5):413–419. doi: 10.1034/j.1398-9995.1999.00089.x. [DOI] [PubMed] [Google Scholar]
- 147.Hu SW, Bigby M. Pityriasis versicolor: a systematic review of interventions. Arch Dermatol. 2010;146(10):1132–1140. doi: 10.1001/archdermatol.2010.259. [DOI] [PubMed] [Google Scholar]
- 148.Helou J, Obeid G, Moutran R, Maatouk I. Pityriasis versicolor: a case of resistance to treatment. Int J Dermatol. 2014;53(2):e114–e116. doi: 10.1111/j.1365-4632.2012.05689.x. [DOI] [PubMed] [Google Scholar]
- 149.Alchorne MM, Paschoalick RC, Forjaz MH. Comparative study of tioconazole and clotrimazole in the treatment of tinea versicolor. Clin Ther. 1987;9(4):360–367. [PubMed] [Google Scholar]
- 150.Aste N, Pau M, Cordaro CI, Biggio P. Double-blind study with fenticonazole or bifonazole lotions in pityriasis versicolor. Int J Clin Pharmacol Res. 1988;8(4):271–273. [PubMed] [Google Scholar]
- 151.Balwada RP, Jain VK, Dayal S. A double-blind comparison of 2% ketoconazole and 1% clotrimazole in the treatment of pityriasis versicolor. Indian J Dermatol Venereol Leprol. 1996;62(5):298–300. [PubMed] [Google Scholar]
- 152.Cantrell WC, Elewksi BE. Can pityriasis versicolor be treated with 2% ketoconazole foam? J Drugs Dermatol. 2014;13(7):855–859. [PubMed] [Google Scholar]
- 153.Clayton R, Du Vivier A, Savage M. Double-blind trial of 1% clotrimazole cream and Whitfield ointment in the treatment of pityriasis versicolor. Arch Dermatol. 1977;113(6):849–850. doi: 10.1001/archderm.1977.01640060145029. [DOI] [PubMed] [Google Scholar]
- 154.del Palacio Hernanz A, López Gómez S, Iglesias Diez L. Short course bifonazole therapy in pityriasis versicolor. Clin Exp Dermatol. 1987;12(4):270–272. doi: 10.1111/j.1365-2230.1987.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 155.Di Fonzo EM, Martini P, Mazzatenta C, Lotti L, Alvino S. Comparative efficacy and tolerability of Ketomousse (ketoconazole foam 1%) and ketoconazole cream 2% in the treatment of pityriasis versicolor: results of a prospective, multicentre, randomised study. Mycoses. 2008;51(6):532–535. doi: 10.1111/j.1439-0507.2008.01508.x. [DOI] [PubMed] [Google Scholar]
- 156.El-Housiny S, Shams Eldeen MA, El-Attar YA, et al. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv. 2018;25(1):78–90. doi: 10.1080/10717544.2017.1413444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gobbato AA, Babadópulos T, Gobbato CA, Ilha Jde O, Gagliano-Jucá T, De Nucci G. A randomized double-blind, non-inferiority Phase II trial, comparing dapaconazole tosylate 2% cream with ketoconazole 2% cream in the treatment of pityriasis versicolor. Expert Opin Investig Drugs. 2015;24(11):1399–1407. doi: 10.1517/13543784.2015.1083009. [DOI] [PubMed] [Google Scholar]
- 158.Hay RJ, Adriaans B, Midgley G, English JS, Zachary CB. A single application of bifonazole 1% lotion in pityriasis versicolor. Clin Exp Dermatol. 1987;12(4):315. doi: 10.1111/j.1365-2230.1987.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 159.Haroon TS, Tareen MI, Hafiz A, Rizwana An open study of tioconazole 1% dermal cream in patients with pityriasis versicolor. J Pak Med Assoc. 1984;34(12):361–362. [PubMed] [Google Scholar]
- 160.Rigopoulos D, Katsambas A, Antoniou C, Polydorou D, Vlachou M, Stratigos J. Tinea versicolor treated with fluconazole shampoo. Int J Dermatol. 1992;31(9):664–665. doi: 10.1111/j.1365-4362.1992.tb03992.x. [DOI] [PubMed] [Google Scholar]
- 161.Sarkar S, Sengupta D, Basak S, Damji SA, Shukla DK, Anurag D. Comparative assessment of the efficacy of topical ketoconazole and topical luliconazole in cases of pityriasis versicolor at a tertiary care hospital in eastern India: a prospective, open, randomized controlled trial. Indian Dermatol Online J. 2016;7(4):335–336. doi: 10.4103/2229-5178.185471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Savin RC, Horwitz SN. Double-blind comparison of 2% ketoconazole cream and placebo in the treatment of tinea versicolor. J Am Acad Dermatol. 1986;15(3):500–503. doi: 10.1016/s0190-9622(86)70200-4. [DOI] [PubMed] [Google Scholar]
- 163.Sharma J, Kaushal J, Aggarwal K. A comparative study of efficacy and safety of eberconazole versus terbinafine in patients of tinea versicolor. Indian J Dermatol. 2018;63(1):53–56. doi: 10.4103/ijd.IJD_126_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spiekermann PH, Young MD. Clinical evaluation of clotrimazole. A broad-spectrum antifungal agent. Arch Dermatol. 1976;112(3):350–352. doi: 10.1001/archderm.112.3.350. [DOI] [PubMed] [Google Scholar]
- 165.Vicik GJ, Mendiones M, Quinones CA, Thorne EG. A new treatment for tinea versicolor using econazole nitrate 1.0 percent cream once a day. Cutis. 1984;33(6):570–571. [PubMed] [Google Scholar]
- 166.Choi FD, Juhasz MLW, Atanaskova Mesinkovska N. Topical ketoconazole: a systematic review of current dermatological applications and future developments. J Dermatolog Treat. 2019;30(8):760–771. doi: 10.1080/09546634.2019.1573309. [DOI] [PubMed] [Google Scholar]
- 167.Elewski BE. Mechanisms of action of systemic antifungal agents. J Am Acad Dermatol. 1993;28(5 Pt 1):S28–S34. doi: 10.1016/s0190-9622(09)80305-8. [DOI] [PubMed] [Google Scholar]
- 168.Beetens JR, Loots W, Somers Y, Coene MC, De Clerck F. Ketoconazole inhibits the biosynthesis of leukotrienes in vitro and in vivo. Biochem Pharmacol. 1986;35(6):883–891. doi: 10.1016/0006-2952(86)90072-9. [DOI] [PubMed] [Google Scholar]
- 169.De Pedrini P, Rapisarda R, Spanò G. The effect of ketoconazole on sebum secretion in patients suffering from acne and seborrhoea. Int J Tissue React. 1988;10(2):111–113. [PubMed] [Google Scholar]
- 170.Van Cutsem J, Van Gerven F, Cauwenbergh G, Odds F, Janssen PA. The antiinflammatory effects of ketoconazole. A comparative study with hydrocortisone acetate in a model using living and killed Staphylococcus aureus on the skin of guinea-pigs. J Am Acad Dermatol. 1991;25(2 Pt 1):257–261. doi: 10.1016/0190-9622(91)70192-5. [DOI] [PubMed] [Google Scholar]
- 171.Bakr E, Abdo H, Abd-Elaziz H, Abd-Elrazek H, Amer M. Adapalene gel 0.1% vs ketoconazole cream 2% and their combination in treatment of pityriasis versicolor: a randomized clinical study. Dermatol Ther. 2020;33(3):e13319. doi: 10.1111/dth.13319. [DOI] [PubMed] [Google Scholar]
- 172.Shi TW, Zhang JA, Tang YB, Yu HX, Li ZG, Yu JB. A randomized controlled trial of combination treatment with ketoconazole 2% cream and adapalene 0.1% gel in pityriasis versicolor. J Dermatolog Treat. 2015;26(2):143–146. doi: 10.3109/09546634.2014.921661. [DOI] [PubMed] [Google Scholar]
- 173.Bseiso EA, Nasr M, Sammour O, Abd El Gawad NA. Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol. 2015;81(5):457–463. doi: 10.4103/0378-6323.162328. [DOI] [PubMed] [Google Scholar]
- 174.Deng P, Teng F, Zhou F, et al. Y-shaped methoxy poly (ethylene glycol)-block-poly (epsilon-caprolactone)-based micelles for skin delivery of ketoconazole: in vitro study and in vivo evaluation. Mater Sci Eng C Mater Biol Appl. 2017;78:296–304. doi: 10.1016/j.msec.2017.04.089. [DOI] [PubMed] [Google Scholar]
- 175.Patel MR, Patel RB, Parikh JR, Solanki AB, Patel BG. Investigating effect of microemulsion components: in vitro permeation of ketoconazole. Pharm Dev Technol. 2011;16(3):250–258. doi: 10.3109/10837451003610845. [DOI] [PubMed] [Google Scholar]
- 176.Souto EB, Müller RH. SLN and NLC for topical delivery of ketoconazole. J Microencapsul. 2005;22(5):501–510. doi: 10.1080/02652040500162436. [DOI] [PubMed] [Google Scholar]
- 177.Lamie C, Elmowafy E, Ragaie MH, Attia DA, Mortada ND. Assessment of antifungal efficacy of itraconazole loaded aspasomal cream: comparative clinical study. Drug Deliv. 2022;29(1):1345–1357. doi: 10.1080/10717544.2022.2067601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aste N, Pau M, Pinna AL, Colombo MD, Biggio P. Clinical efficacy and tolerability of terbinafine in patients with pityriasis versicolor. Mycoses. 1991;34(7–8):353–357. doi: 10.1111/j.1439-0507.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 179.Faergemann J, Hersle K, Nordin P. Pityriasis versicolor: clinical experience with Lamisil cream and Lamisil DermGel. Dermatology. 1997;194(Suppl 1):19–21. doi: 10.1159/000246178. [DOI] [PubMed] [Google Scholar]
- 180.Savin R, Eisen D, Fradin MS, Lebwohl M. Tinea versicolor treated with terbinafine 1% solution. Int J Dermatol. 1999;38(11):863–865. doi: 10.1046/j.1365-4362.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 181.Vermeer BJ, Staats CC. The efficacy of a topical application of terbinafine 1% solution in subjects with pityriasis versicolor: a placebo-controlled study. Dermatology. 1997;194(Suppl 1):22–24. doi: 10.1159/000246179. [DOI] [PubMed] [Google Scholar]
- 182.Rad F, Aala F, Reshadmanesh N, Yaghmaie R. Randomized comparative clinical trial of Artemisia sieberi 5% lotion and clotrimazole 1% lotion for the treatment of pityriasis versicolor. Indian J Dermatol. 2008;53(3):115–118. doi: 10.4103/0019-5154.43209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chopra V, Jain VK. Comparative study of topical terbinafine and topical ketoconazole in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2000;66(6):299–300. [PubMed] [Google Scholar]
- 184.Del Rosso JQ, Kircik LH. Optimizing topical antifungal therapy for superficial cutaneous fungal infections: focus on topical naftifine for cutaneous dermatophytosis. J Drugs Dermatol. 2013;12(Suppl 11):s165–s171. [PubMed] [Google Scholar]
- 185.Albanese G, Giorgetti P, Santagostino L, Di Cintio R, Colombo MD. [Evaluation of the efficacy of a new antimycotic molecule for topical use: naftifine]. G Ital Dermatol Venereol. 1989;124(5):XXXIII–XXXVII. [PubMed] [Google Scholar]
- 186.Gold MH, Bridges T, Avakian E, et al. An open-label study of naftifine hydrochloride 1% gel in the treatment of tinea versicolor. Skinmed. 2011;9(5):283–286. [PubMed] [Google Scholar]
- 187.Hira SK, Abraham MS, Mwinga A, Kamanga J, Schmidt C. Naftifine solution (1%) in the treatment of pityriasis versicolor in Zambia. Mykosen. 1986;29(8):378–381. [PubMed] [Google Scholar]
- 188.Gupta AK. Butenafine: an update of its use in superficial mycoses. Skin Therapy Lett. 2002;7(7):1–2. 5. [PubMed] [Google Scholar]
- 189.Abdul Bari MA. Comparison of superficial mycosis treatment using Butenafine and Bifonazole nitrate clinical efficacy. Glob J Health Sci. 2012;5(1):150–154. doi: 10.5539/gjhs.v5n1p150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Singal A. Butenafine and superficial mycoses: current status. Expert Opin Drug Metab Toxicol. 2008;4(7):999–1005. doi: 10.1517/17425255.4.7.999. [DOI] [PubMed] [Google Scholar]
- 191.Treatment of tinea versicolor with a new antifungal agent, ciclopirox olamine cream 1% Clin Ther. 1985;7(5):574–583. No authors listed. [PubMed] [Google Scholar]
- 192.del Palacio-Hernanz A, Guarro-Artigas J, Figueras-Salvat MJ, Esteban-Moreno J, Lopez-Gomez S. Changes in fungal ultrastructure after short-course ciclopiroxolamine therapy in pityriasis versicolor. Clin Exp Dermatol. 1990;15(2):95–100. doi: 10.1111/j.1365-2230.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 193.Kudriavskaia VM, Rudenko AV, Pyrig LA. [Drug sensitivity of Candida in patients with kidney diseases receiving immunosuppressive therapy]. Antibiot Khimioter. 1989;34(10):769–773. [PubMed] [Google Scholar]
- 194.Gupta AK, Daigle D, Foley KA. Drug safety assessment of oral formulations of ketoconazole. Expert Opin Drug Saf. 2015;14(2):325–334. doi: 10.1517/14740338.2015.983071. [DOI] [PubMed] [Google Scholar]
- 195.Hersle K. Selenium sulphide treatment of tinea versicolor. Acta Derm Venereol. 1971;51(6):476–478. [PubMed] [Google Scholar]
- 196.Sánchez JL, Torres VM. Double-blind efficacy study of selenium sulfide in tinea versicolor. J Am Acad Dermatol. 1984 Aug;11(2 Pt 1):235–238. doi: 10.1016/s0190-9622(84)70155-1. [DOI] [PubMed] [Google Scholar]
- 197.Sánchez JL, Torres VM. Selenium sulfide in tinea versicolor: blood and urine levels. J Am Acad Dermatol. 1984;11(2 Pt 1):238–241. doi: 10.1016/s0190-9622(84)70156-3. [DOI] [PubMed] [Google Scholar]
- 198.Chu AC. Comparative clinical trial of bifonazole solution versus selenium sulphide shampoo in the treatment of pityriasis versicolor. Dermatologica. 1984;169(Suppl 1):81–86. doi: 10.1159/000249644. [DOI] [PubMed] [Google Scholar]
- 199.Katsambas A, Rigopoulos D, Antoniou C, Zachari A, Fragouli E, Stratigos J. Econazole 1% shampoo versus selenium in the treatment of tinea versicolor: a single-blind randomized clinical study. Int J Dermatol. 1996;35(9):667–668. doi: 10.1111/j.1365-4362.1996.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 200.Faergemann J, Fredriksson T. An open trial of the effect of a zinc pyrithione shampoo in tinea versicolor. Cutis. 1980;25(6):667, 669. [PubMed] [Google Scholar]
- 201.Fredriksson T, Faergemann J. Double-blind comparison of a zinc pyrithione shampoo and its shampoo base in the treatment of tinea versicolor. Cutis. 1983;31(4):436–437. [PubMed] [Google Scholar]
- 202.Faergemann J, Fredriksson T. Propylene glycol in the treatment of tinea versicolor. Acta Derm Venereol. 1980;60(1):92–93. [PubMed] [Google Scholar]
- 203.Clayton YM, Connor BL. Comparison of clotrimazole cream, Whitfield’s ointment and Nystatin ointment for the topical treatment of ringworm infections, pityriasis versicolor, erythrasma and candidiasis. Br J Dermatol. 1973;89(3):297–303. doi: 10.1111/j.1365-2133.1973.tb02978.x. [DOI] [PubMed] [Google Scholar]
- 204.Bamford JT. Editorial: tinea versicolor treatment. Arch Dermatol. 1974;110(6):956. doi: 10.1001/archderm.1974.01630120088025. [DOI] [PubMed] [Google Scholar]
- 205.Bamford JT. Treatment of tinea versicolor with sulfur-salicylic shampoo. J Am Acad Dermatol. 1983;8(2):211–213. doi: 10.1016/s0190-9622(83)70026-5. [DOI] [PubMed] [Google Scholar]
- 206.Faergemann J, Fredriksson T. Antimycotic activity of propane-1,2-diol (propylene glycol) Sabouraudia. 1980;18(3):163–166. doi: 10.1080/00362178085380301. [DOI] [PubMed] [Google Scholar]
- 207.Kligman AM, Leyden JJ, Stewart R. New uses for benzoyl peroxide: a broad-spectrum antimicrobial agent. Int J Dermatol. 1977;16(5):413–417. doi: 10.1111/j.1365-4362.1977.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 208.Prestia AE. Topical benzoyl peroxide for the treatment of tinea versicolor. J Am Acad Dermatol. 1983;9(2):277–278. doi: 10.1016/s0190-9622(83)80152-2. [DOI] [PubMed] [Google Scholar]
- 209.Nagpal VB, Jain VK, Aggarwal K. Comparative study of oral and topical ketoconazole therapy in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2003;69(4):287–288. [PubMed] [Google Scholar]
- 210.Hickman JG. A double-blind, randomized, placebo-controlled evaluation of short-term treatment with oral itraconazole in patients with tinea versicolor. J Am Acad Dermatol. 1996;34(5 Pt 1):785–787. doi: 10.1016/s0190-9622(96)90014-6. [DOI] [PubMed] [Google Scholar]
- 211.Karakaş M, Durdu M, Memişoğlu HR. Oral fluconazole in the treatment of tinea versicolor. J Dermatol. 2005;32(1):19–21. doi: 10.1111/j.1346-8138.2005.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 212.Aksoy B. Itraconazole-induced thrombocytopenia. Cutan Ocul Toxicol. 2017;36(3):305–306. doi: 10.1080/15569527.2016.1257995. [DOI] [PubMed] [Google Scholar]
- 213.Lyman CA, Walsh TJ. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs. 1992;44(1):9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 214.Wahab MA, Kamal SB, Shahin MR, et al. Efficacy of itraconazole in the prevention of recurrence of tinea versicolor: a three year follow up. Mymensingh Med J. 2020;29(2):351–356. [PubMed] [Google Scholar]
- 215.Wishart JM. The influence of food on the pharmacokinetics of itraconazole in patients with superficial fungal infection. J Am Acad Dermatol. 1987;17(2 Pt 1):220–223. doi: 10.1016/s0190-9622(87)70194-7. [DOI] [PubMed] [Google Scholar]
- 216.Faergemann J, Gupta AK, Al Mofadi A, Abanami A, Shareaah AA, Marynissen G. Efficacy of itraconazole in the prophylactic treatment of pityriasis (tinea) versicolor. Arch Dermatol. 2002;138(1):69–73. doi: 10.1001/archderm.138.1.69. [DOI] [PubMed] [Google Scholar]
- 217.Cam VT, Van TN, Hau KT, et al. Efficacy of azole antifungal in treatment of pityriasis versicolor. Open Access Maced J Med Sci. 2019;7(2):272–274. doi: 10.3889/oamjms.2019.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Muhammad N, Kamal M, Islam T, Islam N, Shafiquzzaman M. A study to evaluate the efficacy and safety of oral fluconazole in the treatment of tinea versicolor. Mymensingh Med J. 2009;18(1):31–35. [PubMed] [Google Scholar]
- 219.Partap R, Kaur I, Chakrabarti A, Kumar B. Single-dose fluconazole versus itraconazole in pityriasis versicolor. Dermatology. 2004;208(1):55–59. doi: 10.1159/000075047. [DOI] [PubMed] [Google Scholar]
- 220.García Rodríguez LA, Duque A, Castellsague J, Pérez-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48(6):847–852. doi: 10.1046/j.1365-2125.1999.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yan JY, Nie XL, Tao QM, Zhan SY, Zhang YD. Ketoconazole associated hepatotoxicity: a systematic review and meta- analysis. Biomed Environ Sci. 2013;26(7):605–610. doi: 10.3967/0895-3988.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 222.Alberdi E, Gómez C. Successful treatment of Pityriasis Versicolor by photodynamic therapy mediated by methylene blue. Photodermatol Photoimmunol Photomed. 2020;36(4):308–312. doi: 10.1111/phpp.12555. [DOI] [PubMed] [Google Scholar]
- 223.Balevi A, Üstüner P, Kakşi SA, Özdemir M. Narrow-band UV-B phototherapy: an effective and reliable treatment alternative for extensive and recurrent pityriasis versicolor. J Dermatolog Treat. 2018;29(3):252–255. doi: 10.1080/09546634.2017.1364690. [DOI] [PubMed] [Google Scholar]
- 224.Khattab FM, Omran FH. 308-nm excimer laser: a hopeful and optional therapy for pityriasis versicolor. J Dermatolog Treat. 2021;32(7):795–799. doi: 10.1080/09546634.2020.1713972. [DOI] [PubMed] [Google Scholar]
- 225.Kim YJ, Kim YC. Successful treatment of pityriasis versicolor with 5-aminolevulinic acid photodynamic therapy. Arch Dermatol. 2007;143(9):1218–1220. doi: 10.1001/archderm.143.9.1218. [DOI] [PubMed] [Google Scholar]
- 226.Cherniack EP. Bugs as drugs, Part 1: insects: the “new” alternative medicine for the 21st century? Altern Med Rev. 2010;15(2):124–135. [PubMed] [Google Scholar]
- 227.Carmo ES, Pereira Fde O, Cavalcante NM, Gayoso CW, Lima Ede O. Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf - therapeutic pilot study. An Bras Dermatol. 2013;88(3):381–385. doi: 10.1590/abd1806-4841.20131800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mirzaii M, Yaeghoobi M, Afzali M, Amirkhalili N, Mahmoodi M, Sajirani EB. Antifungal activities of quince seed mucilage hydrogel decorated with essential oils of Nigella sativa, Citrus sinensis and Cinnamon verum. Iran J Microbiol. 2021;13(3):352–359. doi: 10.18502/ijm.v13i3.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lone AH, Ahmad T, Anwar M, Sofi G. Clinical efficacy and safety of a pharmacopial polyherbal Unani formulation in pityriasis versicolor: a comparative randomized single-blind study. J Altern Complement Med. 2012;18(10):978–982. doi: 10.1089/acm.2011.0520. [DOI] [PubMed] [Google Scholar]
- 230.Kagisha V, Djang’eing’a RM, Muganga R, et al. Pentas longiflora Oliv. (Rubiaceae), a plant used in the treatment of pityriasis versicolor in Rwanda: chemical composition and standardization of leaves and roots. Fitoterapia. 2021;153:104974. doi: 10.1016/j.fitote.2021.104974. [DOI] [PubMed] [Google Scholar]
- 231.Sherifat KO, Itohan AM, Adeola SO, Adeola KM, Aderemi OL. Antifungal activity of Acalypha wilkesiana: a preliminary study of fungal isolates of clinical significance. Afr J Infect Dis. 2021;16(1):21–30. doi: 10.21010/Ajid.v16i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jowkar F, Jamshidzadeh A, Pakniyat S, Namazi MR. Efficacy of nitric oxide-liberating cream on pityriasis versicolor. J Dermatolog Treat. 2010;21(2):93–96. doi: 10.3109/09546630902887229. [DOI] [PubMed] [Google Scholar]
- 233.Nashwa RK, Ahmed EB, Nemr WA. Comparative study between topically applied irradiated human amniotic membrane in combination with tea tree oil versus topical tioconazole in pityraisis versicolor treatment. Cell Tissue Bank. 2020;21(2):313–320. doi: 10.1007/s10561-020-09824-5. [DOI] [PubMed] [Google Scholar]