Abstract
Introduction
Vascular calcification (VC) is high prevalent and predicts cardiovascular mortality in dialysis patients. The mechanisms are not known clearly. Trimethylamine-N-oxide (TMAO), a gut-microbiota derivate metabolite, is also associated with cardiovascular outcomes in hemodialysis (HD) patients. This study aims to evaluate serum TMAO levels and establish their relation to VC in HD patients.
Methods
Serum TMAO concentrations were measured by high-performance liquid chromatography–mass spectrometry. Vascular calcification was evaluated by abdominal aortic calcification (AAC) scores. Taking the AAC score value 5.5 as the cutoff value, the participants were divided into the high AAC score group and the low AAC score group.
Results
A total of 184 HD patients and 39 healthy controls were enrolled in this cross-sectional study. Serum Ln(TMAO) (the natural logarithm of TMAO) concentrations were significantly higher in HD patients than that of control subjects (1.82 ± 0.62 vs. −1.60 ± 0.77, p < 0.001). Compared with the group with low AAC scores, the HD patients with high AAC scores showed significantly higher serum Ln(TMAO) levels (2.09 ± 0.55 vs. 1.67 ± 0.54, p < 0.001). In the multivariate regression analysis, serum Ln(TMAO), HD vintage, with diabetic mellitus, age and plasma intact parathyroid hormone (iPTH) were independent determinant factors for VC in HD patients.
Conclusions
Higher serum TMAO levels, older age, longer HD vintage, higher plasma iPTH and with diabetes mellitus were independent risk factors for VC in HD patients. The underlying mechanism deserves further investigations and the finding hints at a new target for the treatment of VC.
Keywords: Trimethylamine-N-oxide, abdominal aortic calcification, hemodialysis, vascular calcification, chronic kidney disease, cardiovascular disease
Introduction
Vascular calcification (VC) commonly occurs in patients with end-stage renal disease (ESRD) and significantly contributes to the high cardiovascular morbidity and mortality in dialysis patients [1]. VC is an active, highly regulated, and complex biological process. Medial calcification, not intimal calcification, is highly prevalent and special in patients with ESRD, which is characterized by the differentiation of vascular smooth muscle cells (VSMCs) into osteoblast-like cells [2]. However, the pathogenesis of VC in chronic kidney disease (CKD) state is very complicated and is not fully elucidated.
Presently, it was reported that patients with CKD showed intestinal dysbiosis, an alteration of the gut micro-organism composition and function [3]. Gut dysbiosis was linked to cardiovascular diseases (CVDs), such as hypertension, heart failure in general population [4,5]. Trimethylamine-N-oxide (TMAO) is a small, organic, gut microbiota-derived metabolite, which is emerging as a new and potentially important risk factor for atherosclerosis and CVDs in the general population. To date, there are several studies demonstrating that markedly elevating serum TMAO levels were associated with cardiovascular outcomes in patients receiving dialysis [6–8]. In a United States multicenter study enrolling over 1000 hemodialysis (HD) patients, TMAO concentrations were associated with cardiovascular events [6]. Also in a few relatively smaller studies in China, the positive associations of TMAO levels with cardiovascular, all-cause mortality [8] and the incidence of hospitalization events [9] were reported in HD patients. Although previous studies have observed the relation between serum TMAO and CVD events in dialysis patients, the role of TMAO in the pathogenesis of CVD are still not clear completely. Recently, Lin et al. found that TMAO affected the balance of bone metabolism and accelerated osteoporosis in vitro [10]. VC shares a similar etiology with bone disease in CKD [11].
Thus, we speculate that TMAO may, through increasing VC, contribute to CVD in ESRD patients. In this study, we will investigate the association between serum TMAO and VC in HD patients. Until now, few similar clinical studies have been reported. Based on our study, we want to acquire some clues for the probable therapeutic pathways for VC, such as applying probiotics, in patients with uremia in the future.
Materials and methods
Subjects
This cross-sectional study screened 220 HD patients and enrolled 184 maintenance HD patients and 39 healthy controls in the Peking University Third Hospital between March and May 2019. Patients were eligible for inclusion if they (1) were aged >18 years old and (2) had been on HD for at least 3 months. The exclusion criteria included (1) in the acute phase of infections, heart failure, or some other complications, (2) with significant residual kidney function (urinary urea clearance >1.5 mL/min, according to previous studies [6,12]), and (3) refusal to participate in the study. A flowchart of patient recruitment for the study is shown in Figure 1.
Figure 1.
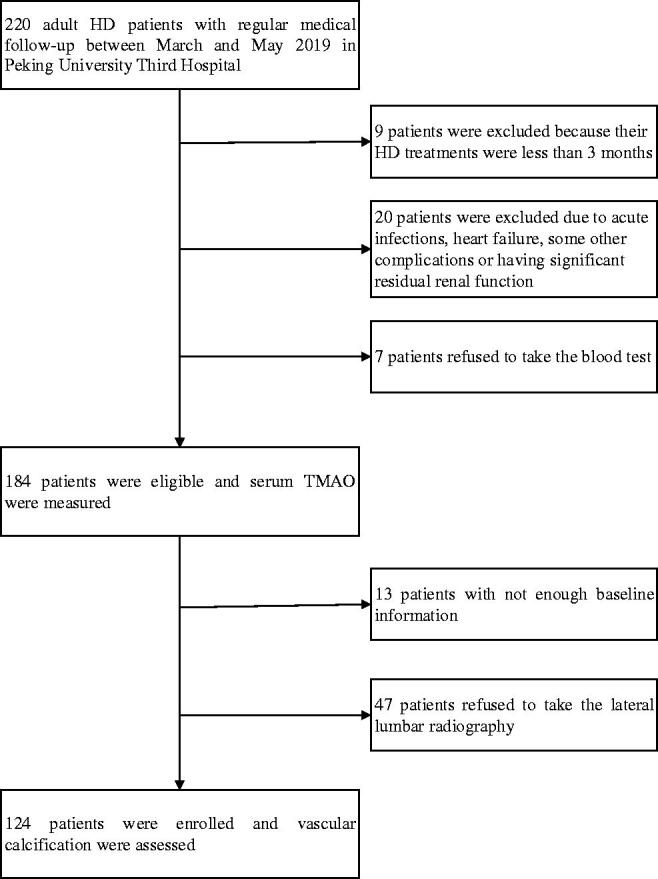
Flowchart of the study. HD: hemodialysis; TMAO: trimethylamine-N-oxide.
The study complied with principles laid down by Declaration of Helsinki and was approved by the Ethics Committee of the Peking University Third Hospital (IRB00006761-M2019003). Written informed consent was provided by all participants. Authors had not access to information that could identify individual participants during or after data collection.
Clinical and biochemical data collection
Data collected included patient demographics such as age, gender, underlying cause of ESRD and HD vintage. Laboratory data collected at baseline included hemoglobin, albumin, corrected calcium, phosphorus, intact parathyroid hormone (iPTH) level, ultrasensitive C-reactive protein (usCRP), and lipid profile. Fractional urea clearance (Kt/V urea) was calculated by the Daugirdas formula.
Measurement of TMAO
Blood samples were collected in vacuum tubes without anticoagulant after fasting for at least 8 h and pre dialysis. Following centrifugation, serum was collected and stored at –80 °C until analysis. Serum TMAO levels were determined using high-performance liquid chromatography–mass spectrometry (HPLC–MS) [13].
Assessment of vascular calcification
Vascular calcification was imaged within 2 weeks of enrollment: abdominal aortic calcification (AAC) by lateral lumbar radiography with Kauppila’s scoring [14]. According to the previous studies [15,16], the point with AAC score of 5.5 was reported to determine the severity of coronary artery disease in the dialysis population. Thus, taking the AAC score value 5.5 as the cutoff value, patients were allocated to the high AAC score group if they had an AAC score of ≥5.5 (moderate or heavy calcification) and the low AAC score group if they had an AAC score of <5.5 (no or minor calcification).
Statistical analysis
Results are expressed as proportions (percentages) for categorical variables, mean ± standard deviation for continuous normally distributed variables, and median with interquartile ranges for continuous non-normally distributed variables. Student’s t-test was used to compare differences between the two groups for normally distributed data, while the Mann–Whitney U-test was used for non-normal data. Categorical data were compared using the Chi-square test. Correlations were expressed as Pearson’s correlation coefficients for two normally distributed variables, and Spearman’s rank correlations were used for non-normally distributed variables. Serum TMAO, the non-normally distributed data, were changed to Ln(TMAO) (the natural logarithm of TMAO) for Pearson’s correlated analysis and Student’s t-test. The multivariate linear regression model (using backward conditional method) was employed to select variables independently related to serum Ln(TMAO). Binary logistic regression analysis (using backward conditional method) was employed to select variables independently related to VC, with all factors related to VC included in the model. Receiver operating characteristic (ROC) curve analysis found the best cutoff value of Ln(TMAO) for VC (a high AAC score ≥ 5.5) prediction. All analyses were two-tailed, and a p < 0.05 was considered statistically significant. SPSS Software, version 16.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
Results
Baseline characteristics of subjects
One hundred and eighty-four HD patients were enrolled according to the inclusion and exclusion criteria. The primary renal diseases were mainly diabetes (50 patients, 27.2%) and chronic glomerulonephritis (61 patients, 33.1%). Other demographic and clinical characteristics of HD patients and 39 healthy controls are shown in Table 1. In HD patients, the serum TMAO concentration was 5.80 (3.96, 9.46) µg/mL, whereas in controls it was 0.18 (0.11, 0.32) µg/mL. Serum Ln(TMAO) were significantly higher in HD patients than that of control subjects (1.82 ± 0.62 vs. −1.60 ± 0.77, p < 0.001). Since age or gender were not matched well between the controls and the patients, in order to adjust age and gender, a multivariate linear regression analysis for Ln(TMAO) was done in all participants (controls and HD patients). Age, gender, different group number (HD patients = 1, controls = 2), and other confounders in Table 1 were included in the analysis. Consequently, different group was independently related to Ln(TMAO) (p < 0.001). It meant that the difference of serum Ln(TMAO) level between HD patients and controls still existed after adjusting age, gender, and some other confounders.
Table 1.
Comparison of clinical parameters between hemodialysis patients and normal controls.
| Characteristics | Hemodialysis patients (n = 184) | Normal controls (n = 39) | t/κ2/Z value | p Value |
|---|---|---|---|---|
| Age (years) | 60.1 ± 14.8 | 44.8 ± 12.6 | 6.002 | <0.001 |
| Gender (male, n, %) | 123,66.8 | 15,38.5 | 10.993 | 0.001 |
| Dialysis vintage (months) | 56.0 (32.6–105.4) | |||
| Hemoglobin (g/L) | 108.2 ± 11.6 | 144.0 ± 15.1 | –13.930 | <0.001 |
| Albumin (g/L) | 40.6 ± 3.4 | 44.8 ± 2.4 | –7.115 | <0.001 |
| usCRP (mg/L) | 2.81 (0.87,6.61) | 0.72 (0.17–2.11) | –3.515 | <0.001 |
| Serum urea (mmol/L) | 26.1 ± 5.6 | 4.7 ± 1.2 | 46.911 | <0.001 |
| Serum creatinine (µmol/L) | 957.9 ± 269.3 | 72.9 ± 12.1 | 44.369 | <0.001 |
| Serum TMAO (µg/mL) | 5.80 (3.96, 9.46) | 0.18 (0.11, 0.32) | –9.782 | <0.001 |
| Ln(TMAO) | 1.82 ± 0.62 | –1.60 ± 0.77 | 29.835 | <0.001 |
usCRP: ultrasensitive C-reactive protein; Ln(TMAO): the natural logarithm of trimethylamine-N-oxide.
Correlation analysis of serum Ln(TMAO) with other parameters in HD patients
Bivariate correlation analysis revealed that serum Ln(TMAO) was positively correlated with dialysis vintage (Spearman’s r = 0.160, p = 0.030), serum urea (Pearson’s r = 0.176, p = 0.017), triglycerides (TGs) (Spearman’s r = 0.272, p < 0.001), and diabetic mellitus (Spearman’s r = 0.168, p = 0.023), while negatively correlated with serum high-density lipoprotein cholesterol (HDL-C) (Pearson’s r=−0.164, p = 0.027). Furthermore, circulating Ln(TMAO) was also positively correlated with AAC scores (Spearman’s r = 0.286, p = 0.002) (see Table 2).
Table 2.
Correlation analysis of serum Ln(TMAO) with other parameters in HD patients (n = 184).
| Variables | r [Ln(TMAO)] | p Value |
|---|---|---|
| Age (years) | 0.092 | 0.214 |
| Gender (male = 1, female = 2) | –0.029 | 0.698 |
| Diabetic mellitus (no = 0, yes = 1) | 0.168 | 0.023 |
| Dialysis vintage (months) | 0.160 | 0.030 |
| Hemoglobin (g/L) | 0.095 | 0.199 |
| Serum albumin (g/L) | 0.116 | 0.116 |
| Serum urea (mmol/L) | 0.176 | 0.017 |
| Serum creatinine (µmol/L) | 0.079 | 0.285 |
| Serum corrected calcium (mmol/L) | 0.072 | 0.329 |
| Serum phosphorus (mmol/L) | 0.087 | 0.239 |
| iPTH (pg/mL) | 0.048 | 0.523 |
| Serum usCRP (mg/L) | 0.102 | 0.176 |
| Serum triglycerides (mmol/L) | 0.272 | <0.001 |
| Serum LDL-C (mmol/L) | 0.084 | 0.260 |
| Serum HDL-C (mmol/L) | –0.164 | 0.027 |
| Kt/V urea | 0.107 | 0.156 |
| AAC score | 0.286 | 0.002 |
Ln(TMAO): the natural logarithm of trimethylamine-N-oxide; HD: hemodialysis; iPTH: intact parathyroid hormone; usCRP: ultrasensitive C-reactive protein; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; Kt/V urea: fractional urea clearance; AAC: abdominal aortic calcification.
In order to remove the impact of confounding factors on Ln(TMAO), multivariate linear regression analysis was done. In the analysis, all the factors related or having the tendency related to Ln(TMAO) in Table 2 were included as candidate variables, such as dialysis vintage, diabetes mellitus, serum urea, serum TG, serum HDL-C, serum usCRP, and serum albumin. At the end, the multivariate regression analysis (backward method) showed that diabetes mellitus, serum urea, and dialysis vintage were independent variables related to serum Ln(TMAO) levels in HD patients (see Table 3).
Table 3.
Independent influencing factors of serum Ln(TMAO) by multivariate linear regression analysis in HD patientsa.
| Variables | First step |
Last step |
||
|---|---|---|---|---|
| B | p Value | B | p Value | |
| Constant | 0.771 | 0.224 | 1.041 | <0.001 |
| Dialysis vintage (months) | 0.003 | 0.002 | 0.003 | 0.001 |
| Diabetic mellitus (no = 0, yes = 1) | 0.285 | 0.005 | 0.309 | 0.002 |
| Serum urea (mmol/L) | 0.017 | 0.047 | 0.019 | 0.018 |
| Serum triglycerides (mmol/L) | 0.027 | 0.414 | Excluded | |
| Serum HDL-C (mmol/L) | –0.139 | 0.355 | Excluded | |
| Serum usCRP(mg/L) | 0.001 | 0.801 | Excluded | |
| Serum albumin (g/L) | 0.010 | 0.485 | Excluded | |
Ln(TMAO): the natural logarithm of trimethylamine-N-oxide; HD: hemodialysis; HDL-C: high-density lipoprotein cholesterol; usCRP: ultrasensitive C-reactive protein; B: partial regression coefficient.
All factors correlated or having the tendency correlated to serum Ln(TMAO) in Table 2, including dialysis vintage, diabetic mellitus, serum urea, triglycerides, HDL-C, usCRP, and serum albumin, were included in the multivariate linear regression model, using backward conditional methods for analysis.
Comparison of serum Ln(TMAO) levels and other established parameters between the high and the low AAC scores group
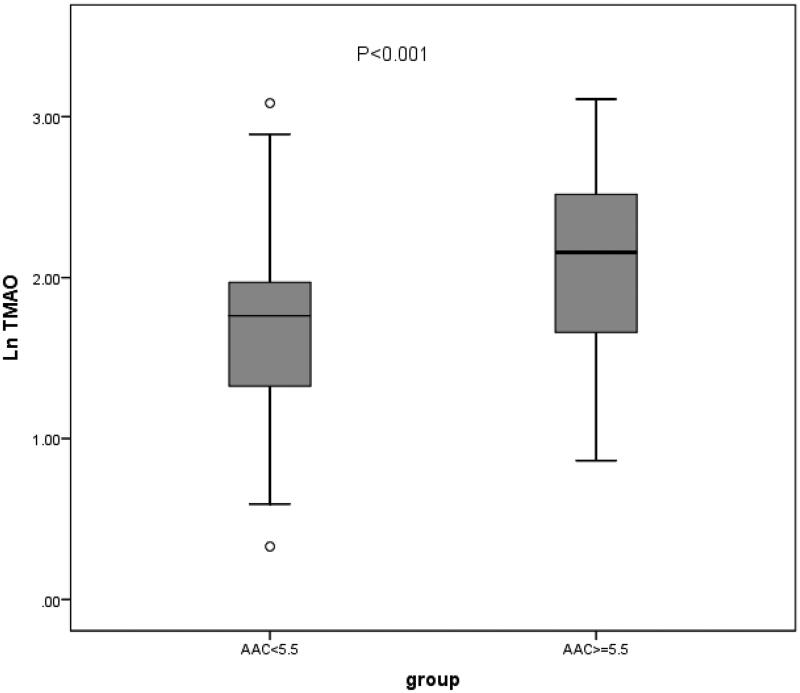
Compared with the low AAC score group, the HD patients with high AAC score showed significantly higher serum Ln(TMAO) levels (2.09 ± 0.55 vs. 1.67 ± 0.54, p < 0.001) (see Table 4 and Figure 2). The HD patients with high AAC score had lower serum creatinine levels than the low AAC score group had (p = 0.002). Meanwhile, there were significantly older age (p < 0.001), longer HD vintage (p < 0.001), higher plasma iPTH levels (p = 0.005), and more diabetes mellitus (p = 0.029) in HD patients with high AAC score than those in the low AAC score group. We can see higher serum usCRP level in the high AAC score group, but it was not statistically significant (p = 0.076). Additionally, there were no differences in serum calcium, phosphate, lipids levels, and gender between the two groups (all p > 0.05) (see Table 4).
Table 4.
Comparison of serum Ln(TMAO) and other parameters between the high AAC score group and the low AAC score group.
| Characteristics | AAC score < 5.5 (n = 67) | AAC score ≥ 5.5 (n = 57) | p Value |
|---|---|---|---|
| Age (years) | 53.3 ± 14.2 | 66.0 ± 11.3 | <0.001 |
| Gender, male, n (%) | 44 (65.7) | 35 (61.4) | 0.622 |
| Dialysis vintage (months) | 53.9 (22.4, 74.3) | 75.2 (50.7, 142.1) | <0.001 |
| Diabetes mellitus, n (%) | 19 (28.4) | 27 (47.4) | 0.029 |
| Hemoglobin (g/L) | 108.6 ± 9.9 | 109.3 ± 11.8 | 0.722 |
| Serum creatinine (µmol/L) | 1044.9 ± 311.8 | 897.5 ± 193.9 | 0.002 |
| Kt/V urea (per week) | 1.28 ± 0.25 | 1.34 ± 0.17 | 0.125 |
| Serum albumin (g/L) | 41.1 ± 3.3 | 40.5 ± 2.9 | 0.351 |
| Serum corrected calcium (mmol/L) | 2.26 ± 0.18 | 2.28 ± 0.21 | 0.655 |
| Serum phosphate (mmol/L) | 1.80 ± 0.52 | 1.77 ± 0.48 | 0.776 |
| Serum usCRP (mg/L) | 2.07 (0.58, 6.56) | 3.39 (1.37, 8.12) | 0.076 |
| Plasma iPTH (pg/mL) | 229.1(112.5, 443.5) | 368.0(212.2, 600.1) | 0.005 |
| Serum CO2CP (mmol/L) | 19.7 ± 3.0 | 19.6 ± 2.2 | 0.844 |
| Serum LDL-C (mmol/L) | 2.02 ± 0.63 | 1.96 ± 0.67 | 0.596 |
| Serum HDL-C (mmol/L) | 1.00 ± 0.34 | 0.96 ± 0.35 | 0.530 |
| Serum triglycerides (mmol/L) | 1.64 (0.92, 2.50) | 1.98 (1.18, 2.89) | 0.123 |
| Serum Ln(TMAO) | 1.67 ± 0.54 | 2.09 ± 0.55 | <0.001 |
| AAC (score) | 2 (0, 3) | 9 (6.5, 13) | <0.001 |
Ln(TMAO): the natural logarithm of trimethylamine-N-oxide; AAC: abdominal aortic calcification; Kt/V urea: fractional urea clearance; usCRP: ultrasensitive C-reactive protein; iPTH: intact parathyroid hormone; CO2CP: carbon dioxide combining power; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol.
Figure 2.
Comparison of serum Ln(TMAO) between the high AAC score group and the low AAC score group. Ln(TMAO): the natural logarithm of trimethylamine-N-oxide; AAC: abdominal aortic calcification.
Independent risk factors of vascular calcification by binary logistic analysis in HD patients
In order to remove the impact of confounding factors on VC, a multivariate logistic regression model was performed to find the independent risk factors of VC in HD patients. The variables which significantly correlated to a high AAC score of ≥5.5 in univariate analysis, including age, dialysis vintage, diabetic mellitus, serum creatinine, plasma iPTH, serum Ln(TMAO), and usCRP which had a tendency of significant association (shown in Table 4) entered the analysis as candidate variables. Finally, age, dialysis vintage, diabetic mellitus, plasma iPTH, and serum Ln(TMAO) were independently associated with a high AAC score in HD patients (all p < 0.05) (see Table 5).
Table 5.
Independent related factors of a high AAC score ≥ 5.5 by multivariate binary logistic analysis in HD patientsa.
| Variables | First step |
Last step |
||||
|---|---|---|---|---|---|---|
| B | p Value | 95% CI for Exp(B)/OR | B | p Value | 95% CI for Exp(B)/OR | |
| Age (years) | 0.093 | 0.002 | 1.035–1.164 | 0.096 | <0.001 | 1.049–1.155 |
| Dialysis vintage (months) | 0.014 | 0.010 | 1.003–1.025 | 0.014 | 0.009 | 1.004–1.025 |
| DM (no = 0, yes = 1, 1 vs. 0) | 1.255 | 0.044 | 1.033–11.910 | 1.318 | 0.026 | 1.172–11.908 |
| Serum creatinine (µmol/L) | –0.0003 | 0.818 | 0.997–1.003 | excluded | ||
| Serum usCRP (mg/L) | –0.013 | 0.692 | 0.926–1.052 | excluded | ||
| Plasma iPTH (pg/mL) | 0.002 | 0.008 | 1.001–1.004 | 0.002 | 0.008 | 1.001–1.004 |
| Serum Ln(TMAO) | 1.261 | 0.014 | 1.296–9.604 | 1.263 | 0.011 | 1.328–9.406 |
| Constant | –10.200 | 0.001 | –10.765 | <0.001 | ||
AAC: abdominal aortic calcification; HD: hemodialysis; DM: diabetes mellitus; usCRP: ultrasensitive C-reactive protein; iPTH: intact parathyroid hormone; Ln(TMAO): the natural logarithm of trimethylamine-N-oxide; B: partial regression coefficient; Exp(B)/OR: odds ratio; CI: confidence interval.
All factors correlated to vascular calcification in Table 4, including age, dialysis vintage, DM, serum creatinine, usCRP, iPTH, and Ln(TMAO), were included in the multivariate logistic regression model, using backward conditional methods for analysis.
ROC curve analysis of serum Ln(TMAO) level and a high AAC score ≥ 5.5 in hemodialysis patients
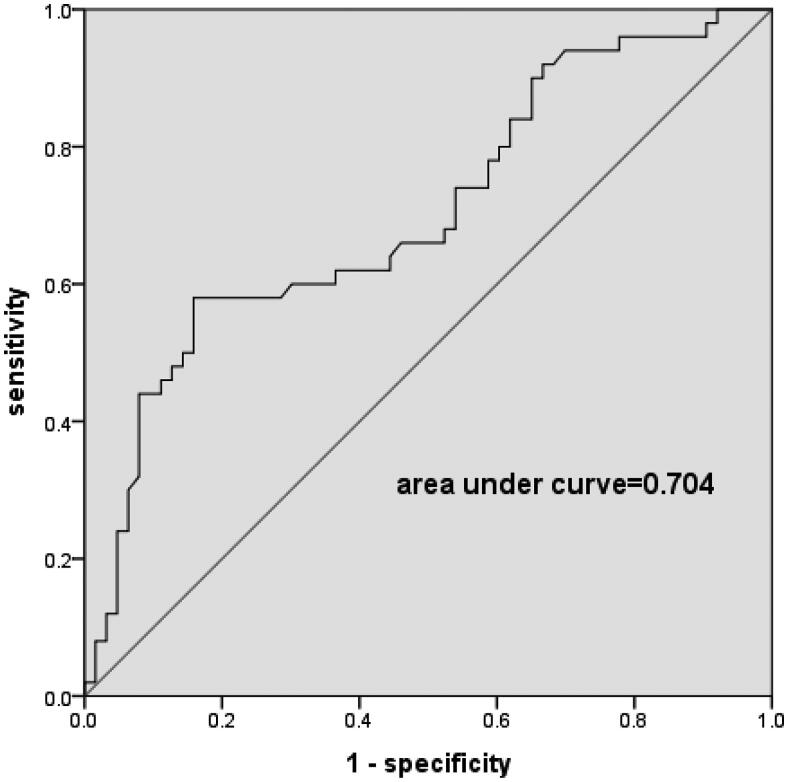
ROC analysis identified the best serum Ln(TMAO) cutoff value for a high AAC score ≥5.5 prediction. It showed that a serum Ln(TMAO) of 2.07 was associated with a high AAC score ≥5.5 with a sensitivity of 0.580 and a specificity of 0.841. The area under curve (AUC) was 0.704 (p < 0.001) (see Figure 3). The Ln(TMAO) value of 2.07 equals the TMAO value of 7.92.
Figure 3.
Receiver operating characteristic (ROC) analysis of serum Ln(TMAO) and the risk of a high AAC score ≥ 5.5 in hemodialysis patients. ROC study showed an area under curve of 0.704 (p < 0.001). A serum Ln(TMAO) greater than 2.07 was associated with a high AAC score ≥ 5.5 occurrence with a sensitivity of 0.580 and a specificity of 0.841. Ln(TMAO): the natural logarithm of trimethylamine-N-oxide; AAC: abdominal aortic calcification.
Discussion
In the current study, our results showed that serum TMAO levels, one of the gut microbiota-derived metabolite, were significantly higher in maintenance HD patients than that in controls. Importantly, we demonstrated for the first time that serum TMAO concentrations were notably higher in the high AAC score group than that in the low AAC score group in HD patients. Moreover, higher serum TMAO was an independent risk factor for VC in HD patients. Thus, these findings supported our hypothesis that through VC, TMAO may contribute to CVD in ESRD patients.
We observed that the circulating TMAO concentrations in HD patients were about 30 times higher than that in healthy controls. It was similar to the data from Shafi et al.’s, Zhang et al.’s, and Tang et al.’s studies [6,8,17]. The mechanisms of a significant increase in circulating TMAO in late-stage CKD could be multifactorial. TMAO is derived primarily from metabolization of trimethylamine (TMA)-containing substrates, such as dietary carnitine, choline, and betaine, through the action of gut microbiota [18]. HD patients had to restrict their diets, take medicine and thus the gut microbiota would alter due to the changed eating habits, all kinds of drugs and uremia state [3]. In addition, TMAO is a low-molecular-weight (75 Da) metabolite, which is excreted in the urine [17]. It also can be removed efficiently like urea during a single HD session. However, it accumulates between HD sessions in HD patients [19]. Hence, both impaired renal function and dysbiosis of the gut microbiota contribute to the elevated serum TMAO levels in HD patients [20]. In this study, the HD patients with significant residual kidney function were excluded to minimize the effect of residual kidney function.
The most important and interesting thing was that we found higher serum TMAO was an independent risk factor for VC in HD patients. It was independent of serum creatinine and dialysis adequacy (Kt/V). As far as we know, it is the first clinical study about the relation between TMAO and AAC in HD patients. Vascular calcification is a special risk factor for high cardiovascular mortality and morbidity of CKD cases [1]. The medial VC promoted by hyperphosphatemia is the key process in CKD patients [2]. TMAO, a gut microbiota-derived metabolite, was reported to relate to adverse cardiovascular events in uremia patients [6]. Our study’s results demonstrated a clinical link between VC and TMAO in HD patients. Further, the underlying mechanisms need to be elucidated. Recently, Zhang et al. [21] revealed that TMAO promoted calcium-induced osteogenic differentiation of VSMCs and also exacerbated VC in rats with CKD, which were involved activation of NLRP3 (nucleotide-binding domain, leucine-rich containing family, pyrin domain-containing-3) inflammasome and nuclear factor κB (NF-κB) signaling pathways. In fact, apart from VSMCs trans-differentiation, VSMCs apoptosis, autophagy, oxidative stress, chronic inflammation, endothelial dysfunction, loss of mineralization inhibitors, release of calcifying extracellular vesicles and multiple signaling pathways are all recognized to drive the VC process now [2,22]. The mechanisms of TMAO promoting VC in CKD deserve to be further studied. Based on these results, changing gut flora and decreasing the TMAO levels maybe a potential therapy for alleviating VC in dialysis patients. However, another clinical study showed that TMAO concentration was not associated with coronary artery calcium incidence in non-CKD young healthy adults [23]. We thought the young healthy adults in that study were very different from HD patients in our study and the results were not comparable between each other. In this study, in consideration of the confounding factor of age, multivariate regression analysis has been done in finding the independent risk factor of VC in HD patients and TMAO was still the risk factor of VC independently.
In addition, by ROC curve analysis, we found serum Ln(TMAO) greater than 2.07 or serum TMAO greater than 7.92 predicted VC (AAC score ≥ 5.5) existence. However, the cutoff value 7.92 of TMAO related to VC needs to be verified in other HD cohort in the future. Previous studies [15,16] reported that the cutoff value 5.5 of AAC score can predict CVD in ESRD patients. Hence in this study, VC group was divided according to the AAC score 5.5. It is also worth further study to clarify whether the cutoff value 7.92 of TMAO can predict the occurrence of CVD in HD patients.
The traditional risk factors of VC in dialysis patients include old age, prolonged dialysis vintage, diabetic mellitus, hyperphosphatemia, high iPTH level, and inflammation [24]. Consistently, our results showed that older age, longer dialysis vintage, with diabetic mellitus and higher iPTH were also independent related factors of AAC in HD patients. However, no significant correlations between VC and serum phosphorus, inflammation were observed in our study, possibly due to the relatively small sample size, the generally well-controlled serum phosphorus or inflammation, and the single serum phosphorus and inflammation biomarker data used in our study.
In general population, diabetes mellitus was associated with higher plasma TMAO levels [25]. Obeid et al. [26] proposed that the elevated plasma TMAO concentrations were likely to reflect a specific metabolic pattern characterized by low high-density lipoprotein (HDL) and phospholipids. Evidence suggested that TMAO played a key regulatory role in glucose and lipid metabolism [25,27]. In HD patients, similar studies were rare. Shafi et al.’s results showed diabetes was associated with higher TMAO concentrations in HD populations [6]. Our present study also revealed the same results and diabetes was an independent factor related to the serum TMAO levels in HD patients. In addition, we found higher serum TGs and lower HDL-C levels were associated with higher TMAO levels in HD patients, although in multivariate linear regression analysis they were not independent influencing factors of TMAO. Hence even in uremia, TMAO still appeared to regulate glucose and lipid homeostasis, which could also act as an important determinant for atherosclerosis and CVD.
We found dialysis vintage was positively associated with serum TMAO independently. Stubbs et al.’s study results also showed a trend across TMAO quintiles, however, without significance statistically [28]. Another study did not found the relation between dialysis vintage and TMAO [6]. It needs further prospective studies to clarify. In our opinion, longer dialysis vintage, more altered gut microbiota and higher serum TMAO level. As everyone knows, dialysis vintage is also a risk factor for VC in dialysis patients [24].
In addition, higher serum urea levels were associated with higher TMAO concentrations independently in our cross-sectional study. It was consistent with the results of Shafi et al.’s study which utilized the data from the Hemodialysis (HEMO) Study, a United States multicenter trial of dialysis dose and flux [6]. Similarly, Stubbs et al.’s study in a subset of the EVOLVE trial also found the higher TMAO quintiles, the higher serum urea concentrations [28]. The relation between TMAO and urea may be explained by the various diets. One of the main precursors of TMA is l-carnitine, an amino acid in red meat, which is also the dietary source for urea. Alternatively, Meersman et al. demonstrated that TMAO could protect living organisms from the protein denaturing effects originated from high levels of urea [29]. Based on these, we can speculate that more protein intake or increased protein catabolism due to dysbiosis of gut microbiota was related to higher urea and TMAO. The processes also meant higher phosphate intake or the inflammation state in HD patients, both of which will accelerate VC [24]. Urea effects on the intestinal epithelium, vascular wall can promote systemic inflammation, VC [30].
Several studies have shown a positive association between the serum concentration of TMAO and inflammation biomarkers, such as usCRP, IL-1β, and so on, in general population or patients with stable angina [31,32]. However, we found no association between TMAO and usCRP in 184 HD patients, consistent with Wilson Tang et al.’s study in 521 CKD patients [17]. In contrast, Missailidis et al.’s study observed a weak positive correlation between TMAO and usCRP, which was disappeared when GFR was taken into account in non-HD CKD patients [7], whereas Kaysen et al. reported a paradoxical inverse association between TMAO and usCRP in HD patients [33]. Though we cannot explain the different outcomes in these studies, it may be related to many confounding factors, such as different inflammation state in those groups, different GFRs, with or without HD patients, relatively small sample sizes and so on. Therefore, the relationship between TMAO and usCRP in CKD patients needs to be further determined in larger samples. It is worth noting that in vitro studies performed in cultured endothelial progenitor cells (EPCs) showed that TMAO promoted cellular inflammation and increased oxidative stress [32].
Our study had several limitations. This was a cross-sectional observational study; thus it cannot provide a causal relationship to the results of the study. The sample size of the study was relatively small and it was a single center study. There were still some confounding factors such as diets, drugs, and so on that may affect the results. Controls were not matched well and AAC score was not measured in control group. TMAO was measured only once and changes in diet and gut microbiome over time may change serum TMAO concentrations.
Conclusions
Our study provided the clinical evidence for the first time that TMAO, the gut microbiota-derived metabolite, was an independent related factor of VC in HD patients. Higher serum TMAO, older age, longer dialysis vintage, higher plasma iPTH and with diabetes mellitus were related to increased AAC in HD patients independently. Serum TMAO were associated with glucose and lipid metabolism in HD group. The underlying mechanism about TMAO and VC is worth to be further explored. These findings may hint at a new therapeutic pathway for VC.
Acknowledgements
The invaluable support of all staff of the HD Center in Peking University Third Hospital is gratefully acknowledged.
Funding Statement
The work was supported by the National Natural Science Foundation (Grant No. 81873619, Grant No. 81570663) to Aihua Zhang, Research Initiation Fund for the Excellent Returned Overseas Scholars of Peking University Third Hospital (Grant No. BYSYLXHG2019007) to Wenling Yang, and the Key Program of Peking University Third Hospital (Grant No. BYSY2018024) to Lian He.
Disclosure statement
The authors have no conflicts of interest to declare.
Data availability statement
All the data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Block GA, Klassen PS, Lazarus JM, et al. . Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Lee I-K, Jeon J-H.. Vascular calcification—new insights into its mechanism. Int J Mol Sci. 2020;21(8):2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaziri ND, Wong J, Pahl M, et al. . Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. [DOI] [PubMed] [Google Scholar]
- 4.Yang T, Santisteban MM, Rodriguez V, et al. . Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasini E, Aquilani R, Testa C, et al. . Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3):220–227. [DOI] [PubMed] [Google Scholar]
- 6.Shafi T, Powe NR, Meyer TW, et al. . Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol. 2017;28(1):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Missailidis C, Hällqvist J, Qureshi AR, et al. . Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLOS One. 2016;11(1):e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Zou JZ, Chen J, et al. . Association of trimethylamine N-oxide with cardiovascular and all-cause mortality in hemodialysis patients. Ren Fail. 2020;42(1):1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, Tang Z, You L, et al. . Trimethylamine-N-oxide is an independent risk factor for hospitalization events in patients receiving maintenance hemodialysis. Ren Fail. 2020;42(1):580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H, Liu T, Li X, et al. . The role of gut microbiota metabolite trimethylamine N-oxide in functional impairment of bone marrow mesenchymal stem cells in osteoporosis disease. Ann Transl Med. 2020;8(16):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreto DV, Barreto FdC, Carvalho ABd, et al. . Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis. 2008;52(6):1139–1150. [DOI] [PubMed] [Google Scholar]
- 12.Eknoyan G, Beck GJ, Cheung AK, et al. . Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. [DOI] [PubMed] [Google Scholar]
- 13.Yang P, Li X, Yang W, et al. . A simple liquid chromatography/differential ion mobility spectrometry tandem mass spectrometry method for the determination of trimethylamine-N-oxide in human serum: an application in dialysis patients. Rapid Commun Mass Spectrom. 2021;35(1):e8965. [DOI] [PubMed] [Google Scholar]
- 14.Kauppila LI, Polak JF, Cupples LA, et al. . New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–250. [DOI] [PubMed] [Google Scholar]
- 15.Chen HC, Wang WT, Hsi CN, et al. . Abdominal aortic calcification score can predict future coronary artery disease in hemodialysis patients: a 5-year prospective cohort study. BMC Nephrol. 2018;19(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen HC, Chou CY, Lin HJ, et al. . The abdominal aortic calcification score predicts the occurrence of coronary artery disease in Middle-aged peritoneal dialysis patients. Nephrology. 2019;24(3):336–340. [DOI] [PubMed] [Google Scholar]
- 17.Wilson Tang WH, Wang Z, Kennedy DJ, et al. . Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho CE, Caudill MA.. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab. 2017;28(2):121–130. [DOI] [PubMed] [Google Scholar]
- 19.Bain MA, Faull R, Fornasini G, et al. . Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21(5):1300–1304. [DOI] [PubMed] [Google Scholar]
- 20.Xu KY, Xia GH, Lu JQ, et al. . Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep. 2017;7(1):1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Yining L, Yang P, et al. . Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler Thromb Vasc Biol. 2020;40(3):751–765. [DOI] [PubMed] [Google Scholar]
- 22.Zununi Vahed S, Mostafavi S, Hosseiniyan Khatibi SM, et al. . Vascular calcification: an important understanding in nephrology. Vasc Health Risk Manag. 2020;16:167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer KA, Benton TZ, Bennett BJ, et al. . Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc. 2016;5:e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Disthabanchong S. Vascular calcification in chronic kidney disease: pathogenesis and clinical implication. World J Nephrol. 2012;1(2):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dambrova M, Latkovskis G, Kuka J, et al. . Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes. 2016;124(4):251–256. [DOI] [PubMed] [Google Scholar]
- 26.Obeid R, Awwad HM, Rabagny Y, et al. . Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103(3):703–711. [DOI] [PubMed] [Google Scholar]
- 27.Janeiro MH, Ramirez MJ, Milagro FI, et al. . Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stubbs JR, Stedman MR, Liu S III, et al. . Trimethylamine N-oxide and cardiovascular outcomes in patients with ESKD receiving maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14(2):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meersman F, Bowron D, Soper AK, et al. . Counteraction of urea by trimethylamine N-oxide is due to direct interaction. Biophys J. 2009;97(9):2559–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau WL, Vaziri ND.. Urea, a true uremic toxin: the empire strikes back. Clin Sci. 2017;131(1):3–12. [DOI] [PubMed] [Google Scholar]
- 31.Rohrmann S, Linseisen J, Allenspach M, et al. . Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J Nutr. 2016;146(2):283–289. [DOI] [PubMed] [Google Scholar]
- 32.Chou RH, Chen CY, Chen IC, et al. . Trimethylamine N-oxide, circulating endothelial progenitor cells, and endothelial function in patients with stable angina. Sci Rep. 2019;9(1):4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaysen GA, Johansen KL, Chertow GM, et al. . Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25(4):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.